Summary
Neurogenesis, the production of new neurons from less differentiated precursor cells, normally occurs in adult brains in the subventricular zone (SVZ) of the lateral ventricles and the subgranular zone of the hippocampal dentate gyrus. Neurogenesis declines with aging. In previous studies, neurogenesis was stimulated by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine hydrochloride (MPTP) in young animals. In this study, we examined the effect of acute MPTP administration and mutant α-synuclein A53T on neurogenesis and migration of newborn neurons in the aged (23-month) vs. young (2-month) rodent brain. Cell proliferation and neurogenesis were assessed via bromodeoxyuridine labeling and immunostaining for cell type-specific markers. In the aged brain, neural precursor cells in the rostral SVZ retained the capacity for proliferation and migration in response to MPTP-induced Parkinsonism, although the response is less robust than in younger animals. Furthermore, in transgenic mice that overexpress mutant α-synuclein (A53T), brains examined day 21 after MPTP administration showed markedly decreased olfactory bulb and substantia nigra neurogenesis. Our data suggest that in addition to aging effects associated with decline in the number of newly generated cells, mutant α-synuclein reduces MPTP-induced neurogenesis. This could provide a novel therapeutic target for chronic brain repair in this condition.
Keywords: Aging, Parkinson’s disease, proliferation, progenitor, substantia nigra, α-synuclein
Introduction
Parkinson’s disease (PD) is a common age-related neurodegenerative disorder that is pathologically characterized by the selective loss of nigrostriatal dopaminergic neurons in the substantia nigra pars compacta (SNpc) region of the ventral midbrain and the presence of ubiquinated protein deposits in residual neurons (Lewy bodies) (Forno, 1996; Lang & Lozano, 1998; Dauer & Przedborski, 2003). It affects approximately 1% of the population by the age of 65 years, increasing to 4–5% of the population by the age of 85 years (Forno, 1996; Lang & Lozano, 1998; Dauer & Przedborski, 2003). Aging is the most prevalent risk factor for the disease.
α-Synuclein (PARK 1) protein is present at high levels in Lewy bodies, intracytoplasmic inclusions that are characteristic of both sporadic and inherited forms of PD (Spillantini et al., 1997). Three point mutations in α-synuclein linked to autosomal dominant early-onset PD have been described, A53T (Polymeropoulos et al., 1997), A30P (Kruger et al., 1998), and E46K (Zarranz et al., 2004). The clinical signs and symptoms are heterogeneous but resemble sporadic PD with an average age of onset in the late 50s and 60s but with a more benign prognosis and less dementia. Post-mortem studies suggest that temporal patterns of α-synuclein-containing Lewy body accumulation within various brain regions track with disease progression and could contribute to both motor and non-motor features of the disorder (Braak et al., 2003). Recent gene-wide association studies have identified common gene polymorphisms in the α-synuclein gene as potential susceptibility factors for idiopathic PD (Simon-Sanchez et al., 2009).
Neurogenesis occurs in select regions of the brain of adult rodents including the rostral subventricular zone (SVZ) of the lateral ventricles (Luskin, 1993) and the subgranular zone (SGZ) of the dentate gyrus (DG) (Kaplan & Hinds, 1977). Slow dividing stem cells (type B cells) in the SVZ give first rise to transit amplifying cells (type C cells), which generate fast dividing neuroblasts (type A cells) that migrate tangentially within glial tubes along the rostral migratory stream (RMS) to the olfactory bulb (OB), where they radially migrate and differentiate into interneurons of the granule cell layer (GCL) and glomerular layer (GL) (Luskin, 1993; Lois et al., 1996). Neurogenesis in the adult brain is found to be increased after injury (Gould & Tanapat, 1997; Snyder et al., 1997). Neurogenesis is increased in PD animal models including following 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine hydrochloride (MPTP) (Kay & Blum, 2000; Mao et al., 2001; Zhao et al., 2003; Peng et al., 2008) and 6-hydroxydopamine (6-OHDA) administration (Fallon et al., 2000; Cooper & Isacson, 2004; Hoglinger et al., 2004; Mohapel et al., 2005; Van Kampen & Eckman, 2006; de Chevigny et al., 2008). Whether the aged brain can continue to respond to a destructive injury with increased neurogenesis in PD is not clear.
In this study, we examined the effect of acute MPTP administration and mutant α-synuclein A53T on neurogenesis and migration of newborn neurons in the aged vs. young rodent brain. Cell proliferation and neurogenesis were assessed via bromodeoxyuridine (BrdU) labeling and immunostaining for cell type-specific markers. In the aged brain, neural precursor cells in the rostral SVZ were found to retain the ability to proliferate and migrate in response to MPTP-induced Parkinsonism, although the response was found to be less robust than in younger animals. Furthermore, in transgenic mice that overexpress mutant α-synuclein (A53T), markedly decreased neurogenesis was observed in the OB and substantia nigra following MPTP treatment. Our data suggest that in addition to aging effects associated with decline in the number of newly generated cells, mutant α-synuclein also reduces MPTP-induced neurogenesis.
Results
Neurogenesis is decreased in the rostral subventricular zone of aged mice
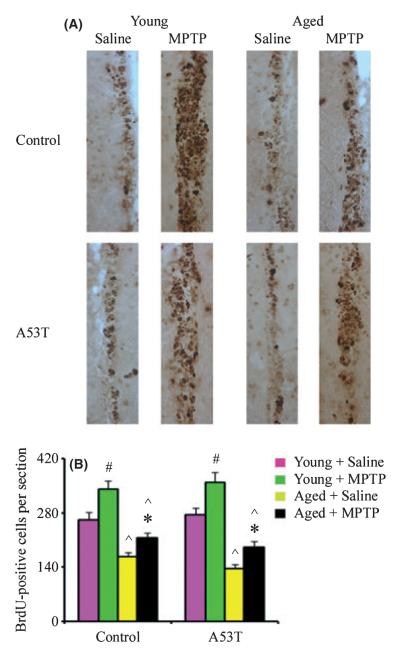
Neurogenesis continues in the brains of adult mammals, producing cells that can proliferate and migrate into damaged brain regions following MPTP lesions (Kay & Blum, 2000; Mao et al., 2001; Zhao et al., 2003; Yamada et al., 2004; Peng et al., 2008). To evaluate the neurogenic response to MPTP lesions in the aged mouse brain, proliferating cells from mice following MPTP administration were labeled with BrdU over a 5-day period, beginning on day 1 following the last MPTP injection. As shown in Fig. 1, basal neurogenesis was decreased in the SVZ of both aged wild-type and A53T transgenics compared with young adult mouse brains, consistent with previous findings in pan-neuronally expressing lines (Winner et al., 2008). It should be noted that in this previous study, animals were assessed at middle age (15 months) whereas our studies have extended the analyses out to older ages (23 months). As previously shown by our laboratory, acute MPTP administration was found to stimulate neurogenesis in the SVZ of young wild-type mice (Peng et al., 2008) and also in the A53T transgenics (Fig. 1). MPTP lesions were found also to increase the incorporation of BrdU into cells in the SVZ of aged wild-type and A53T transgenics, although the magnitude of the increase was slightly less than that in young adult mice. To quantify these changes, BrdU-labeled nuclei were counted and found to be decreased by 37% and 46% in the SVZ of aged wild-type and A53T transgenics compared with young animals, respectively (Fig. 1).
Fig. 1.
Decreased neurogenesis in the subventricular zone (SVZ) of MPTP-treated aged mice. (A) Immunocytochemistry demonstrates a decrease in bromodeoxyuridine (BrdU)-positive cells in aged compared with young adult brains. (B) Quantitation of BrdU-positive cells in the SVZ. Data are mean values ± SE, n = 4–5. #P < 0.01, significantly from saline young group; *P < 0.05, significantly from saline aged group; and ^P < 0.01, significantly from matched young group.
Relationship between BrdU labeling and DCX immunoreactivity
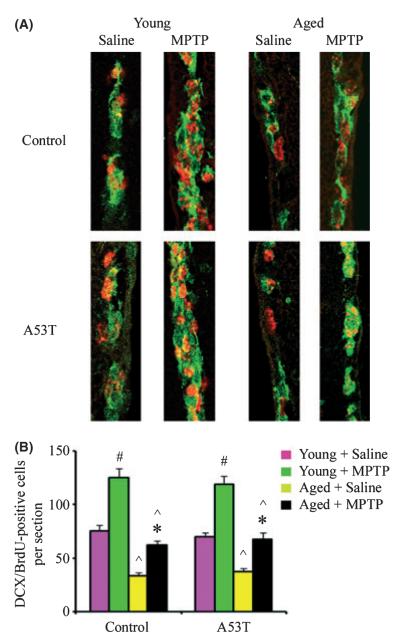
To investigate whether BrdU labeling following acute MPTP challenge correlates with labeling of neuronal precursors in the proliferation zone, we double-labeled brain sections with antibodies against BrdU and the developmentally regulated marker doublecortin (DCX), a microtubule-associated protein found in the soma and processes of newborn and migrating neurons during development (Gleeson et al., 1999) and in lesion-induced adult neurogenesis (Magavi et al., 2000). Bromodeoxyuridine-labeled cells co-expressed both DCX and proliferating cell nuclear antigen (not shown) suggesting that they are nascent neurons. Compared with saline controls, there were more BrdU-labeled cells expressing DCX in the SVZ of both MPTP-injured young adult and aged mouse brains vs. saline-treated controls (Fig. 2). However, the number of the BrdU/DCX-positive cells decreased with age in the SVZ of both wild-type and A53T transgenic mice, further consistent with an age-related decline in neurogenesis.
Fig. 2.
Relationship between bromodeoxyuridine (BrdU) incorporation and doublecortin (DCX) expression in the subventricular zone (SVZ). (A) Immunocytochemistry demonstrates a decrease in DCX (green)/BrdU (red)-positive cells in aged compared with young adult brains. (B) Quantitation of DCX/BrdU-positive cells in the SVZ. Data are mean values ± SE, n = 4. #P < 0.01, significantly from saline young group; *P < 0.05, significantly from saline aged group; and ^P < 0.01, significantly from matched young group.
Aged mice exhibit decreases in neurogenesis in the striatum
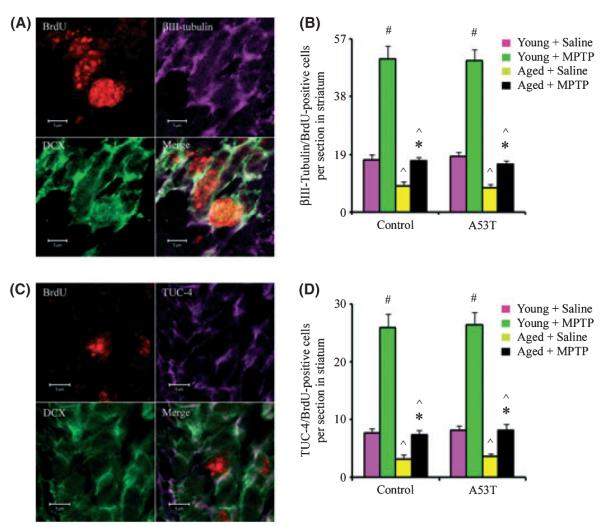
Although pathological processes can enhance neurogenesis in the adult brain, the fate of the newborn neurons that are produced and their role in aged brain repair are not well understood. MPTP-induced proliferation of neuronal precursors in the SVZ can be followed by migration of newborn neurons into the adjacent striatum (Peng et al., 2008). To determine whether acute MPTP-induced neuronal proliferation is associated with migration of nascent neurons from proliferation zones toward the injury site in aged mice, we performed triple-labeling with antibodies against BrdU, DCX, and two neuronal lineage markers: βIII-tubulin and the TOAD/Ulip/CRMP family protein 4 (TUC-4). Markers of new neurons including both βIII-tubulin and TUC-4 were detected in cells that also expressed DCX. As shown in Fig. 3, we found that BrdU/DCX-labeled cells were abundant in the striatum of MPTP-treated mice suggesting that new neurons either arose in or migrated to the striatum. In wild-type or A53T transgenic young mice that underwent MPTP injury, this was evidenced by an increase in the number of BrdU/βIII-tubulin- and BrdU/TUC-4-positive cells in the stratum. However, these positive cells were decreased in the striatum of aged MPTP-treated mice (Fig. 3). Thus, aging was found to reduce not only baseline but also MPTP-induced neurogenesis and subsequent neuromigration.
Fig. 3.
Decreased neurogenesis in the striatum of MPTP-treated aged mice. (A) Bromodeoxyuridine (BrdU) (red nucleus), βIII-tubulin (purple cytoplasm), and doublecortin (DCX) (green cytoplasm) co-localized within a single cell in the striatum. Scale bar, 5 μm. (B) Quantitation of βIII-tubulin/BrdU-positive cells in the striatum. (C) BrdU (red nucleus), TOAD/Ulip/CRMP family protein 4 (TUC-4) (purple cytoplasm), and DCX (green cytoplasm) co-localized within a single cell in the striatum. Scale bar, 5 μm. (D) Quantitation of TUC-4/BrdU-positive cells in the striatum. Data are mean values ± SE, n = 4. #P < 0.01, significantly from saline young group; *P < 0.05, significantly from saline aged group; and ^P < 0.01, significantly from matched young group.
Aging and mutant α-synuclein expression reduce neurogenesis in the olfactory bulb and substantia nigra
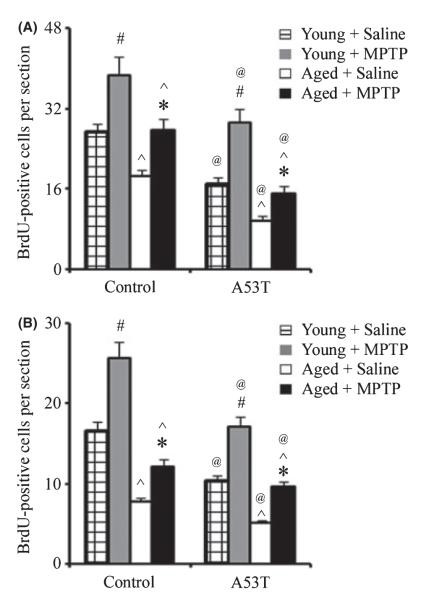
To determine the effect of aging and transgenic overexpression of α-synuclein on neurogenesis in the OB and substantia nigra (SN), we examined sections stained for BrdU (Fig. 4). Similar to the SVZ, MPTP administration induced neurogenesis in the OB and SN of both wild-type and A53T α-synuclein-expressing transgenic mice. The aged mice had fewer BrdU-labeled cells in both the glomerular layer of the OB and SN than in young mice. Furthermore, the number of BrdU-positive cells in the glomerular layer of the OB and SN was lower in A53T α-synuclein-expressing transgenic mice than in controls. This is to our knowledge the first study performed assessing the combined impact of aging and familial genetic mutations on this parameter.
Fig. 4.
Decreased neurogenesis in the olfactory bulb (OB) and the substantia nigra of A53T and aged mice. Quantitation of bromodeoxyuridine-positive profiles in the OB (A) and the substantia nigra (B). Data are mean values ± SE, n = 4. #P < 0.01, significantly from saline young group; *P < 0.05, significantly from saline aged group; ^P < 0.01, significantly from matched young group; and @P < 0.05, significantly from matched control group.
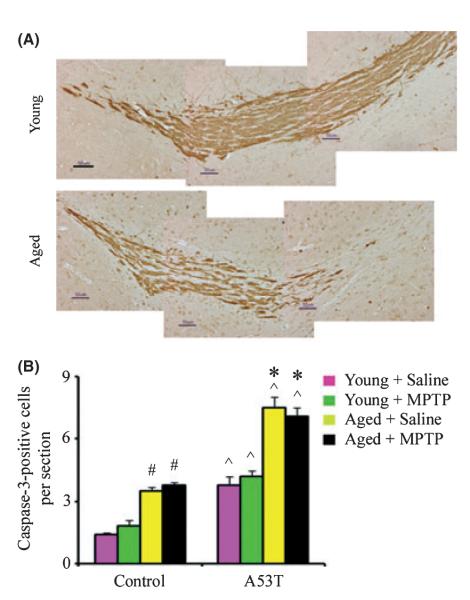
Labeling of newborn neurons with [3H] thymidine, BrdU, or retroviral vectors has shown that neurogenesis occurs primarily in two areas of adult mammalian brain, the SGZ of dentate gyrus and the rostral SVZ, and that the RMS transports newborn neurons from SVZ to the OB (Luskin, 1993; Lois et al., 1996). To test further whether aging affects normal migration of newborn neurons in the adult brain, we labeled brain sections with antibody against DCX. As found in previous studies (Luskin, 1993; Lois et al., 1996), newborn cells of neuronal lineage identified by immunoreactivity for DCX migrated from the SVZ via the RMS, into the OB of young adult brain. However, the migration of these cells was reduced in the aged brain, suggesting an age-dependent defect in the migration of newborn cells into regions of brain injury (Fig. 5).
Fig. 5.
Aging decreases cell migration in the rostral migratory stream (RMS) and increases cell death in the olfactory bulb (OB). (A) Immunohistochemical expression of doublecortin (brown) in sagittal sections through RMS of 2-month- and 23-month-old mouse brain. Scale bar, 50 μm. (B) Quantitation of caspase-3-positive profiles in the OB. Data are mean values ± SE, n = 4. #P < 0.05, significantly from matched control young group; *P < 0.05, significantly from marched A53T young group; and ^P < 0.05, significantly from matched control group.
Accumulation of mutant α-synuclein has been reported to result in reduced neurogenesis and survival of neural precursor cells in the OB (Winner et al., 2008; Marxreiter et al., 2009). Therefore, to determine whether the decrease of neurogenesis in the OB is attributed to increased levels of apoptosis, we analyzed cell death by counting caspase-3-positive cells in the glomerular layer of the OB. As shown in Fig. 5, we found a significant increase in caspase-3-positive cells in A53T transgenic mice compared with wild-type mice. Furthermore, the number of caspase-3-positive cells in the glomerular layer of the OB was higher in aged than in young animals.
Discussion
The potential role of variations in environmental exposures combined with genetic susceptibility factors in PD remains elusive especially in the context of aging, the single major risk factor for the disease. As PD is likely to be a multifactorial disorder, the use of in vivo models to explore the additive or synergistic effects of combined risk factors especially in the context of aging are likely to be of great importance in understanding neurogenesis. Our study extends results from previous studies by demonstrating that age-related reduction in α-synuclein-induced neurogenesis is aggravated further by environmental factor, in this case MPTP administration. In this study, we used transgenic mice in which the expression of mutant human α-synuclein protein was restricted to tyrosine hydroxylase-positive cells, allowing accumulation of levels of human α-synuclein specifically in the OB and SN (Matsuoka et al., 2001). This allowed us to examine the impact of α-synuclein pathology specifically within these brain regions on age-related neurogenesis. We examined the impact at older ages than had been previously examined i.e. in old vs. middle aged animals (23 vs. 15 months, Winner et al., 2008).
As others have previously shown, our data are consistent with the notion that adult neurogenesis in the SVZ undergoes a substantial decline with advancing age. The numbers of newly generated cells were significantly decreased in aged mice and the percentage of BrdU-labeled cells in the SVZ expressing a marker of immature neuronal phenotype (DCX) were also decreased with age. These findings are consistent with previous reports in rats (Kuhn et al., 1996), mice (Kempermann et al., 1998), dogs (Siwak-Tapp et al., 2007), macaque monkeys (Gould et al., 1999), and humans (Eriksson et al., 1998), suggesting that although neurogenesis continues throughout life, its rate declines with advancing age. The reduction in neurogenesis with age may be attributed to a decrease in neural precursor proliferation or a decrease in new neuron survival (Kuhn et al., 1996) or both. However, the factors that control the decrease in adult neurogenesis in the aged brain remain unknown. Changes in any of several environmental cues may contribute to age-related decline in adult neurogenesis. For example growth factors such as fibroblast growth factor-2 (FGF-2), insulin-like growth factor-1 (IGF-1), brain-derived neurotrophic factor (BDNF), heparin-binding epidermal growth factor-like growth factor (HB-EGF), and vascular endothelial growth factor (VEGF) decrease considerably by middle age and have been linked to reduced production of new cells in the SVZ and dentate gyrus with aging. Furthermore, central administration of several of these neurotrophic factors stimulates neurogenesis in both the SVZ and dentate gyrus of aged animals (Jin et al., 2003; Hattiangady et al., 2005; Shetty et al., 2005).
Whether injury-induced neurogenesis contributes by replacing lost cells, secreting trophic factors or other means, its effectiveness is likely to require migration of newly generated cells to the vicinity of the injury. Similar to the SVZ, fewer newborn cells entered the MPTP-damaged striatum, OB, and SN in aged vs. young adult mice, which would predict less effective self-repair in the aged brain. The significance of injury-induced neurogenesis in the adult brain is uncertain. The outcome from ischemia appears to be worse when neurogenesis is inhibited by cytosine-β-d-arabinofuranoside (Ara-C) (Arvidsson et al., 2002), x-irradiation (Raber et al., 2004), or ablation of neuronal precursors (Jin et al., 2010), suggesting that ischemia-induced neurogenesis helps mitigate histological and neurobehavioral deficits in the early aftermath of experimental stroke. In addition, the upregulating effects of environmental enrichment on adult hippocampal neurogenesis are paralleled by an improvement on a hippocampal learning task (Kempermann et al., 1997).
Accumulation of human wild-type or mutant (A53T, A30P) α-synuclein in the CNS of transgenic mice has been reported to result in reduced neurogenesis and survival of neural precursor cells in the SGZ and OB (Winner et al., 2004, 2008; Marxreiter et al., 2009). There also appears to be a significant (40–50%) decrease in the number of surviving BrdU-positive neural precursor cells in the SGZ of the dentate gyrus in these mice. This was reported to be accompanied by a reduction in the numbers of DCX-positive neuroblasts that was more severe in the mutant (A53T) vs. wild-type α-synuclein expressing mice (Crews et al., 2008). A recent study has demonstrated that α-synuclein may impair neurogenesis via decreased Notch-1, NICD, and Hes-5 expression (Crews et al., 2008). Notch-1 is implicated in not only the survival of neural precursor cells but also in neuronal and glial differentiation (Oishi et al., 2004; Louvi & Artavanis-Tsakonas, 2006). In this current study, the decrease of neurogenesis in the OB of the A53T transgenics vs. wild-type controls was found to be accompanied by increased levels of apoptosis as assessed by significant increases in caspase-3-positive cells. Furthermore, the number of caspase-3-positive cells in the glomerular layer of the OB was found to be higher in aged vs. young mice. These data suggest that the negative effects of mutant α-synuclein on neurogenesis are as a consequence of increases in the apoptotic cell death of nascent neurons.
The possibility that cell replacement therapy might be exploited to improve outcome in neurodegenerative diseases such as PD is of great current interest. As the disease tends to occur with advancing age and since aging also affects cell proliferation and related events required for cell replacement, it is very important to consider age as a factor in preclinical studies. Here we report that MPTP-induced neurogenesis previously seen in young animals persists in aged animals as well, although its magnitude is diminished. The observation that the aged brain continues to respond to pathological as well as physiological stimuli by stepping up the production of newborn cells is encouraging, in so far that it is consistent with the prospect of stimulating neurogenesis further for therapeutic purposes.
Experimental procedures
MPTP administration
Generation of vector and human mutant α-synuclein A53T transgenic mice has been described elsewhere (Matsuoka et al., 2001). Mice aged 2 and 23 months of age were intraperitoneally injected with saline, 20 mg kg−1 MPTP (free base; Sigma, St Louis, MO, USA) (2 months of age), or 9 mg kg−1 MPTP (23 months of age) every 2 h for four doses. Mice used as controls received an equivalent volume of saline. Experimental protocols were in accordance with the National Institutes of Health Guidelines for Use of Live Animals and were approved by the Animal Care and Use Committee at the Buck Institute. All possible efforts were made to minimize the number of animals used and their suffering.
BrdU labeling and counting
Bromodeoxyuridine (50 mg kg−1; Sigma) dissolved in saline was given intraperitoneally twice daily, at 8-h intervals, on consecutive days (days 1–5 after MPTP or saline administration). Mice were sacrificed on day 21 after MPTP or saline administration. Brains were removed after perfusion with saline and 4% paraformaldehyde in phosphate buffered saline (PBS). Adjacent 50-μm sections were cut with a cryostat and stored at −80°C. The sections were pretreated with 50% formamide, 280 mm NaCl, and 30 mm sodium citrate at 65°C for 2 h, incubated in 2 m HCl at 37°C for 30 min, and rinsed in 0.1 m boric acid, pH 8.5, at room temperature for 10 min. The sections were incubated in 1% H2O2 in PBS for 15 min, in blocking solution (2% goat serum, 0.3% Triton X-100, and 0.1% bovine serum albumin in PBS) for 2 h at room temperature, and with 2 μg mL−1 of mouse monoclonal anti-BrdU antibody (Roche, Indianapolis, IN, USA) at 4°C overnight. The sections were washed with PBS, incubated with biotinylated goat anti-mouse secondary antibody (1:200; Vector, Burlingame, CA, USA) for 2 h at 25°C, washed, and placed in avidin-peroxidase conjugate (Vector) solution for 1 h. The horseradish peroxidase reaction was detected with 0.05% diaminobenzidine (DAB) and 0.03% H2O2. Processing was stopped with H2O, and the sections were dehydrated through graded alcohols, cleared in xylene, and coverslipped in permanent mounting medium (Vector). Bromodeoxyuridine-positive cells in the SVZ, OB, and substantia nigra were counted blindly in five DAB-stained, 50-μm coronal sections per mouse, spaced 200 μm apart. The cells were counted under high-power (200 ×) on a Nikon E300 microscope with a Magnifire digital camera and the image was displayed on a computer monitor. Results were expressed as the average number of BrdU-positive cells per section.
Fluorescence immunohistochemistry
The sections were fixed with 4% paraformaldehyde in PBS for 1 h at room temperature, washed twice with PBS, and incubated in 2 m HCl at 37°C for 1 h. After washing again, the sections were incubated with blocking solution, then with primary antibodies at 4°C overnight, and with secondary antibodies in blocking solution at room temperature for 2 h. Primary antibodies used included mouse monoclonal anti-BrdU (2 μg mL−1; Roche), sheep polyclonal anti-BrdU (25 μg mL−1; Biodesign, Saco, ME, USA), affinity-purified goat polyclonal anti-doublecortin (DCX) (1:200; Santa Cruz Biotechnology, Santa Cruz, CA. USA), rabbit polyclonal anti-TUC-4 (1:1000; Chemicon, Temecula, CA, USA ), mouse monoclonal anti-βIII-tubulin (1:400; Caltag Laboratories, Burlingame, CA, USA), and rabbit polyclonal anticleaved caspase-3 (1:100; Cell Signaling Technology, Danvers, MA, USA). Secondary antibodies included Alexa Fluro 488- or 594-conjugated donkey anti-sheep, anti-mouse, anti-goat, or anti-rabbit IgG (1:200; Molecular Probes, Carlsbad, CA, USA). Fluorescence signals were detected with an LSM 510 NLO Confocal Scanning System mounted on an Axiovert 200 inverted microscope (Carl Zeiss Ltd., Thornwood, NY, USA) equipped with a two-photon Chameleon laser (Coherent Inc., Santa Clara, CA, USA). Three-color images were scanned using Argon and 543 HeNe lasers. imaris (Bitplane AG, Saint Paul, MN, USA) imaging software was used for three-dimensional image reconstruction. Images were acquired using lsm 510 Imaging Software (Carl Zeiss Ltd) as described previously (Peng et al., 2007). Bromodeoxyuridine single-labeled cells, and cells double labeled for BrdU and specific markers, were counted in three 50-μm coronal sections per mouse, spaced 200 μm apart. Controls included omitting or preabsorbing primary or omitting secondary antibodies.
Statistical analysis
All data are expressed as mean ± SE for the number (n) of mice per group. Differences among the means for all experiments described were analyzed using two-way anova with time or treatment as the independent factor. Newman–Keuls post hoc test was employed when differences were observed using analysis of variance testing (P < 0.05).
Acknowledgments
We are grateful to May Lin Oo for technical assistance.
References
- Arvidsson A, Collin T, Kirik D, Kokaia Z, Lindvall O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat. Med. 2002;8:963–970. doi: 10.1038/nm747. [DOI] [PubMed] [Google Scholar]
- Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol. Aging. 2003;24:197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- de Chevigny A, Cooper O, Vinuela A, Reske-Nielsen C, Lagace DC, Eisch AJ, Isacson O. Fate mapping and lineage analyses demonstrate the production of a large number of striatal neuroblasts after TGF{alpha} and noggin striatal infusions into the dopamine-depleted striatum. Stem Cells. 2008;12:12. doi: 10.1634/stemcells.2008-0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper O, Isacson O. Intrastriatal transforming growth factor alpha delivery to a model of Parkinson’s disease induces proliferation and migration of endogenous adult neural progenitor cells without differentiation into dopaminergic neurons. J. Neurosci. 2004;24:8924–8931. doi: 10.1523/JNEUROSCI.2344-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews L, Mizuno H, Desplats P, Rockenstein E, Adame A, Patrick C, Winner B, Winkler J, Masliah E. Alpha-synuclein alters Notch-1 expression and neurogenesis in mouse embryonic stem cells and in the hippocampus of transgenic mice. J. Neurosci. 2008;28:4250–4260. doi: 10.1523/JNEUROSCI.0066-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauer W, Przedborski S. Parkinson’s disease: mechanisms and models. Neuron. 2003;39:889–909. doi: 10.1016/s0896-6273(03)00568-3. [DOI] [PubMed] [Google Scholar]
- Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH. Neurogenesis in the adult human hippocampus. Nat. Med. 1998;4:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- Fallon J, Reid S, Kinyamu R, Opole I, Opole R, Baratta J, Korc M, Endo TL, Duong A, Nguyen G, Karkehabadhi M, Twardzik D, Patel S, Loughlin S. In vivo induction of massive proliferation, directed migration, and differentiation of neural cells in the adult mammalian brain. Proc. Natl. Acad. Sci. USA. 2000;97:14686–14691. doi: 10.1073/pnas.97.26.14686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forno LS. Neuropathology of Parkinson’s disease. J. Neuropathol. Exp. Neurol. 1996;55:259–272. doi: 10.1097/00005072-199603000-00001. [DOI] [PubMed] [Google Scholar]
- Gleeson JG, Lin PT, Flanagan LA, Walsh CA. Doublecortin is a microtubule-associated protein and is expressed widely by migrating neurons. Neuron. 1999;23:257–271. doi: 10.1016/s0896-6273(00)80778-3. [DOI] [PubMed] [Google Scholar]
- Gould E, Tanapat P. Lesion-induced proliferation of neuronal progenitors in the dentate gyrus of the adult rat. Neuroscience. 1997;80:427–436. doi: 10.1016/s0306-4522(97)00127-9. [DOI] [PubMed] [Google Scholar]
- Gould E, Reeves AJ, Fallah M, Tanapat P, Gross CG, Fuchs E. Hippocampal neurogenesis in adult Old World primates. Proc. Natl. Acad. Sci. USA. 1999;96:5263–5267. doi: 10.1073/pnas.96.9.5263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattiangady B, Rao MS, Shetty GA, Shetty AK. Brain-derived neurotrophic factor, phosphorylated cyclic AMP response element binding protein and neuropeptide Y decline as early as middle age in the dentate gyrus and CA1 and CA3 subfields of the hippocampus. Exp. Neurol. 2005;195:353–371. doi: 10.1016/j.expneurol.2005.05.014. [DOI] [PubMed] [Google Scholar]
- Hoglinger GU, Rizk P, Muriel MP, Duyckaerts C, Oertel WH, Caille I, Hirsch EC. Dopamine depletion impairs precursor cell proliferation in Parkinson disease. Nat. Neurosci. 2004;7:726–735. doi: 10.1038/nn1265. [DOI] [PubMed] [Google Scholar]
- Jin K, Sun Y, Xie L, Batteur S, Mao XO, Smelick C, Logvinova A, Greenberg DA. Neurogenesis and aging: FGF-2 and HB-EGF restore neurogenesis in hippocampus and subventricular zone of aged mice. Aging Cell. 2003;2:175–183. doi: 10.1046/j.1474-9728.2003.00046.x. [DOI] [PubMed] [Google Scholar]
- Jin K, Wang X, Xie L, Mao XO, Greenberg DA. Transgenic ablation of doublecortin-expressing cells suppresses adult neurogenesis and worsens stroke outcome in mice. Proc. Natl. Acad. Sci. USA. 2010;107:7993–7998. doi: 10.1073/pnas.1000154107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan MS, Hinds JW. Neurogenesis in the adult rat: electron microscopic analysis of light radioautographs. Science. 1977;197:1092–1094. doi: 10.1126/science.887941. [DOI] [PubMed] [Google Scholar]
- Kay JN, Blum M. Differential response of ventral midbrain and striatal progenitor cells to lesions of the nigrostriatal dopaminergic projection. Dev. Neurosci. 2000;22:56–67. doi: 10.1159/000017427. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Kuhn HG, Gage FH. More hippocampal neurons in adult mice living in an enriched environment. Nature. 1997;386:493–495. doi: 10.1038/386493a0. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Kuhn HG, Gage FH. Experience-induced neurogenesis in the senescent dentate gyrus. J. Neurosci. 1998;18:3206–3212. doi: 10.1523/JNEUROSCI.18-09-03206.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruger R, Kuhn W, Muller T, Woitalla D, Graeber M, Kosel S, Przuntek H, Epplen JT, Schols L, Riess O. Ala30Pro mutation in the gene encoding alpha-synuclein in Parkinson’s disease. Nat. Genet. 1998;18:106–108. doi: 10.1038/ng0298-106. [DOI] [PubMed] [Google Scholar]
- Kuhn HG, Dickinson-Anson H, Gage FH. Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. J. Neurosci. 1996;16:2027–2033. doi: 10.1523/JNEUROSCI.16-06-02027.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang AE, Lozano AM. Parkinson’s disease. First of two parts. N. Engl. J. Med. 1998;339:1044–1053. doi: 10.1056/NEJM199810083391506. [DOI] [PubMed] [Google Scholar]
- Lois C, Garcia-Verdugo JM, Alvarez-Buylla A. Chain migration of neuronal precursors. Science. 1996;271:978–981. doi: 10.1126/science.271.5251.978. [DOI] [PubMed] [Google Scholar]
- Louvi A, Artavanis-Tsakonas S. Notch signalling in vertebrate neural development. Nat. Rev. Neurosci. 2006;7:93–102. doi: 10.1038/nrn1847. [DOI] [PubMed] [Google Scholar]
- Luskin MB. Restricted proliferation and migration of postnatally generated neurons derived from the forebrain subventricular zone. Neuron. 1993;11:173–189. doi: 10.1016/0896-6273(93)90281-u. [DOI] [PubMed] [Google Scholar]
- Magavi SS, Leavitt BR, Macklis JD. Induction of neurogenesis in the neocortex of adult mice. Nature. 2000;405:951–955. doi: 10.1038/35016083. [DOI] [PubMed] [Google Scholar]
- Mao L, Lau YS, Petroske E, Wang JQ. Profound astrogenesis in the striatum of adult mice following nigrostriatal dopaminergic lesion by repeated MPTP administration. Brain Res. Dev. Brain Res. 2001;131:57–65. doi: 10.1016/s0165-3806(01)00260-7. [DOI] [PubMed] [Google Scholar]
- Marxreiter F, Nuber S, Kandasamy M, Klucken J, Aigner R, Burgmayer R, Couillard-Despres S, Riess O, Winkler J, Winner B. Changes in adult olfactory bulb neurogenesis in mice expressing the A30P mutant form of alpha-synuclein. Eur. J. Neurosci. 2009;29:879–890. doi: 10.1111/j.1460-9568.2009.06641.x. [DOI] [PubMed] [Google Scholar]
- Matsuoka Y, Vila M, Lincoln S, McCormack A, Picciano M, LaFrancois J, Yu X, Dickson D, Langston WJ, McGowan E, Farrer M, Hardy J, Duff K, Przedborski S, Di Monte DA. Lack of nigral pathology in transgenic mice expressing human alpha-synuclein driven by the tyrosine hydroxylase promoter. Neurobiol. Dis. 2001;8:535–539. doi: 10.1006/nbdi.2001.0392. [DOI] [PubMed] [Google Scholar]
- Mohapel P, Frielingsdorf H, Haggblad J, Zachrisson O, Brundin P. Platelet-derived growth factor (PDGF-BB) and brain-derived neurotrophic factor (BDNF) induce striatal neurogenesis in adult rats with 6-hydroxydopamine lesions. Neuroscience. 2005;132:767–776. doi: 10.1016/j.neuroscience.2004.11.056. [DOI] [PubMed] [Google Scholar]
- Oishi K, Kamakura S, Isazawa Y, Yoshimatsu T, Kuida K, Nakafuku M, Masuyama N, Gotoh Y. Notch promotes survival of neural precursor cells via mechanisms distinct from those regulating neurogenesis. Dev. Biol. 2004;276:172–184. doi: 10.1016/j.ydbio.2004.08.039. [DOI] [PubMed] [Google Scholar]
- Peng J, Peng L, Stevenson FF, Doctrow SR, Andersen JK. Iron and paraquat as synergistic environmental risk factors in sporadic Parkinson’s disease accelerate age-related neurodegeneration. J. Neurosci. 2007;27:6914–6922. doi: 10.1523/JNEUROSCI.1569-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng J, Xie L, Jin K, Greenberg DA, Andersen JK. Fibroblast growth factor 2 enhances striatal and nigral neurogenesis in the acute 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine model of Parkinson’s disease. Neuroscience. 2008;153:664–670. doi: 10.1016/j.neuroscience.2008.02.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, Pike B, Root H, Rubenstein J, Boyer R, Stenroos ES, Chandrasekharappa S, Athanassiadou A, Papapetropoulos T, Johnson WG, Lazzarini AM, Duvoisin RC, Di Iorio G, Golbe LI, Nussbaum RL. Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease. Science. 1997;276:2045–2047. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- Raber J, Fan Y, Matsumori Y, Liu Z, Weinstein PR, Fike JR, Liu J. Irradiation attenuates neurogenesis and exacerbates ischemia-induced deficits. Ann. Neurol. 2004;55:381–389. doi: 10.1002/ana.10853. [DOI] [PubMed] [Google Scholar]
- Shetty AK, Hattiangady B, Shetty GA. Stem/progenitor cell proliferation factors FGF-2, IGF-1, and VEGF exhibit early decline during the course of aging in the hippocampus: role of astrocytes. Glia. 2005;51:173–186. doi: 10.1002/glia.20187. [DOI] [PubMed] [Google Scholar]
- Simon-Sanchez J, Schulte C, Bras JM, Sharma M, Gibbs JR, Berg D, Paisan-Ruiz C, Lichtner P, Scholz SW, Hernandez DG, Kruger R, Federoff M, Klein C, Goate A, Perlmutter J, Bonin M, Nalls MA, Illig T, Gieger C, Houlden H, Steffens M, Okun MS, Racette BA, Cookson MR, Foote KD, Fernandez HH, Traynor BJ, Schreiber S, Arepalli S, Zonozi R, Gwinn K, van der Brug M, Lopez G, Chanock SJ, Schatzkin A, Park Y, Hollenbeck A, Gao J, Huang X, Wood NW, Lorenz D, Deuschl G, Chen H, Riess O, Hardy JA, Singleton AB, Gasser T. Genome-wide association study reveals genetic risk underlying Parkinson’s disease. Nat. Genet. 2009;41:1308–1312. doi: 10.1038/ng.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siwak-Tapp CT, Head E, Muggenburg BA, Milgram NW, Cotman CW. Neurogenesis decreases with age in the canine hippocampus and correlates with cognitive function. Neurobiol. Learn. Mem. 2007;88:249–259. doi: 10.1016/j.nlm.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder EY, Yoon C, Flax JD, Macklis JD. Multipotent neural precursors can differentiate toward replacement of neurons undergoing targeted apoptotic degeneration in adult mouse neocortex. Proc. Natl. Acad. Sci. USA. 1997;94:11663–11668. doi: 10.1073/pnas.94.21.11663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M. Alpha-synuclein in Lewy bodies. Nature. 1997;388:839–840. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- Van Kampen JM, Eckman CB. Dopamine D3 receptor agonist delivery to a model of Parkinson’s disease restores the nigrostriatal pathway and improves locomotor behavior. J. Neurosci. 2006;26:7272–7280. doi: 10.1523/JNEUROSCI.0837-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winner B, Lie DC, Rockenstein E, Aigner R, Aigner L, Masliah E, Kuhn HG, Winkler J. Human wild-type alpha-synuclein impairs neurogenesis. J. Neuropathol. Exp. Neurol. 2004;63:1155–1166. doi: 10.1093/jnen/63.11.1155. [DOI] [PubMed] [Google Scholar]
- Winner B, Rockenstein E, Lie DC, Aigner R, Mante M, Bogdahn U, Couillard-Despres S, Masliah E, Winkler J. Mutant alpha-synuclein exacerbates age-related decrease of neurogenesis. Neurobiol. Aging. 2008;29:913–925. doi: 10.1016/j.neurobiolaging.2006.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada M, Onodera M, Mizuno Y, Mochizuki H. Neurogenesis in olfactory bulb identified by retroviral labeling in normal and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-treated adult mice. Neuroscience. 2004;124:173–181. doi: 10.1016/j.neuroscience.2003.10.040. [DOI] [PubMed] [Google Scholar]
- Zarranz JJ, Alegre J, Gomez-Esteban JC, Lezcano E, Ros R, Ampuero I, Vidal L, Hoenicka J, Rodriguez O, Atares B, Llorens V, Gomez Tortosa E, del Ser T, Munoz DG, de Yebenes JG. The new mutation, E46K, of alpha-synuclein causes Parkinson and Lewy body dementia. Ann. Neurol. 2004;55:164–173. doi: 10.1002/ana.10795. [DOI] [PubMed] [Google Scholar]
- Zhao M, Momma S, Delfani K, Carlen M, Cassidy RM, Johansson CB, Brismar H, Shupliakov O, Frisen J, Janson AM. Evidence for neurogenesis in the adult mammalian substantia nigra. Proc. Natl. Acad. Sci. USA. 2003;100:7925–7930. doi: 10.1073/pnas.1131955100. [DOI] [PMC free article] [PubMed] [Google Scholar]