Abstract
The pathogenic yeast Cryptococcus gattii, which is causing an outbreak in the Pacific Northwest region of North America, causes life-threatening pulmonary infections and meningoencephalitis in healthy individuals, unlike Cryptococcus neoformans, which commonly infects immunocompromised patients. In addition to a greater predilection for C. gattii to infect healthy hosts, the C. gattii genome sequence project revealed extensive chromosomal rearrangements compared with C. neoformans, showing genomic differences between the two Cryptococcus species. We investigated the roles of C. gattii calcineurin in three molecular types: VGIIa (R265), VGIIb (R272), and VGI (WM276). We found that calcineurin exhibits a differential requirement for growth on solid medium at 37°, as calcineurin mutants generated from R265 were more thermotolerant than mutants from R272 and WM276. We demonstrated that tolerance to calcineurin inhibitors (FK506, CsA) at 37° is linked with the VGIIa molecular type. The calcineurin mutants from the R272 background showed the most extensive growth and morphological defects (multivesicle and larger ring-like cells), as well as increased fluconazole susceptibility. Our cellular architecture examination showed that C. gattii and C. neoformans calcineurin mutants exhibit plasma membrane disruptions. Calcineurin in the C. gattii VGII molecular type plays a greater role in controlling cation homeostasis compared with that in C. gattii VGI and C. neoformans H99. Importantly, we demonstrate that C. gattii calcineurin is essential for virulence in a murine inhalation model, supporting C. gattii calcineurin as an attractive antifungal drug target.
Keywords: temperature sensitivity, pathogenicity, fluconazole tolerance, FK506, cyclosporin A, cation homeostasis, melanin, capsule
Cryptococcus gattii and Cryptococcus neoformans are closely related basidiomycetous yeast species that cause the disease cryptococcosis after inhalation by the host. Cryptococcosis can cause pneumonia and disseminates throughout the body to infect other tissues, especially the central nervous system, causing meningoencephalitis (Perfect and Casadevall 2012). Cryptococcal meningoencephalitis is 100% fatal if untreated, and in certain human immunodeficiency (HIV)-infected populations, mortality rates of 100% have been reported within 2 wk after clinical presentation to health care facilities (French et al., 2002). It is estimated that cryptococcosis causes more than 600,000 deaths per year (Park et al., 2009). Unlike C. neoformans, which predominantly causes disease in immunosuppressed persons such as organ transplant recipients or HIV/acquired immunodeficiency syndrome (AIDS) patients, C. gattii frequently causes disease in both immunocompetent and immunosuppressed hosts (Chaturvedi and Chaturvedi 2011; Sorrell 2001). Although C. neoformans is distributed worldwide, it was previously thought that C. gattii was restricted to tropical regions of the world (Kwon-Chung and Bennett 1984). However, C. gattii emerged on Vancouver Island, Canada, in 1999, afflicting both residents and animals of the island. In 2006, the first case in the United States of infection by a C. gattii strain indistinguishable from the Vancouver Island VGIIa/major outbreak strain was identified in a patient from Orcas Island in Washington state (Upton et al. 2007; Datta et al. 2009). C. gattii has continued to spread throughout the Pacific Northwest and down the western coast and has emerged as a primary pathogen in the northwestern United States (Byrnes et al. 2010).
C. gattii is divided into four molecular types: VGI, VGII, VGIII, and VGIV (Bovers et al. 2008). Types VGI and VGII cause the majority of infections that occur in otherwise-healthy individuals, whereas VGIII and VGIV more commonly infect immunosuppressed HIV/AIDS patients (Byrnes et al. 2011). VGI is the most commonly isolated molecular type worldwide (Sorrell 2001), whereas the VGII molecular type is the cause of the Vancouver Island/Pacific Northwest outbreak (Fraser et al. 2005; Byrnes et al. 2010; Kidd et al. 2004). Type VGII can be further subdivided in VGIIa/major and VGIIb/minor, which were identified in the Vancouver Island outbreak (Kidd et al. 2004), as well as VGIIc/novel, which has emerged in Oregon and is causing illness in the region, along with isolates of the VGIIa and VGIIb genotypes (Byrnes et al. 2010).
Virulence studies have revealed both shared and unique molecular virulence attributes for C. neoformans and C. gattii. For example, the superoxide dismutase Sod1 is a prominent antioxidant that is required for virulence of C. gattii but not C. neoformans (Narasipura et al. 2003). Trehalose functions as an antioxidant and stress protectant and is produced by the enzyme trehalose-6-phosphate synthase, which is encoded by the TPS1 and TPS2 genes. Both Tps1 and Tps2 were found to be critical for thermotolerance, pathogenicity, and other virulence attributes (capsule and melanin production) in C. gattii (Ngamskulrungroj et al. 2009). However, the homologous genes in C. neoformans are required for thermotolerance but not for capsule or melanin production (Petzold et al. 2006). An interesting evolutionary switch of the cAMP-activated protein kinase A (Pka1 and Pka2) has been found in C. gattii and C. neoformans. Pkal governs mating, virulence, capsule, and melanin production in C. neoformans but only controls capsule production in C. gattii (D’Souza et al. 2001; Hicks and Heitman 2007). Pka2 does not regulate mating, capsule, or melanin production in C. neoformans, whereas the corresponding protein regulates these functions in C. gattii (D’Souza et al. 2001; Hicks and Heitman 2007). Ste12α is a transcription factor that regulates melanin, mating, virulence, and ecological fitness in C. gattii but only regulates mating and capsule size in C. neoformans (Ren et al. 2006; Yue et al. 1999). Finally, Gat1 is a GATA transcription factor required for virulence in C. gattii but not in C. neoformans (Ngamskulrungroj et al. 2012a). Meanwhile, C. neoformans Gat1 plays a greater role in the use of nitrogen sources such as glycine and creatinine compared with C. gattii Gat1 (Ngamskulrungroj et al. 2012a).
Calcineurin is a calcium-calmodulin activated serine-threonine specific protein phosphatase composed of a catalytic A (Cna1) and regulatory B calcium-binding subunit (Cnb1). Active calcineurin is an AB heterodimer, and loss of the B subunit can result in destabilization of the A subunit, causing calcineurin malfunction (Chen et al. 2010a). Pathogenic fungi require calcineurin for establishing infection via distinct mechanisms: (1) growth at body temperature (C. neoformans and C. glabrata) (Chen et al. 2012; Odom et al. 1997b); (2) survival in serum (C. albicans) (Blankenship et al. 2003); (3) filamentous growth (A. fumigatus and C. dubliniensis) (Chen et al. 2011; Steinbach et al. 2006); and (4) appressorial formation (Magnaporthe oryzae) (Choi et al. 2009). In this study we tested the pathogenic roles of calcineurin of C. gattii compared with C. neoformans, particularly with respect to the relevance to virulence and antifungal drug resistance of the C. gattii isolates causing the expanding Pacific Northwest outbreak.
Materials and Methods
Yeast strains, growth media, and chemicals
Yeast strains used in this study are listed in Table 1. The following media were used in this study: yeast extract peptone dextrose (YPD; 1% yeast extract, 2% peptone, 2% glucose) liquid medium and agar (2%) plates. YPD medium containing 100 µg/mL nourseothricin was used to select transformants. The following supplements were added to the media at the concentrations indicated: FK506 (Astellas Pharma Inc.), cyclosporin A (CsA; LC Laboratories), sodium dodecyl sulfate (Fisher), calcofluor white (fluorescent brightener 28) (Sigma-Aldrich), Congo red (Sigma-Aldrich), tunicamycin (Sigma-Aldrich), and fluconazole (Bedford Laboratories). Niger seed (Guizotia abyssinica) agar plates was made with 70 g of ground niger seed in 350 mL of distilled water (dH2O), filtered through cheese cloth with the addition of 1 g of dextrose, 20 g of Bacto agar, and dH2O added to 1 L. L-DOPA agar plates was made with 20 g of Bacto agar autoclaved in 900 mL of dH2O followed by addition of 1 g of L-asparagine, 1 g of dextrose, 3 g of KH2PO4, 0.25 g of MgSO4, 100 mg of L-DOPA, and 1 mg of thiamine. The pH was adjusted to 5.6, and dH2O was added to 1 L. Low iron media was made with 5 g of dextrose, 5 g of asparagine, 400 mg of K2HPO4, 80 mg of MgSO4, 250 mg of CaCl2, and 20 mg of ethylenediamine-N,N′-bis(2-hydroxyphenylacetic acid) followed by the adjustment of pH to 7.4 in 1 L of dH2O; then, 1 mL of 1000× vitamin-mineral mixture (400 mg of thiamine, 57 mg of boric acid, 5 mg of CuSO4, 10 mg of MnCl2, 2000 mg of ZnSO4, and 460 mg of sodium molybdate in 1 L of dH2O) was added after autoclaving.
Table 1. Cryptococcus gattii and Cryptococcus neoformans strains.
| Strain | Parent | Genotype | Source of Reference |
|---|---|---|---|
| C. gattii | |||
| R265 | Clinical isolate | Wild-type VGIIa MATα, serotype B | (Kidd et al. 2004) |
| YL136 | R265 | cna1Δ::NAT | This study |
| YL137 | R265 | cna1Δ::NAT | This study |
| R272 | Clinical isolate | Wild-type VGIIb MATα, serotype B | (Kidd et al. 2004) |
| YL165 | R272 | cna1Δ::NAT | This study |
| YL169 | R272 | cna1Δ::NAT | This study |
| YC875 | YL165 | cna1Δ::CNA1 (native locus) | This study |
| YC879 | YL165 | cna1Δ::NAT CNA1 (ectopic) | This study |
| YC883 | YL165 | cna1Δ::NAT suppressor mutation | This study |
| YC886 | YL165 | cna1Δ::NAT suppressor mutation | This study |
| WM276 | Environmental isolate | Wild-type VGI MATα, serotype B | (Kidd et al. 2005) |
| YL277 | WM276 | cna1Δ::NAT | This study |
| YL344 | WM276 | cna1Δ::NAT | This study |
| C. neoformans | |||
| H99 | Clinical isolate | Wild-type MATα, serotype A | (Perfect et al. 1993) |
| KK1 | H99 | cna1Δ::NAT | (Kojima et al. 2006) |
| KK5 | KK1 | cna1Δ::NAT CNA1::NEO | (Kojima et al. 2006) |
| JLCN146 | H99 | lac1Δ::HYG | (Nelson et al. 2003) |
Identification of C. gattii calcineurin gene orthologs
The C. gattii orthologs of the gene encoding the C. neoformans calcineurin catalytic subunit (Cna1) were identified by reciprocal BLAST searches between the two species, with the reciprocal best BLAST hit orthologs in C. gattii being CNA1 (CNBG_5632 from R265; CGB_I3140C from WM276). C. gattii R265 Cna1 shares 99.4% and 97.0% identity over the full protein length with its corresponding C. gattii WM276 and C. neoformans H99 orthologs, respectively (Supporting Information, Figure S1A). In addition to the catalytic domain, C. gattii Cna1 has the conserved calcineurin B binding, calmodulin binding, and autoinhibitory domains (Cyert et al. 1991; Rusnak and Mertz 2000) (Figure S1).
Disruption of the C. gattii calcineurin genes
All deletion strains were generated from the prototrophic C. gattii R265 (VGIIa), R272 (VGIIb), and WM276 (VGI) strains. The nourseothricin (NAT) resistance gene from plasmid pAI3 was used as a dominant selectable marker in a biolistic transformation protocol with a Bio-Rad model PDS-1000/He biolistic particle delivery system (Toffaletti et al. 1993). All primers used in strain construction are listed in Table S1. The cna1Δ mutant was generated in the C. gattii strains R265 (VGIIa), R272 (VGIIb), and WM276 (VGI) by overlap PCR as previously described (Davidson et al. 2002). For VGII strains, the 5′ and 3′ noncoding regions of the CNA1 gene were amplified with primers JC174/JC175 and JC176/JC177 from R265 or R272 genomic DNA, and the dominant selectable marker (NAT) was amplified with the M13 universal primers (JC65/JC66) from plasmid pAI3. The CNA1 gene replacement cassettes were generated by overlap PCR with flanking primers JC178/JC179, precipitated onto gold microcarrier beads (0.6 µm; Bio-Rad Laboratories, Inc., Hercules, CA), and the VGII strains R265 and R272 were biolistically transformed.
For the VGI strain WM276, the 5′ and 3′ regions of the CNA1 gene were amplified with primers JC207/JC208 and JC209/JC210 from genomic DNA. The CNA1 replacement cassette was generated by overlap PCR with primers JC211/JC212, and WM276 was biolistically transformed. Stable transformants were selected on YPD medium containing 100 µg/mL nourseothricin. Two independent nourseothricin-resistant cna1 mutants (YL136 and YL137 from R265; YL165 and YL169 from R272; and YL277 and YL344 from WM276) derived from two separate transformations were obtained (Table 1). Mutants were confirmed by PCR and Southern blot analysis.
Complementation of R272 calcineurin mutant and isolation of suppressor mutations at 38°
C. gattii R272 cna1Δ::NAT mutant YL165 was grown overnight in 50 mL of YPD medium containing 100 μg/mL nourseothricin, washed with dH2O, and resuspended in 12.5 mL of dH2O. Three-hundred microliters were spread to YPD medium containing 1 M sorbitol and incubated at 24° for 4 hr, and biolistic transformation was then performed as described previously. The 4.6-kb complementation DNA fragment used for biolistic transformation was amplified with primers JC174/JC177 from R272 genomic DNA and contained the 5′ noncoding region (NCR), CNA1 gene, and 3′NCR. Because calcineurin is essential for growth at 37°, we screened transformants in YPD medium at a slightly more stringent condition of 38°. Transformants able to grow at 38° were colony purified and replica-plated to YPD medium containing 100 μg/mL nourseothricin. Both nourseothricin-sensitive and -resistant colonies were observed. The nouseothricin-sensitive colony YC875 (Table 1; Figure S7) represents native locus complementation from which the NAT selectable marker within the cna1 mutant YL165 was replaced by wild-type CNA1 gene (thus resulting in nourseothricin sensitivity), whereas nouseothricin resistant colonies represent ectopic CNA1 integration (YC879; Table 1; Figure S7), or suppressor mutations (YC883 and YC886; two independently derived mutants; Table 1; Figure S7).
Transmission electron microscopy
Cells were pelleted and rinsed in PBS buffer and resuspended in 3% glutaraldehyde in 0.1 M sodium cacodylate buffer (pH = 6.8) at room temperature for 1 hr. The cells were then transferred to 4° and incubated in fixative for several weeks. The cells were rinsed with three changes of cold 0.1 M sodium cacodylate buffer (pH = 6.8) by centrifugation and resuspended in fresh buffer and then postfixed with 2% OsO4 in the same buffer for 2 hr on ice in the dark. Cells were again rinsed with three changes of buffer as described previously. After the last buffer wash, the cells were centrifuged and the pellet was embedded in warm (60°) 2% agarose prepared in the same buffer. The tubes were spun for 5 min while the agarose was warm enough to pellet the cells before the agarose gelled. After 1 hr on ice, the gelled pellets were removed from the tubes and cut into 1-mm3 blocks in dH2O at room temperature. Blocks were placed in buffer at room temperature and then rinsed once with room temperature dH2O before en bloc staining with 1% uranyl acetate in dH2O in the dark for 1 hr at room temperature. Samples were rinsed once with room temperature dH2O and then dehydrated through a graded ethanol series (30%, 50%, 70%, 95%, 3× 100%) for 1 hr each change and then infiltrated with Spurr’s resin (1:1 Spurr’s:ethanol, 3:1 Spurr’s:ethanol, and three changes of 100% Spurr’s resin in vacuum) for at least 6 hr each. Individual pieces were embedded in fresh 100% Spurr’s in BEEM capsules at 70° for 2 d. Thin sections were cut with an LKB NOVA Ultrotome III (Leica, Brannockburn IL), collected on 200-mesh grids, and then stained with 4% aqueous uranyl acetate for 1 hr and Reynold’s lead citrate for 4 min. Grids were viewed using a JEOL 1200EX transmission electron microscope (JEOL USA Inc,, Peabody, MA). Images were recorded on Kodak 4489 film (Eastman Kodak Co., Rochester NY) that was then scanned using an Epson 4870 (Epson America, Inc. Long Beach, CA) flatbed scanner at 1200 dpi. Digital negatives were processed to positives and labeled using Photoshop CS4.
Melanin production assay
Niger seed and L-DOPA plates were used to determine C. gattii melanin production. Strains were grown overnight at 24°, washed twice with dH2O, diluted to 1 OD600/mL, and then 5 µL of cell suspension was spotted on Niger seed and L-DOPA agar plates and incubated for 72 hr at 24°.
Capsule production assay
Strains were cultured in YPD media overnight at 24°, washed twice with dH2O, and diluted to 0.2 OD600/ml (5 mL) in low iron media for growth at 24° for 72 hr. Three microliters of India ink (Gibson Laboratories, Inc) was added to 97 µL of cell suspension. The images were taken at 1000× magnification.
Mouse infection studies
Animals studies conducted in the Division of Laboratory Animal Resources facilities at Duke University Medical Center (DUMC) were conducted in good practice as defined by the United States Animal Welfare Act and in full compliance with the guidelines of the DUMC Institutional Animal Care and Use Committee. The vertebrate experiments were reviewed and approved by the DUMC Institutional Animal Care and Use Committee under protocol number A217-11-08.
Seven- to eight-week-old female A/Jcr mice (Jackson Laboratory, 18−22 g) were used in this study. For infection, strains were cultured in YPD broth overnight at 24° and washed twice with sterile phosphate-buffered saline (PBS). Cells were counted with a hemocytometer and resuspended in sterile PBS at 106 cells per milliliter. Dilutions of the cells were plated onto YPD and incubated at 24° for 48 hr to determine CFU and viability. Groups of 10 mice (excluding R265, which had eight mice; two mice died due to pentobarbital treatment) per strain were anesthetized with pentobarbital (Lundbeck Inc, Deerfield, IL), and inoculated with C. gattii via intranasal instillation of an inocula of 5 × 104 cells (in 50 µL). Survival was monitored 1 to 2 times daily, and moribund mice were killed with CO2. Kaplan-Meier survival curves were generated with Prism 5.03 (GraphPad software, La Jolla, CA), and P values were evaluated by a Log-rank (Mantel-Cox) test. A P value of <0.05 was considered significant.
To determine fungal burden, the lungs and brains of C. gattii infected mice (n = 5 for each strain) were dissected at day 14 after infection. Half organ portions were weighed, transferred to a 15-mL Falcon tube filled with 5 ml PBS, and homogenized for 10 sec at 13,600 rpm/min (Power Gen 500, Fisher Scientific). Tissue homogenates were serially diluted, and 100 µL was plated onto a YPD plate containing 100 µg/mL chloramphenicol. The plates were incubated at 24° for 48 to 72 hr to determine CFU per gram of lung or brain. The significance of differences in fungal burden was determined using one-way analysis of variance (ANOVA) and Dunnett’s multiple comparison test. For histopathological analysis, half organ samples of lung and brain were fixed in 10% phosphate-buffered formalin (Fisher Scientific), and mucicarmine and hematoxylin & eosin (H&E) stainings were performed by technicians at the Department of Pathology at Duke University. After slide preparation, each sample was examined thoroughly by microscopy for analysis of Cryptococcus colonization (mucicarmine) and tissue necrosis (H&E). Images were captured using an Olympus Vanox microscope (PhotoPath; Duke University Medical Center).
Wax moth infection studies
Wax moths (Galleria mellonella) of the final instar larval stage (~0.3 g) from Vanderhorst Wholesale, Inc. (St. Marys, OH) were used (10 per strain) within 7 d from the day of shipment. The larval infection protocol was adapted from previously described methods for C. neoformans (Mylonakis et al. 2005) with minor modifications. Each larva was infected with 5 × 104 or 1 × 105 C. gattii cells in 5 µL of PBS by injection into the last pseudopod followed by incubation at 24° in a Petri dish with wood shavings. Larvae showing signs of severe morbidity, such as changes in body color and no response to touch were killed by cold treatment at −20°. The number of surviving wax moths was monitored and recorded daily.
Results
Calcineurin is critical for thermotolerance of C. gattii
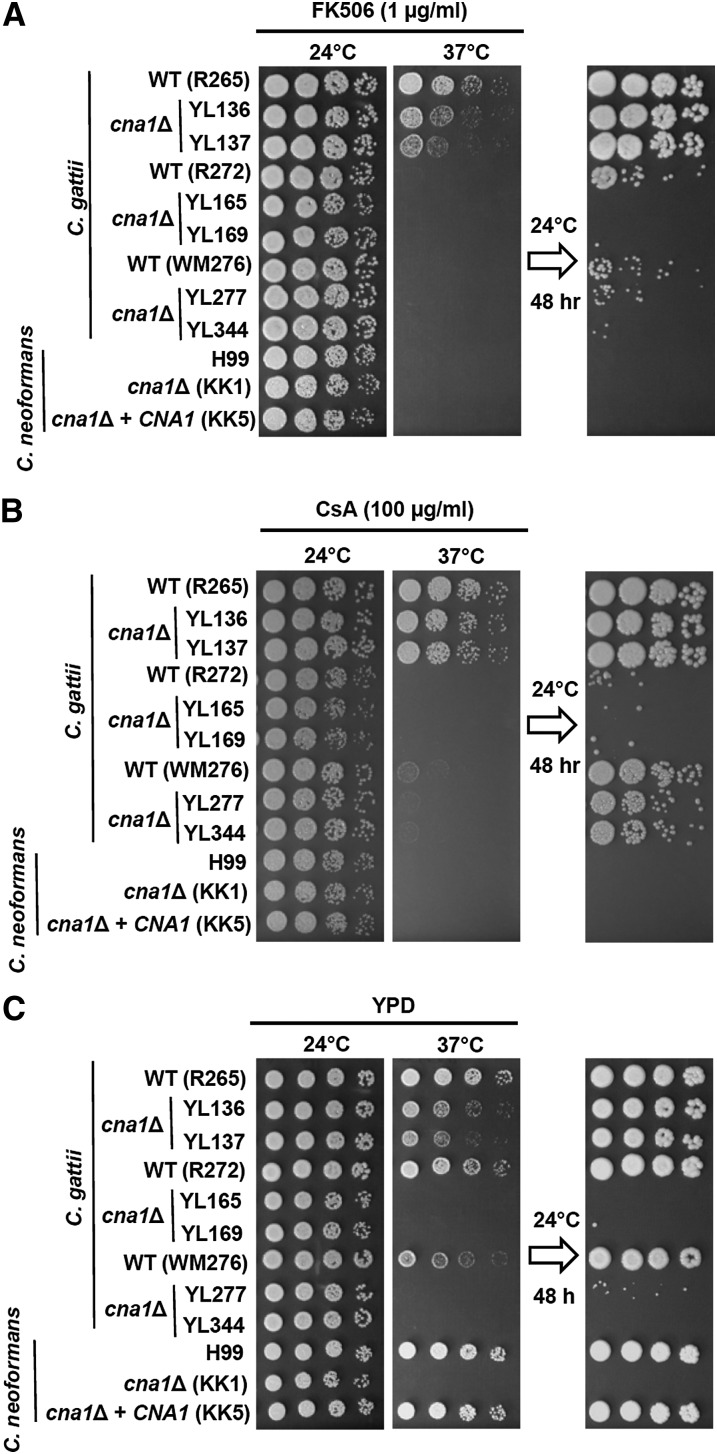
The ability to grow at host body temperature (37°) is a critical virulence factor for pathogens to establish infection. Previous studies on Sod1, Tps1, Tps2, Pka1, Pka2, Ste12α, and Gat1 have revealed examples of divergent functions between C. gattii and C. neoformans. Here, we examined whether C. gattii calcineurin has divergent or conserved functions compared with C. neoformans. We first tested the effect of the calcineurin inhibitors FK506 and cyclosporin A (CsA) on C. gattii growth at room (24°) and body (37°) temperatures. We found that C. gattii R265, R272, WM276, and C. neoformans H99 wild-type strains exhibited different responses at 37° in the presence of FK506 or CsA (Figure 1, A and B). C. gattii R265 was more tolerant to growth at 37° in the presence of a calcineurin inhibitor compared with the R272, WM276, and H99 strains (Figure 1, A and B). This finding suggested that (1) calcineurin could be less important for growth at 37° in the C. gattii R265 isolate; (2) FK506 is either not efficiently imported into the cells, or is being rapidly exported; or (3) FKBP12 is not sufficiently abundant to inhibit all calcineurin activity.
Figure 1 .
Calcineurin is critical for thermotolerance in C. gattii. (A and B) Inhibition of calcineurin via calcineurin inhibitors (FK506, CsA). Cells were grown overnight in YPD at 24°, fivefold serially diluted, spotted onto YPD medium containing FK506 (A) or cyclosporin A (CsA) (B), and incubated at the indicated temperatures for 48 hr. The plate incubated at 37° was transferred to 24° for 48-hr incubation to test viability of the strains after FK506 or CsA inhibition at 37°. (C) Deletion of C. gattii calcineurin catalytic A subunit results in temperature sensitivity. Cells were grown overnight in YPD at 24°, fivefold serially diluted, spotted onto YPD medium, and incubated at the indicated temperatures for 48 hr. The plate incubated at 37° was transferred to 24° for 48-hr incubation to test whether the temperature-sensitive calcineurin mutants survived exposure to the nonpermissive temperature.
To test these models and determine whether the phenotypes observed are due to pharmacological inhibition of calcineurin, we genetically disrupted the calcineurin catalytic subunit A gene from the three C. gattii isolates representing the VGIIa (R265), VGIIb (R272), and VGI (WM276) molecular genotypes. Although R265 cna1Δ mutants were more tolerant to growth on solid medium at 37° compared with cna1Δ mutants generated from C. gattii R272, WM276, and C. neoformans H99 strains (Figure 1C), the R265 cna1Δ mutants did exhibit a clear growth defect at 37° that was more severe than the modest impact of the calcineurin inhibitor FK506 or CsA on strain R265 growth at 37°. Thus, either drug import is limiting, drug export is more prominent, or FKBP12 or cyclophilin A is limiting. In addition, we noted that the R265 cna1Δ mutants exhibited growth defects in the presence of FK506 (but not CsA) compared with R265 wild-type in the presence of FK506 (Figures 1, A and B), providing evidence that FK506 may have target(s) in addition to calcineurin upon exposure to 37° in C. gattii R265.
To test whether loss of calcineurin function exerts a fungicidal effect at 37°, we shifted cells to 24° after exposure at 37°. We found that most calcineurin mutant cells from C. gattii R272, WM276, and C. neoformans H99 grown at 37° for 48 hr were inviable after transferring to 24° for 48 hr growth, whereas most calcineurin mutant cells from R265 were viable (Figure 1). Previous studies demonstrated that Candida glabrata calcineurin mutants are also temperature sensitive and the growth defects at elevated temperature can be rescued with an osmotic stabilizer (1 M sorbitol) (Chen et al. 2012). We then tested whether sorbitol can rescue growth defects of C. gattii and C. neoformans calcineurin mutants at 37°. We found that sorbitol could not rescue the growth defects of C. gattii or C. neoformans calcineurin mutants at 37° (data not shown), suggesting divergent roles of calcineurin for controlling osmotic responses between Cryptococcus species and C. glabrata.
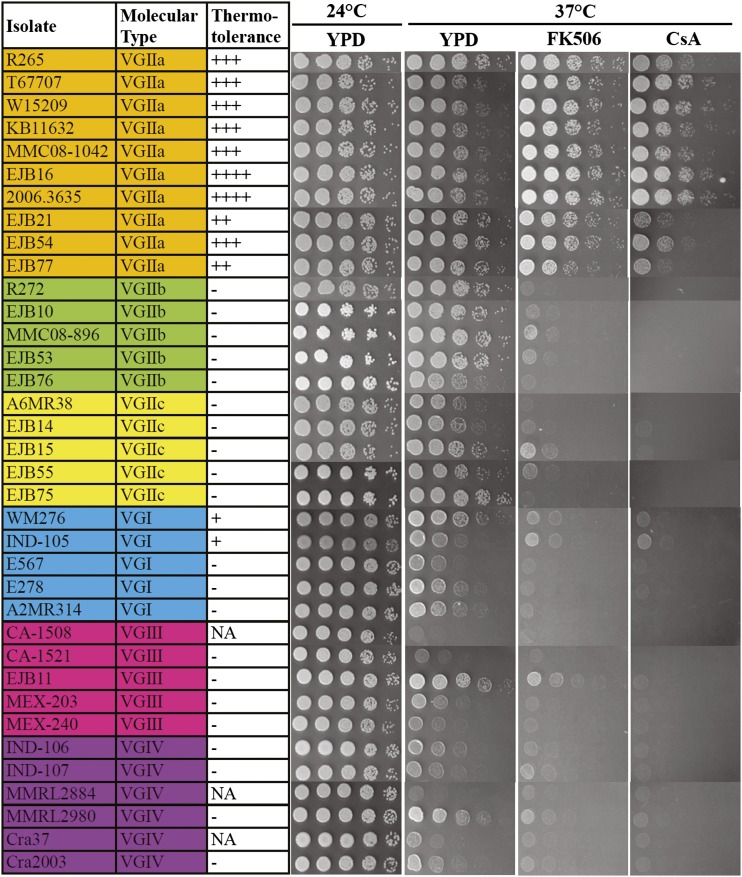
Roles of calcineurin in thermotolerance are distinct in the C. gattii outbreak isolate R265 compared with the C. gattii R272 and WM276 or C. neoformans H99 isolates (Figure 1). We hypothesize that tolerance for growth at 37° despite loss of calcineurin is specific to VGIIa. We therefore investigated 36 C. gattii isolates representing all four molecular types, VGI through VGIV. In VGII, representatives of the Pacific Northwest outbreak VGIIa/major, VGIIb/minor, and VGIIc/novel genotypes were also included. Our hypothesis is supported by the findings shown in Figure 2 as all VGIIa isolates (100%; 10/10) were more tolerant to growth on solid agar medium at 37° in the presence of FK506 or CsA compared with isolates of the VGI, VGIIb, VGIIc, VGIII, and VGIV molecular types, suggesting a divergent role for calcineurin in controlling thermotolerance among different molecular types of C. gattii.
Figure 2 .
Molecular-type VGIIa isolates are more tolerant to calcineurin inhibitors at 37°. Thirty-six randomly selected C. gattii isolates covering four molecular types [VGI, VGII(a,b,c), VGIII, and VGIV] were grown overnight in YPD at 30°, fivefold serially diluted, spotted onto YPD medium in the absence or presence of FK506 (1 µg/mL) and CsA (100 µg/mL), and incubated for 48 hr at 24° and 37°. FK506 and CsA have no obvious effects on cryptococcal growth at 24°, and thus data are not shown. The calcineurin inhibitor tolerance of R265 was set as a control scale with three pluses (+++). The isolates that were hypersensitive to calcineurin inhibitor compared with R265 were labeled with ++ or + symbol, whereas isolates that were extremely sensitive to FK506 or CsA were given a – symbol, indicating that calcineurin is essential in the isolate. NA indicates not available due to the wild-type strain being hypersensitive to growth at 37° in the absence of either calcineurin inhibitor.
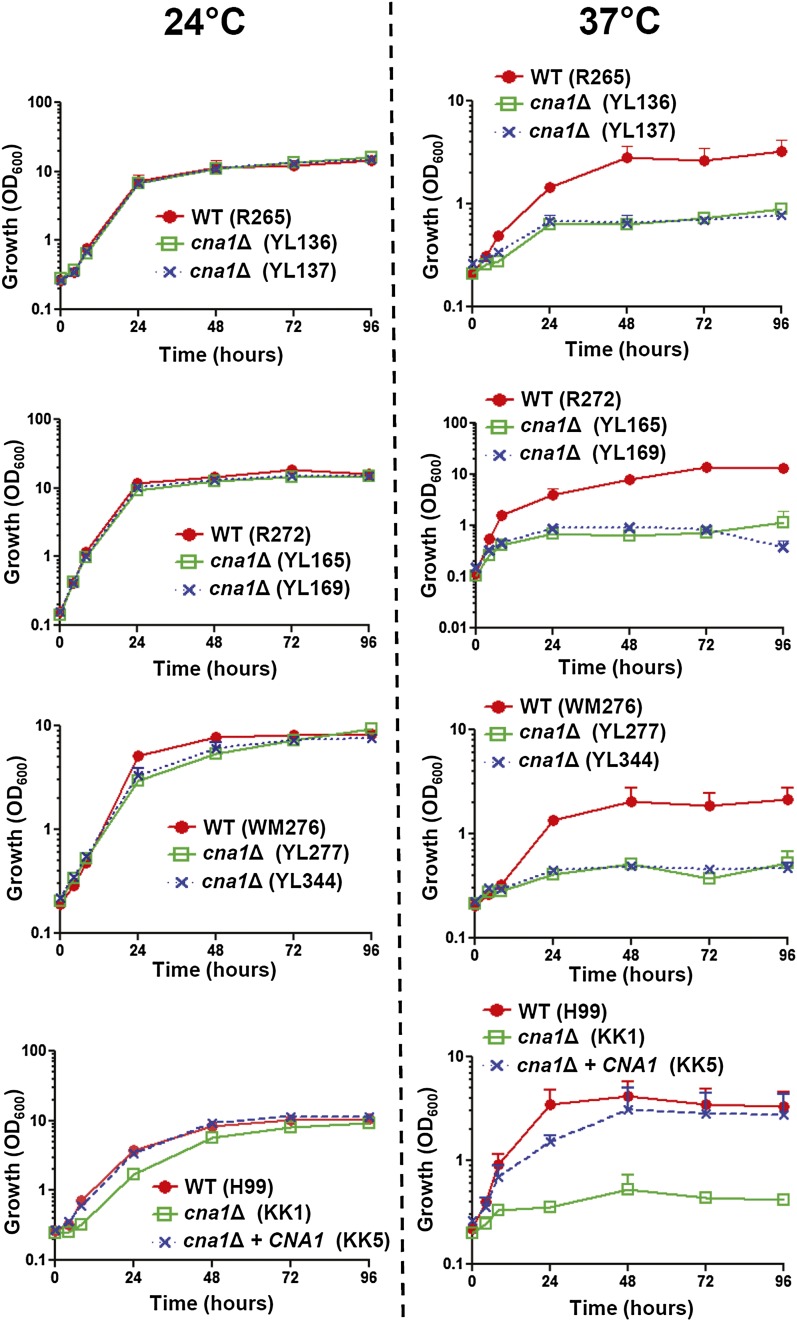
The growth kinetics (in liquid medium) of wild-type and calcineurin mutants from three C. gattii molecular type backgrounds were similar at 24°, whereas C. neoformans calcineurin mutants exhibited a slightly slower growth rate compared with the wild-type or complemented strains (Figure 3, left panel). At 37°, C. gattii and C. neoformans calcineurin mutants exhibited a slower growth rate compared with the wild-type (Figure 3, right panel), including calcineurin mutants of the VGIIa outbreak strain R265.
Figure 3 .
Growth kinetics of wild-type and calcineurin mutants in response to thermal stress in C. gattii and C. neoformans. Cells were grown overnight at 24°, washed twice with dH2O, diluted to 0.2 OD600/mL in fresh YPD medium, and incubated at 24° (left) and 37° (right) with shaking. The OD600 of cultures was measured at 0, 4, 8, 24, 48, 72, and 96 hr. The experiments were performed in triplicate, and data were plotted using Prism 5.03.
Complementation of cna1 mutant and isolation of suppressor mutations
Because R272 cna1 mutants exhibit severe growth defects at 37°, this phenotype led us to isolate (1) CNA1 complemented strains and (2) suppressed mutants via screening surviving colonies at a stringent cna1 non-permissive condition (38°) after biolistic transformation. We used the R272 CNA1 ORF flanked by ~1 kb of the 5′ NCR and 3′ NCR regions (~4.6 kb in total) to complement the cna1 mutation in strain YL165 and isolated three types of transformants able to grow at 38°, including: *1) CNA1 native-locus integration (YC875; CNA1 positive, NAT negative), (2) CNA1 ectopic integration (YC879; CNA1 and NAT positive), and (3) suppressor mutations (YC883 and YC886; CNA1 negative, NAT positive) (Figure S7).
Although all three were restored to growth at 37°, the three types of transformants exhibited different responses to SDS and fluconazole. In the native-locus complemented strain YC875 the growth defects conferred by the cna1 mutation were fully rescued on YPD medium containing SDS or fluconazole, while ectopic complementation (strain YC879) partially rescued the growth defects of the cna1 mutant on YPD medium containing SDS, but not fluconaole (Figure S7B). On the other hand, suppressor mutants YC883 and YC886 were only rescued for the growth defects conferred by the cna1 mutation at 38° but not on YPD medium containing SDS or fluconazole. This suppressor analysis suggests a possible bifurcation of the calcineurin pathway, with one branch responding to temperature stress and another to cell wall/membrane stress. To decipher the nature of these suppressor mutations, we plan to conduct whole genome sequencing in the future.
Calcineurin is essential for virulence in C. gattii
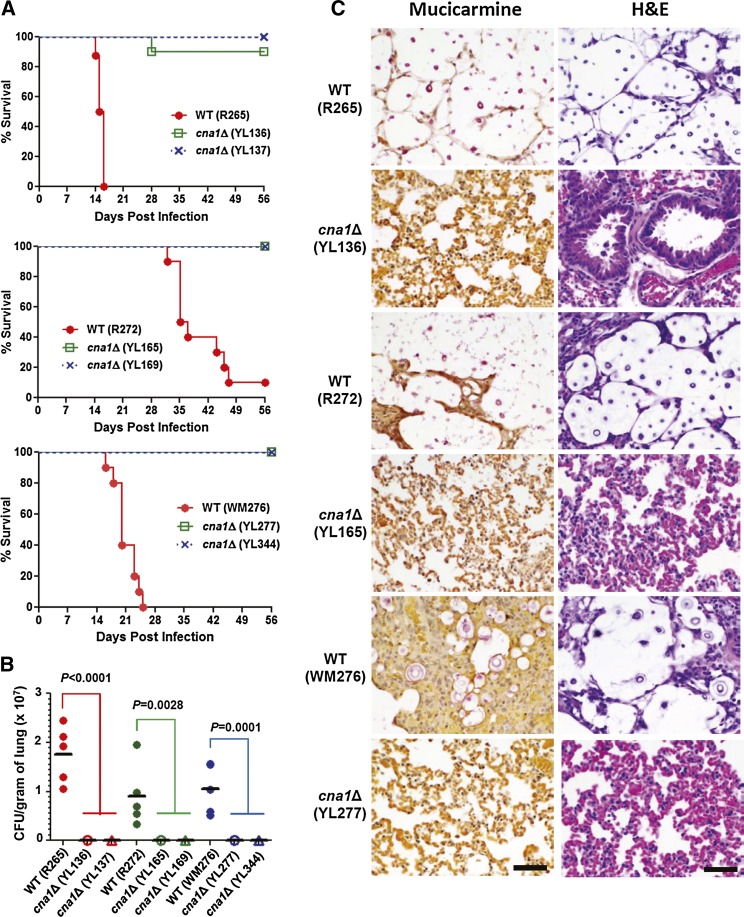
Given the growth defects of calcineurin mutants at mammalian body temperature, we tested the hypothesis that calcineurin would be required for C. gattii virulence in a murine inhalation model. Previous studies have shown that C. neoformans calcineurin is required for virulence in both rabbit and murine models (Cruz et al. 2000; Fox et al. 2001; Odom et al. 1997a). However, the roles of calcineurin in virulence have not yet been studied in C. gattii. We found that the three wild-type strains differ in pathogenicity. R265 VGIIa wild type (median survival: 15.5 days) is more virulent than both WM276 (median survival: 20 days; P = 0.0001; log-rank test) and R272 (median survival: 36 days; P < 0.0001), whereas WM276 is more virulent than the R272 strain (P < 0.0001; Figure 4A and Figure S4A). This finding suggests that a C. gattii environmental strain (WM276) can be as virulent as clinical isolates (R265 and R272). Because C. gattii calcineurin mutants from the three wild-type strains exhibit different responses to temperature, it was of interest to determine whether these mutants are still able to cause infection in a murine inhalation model. We found that C. gattii calcineurin mutants from all three molecular genotype isolates were avirulent or strongly attenuated for virulence (P < 0.0001 as compared to each wild type; Figure 4A).
Figure 4 .
C. gattii calcineurin mutants are compromised for virulence in a murine inhalation infection model. (A) Groups of 10 A/Jcr mice were each infected via intranasal instillation with an infectious inocula of 5 × 104 cells (in 50 µL) of the wild-type and two independent cna1Δ mutants. Survival percentage was monitored for 8 wk postinfection. (B) The fungal burden in the lungs was determined at 14 d postinfection (5 × 104 cells per mouse). Five mice per strain were used. P values were determined by ANOVA and Dunnett’s multiple comparison test. (C) Histopathological sections of lungs dissected from mice infected with wild-type and mutant from R265, R272, and WM276 strains. The mice were infected with 5 × 104 yeast cells and sacrificed at day 14. Mucicarmine and H&E stains were used to observe C. gattii colonization and tissue necrosis, respectively. Scale bar = 50 µm.
The strongly attenuated virulence of calcineurin mutants might be due to reduced or loss of in vivo proliferation in the murine host. Studies in experimental pathobiology and clinical epidemiology have suggested that C. gattii preferentially causes pulmonary infection, whereas C. neoformans preferentially causes disease of the central nervous system (Ngamskulrungroj et al. 2012b; Perfect 2012; Sorrell et al. 2012). To determine colonization ability, we performed fungal burden analysis in the lungs and brains of animals infected with wild type and cna1Δ mutants. We found that calcineurin mutants exhibited strongly reduced fungal burden in the lung tissues compared with the respective wild type isolates (P < 0.01; ANOVA and Dunnett’s multiple comparison test; Figure 4B). Calcineurin mutants exhibited little or no fungal burden in lung tissues, whereas wild-type strains proliferated well (Figure 4B). With regard to brain tissue, we did not recover any calcineurin mutant cells from R265, R272, and WM276, whereas we were able to recover wild-type cells from several, but not all, infected mice (Figure S2).
Histopathological mucicarmine staining of tissues revealed that the wild-type R265, R272, and WM276 strains proliferated extensively in lung tissues, whereas calcineurin mutants from each wild type were not observed (Figure 4C; left panels), suggesting that calcineurin is required for proliferation in the lung. In H&E staining, alveolar damage or tissue necrosis in lung tissues was only observed in animals infected with the wild-type strains but not with the calcineurin mutants (Figure 4C; right panels). The histopathological analysis was well correlated with fungal burden in the lung tissues. We did not observe mucicarmine-stained or damaged tissues in the brain tissues of animals infected with any strain at the time point examined (data not shown). We further test whether C. gattii calcineurin is required for melanin and capsule production. We found that C. gattii calcineurin played no roles in melanin production (Figure S3 and File S1), but minor roles in capsule production (Figure S6).
Calcineurin mutants exhibit multiple vesicle phenotypes and plasmamembrane disruptions upon thermal stress in C. gattii and C. neoformans
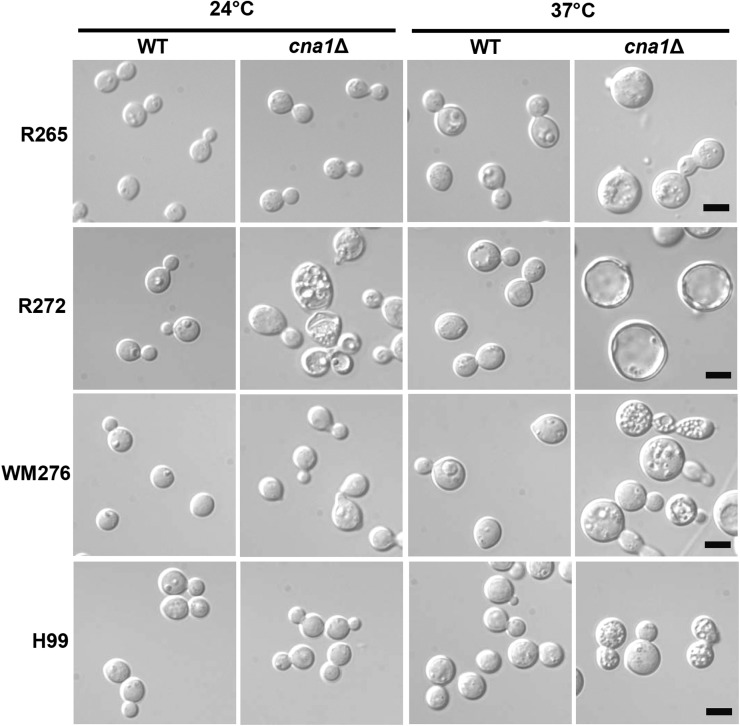
We investigated whether calcineurin mutants exhibit morphological changes when exposed to 24° and 37°. We found that C. gattii calcineurin mutants from R265 and WM276 and C. neoformans H99 exhibited multiple vesicle phenotypes at 37°, whereas these mutants exhibited a normal wild-type morphology at 24° (Figure 5). Interestingly, C. gattii calcineurin mutants from the R272 isolate exhibited more severe phenotypes such as larger ring-like cells in addition to multiple vesicle phenotypes at 37° (Figure 5). In contrast to calcineurin mutants from C. gattii R265 and WM276, and C. neoformans H99 isolates, C. gattii calcineurin mutants from R272 also showed multiple vesicle phenotypes at 24° (Figure 5).
Figure 5 .
Calcineurin mutants exhibit multivesicular phenotypes at 37°. Strains were grown in liquid YPD medium overnight at 24°. Cells were washed twice, diluted into two tubes of 0.4 OD600/mL, and incubated for 5 hr at 24° and 37° to reach log phase. The cells were spun and washed twice with PBS buffer, then resuspended in 5 mL of fixative buffer [1:1 ratio of 0.2 M Sodium cacodylate (pH = 6.8) and 6% glutaraldehyde in dH2O]. The cna1Δ mutants tested were YL136 (from R265), YL165 (from R272), YL277 (from WM276), and KK1 (from H99). Cells were viewed by light microscopy at 1000× magnification and photographed. Scale bar = 5 µm.
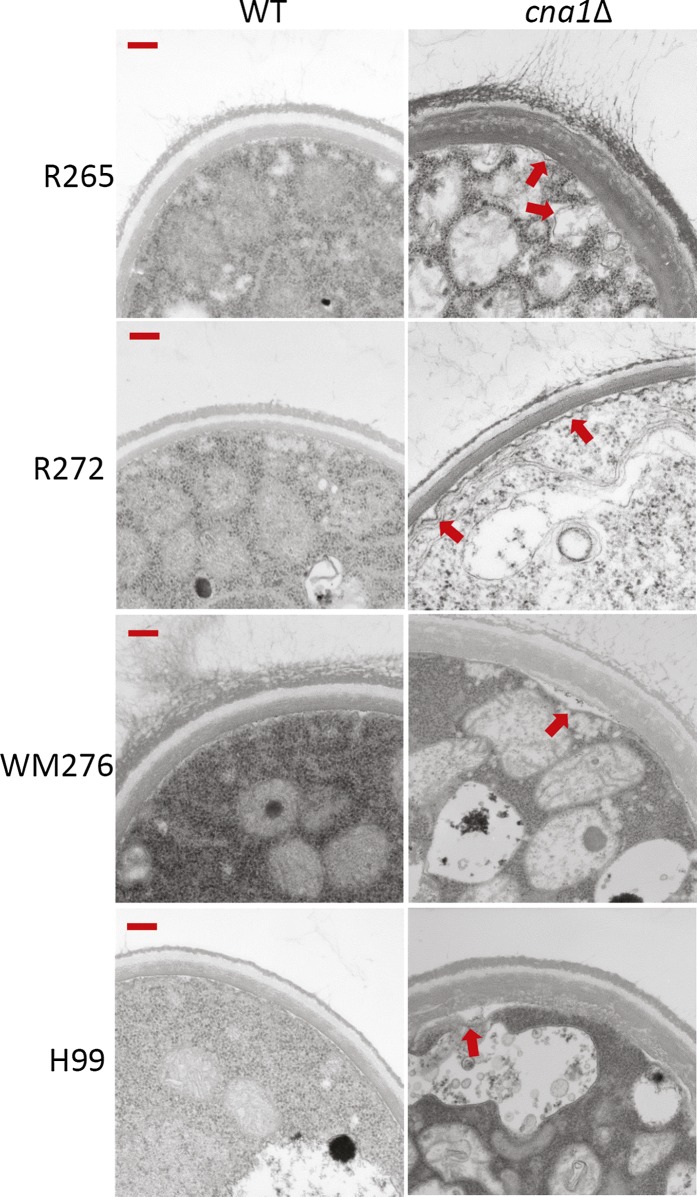
The intracellular defects of C. neoformans calcineurin mutants have not been investigated (Kojima et al. 2006; Odom et al. 1997b). We therefore examined intracellular defects via transmission electron microscopy of C. gattii and C. neoformans calcineurin mutants exposed to 24° and 37°. We found that C. gattii and C. neoformans calcineurin mutants exhibited plasma membrane disruptions at 37° (Figure 6, right panels) but not at 24° (data not shown), whereas wild-type isolates had intact plasma membranes (Figure 6, left panels).
Figure 6 .
Calcineurin mutants exhibit plasma membrane disruptions at 37° (indicated by red arrows). Cell growth and fixation conditions are described in Materials and Methods. Transmission electron microscopy was used to observe intracellular architecture. Only cells grown at 37° are shown. Images were taken at 25,000× magnification. Scale bar = 200 nm.
Divergent roles of calcineurin in cell membrane and cell wall integrity and endoplasmic reticulum (ER) stress responses
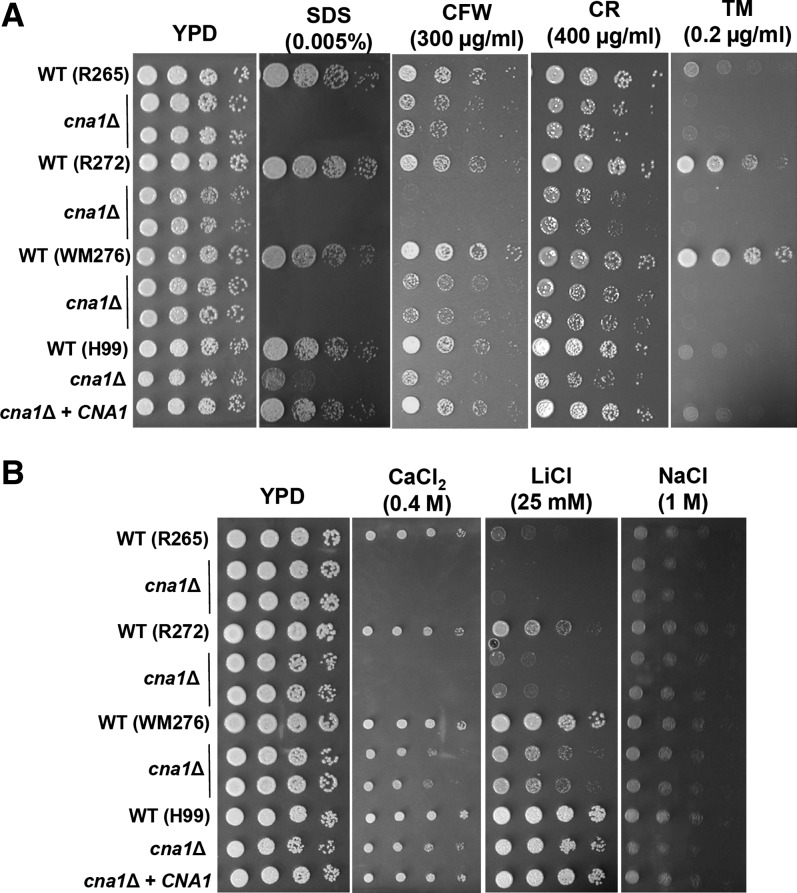
The roles of C. neoformans calcineurin on cell membrane and cell wall integrity remain elusive (Kojima et al. 2006; Odom et al. 1997b). Here, we demonstrate that in C. gattii and C. neoformans, calcineurin is required for cell membrane integrity as evidenced by the fact that calcineurin mutants are more sensitive to sodium dodecyl sulfate (SDS) (Figure 7A), a reagent which compromises cell membrane integrity. In C. gattii and C. neoformans calcineurin plays minor roles in response to the cell wall-disturbing agents calcofluor white and Congo red compared with SDS (Figure 7A).
Figure 7 .
Divergent roles of calcineurin in cell wall integrity, ER stress response, and cation homeostasis. (A) Cells were grown overnight in YPD at 24°, fivefold serially diluted, and spotted onto YPD medium containing SDS, calcofluor white (CFW), Congo red (CR), and tunicamycin (TM) at the indicated concentrations and incubated at 24° for 48 hr. (B) In C. gattii calcineurin plays a larger role than in C. neoformans in controlling cation homeostasis. Cells were grown overnight in YPD at 24°; fivefold serially diluted; spotted onto YPD medium containing CaCl2, LiCl, or NaCl at the indicated concentrations; and incubated at 24°C for 48 hr.
Recently, Kozubowski et al. demonstrated that C. neoformans calcineurin (Cna1) interacts with the COPI component Sec28 and the COPII component Sec13 (Kozubowski et al. 2011), which are involved in Golgi-to-ER protein traffic and vesicle formation during ER-to-Golgi transport, respectively. We were interested in investigating the roles of calcineurin in response to the ER stress inducer tunicamycin, a chemical that blocks the synthesis of N-linked glycoproteins and induces the unfolded protein response. We found that in C. gattii and C. neoformans calcineurin is essential for ER stress responses because calcineurin mutants are sensitive compared to wild-type isolates (Figure 7A). Interestingly, we found that Cryptococcus wild-type isolates tolerate tunicamycin differently, with C. gattii WM276 > R272 > R265 = C. neoformans H99 (Figure 7A), indicating that the unfolded protein response pathway might be evolutionary diverged between C. gattii and C. neoformans.
Divergent roles of calcineurin in Ca2+ and Li+ homeostasis
The role of C. neoformans calcineurin in response to Ca2+ cation remains elusive (Cruz et al. 2000; Kojima et al. 2006; Odom et al. 1997b). Calcineurin plays a role as a positive regulator of Ca2+ tolerance in C. albicans, C. dubliniensis, and C. lusitaniae (Chen et al. 2011; Sanglard et al. 2003; Zhang et al. 2012b), whereas it acts as a negative regulator of Ca2+ tolerance in S. cerevisiae and C. glabrata (Chen et al. 2012; Withee et al. 1998), suggesting divergent functions of calcineurin in controlling Ca2+ homeostasis in fungi. We demonstrated that C. gattii requires calcineurin for optimal growth in the presence of Ca2+ (Figure 7B). In contrast to C. gattii calcineurin mutants, those of C. neoformans do not show obvious growth defects in response to Ca2+ compared with the wild type (Figure 7B), indicating a divergent role of calcineurin in controlling Ca2+ homeostasis between C. gattii and C. neoformans. Here, we also examined the role of calcineurin in response to Li+ cations and found that in both C. gattii and C. neoformans calcineurin plays a role similar to the Ca2+ response (Figure 7B), indicating that individual C. gattii or C. neoformans isolates have similar mechanisms in controlling Ca2+ and Li+ homeostasis. A previous study has been shown that calcineurin is required for Na+ homeostasis in C. neoformans (Cruz et al. 2000). Our data revealed that calcineurin plays a minor role in controlling Na+ homeostasis of C. neoformans H99 whereas calcineurin is not required for Na+ homeostasis in C. gattii R265, R272, and WM276 (Figure 7B).
Divergent roles of C. gattii and C. neoformans calcineurin in fluconazole tolerance
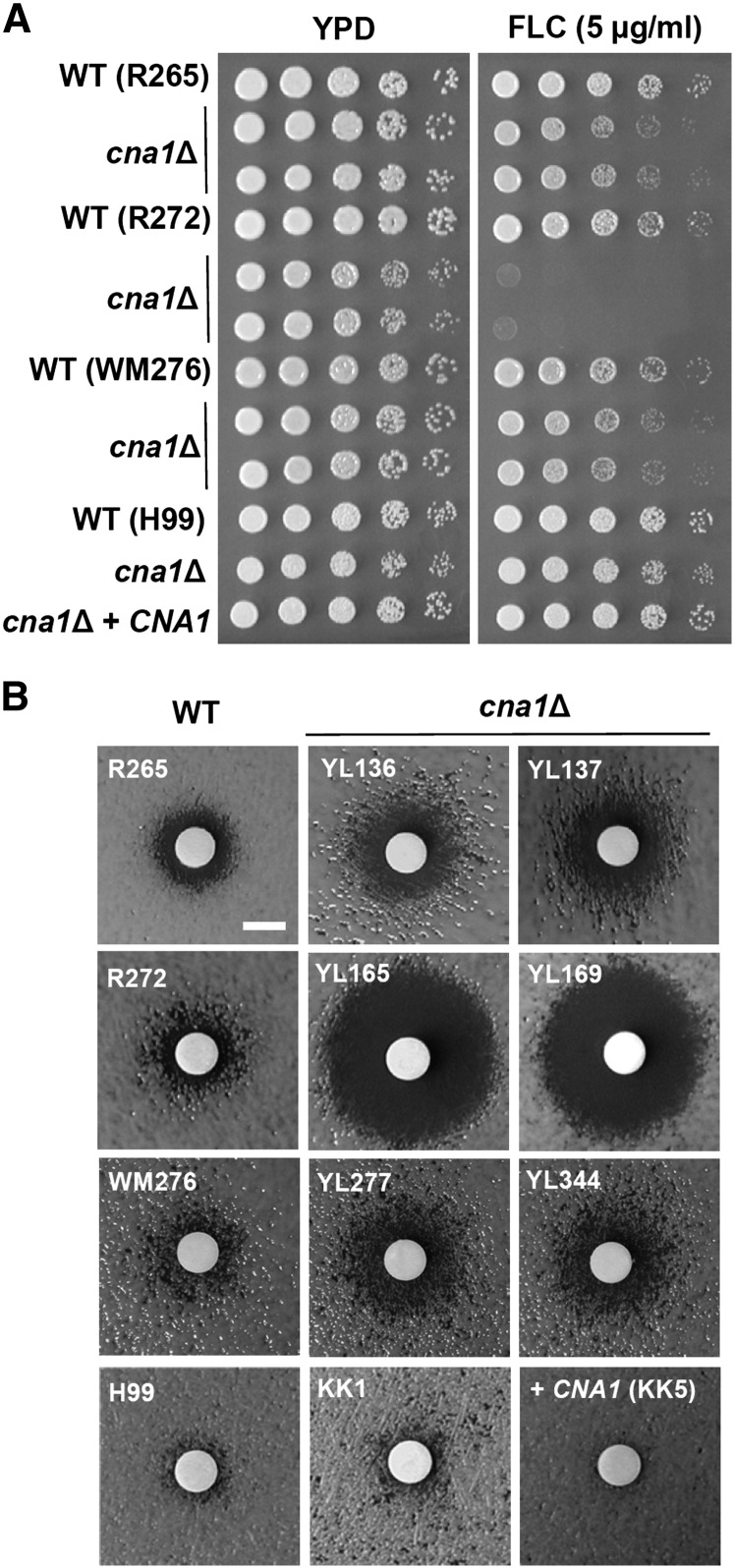
The primary therapy for patients with cryptococcal meningoencephalitis is amphotericin B (0.7 ~1 mg/kg/day, intravenously) plus flucytosine (100 mg/kg/day, orally) in four divided doses for at least 2 wk, followed by fluconazole (6 mg/kg/day, orally) for a minimum of 8 wk (Perfect et al. 2010). Although calcineurin is required for fluconazole tolerance in Candida species (Chen et al. 2011, 2012; Cruz et al. 2002), its role remains unclear in C. neoformans. Del Poeta et al. (2000) reported that FK506 exhibits in vitro synergistic antifungal activity with fluconazole against C. neoformans wild-type H99, the cna1Δ mutant, and the frr1Δ mutant (as evidence of FIC = 0.25), suggesting that FK506 shows synergistic antifungal activity with fluconazole via a mechanism that is independent of the FK506 target proteins calcineurin and FKBP12 (Del Poeta et al. 2000). Here, we demonstrate that C. neoformans H99 calcineurin is not required for fluconazole tolerance, as shown by the fact that calcineurin mutants exhibited similar growth via spotting assays (Figure 8A), inhibition halos via disk diffusion assays (Figure 8B), or MIC values via E-test (Table 2) compared with the wild-type in the presence of fluconazole. In contrast to C. neoformans calcineurin, we found that C. gattii calcineurin plays a greater role in fluconazole tolerance, especially in the R272 background, as evidenced by the fact that calcineurin mutants exhibited strong growth defects in spotting assays (Figure 8A), larger inhibition halos in disk diffusion assays (Figure 8B), and an eightfold increased fluconazole susceptibility compared with the wild type (Table 2). Calcineurin mutants from the C. gattii R265 and WM276 strains exhibited modest sensitivity to fluconazole compared with their corresponding wild-type isolates (Figure 8 and Table 2).
Figure 8 .
Divergent roles of calcineurin in fluconazole tolerance between C. gattii and C. neoformans. (A) Cells were grown overnight in YPD medium at 24°, fivefold serially diluted, and spotted onto YPD medium in the absence or presence of fluconazole (5 µg/mL). The plates were incubated at 24° for 48 hr. (B) Disk diffusion halo assays were used to determine fluconazole susceptibility of wild-type and mutant strains. Cells were grown overnight at 24°, and 100 µL of 0.1 OD600/mL was spread on the surface of YPD medium. A disk was placed on the surface of the medium, and fluconazole (20 µg) was added to each disk. The plates were incubated at 24° for 48 hr and photographed. Scale bar = 6 mm.
Table 2. Drug susceptibility of wild-type and calcineurin mutants of C. gattii and C. neoformans.
| Strains | Fluconazole | Voriconazole | Amphotericin B | Caspofungin |
|---|---|---|---|---|
| WT (R265) | 32~48a | 0.38 | 0.125~0.19 | >32 |
| cna1Δ (YL136) | 16 | 0.125 | 0.38 | >32 |
| cna1Δ (YL137) | 16 | 0.125 | 0.38 | >32 |
| WT (R272) | 64 | 0.25 | 0.25 | >32 |
| cna1Δ (YL165) | 8 | 0.064 | 0.38 | >32 |
| cna1Δ (YL169) | 8 | 0.064 | 0.38 | >32 |
| WT (WM276) | 32~48 | 0.25 | 0.25 | >32 |
| cna1Δ (YL277) | 16 | 0.125 | 0.38 | >32 |
| cna1Δ (YL344) | 16 | 0.125 | 0.38 | >32 |
| WT (H99) | 128 | 0.5 | 0.25 | >32 |
| cna1Δ (KK1) | 96 | 0.25 | 0.38 | >32 |
| cna1Δ + CNA1 (KK5) | 96 | 0.5 | 0.25 | >32 |
WT, wild type; YPD, yeast extract peptone dextrose; dH2O, distilled water; OD, optical density.
Cells were grown overnight in YPD and washed twice with sterile dH2O before diluting to 1 OD600/mL. A total of 0.5 mL (= 0.5 OD) of cells were spread on RPMI-1640 medium (Remel; w/MOPS and 2% glucose; pH 7.3 ± 0.1) and incubated at 24° for 48 hr before reading the E-test values. The numbers indicate minimum inhibitory concentrations (µg/mL).
Discussion
Roles of calcineurin in thermotolerance and virulence in C. gattii and C. neoformans
In this study, we demonstrate that C. gattii requires calcineurin (Cna1) for thermotolerance and virulence in a murine inhalation model. Although calcineurin mutants from C. gattii R265 are more tolerant to growth at 37° (Figure 1), 38°, and 39° (data not shown) on solid medium compared with mutants from R272 and WM276, they exhibited similar sensitivity at 37° in liquid medium compared with calcineurin mutants from R272 and WM276 (Figure 3). The growth defects of C. gattii calcineurin mutants at 37° were strongly correlated with attenuated virulence, suggesting that C. gattii uses calcineurin for survival and infection at body temperature. Therefore, the mechanism via which calcineurin promotes pathogenicity involves thermotolerance and is conserved between C. gattii and C. neoformans. Similarly, previous studies have shown that Candida glabrata and the protozoan parasite Leishmania major use calcineurin to cause infection via a thermotolerance mechanism (Chen et al. 2012; Naderer et al. 2011), suggesting convergent evolution of calcineurin roles in thermotolerance and virulence in basidiomycete and ascomycete yeast pathogens as well as a common parasite. However, in contrast to Cryptococcus species and C. glabrata, other pathogenic fungi use calcineurin in different mechanisms for pathogenicity: C. albicans (serum survival), C. dubliniensis (hyphal growth), C. lusitaniae (serum growth), A. fumigatus (filamentous growth), and M. oryzae (appresorial formation) (Chen et al. 2011; Choi et al. 2009; Cruz et al. 2002; Steinbach et al. 2006; Zhang et al. 2012b), suggesting divergent roles of fungal calcineurin for pathogenesis.
Other mechanisms that C. gattii and C. neoformans calcineurin may employ during infection
Because calcineurin mutants have pleotropic phenotypes linked to virulence, C. gattii and C. neoformans might use calcineurin via other functions that enable pathogenicity. For example, C. gattii calcineurin mutants exhibit defects in cell membrane and cell wall integrity, as well as in ER stress responses (Figure 7). The fungal cells with defects in cell wall integrity and/or ER stress responses often exhibit strongly attenuated virulence in murine infection models (Chen et al. 2010b; Cheon et al. 2011). The wax moth infection model has been widely used as an alternative heterologous host virulence model for cryptococcal infection (Mylonakis et al. 2005). We demonstrated that C. gattii R265, R272, and WM276 wild-type strains exhibited similar virulence between murine inhalation and wax moth infection models (Figure S4). Using a wax moth infection model incubated at 24°, we demonstrated that C. gattii calcineurin is required for virulence in the R265 and WM276 strain background (Figure S5; P < 0.01; log-rank test), indicating that calcineurin also employs a mechanism other than thermotolerance for pathogenicity in the wax moth model. Interestingly, in the C. gattii R272 background calcineurin is only required for virulence in murine inhalation (37°), but not in a wax moth model (24°) (Figure S5), indicating that in C. gattii R272 the role of calcineurin in thermotolerance may be the major mechanism promoting infection in the murine inhalation model. However, the fact that the overall level of virulence of the R272 strain is reduced in the wax moth is a caveat to this conclusion.
Role of calcineurin in azole tolerance of C. gattii
Azoles target fungal 14α-demethylase encoded by ERG11, which is involved in ergosterol biosynthesis and cell membrane/wall integrity (Vanden Bossche et al. 1998). Cell membrane perturbation by fluconazole can enhance uptake and toxicity of calcineurin inhibitors in Candida albicans (Cruz et al. 2002). Conversely, it is also possible that cell membrane defects caused by the cna1Δ mutation results in increased azole uptake, leading to synergistic fungicidal activity. Calcineurin has been demonstrated to be required for azole tolerance and/or resistance in the ascomycetous yeast pathogens C. albicans, C. dubliniensis, C. glabrata, and C. lusitaniae (Chen et al. 2011, 2012; Sanglard et al. 2003; Zhang et al. 2012a,b), but whether calcineurin functions in an analogous role in the basidiomycetous yeasts C. neoformans and C. gattii has been less clear. Previous studies demonstrated that the calcineurin inhibitor FK506 exhibits synergistic antifungal activity with fluconazole against C. neoformans strain H99, but the synergistic mechanism was found to be FKBP12- and calcineurin-independent (Del Poeta et al. 2000), indicating that FK506 might have additional targets in C. neoformans. Our data demonstrating that calcineurin is required for azole tolerance in C. gattii but not in C. neoformans support the findings by Del Poeta et al. (2000) and provide evidence for a divergent role of calcineurin in azole tolerance between C. gattii and C. neoformans. Even in C. gattii, three molecular types (VGIIa, VGIIb, and VGI) exhibit different levels of azole tolerance. For example, R272 cna1Δ mutants are hypersensitive to fluconazole compared with R265 and WM272 cna1Δ mutants (Figure 8 and Table 2). Because R272 cna1Δ mutants exhibit hypersensitivity to cell membrane/wall disturbing agents (Figure 7) that is correlated with increased azole susceptibility (Figure 8), we suggest that cell membrane defects caused by cna1Δ mutation may result in azole hypersensitivity.
In summary, our data suggest that in C. gattii calcineurin promotes thermotolerance and other mechanisms during infection in both the murine inhalation and wax moth models, in addition to a requirement for calcineurin in azole tolerance of C. gattii. Therefore, our studies provide support for C. gattii calcineurin as an attractive target for developing calcineurin inhibitors that retain antifungal activity but are reduced for immunosuppressive activity.
Supplementary Material
Acknowledgments
We thank Valerie Lapham (North Carolina State University) for assistance with transmission electron microscopy, Laura Simone for wax moth infection experiments, the Duke Pathology department for Mucicarmine and H&E staining, and the “Cryptococcus gattii Sequencing Project, Broad Institute of Harvard and MIT (http://www.broadinstitute.org/)” for DNA and protein sequences. This work was supported in part by the Duke University Center for AIDS Research (CFAR), assistance with National Institutes of Health (NIH)-funded program (2P30 AI064518-06 to Y.-L.C.) and NIH/NIAID R01 grant AI50438 (J.H.). This work was also supported by Pilot funds from Astellas Pharma, Inc. and Merck & Co., Inc. (J.H. and Y. -L. C.). The funders had no role in study design, data collection, analysis, decision to publish, or preparation of the manuscript.
Footnotes
Communicating editor: R. B. Brem
Literature Cited
- Blankenship J. R., Wormley F. L., Boyce M. K., Schell W. A., Filler S. G., et al. , 2003. Calcineurin is essential for Candida albicans survival in serum and virulence. Eukaryot. Cell 2: 422–430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bovers M., Hagen F., Kuramae E. E., Boekhout T., 2008. Six monophyletic lineages identified within Cryptococcus neoformans and Cryptococcus gattii by multi-locus sequence typing. Fungal Genet. Biol. 45: 400–421 [DOI] [PubMed] [Google Scholar]
- Byrnes E. J., Li W., Lewit Y., Ma H., Voelz K., et al. , 2010. Emergence and pathogenicity of highly virulent Cryptococcus gattii genotypes in the Northwest United States. PLoS Pathog. 6: e1000850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrnes E. J., III, Li W., Ren P., Lewit Y., Voelz K., et al. , 2011. A diverse population of Cryptococcus gattii molecular type VGIII in southern Californian HIV/AIDS patients. PLoS Pathog. 7: e1002205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaturvedi V., Chaturvedi S., 2011. Cryptococcus gattii: a resurgent fungal pathogen. Trends Microbiol. 19: 564–571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y. L., Kozubowski L., Cardenas M. E., Heitman J., 2010a On the roles of calcineurin in fungal growth and pathogenesis. Curr Fungal Infect Rep 4: 244–255 [Google Scholar]
- Chen Y. L., Montedonico A. E., Kauffman S., Dunlap J. R., Menn F. M., et al. , 2010b Phosphatidylserine synthase and phosphatidylserine decarboxylase are essential for cell wall integrity and virulence in Candida albicans. Mol. Microbiol. 75: 1112–1132 [DOI] [PubMed] [Google Scholar]
- Chen Y. L., Brand A., Morrison E. L., Silao F. G., Bigol U. G., et al. , 2011. Calcineurin controls drug tolerance, hyphal growth, and virulence in Candida dubliniensis. Eukaryot. Cell 10: 803–819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y. L., Konieczka J. H., Springer D. J., Bowen S. E., Zhang J., et al. , 2012. Convergent evolution of calcineurin pathway roles in thermotolerance and virulence in Candida glabrata. G3: Genes, Genomes, Genetics 2: 675–691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheon S. A., Jung K. W., Chen Y. L., Heitman J., Bahn Y. S., et al. , 2011. Unique evolution of the UPR pathway with a novel bZIP transcription factor, Hxl1, for controlling pathogenicity of Cryptococcus neoformans. PLoS Pathog. 7: e1002177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J. H., Kim Y., Lee Y. H., 2009. Functional analysis of MCNA, a gene encoding a catalytic subunit of calcineurin, in the rice blast fungus Magnaporthe oryzae. J. Microbiol. Biotechnol. 19: 11–16 [PubMed] [Google Scholar]
- Cruz M. C., Sia R. A., Olson M., Cox G. M., Heitman J., 2000. Comparison of the roles of calcineurin in physiology and virulence in serotype D and serotype A strains of Cryptococcus neoformans. Infect. Immun. 68: 982–985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz M. C., Goldstein A. L., Blankenship J. R., Del Poeta M., Davis D., et al. , 2002. Calcineurin is essential for survival during membrane stress in Candida albicans. EMBO J. 21: 546–559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyert M. S., Kunisawa R., Kaim D., Thorner J., 1991. Yeast has homologs (CNA1 and CNA2 gene products) of mammalian calcineurin, a calmodulin-regulated phosphoprotein phosphatase. Proc. Natl. Acad. Sci. USA 88: 7376–7380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta K., Bartlett K. H., Baer R., Byrnes E., Galanis E., et al. , 2009. Spread of Cryptococcus gattii into Pacific Northwest region of the United States. Emerg. Infect. Dis. 15: 1185–1191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson R. C., Blankenship J. R., Kraus P. R., de Jesus Berrios M., Hull C. M., et al. , 2002. A PCR-based strategy to generate integrative targeting alleles with large regions of homology. Microbiology 148: 2607–2615 [DOI] [PubMed] [Google Scholar]
- Del Poeta M., Cruz M. C., Cardenas M. E., Perfect J. R., Heitman J., 2000. Synergistic antifungal activities of bafilomycin A(1), fluconazole, and the pneumocandin MK-0991/caspofungin acetate (L-743,873) with calcineurin inhibitors FK506 and L-685,818 against Cryptococcus neoformans. Antimicrob. Agents Chemother. 44: 739–746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Souza C. A., Alspaugh J. A., Yue C., Harashima T., Cox G. M., et al. , 2001. Cyclic AMP-dependent protein kinase controls virulence of the fungal pathogen Cryptococcus neoformans. Mol. Cell. Biol. 21: 3179–3191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox D. S., Cruz M. C., Sia R. A., Ke H., Cox G. M., et al. , 2001. Calcineurin regulatory subunit is essential for virulence and mediates interactions with FKBP12–FK506 in Cryptococcus neoformans. Mol. Microbiol. 39: 835–849 [DOI] [PubMed] [Google Scholar]
- Fraser J. A., Giles S. S., Wenink E. C., Geunes-Boyer S. G., Wright J. R., et al. , 2005. Same-sex mating and the origin of the Vancouver Island Cryptococcus gattii outbreak. Nature 437: 1360–1364 [DOI] [PubMed] [Google Scholar]
- French N., Gray K., Watera C., Nakiyingi J., Lugada E., et al. , 2002. Cryptococcal infection in a cohort of HIV-1-infected Ugandan adults. AIDS 16: 1031–1038 [DOI] [PubMed] [Google Scholar]
- Hicks J. K., Heitman J., 2007. Divergence of protein kinase A catalytic subunits in Cryptococcus neoformans and Cryptococcus gattii illustrates evolutionary reconfiguration of a signaling cascade. Eukaryot. Cell 6: 413–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidd S. E., Hagen F., Tscharke R. L., Huynh M., Bartlett K. H., et al. , 2004. A rare genotype of Cryptococcus gattii caused the cryptococcosis outbreak on Vancouver Island (British Columbia, Canada). Proc. Natl. Acad. Sci. USA 101: 17258–17263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidd S. E., Guo H., Bartlett K. H., Xu J., Kronstad J. W., 2005. Comparative gene genealogies indicate that two clonal lineages of Cryptococcus gattii in British Columbia resemble strains from other geographical areas. Eukaryot. Cell 4: 1629–1638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima K., Bahn Y. S., Heitman J., 2006. Calcineurin, Mpk1 and Hog1 MAPK pathways independently control fludioxonil antifungal sensitivity in Cryptococcus neoformans. Microbiology 152: 591–604 [DOI] [PubMed] [Google Scholar]
- Kozubowski L., Thompson J. W., Cardenas M. E., Moseley M. A., Heitman J., 2011. Association of calcineurin with the COPI protein Sec28 and the COPII protein Sec13 revealed by quantitative proteomics. PLoS ONE 6: e25280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon-Chung K. J., Bennett J. E., 1984. High prevalence of Cryptococcus neoformans var. gattii in tropical and subtropical regions. Zentralbl. Bakteriol. Mikrobiol. Hyg. [A] 257: 213–218 [PubMed] [Google Scholar]
- Mylonakis E., Moreno R., El Khoury J. B., Idnurm A., Heitman J., et al. , 2005. Galleria mellonella as a model system to study Cryptococcus neoformans pathogenesis. Infect. Immun. 73: 3842–3850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naderer T., Dandash O., McConville M. J., 2011. Calcineurin is required for Leishmania major stress response pathways and for virulence in the mammalian host. Mol. Microbiol. 80: 471–480 [DOI] [PubMed] [Google Scholar]
- Narasipura S. D., Ault J. G., Behr M. J., Chaturvedi V., Chaturvedi S., 2003. Characterization of Cu, Zn superoxide dismutase (SOD1) gene knock-out mutant of Cryptococcus neoformans var. gattii: role in biology and virulence. Mol. Microbiol. 47: 1681–1694 [DOI] [PubMed] [Google Scholar]
- Nelson R. T., Pryor B. A., Lodge J. K., 2003. Sequence length required for homologous recombination in Cryptococcus neoformans. Fungal Genet. Biol. 38: 1–9 [DOI] [PubMed] [Google Scholar]
- Ngamskulrungroj P., Himmelreich U., Breger J. A., Wilson C., Chayakulkeeree M., et al. , 2009. The trehalose synthesis pathway is an integral part of the virulence composite for Cryptococcus gattii. Infect. Immun. 77: 4584–4596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngamskulrungroj P., Chang Y., Roh J., Kwon-Chung K. J., 2012a Differences in nitrogen metabolism between Cryptococcus neoformans and C. gattii, the two etiologic agents of cryptococcosis. PLoS ONE 7: e34258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngamskulrungroj P., Chang Y., Sionov E., Kwon-Chung K. J., 2012b The primary target organ of Cryptococcus gattii is different from that of Cryptococcus neoformans in a murine model. mBio 3: e00103–00112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odom A., Del Poeta M., Perfect J., Heitman J., 1997a The immunosuppressant FK506 and its nonimmunosuppressive analog L-685,818 are toxic to Cryptococcus neoformans by inhibition of a common target protein. Antimicrob. Agents Chemother. 41: 156–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odom A., Muir S., Lim E., Toffaletti D. L., Perfect J., et al. , 1997b Calcineurin is required for virulence of Cryptococcus neoformans. EMBO J. 16: 2576–2589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park B. J., Wannemuehler K. A., Marston B. J., Govender N., Pappas P. G., et al. , 2009. Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. AIDS 23: 525–530 [DOI] [PubMed] [Google Scholar]
- Perfect J.R. 2012. The triple threat of Cryptococcosis: it’s the body site, the strain, and/or the host. mBio 3: e00165–00112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perfect J. R., Casadevall A., 2012. The history of Cryptococcus and Cryptococcosis, pp. 17–26 in Cryptococcus: From Human Pathogen to Model Yeast, edited by Heitman J., Kozel T. R., Kwon-Chung K. J., Perfect J. R., Casadevall A. American Society for Microbiology, Washington, DC [Google Scholar]
- Perfect J. R., Ketabchi N., Cox G. M., Ingram C. W., Beiser C. L., 1993. Karyotyping of Cryptococcus neoformans as an epidemiological tool. J. Clin. Microbiol. 31: 3305–3309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perfect J. R., Dismukes W. E., Dromer F., Goldman D. L., Graybill J. R., et al. , 2010. Clinical practice guidelines for the management of cryptococcal disease: 2010 update by the infectious diseases society of america. Clin. Infect. Dis. 50: 291–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petzold E. W., Himmelreich U., Mylonakis E., Rude T., Toffaletti D., et al. , 2006. Characterization and regulation of the trehalose synthesis pathway and its importance in the pathogenicity of Cryptococcus neoformans. Infect. Immun. 74: 5877–5887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren P., Springer D. J., Behr M. J., Samsonoff W. A., Chaturvedi S., et al. , 2006. Transcription factor STE12α has distinct roles in morphogenesis, virulence, and ecological fitness of the primary pathogenic yeast Cryptococcus gattii. Eukaryot. Cell 5: 1065–1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusnak F., Mertz P., 2000. Calcineurin: form and function. Physiol. Rev. 80: 1483–1521 [DOI] [PubMed] [Google Scholar]
- Sanglard D., Ischer F., Marchetti O., Entenza J., Bille J., 2003. Calcineurin A of Candida albicans: involvement in antifungal tolerance, cell morphogenesis and virulence. Mol. Microbiol. 48: 959–976 [DOI] [PubMed] [Google Scholar]
- Sorrell T. C., 2001. Cryptococcus neoformans variety gattii. Med. Mycol. 39: 155–168 [PubMed] [Google Scholar]
- Sorrell T. C., Chen S. C.-A., Phillips P., Marr K. A., 2012. Clinical perspectives on Cryptococcus neoformans and Cryptococcus gattii: implications for diagnosis and management, pp. 595–606 in Cryptococcus: From Human Pathogen to Model Yeast, edited by Heitman J., Kozel T. R., Kwon-Chung K. J., Perfect J. R., Casadevall A. American Society for Microbiology, Washington, DC [Google Scholar]
- Steinbach W. J., Cramer R. A., Jr, Perfect B. Z., Asfaw Y. G., Sauer T. C., et al. , 2006. Calcineurin controls growth, morphology, and pathogenicity in Aspergillus fumigatus. Eukaryot. Cell 5: 1091–1103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toffaletti D. L., Rude T. H., Johnston S. A., Durack D. T., Perfect J. R., 1993. Gene transfer in Cryptococcus neoformans by use of biolistic delivery of DNA. J. Bacteriol. 175: 1405–1411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upton A., Fraser J. A., Kidd S. E., Bretz C., Bartlett K. H., et al. , 2007. First contemporary case of human infection with Cryptococcus gattii in Puget Sound: evidence for spread of the Vancouver Island outbreak. J. Clin. Microbiol. 45: 3086–3088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanden Bossche H., Dromer F., Improvisi I., Lozano-Chiu M., Rex J. H., et al. , 1998. Antifungal drug resistance in pathogenic fungi. Med. Mycol. 36(Suppl 1): 119–128 [PubMed] [Google Scholar]
- Withee J. L., Sen R., Cyert M. S., 1998. Ion tolerance of Saccharomyces cerevisiae lacking the Ca2+/CaM-dependent phosphatase (calcineurin) is improved by mutations in URE2 or PMA1. Genetics 149: 865–878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue C., Cavallo L. M., Alspaugh J. A., Wang P., Cox G. M., et al. , 1999. The STE12α homolog is required for haploid filamentation but largely dispensable for mating and virulence in Cryptococcus neoformans. Genetics 153: 1601–1615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Heitman J., Chen Y. L., 2012a Comparative analysis of calcineurin signaling between Candida dubliniensis and Candida albicans. Commun. Integr. Biol. 5: 122–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Silao F. G., Bigol U. G., Bungay A. A., Nicolas M. G., et al. , 2012b Calcineurin is required for pseudohyphal growth, virulence, and drug resistance in Candida lusitaniae. PLoS ONE 7(8): e44192. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.