Abstract
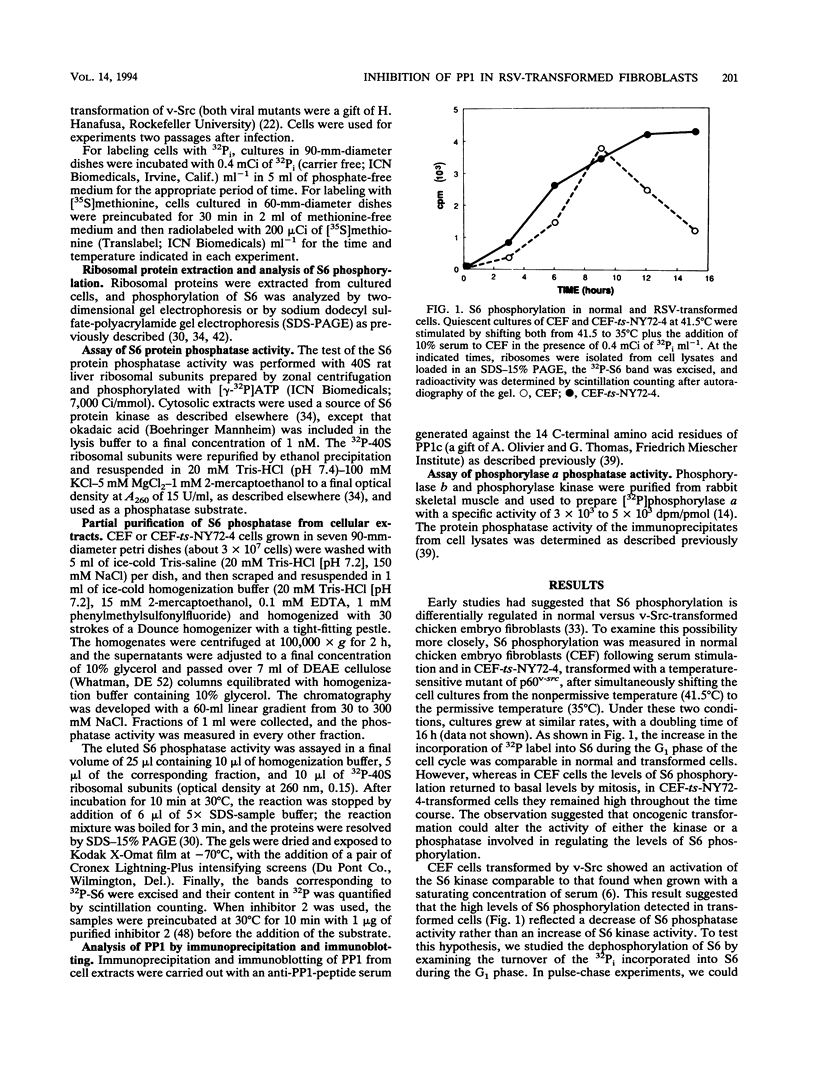
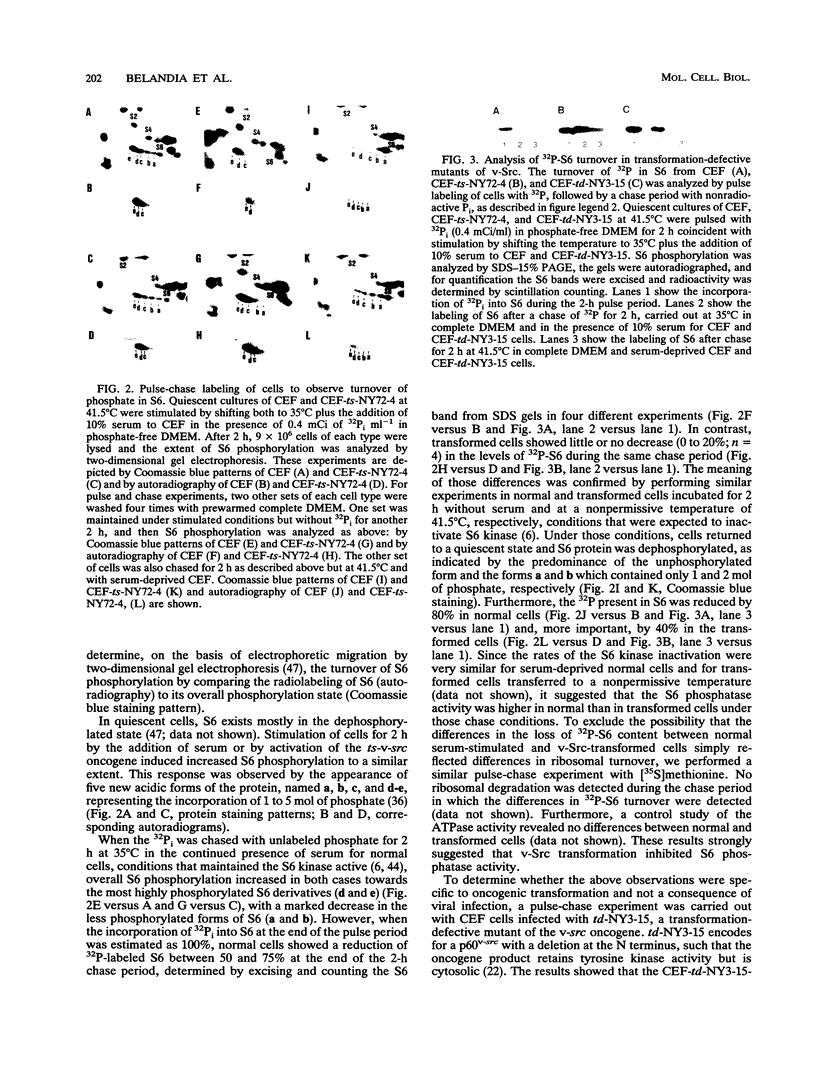
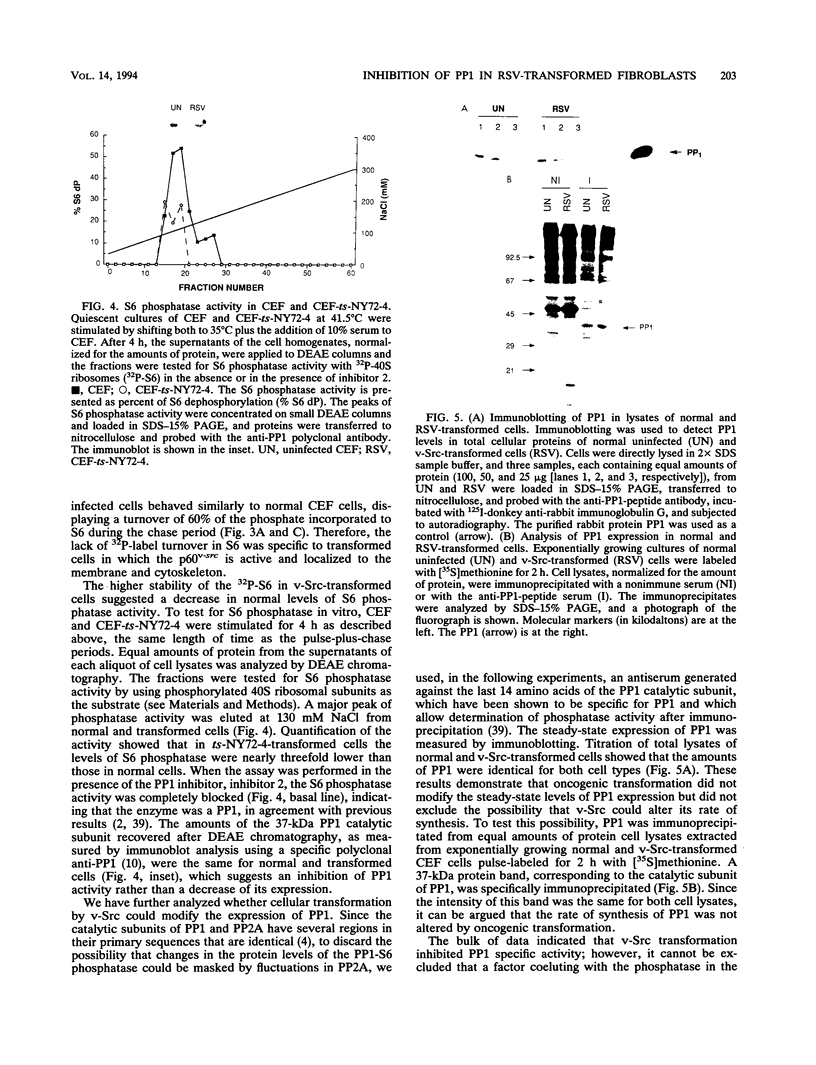
In chicken embryo fibroblasts, phosphorylation of the 40S ribosomal protein S6 increases during G1 but returns to basal level by mitosis. In contrast, in Rous sarcoma virus (RSV)-transformed fibroblasts, S6 remains highly phosphorylated throughout mitosis. This study investigated the mechanism by which RSV alters the pattern of S6 phosphorylation. Pulse-chase experiments demonstrate that phosphate turnover in S6 is rapid in normal cells and in cells infected with an RSV transformation-defective virus. In contrast, phosphate turnover in S6 is severely reduced in cells infected with temperature-sensitive RSV at a temperature permissive for transformation, indicating a diminished S6 phosphatase activity. Fractionation of cell lysates by DEAE chromatography showed an almost threefold lower S6 phosphatase activity in RSV-transformed versus normal cells. The S6 phosphatase was sensitive to inhibitor 2 and specifically recognized by an antibody to type 1 phosphatase (PP1). The S6 phosphatase activity recovered by immunoprecipitation of PP1 was threefold lower in transformed cells, but the steady-state level of expression and the rate of synthesis of PP1 were not altered by oncogenic transformation. Together, the results show that transformation by RSV reduced the S6-PP1 activity.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alessi D., MacDougall L. K., Sola M. M., Ikebe M., Cohen P. The control of protein phosphatase-1 by targetting subunits. The major myosin phosphatase in avian smooth muscle is a novel form of protein phosphatase-1. Eur J Biochem. 1992 Dec 15;210(3):1023–1035. doi: 10.1111/j.1432-1033.1992.tb17508.x. [DOI] [PubMed] [Google Scholar]
- Andres J. L., Maller J. L. Purification and characterization of a novel protein phosphatase highly specific for ribosomal protein S6. J Biol Chem. 1989 Jan 5;264(1):151–156. [PubMed] [Google Scholar]
- Ballou L. M., Jenö P., Thomas G. Protein phosphatase 2A inactivates the mitogen-stimulated S6 kinase from Swiss mouse 3T3 cells. J Biol Chem. 1988 Jan 25;263(3):1188–1194. [PubMed] [Google Scholar]
- Berndt N., Campbell D. G., Caudwell F. B., Cohen P., da Cruz e Silva E. F., da Cruz e Silva O. B., Cohen P. T. Isolation and sequence analysis of a cDNA clone encoding a type-1 protein phosphatase catalytic subunit: homology with protein phosphatase 2A. FEBS Lett. 1987 Nov 2;223(2):340–346. doi: 10.1016/0014-5793(87)80316-2. [DOI] [PubMed] [Google Scholar]
- Blenis J., Erikson R. L. Phosphorylation of the ribosomal protein S6 is elevated in cells transformed by a variety of tumor viruses. J Virol. 1984 Jun;50(3):966–969. doi: 10.1128/jvi.50.3.966-969.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blenis J., Erikson R. L. Regulation of a ribosomal protein S6 kinase activity by the Rous sarcoma virus transforming protein, serum, or phorbol ester. Proc Natl Acad Sci U S A. 1985 Nov;82(22):7621–7625. doi: 10.1073/pnas.82.22.7621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollen M., Stalmans W. The structure, role, and regulation of type 1 protein phosphatases. Crit Rev Biochem Mol Biol. 1992;27(3):227–281. doi: 10.3109/10409239209082564. [DOI] [PubMed] [Google Scholar]
- Booher R., Beach D. Involvement of a type 1 protein phosphatase encoded by bws1+ in fission yeast mitotic control. Cell. 1989 Jun 16;57(6):1009–1016. doi: 10.1016/0092-8674(89)90339-5. [DOI] [PubMed] [Google Scholar]
- Brautigan D. L. Great expectations: protein tyrosine phosphatases in cell regulation. Biochim Biophys Acta. 1992 Sep 14;1114(1):63–77. doi: 10.1016/0304-419x(92)90007-l. [DOI] [PubMed] [Google Scholar]
- Brautigan D. L., Gruppuso P. A., Mumby M. Protein phosphatase type-1 and type-2 catalytic subunits both bind inhibitor-2 and monoclonal immunoglobulins. J Biol Chem. 1986 Nov 15;261(32):14924–14928. [PubMed] [Google Scholar]
- Chan C. P., McNall S. J., Krebs E. G., Fischer E. H. Stimulation of protein phosphatase activity by insulin and growth factors in 3T3 cells. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6257–6261. doi: 10.1073/pnas.85.17.6257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Martin B. L., Brautigan D. L. Regulation of protein serine-threonine phosphatase type-2A by tyrosine phosphorylation. Science. 1992 Aug 28;257(5074):1261–1264. doi: 10.1126/science.1325671. [DOI] [PubMed] [Google Scholar]
- Cohen P. T., Brewis N. D., Hughes V., Mann D. J. Protein serine/threonine phosphatases; an expanding family. FEBS Lett. 1990 Aug 1;268(2):355–359. doi: 10.1016/0014-5793(90)81285-v. [DOI] [PubMed] [Google Scholar]
- Cohen P., Alemany S., Hemmings B. A., Resink T. J., Strålfors P., Tung H. Y. Protein phosphatase-1 and protein phosphatase-2A from rabbit skeletal muscle. Methods Enzymol. 1988;159:390–408. doi: 10.1016/0076-6879(88)59039-0. [DOI] [PubMed] [Google Scholar]
- Cohen P., Cohen P. T. Protein phosphatases come of age. J Biol Chem. 1989 Dec 25;264(36):21435–21438. [PubMed] [Google Scholar]
- Cohen P. The structure and regulation of protein phosphatases. Annu Rev Biochem. 1989;58:453–508. doi: 10.1146/annurev.bi.58.070189.002321. [DOI] [PubMed] [Google Scholar]
- Cross F. R., Garber E. A., Pellman D., Hanafusa H. A short sequence in the p60src N terminus is required for p60src myristylation and membrane association and for cell transformation. Mol Cell Biol. 1984 Sep;4(9):1834–1842. doi: 10.1128/mcb.4.9.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doonan J. H., Morris N. R. The bimG gene of Aspergillus nidulans, required for completion of anaphase, encodes a homolog of mammalian phosphoprotein phosphatase 1. Cell. 1989 Jun 16;57(6):987–996. doi: 10.1016/0092-8674(89)90337-1. [DOI] [PubMed] [Google Scholar]
- Durfee T., Becherer K., Chen P. L., Yeh S. H., Yang Y., Kilburn A. E., Lee W. H., Elledge S. J. The retinoblastoma protein associates with the protein phosphatase type 1 catalytic subunit. Genes Dev. 1993 Apr;7(4):555–569. doi: 10.1101/gad.7.4.555. [DOI] [PubMed] [Google Scholar]
- Erikson R. L. Structure, expression, and regulation of protein kinases involved in the phosphorylation of ribosomal protein S6. J Biol Chem. 1991 Apr 5;266(10):6007–6010. [PubMed] [Google Scholar]
- Fernandez A., Brautigan D. L., Lamb N. J. Protein phosphatase type 1 in mammalian cell mitosis: chromosomal localization and involvement in mitotic exit. J Cell Biol. 1992 Mar;116(6):1421–1430. doi: 10.1083/jcb.116.6.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez N., Cohen P. Dissection of the protein kinase cascade by which nerve growth factor activates MAP kinases. Nature. 1991 Sep 12;353(6340):170–173. doi: 10.1038/353170a0. [DOI] [PubMed] [Google Scholar]
- Hollingsworth R. E., Jr, Hensey C. E., Lee W. H. Retinoblastoma protein and the cell cycle. Curr Opin Genet Dev. 1993 Feb;3(1):55–62. doi: 10.1016/s0959-437x(05)80341-7. [DOI] [PubMed] [Google Scholar]
- Hunter T., Karin M. The regulation of transcription by phosphorylation. Cell. 1992 Aug 7;70(3):375–387. doi: 10.1016/0092-8674(92)90162-6. [DOI] [PubMed] [Google Scholar]
- Hunter T. Protein-tyrosine phosphatases: the other side of the coin. Cell. 1989 Sep 22;58(6):1013–1016. doi: 10.1016/0092-8674(89)90496-0. [DOI] [PubMed] [Google Scholar]
- Hunter T., Sefton B. M. Transforming gene product of Rous sarcoma virus phosphorylates tyrosine. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1311–1315. doi: 10.1073/pnas.77.3.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen J. W., Ingebritsen T. S. Effects of phosphorylation of protein phosphatase 1 by pp60v-src on the interaction of the enzyme with substrates and inhibitor proteins. Biochim Biophys Acta. 1987 Apr 2;928(1):63–75. doi: 10.1016/0167-4889(87)90086-3. [DOI] [PubMed] [Google Scholar]
- Johansen J. W., Ingebritsen T. S. Phosphorylation and inactivation of protein phosphatase 1 by pp60v-src. Proc Natl Acad Sci U S A. 1986 Jan;83(2):207–211. doi: 10.1073/pnas.83.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamibayashi C., Estes R., Slaughter C., Mumby M. C. Subunit interactions control protein phosphatase 2A. Effects of limited proteolysis, N-ethylmaleimide, and heparin on the interaction of the B subunit. J Biol Chem. 1991 Jul 15;266(20):13251–13260. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Ludlow J. W., Glendening C. L., Livingston D. M., DeCarprio J. A. Specific enzymatic dephosphorylation of the retinoblastoma protein. Mol Cell Biol. 1993 Jan;13(1):367–372. doi: 10.1128/mcb.13.1.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Pérez J., Rudkin B. B., Siegmann M., Thomas G. Activation of ribosomal protein S6 phosphorylation during meiotic maturation of Xenopus laevis oocytes: in vitro ordered appearance of S6 phosphopeptides. EMBO J. 1986 Apr;5(4):725–731. doi: 10.1002/j.1460-2075.1986.tb04274.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Pérez J., Thomas G. Ordered phosphorylation of 40S ribosomal protein S6 after serum stimulation of quiescent 3T3 cells. Proc Natl Acad Sci U S A. 1983 Feb;80(4):926–930. doi: 10.1073/pnas.80.4.926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin B. L., Shriner C. L., Brautigan D. L. Modulation of type-1 protein phosphatase by synthetic peptides corresponding to the carboxyl terminus. FEBS Lett. 1991 Jul 8;285(1):6–10. doi: 10.1016/0014-5793(91)80712-c. [DOI] [PubMed] [Google Scholar]
- Ohkura H., Kinoshita N., Miyatani S., Toda T., Yanagida M. The fission yeast dis2+ gene required for chromosome disjoining encodes one of two putative type 1 protein phosphatases. Cell. 1989 Jun 16;57(6):997–1007. doi: 10.1016/0092-8674(89)90338-3. [DOI] [PubMed] [Google Scholar]
- Olivier A. R., Ballou L. M., Thomas G. Differential regulation of S6 phosphorylation by insulin and epidermal growth factor in Swiss mouse 3T3 cells: insulin activation of type 1 phosphatase. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4720–4724. doi: 10.1073/pnas.85.13.4720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivier A. R., Thomas G. Three forms of phosphatase type 1 in Swiss 3T3 fibroblasts. Free catalytic subunit appears to mediate s6 dephosphorylation. J Biol Chem. 1990 Dec 25;265(36):22460–22466. [PubMed] [Google Scholar]
- Pot D. A., Dixon J. E. A thousand and two protein tyrosine phosphatases. Biochim Biophys Acta. 1992 Jul 22;1136(1):35–43. doi: 10.1016/0167-4889(92)90082-m. [DOI] [PubMed] [Google Scholar]
- Schlessinger J., Ullrich A. Growth factor signaling by receptor tyrosine kinases. Neuron. 1992 Sep;9(3):383–391. doi: 10.1016/0896-6273(92)90177-f. [DOI] [PubMed] [Google Scholar]
- Siegmann M., Thomas G. Separation of multiple phosphorylated forms of 40 S ribosomal protein S6 by two-dimensional polyacrylamide gel electrophoresis. Methods Enzymol. 1987;146:362–369. doi: 10.1016/s0076-6879(87)46037-0. [DOI] [PubMed] [Google Scholar]
- Stone E. M., Yamano H., Kinoshita N., Yanagida M. Mitotic regulation of protein phosphatases by the fission yeast sds22 protein. Curr Biol. 1993 Jan;3(1):13–26. doi: 10.1016/0960-9822(93)90140-j. [DOI] [PubMed] [Google Scholar]
- Sweet L. J., Alcorta D. A., Erikson R. L. Two distinct enzymes contribute to biphasic S6 phosphorylation in serum-stimulated chicken embryo fibroblasts. Mol Cell Biol. 1990 Jun;10(6):2787–2792. doi: 10.1128/mcb.10.6.2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas G. MAP kinase by any other name smells just as sweet. Cell. 1992 Jan 10;68(1):3–6. doi: 10.1016/0092-8674(92)90199-m. [DOI] [PubMed] [Google Scholar]
- Thomas G., Martin-Pérez J., Siegmann M., Otto A. M. The effect of serum, EGF, PGF2 alpha and insulin on S6 phosphorylation and the initiation of protein and DNA synthesis. Cell. 1982 Aug;30(1):235–242. doi: 10.1016/0092-8674(82)90029-0. [DOI] [PubMed] [Google Scholar]
- Tonks N. K., Cohen P. The protein phosphatases involved in cellular regulation. Identification of the inhibitor-2 phosphatases in rabbit skeletal muscle. Eur J Biochem. 1984 Nov 15;145(1):65–70. doi: 10.1111/j.1432-1033.1984.tb08522.x. [DOI] [PubMed] [Google Scholar]
- Villa-Moruzzi E., Dalla Zonca P., Crabb J. W. Phosphorylation of the catalytic subunit of type-1 protein phosphatase by the v-abl tyrosine kinase. FEBS Lett. 1991 Nov 18;293(1-2):67–71. doi: 10.1016/0014-5793(91)81154-z. [DOI] [PubMed] [Google Scholar]