Abstract
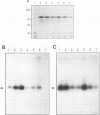
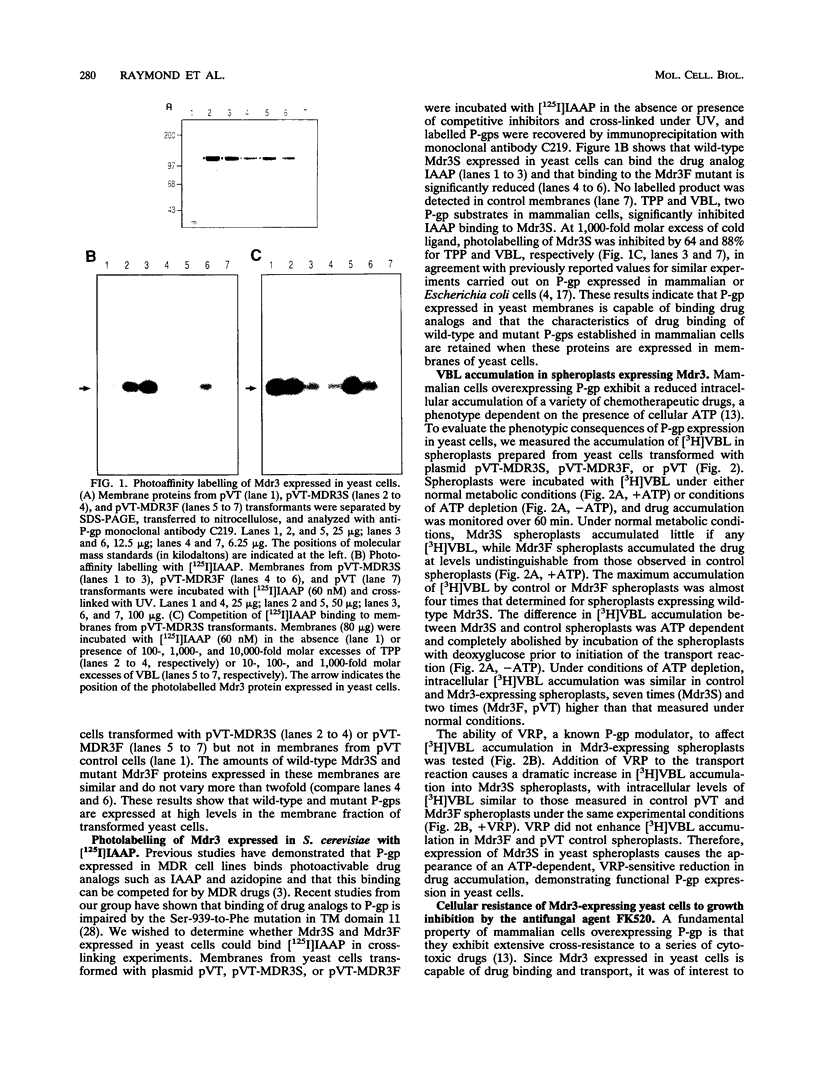
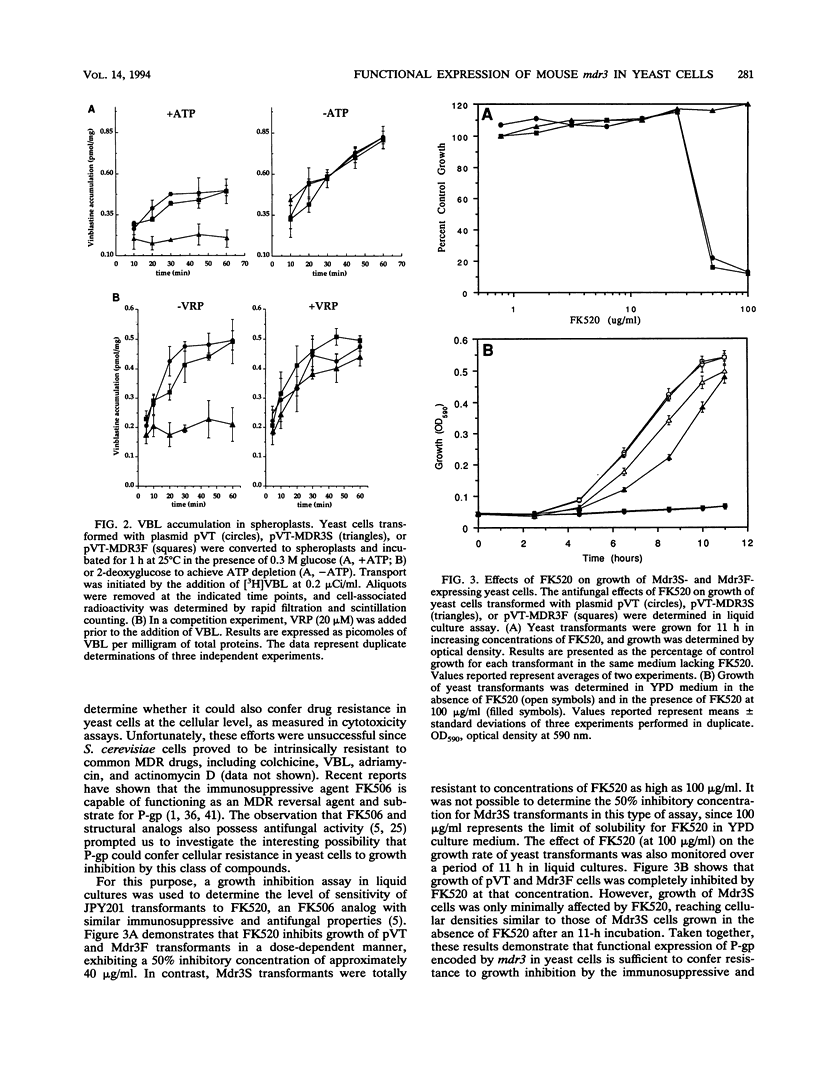
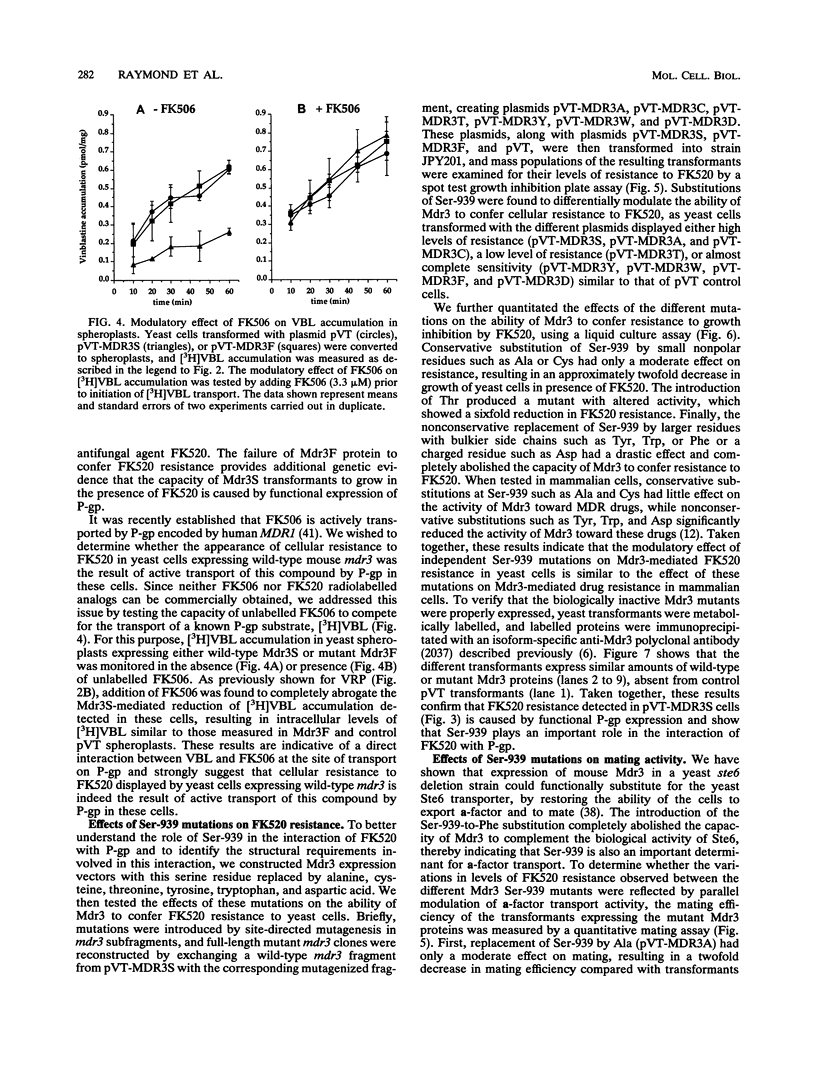
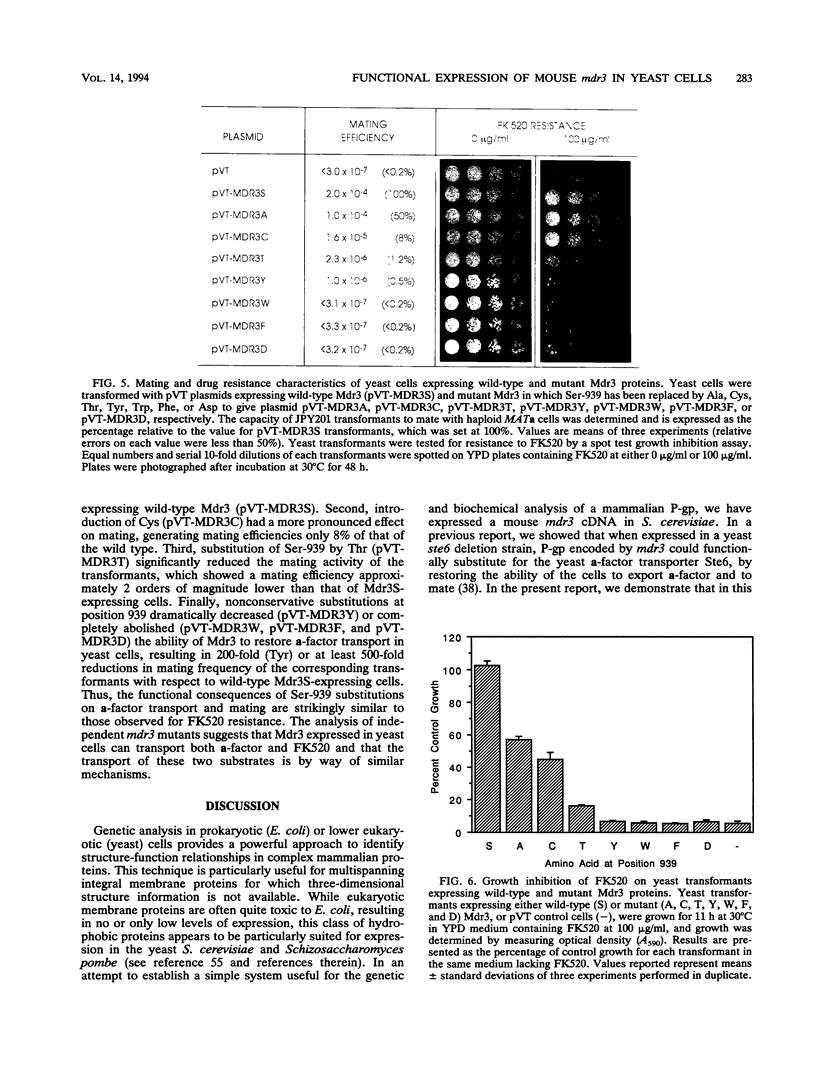
We have recently reported that expression in yeast cells of P-glycoprotein (P-gp) encoded by the mouse multidrug resistance mdr3 gene (Mdr3) can complement a null ste6 mutation (M. Raymond, P. Gros, M. Whiteway, and D. Y. Thomas, Science 256:232-234, 1992). Here we show that Mdr3 behaves as a fully functional drug transporter in this heterologous expression system. Photolabelling experiments indicate that Mdr3 synthesized in yeast cells binds the drug analog [125I]iodoaryl azidoprazosin, this binding being competed for by vinblastine and tetraphenylphosphonium bromide, two known multidrug resistance drugs. Spheroplasts expressing wild-type Mdr3 (Ser-939) exhibit an ATP-dependent and verapamil-sensitive decreased accumulation of [3H]vinblastine as compared with spheroplasts expressing a mutant form of Mdr3 with impaired transport activity (Phe-939). Expression of Mdr3 in yeast cells can confer resistance to growth inhibition by the antifungal and immunosuppressive agent FK520, suggesting that this compound is a substrate for P-gp in yeast cells. Replacement of Ser-939 in Mdr3 by a series of amino acid substitutions is shown to modulate both the level of cellular resistance to FK520 and the mating efficiency of yeast mdr3 transformants. The effects of these mutations on the function of Mdr3 in yeast cells are similar to those observed in mammalian cells with respect to drug resistance and transport, indicating that transport of a-factor and FK520 in yeast cells is mechanistically similar to drug transport in mammalian cells. The ability of P-gp to confer cellular resistance to FK520 in yeast cells establishes a dominant phenotype that can be assayed for the positive selection of intragenic revertants of P-gp inactive mutants, an important tool for the structure-function analysis of mammalian P-gp in yeast cells.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arceci R. J., Stieglitz K., Bierer B. E. Immunosuppressants FK506 and rapamycin function as reversal agents of the multidrug resistance phenotype. Blood. 1992 Sep 15;80(6):1528–1536. [PubMed] [Google Scholar]
- Beck W. T., Qian X. D. Photoaffinity substrates for P-glycoprotein. Biochem Pharmacol. 1992 Jan 9;43(1):89–93. doi: 10.1016/0006-2952(92)90665-6. [DOI] [PubMed] [Google Scholar]
- Beck W. T. Strategies to circumvent multidrug resistance due to P-glycoprotein or to altered DNA topoisomerase II. Bull Cancer. 1990;77(11):1131–1141. [PubMed] [Google Scholar]
- Bibi E., Gros P., Kaback H. R. Functional expression of mouse mdr1 in Escherichia coli. Proc Natl Acad Sci U S A. 1993 Oct 1;90(19):9209–9213. doi: 10.1073/pnas.90.19.9209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brizuela L., Chrebet G., Bostian K. A., Parent S. A. Antifungal properties of the immunosuppressant FK-506: identification of an FK-506-responsive yeast gene distinct from FKB1. Mol Cell Biol. 1991 Sep;11(9):4616–4626. doi: 10.1128/mcb.11.9.4616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buschman E., Arceci R. J., Croop J. M., Che M., Arias I. M., Housman D. E., Gros P. mdr2 encodes P-glycoprotein expressed in the bile canalicular membrane as determined by isoform-specific antibodies. J Biol Chem. 1992 Sep 5;267(25):18093–18099. [PubMed] [Google Scholar]
- Buschman E., Gros P. Functional analysis of chimeric genes obtained by exchanging homologous domains of the mouse mdr1 and mdr2 genes. Mol Cell Biol. 1991 Feb;11(2):595–603. doi: 10.1128/mcb.11.2.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C. J., Chin J. E., Ueda K., Clark D. P., Pastan I., Gottesman M. M., Roninson I. B. Internal duplication and homology with bacterial transport proteins in the mdr1 (P-glycoprotein) gene from multidrug-resistant human cells. Cell. 1986 Nov 7;47(3):381–389. doi: 10.1016/0092-8674(86)90595-7. [DOI] [PubMed] [Google Scholar]
- Dalton W. S., Grogan T. M., Meltzer P. S., Scheper R. J., Durie B. G., Taylor C. W., Miller T. P., Salmon S. E. Drug-resistance in multiple myeloma and non-Hodgkin's lymphoma: detection of P-glycoprotein and potential circumvention by addition of verapamil to chemotherapy. J Clin Oncol. 1989 Apr;7(4):415–424. doi: 10.1200/JCO.1989.7.4.415. [DOI] [PubMed] [Google Scholar]
- Devault A., Gros P. Two members of the mouse mdr gene family confer multidrug resistance with overlapping but distinct drug specificities. Mol Cell Biol. 1990 Apr;10(4):1652–1663. doi: 10.1128/mcb.10.4.1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devine S. E., Ling V., Melera P. W. Amino acid substitutions in the sixth transmembrane domain of P-glycoprotein alter multidrug resistance. Proc Natl Acad Sci U S A. 1992 May 15;89(10):4564–4568. doi: 10.1073/pnas.89.10.4564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhir R., Grizzuti K., Kajiji S., Gros P. Modulatory effects on substrate specificity of independent mutations at the serine939/941 position in predicted transmembrane domain 11 of P-glycoproteins. Biochemistry. 1993 Sep 14;32(36):9492–9499. doi: 10.1021/bi00087a030. [DOI] [PubMed] [Google Scholar]
- Endicott J. A., Ling V. The biochemistry of P-glycoprotein-mediated multidrug resistance. Annu Rev Biochem. 1989;58:137–171. doi: 10.1146/annurev.bi.58.070189.001033. [DOI] [PubMed] [Google Scholar]
- Endicott J. A., Sarangi F., Ling V. Complete cDNA sequences encoding the Chinese hamster P-glycoprotein gene family. DNA Seq. 1991;2(2):89–101. doi: 10.3109/10425179109039677. [DOI] [PubMed] [Google Scholar]
- Foote S. J., Thompson J. K., Cowman A. F., Kemp D. J. Amplification of the multidrug resistance gene in some chloroquine-resistant isolates of P. falciparum. Cell. 1989 Jun 16;57(6):921–930. doi: 10.1016/0092-8674(89)90330-9. [DOI] [PubMed] [Google Scholar]
- Greenberger L. M. Major photoaffinity drug labeling sites for iodoaryl azidoprazosin in P-glycoprotein are within, or immediately C-terminal to, transmembrane domains 6 and 12. J Biol Chem. 1993 May 25;268(15):11417–11425. [PubMed] [Google Scholar]
- Greenberger L. M., Yang C. P., Gindin E., Horwitz S. B. Photoaffinity probes for the alpha 1-adrenergic receptor and the calcium channel bind to a common domain in P-glycoprotein. J Biol Chem. 1990 Mar 15;265(8):4394–4401. [PubMed] [Google Scholar]
- Gros P., Croop J., Housman D. Mammalian multidrug resistance gene: complete cDNA sequence indicates strong homology to bacterial transport proteins. Cell. 1986 Nov 7;47(3):371–380. doi: 10.1016/0092-8674(86)90594-5. [DOI] [PubMed] [Google Scholar]
- Gros P., Dhir R., Croop J., Talbot F. A single amino acid substitution strongly modulates the activity and substrate specificity of the mouse mdr1 and mdr3 drug efflux pumps. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):7289–7293. doi: 10.1073/pnas.88.16.7289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gros P., Raymond M., Bell J., Housman D. Cloning and characterization of a second member of the mouse mdr gene family. Mol Cell Biol. 1988 Jul;8(7):2770–2778. doi: 10.1128/mcb.8.7.2770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada H., Tsuruo T. Purification of the 170- to 180-kilodalton membrane glycoprotein associated with multidrug resistance. 170- to 180-kilodalton membrane glycoprotein is an ATPase. J Biol Chem. 1988 Jan 25;263(3):1454–1458. [PubMed] [Google Scholar]
- Heitman J., Koller A., Kunz J., Henriquez R., Schmidt A., Movva N. R., Hall M. N. The immunosuppressant FK506 inhibits amino acid import in Saccharomyces cerevisiae. Mol Cell Biol. 1993 Aug;13(8):5010–5019. doi: 10.1128/mcb.13.8.5010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heitman J., Movva N. R., Hall M. N. Proline isomerases at the crossroads of protein folding, signal transduction, and immunosuppression. New Biol. 1992 May;4(5):448–460. [PubMed] [Google Scholar]
- Heitman J., Movva N. R., Hall M. N. Targets for cell cycle arrest by the immunosuppressant rapamycin in yeast. Science. 1991 Aug 23;253(5022):905–909. doi: 10.1126/science.1715094. [DOI] [PubMed] [Google Scholar]
- Heitman J., Movva N. R., Hiestand P. C., Hall M. N. FK 506-binding protein proline rotamase is a target for the immunosuppressive agent FK 506 in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1991 Mar 1;88(5):1948–1952. doi: 10.1073/pnas.88.5.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins C. F. ABC transporters: from microorganisms to man. Annu Rev Cell Biol. 1992;8:67–113. doi: 10.1146/annurev.cb.08.110192.000435. [DOI] [PubMed] [Google Scholar]
- Ito H., Fukuda Y., Murata K., Kimura A. Transformation of intact yeast cells treated with alkali cations. J Bacteriol. 1983 Jan;153(1):163–168. doi: 10.1128/jb.153.1.163-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajiji S., Talbot F., Grizzuti K., Van Dyke-Phillips V., Agresti M., Safa A. R., Gros P. Functional analysis of P-glycoprotein mutants identifies predicted transmembrane domain 11 as a putative drug binding site. Biochemistry. 1993 Apr 27;32(16):4185–4194. doi: 10.1021/bi00067a005. [DOI] [PubMed] [Google Scholar]
- Koltin Y., Faucette L., Bergsma D. J., Levy M. A., Cafferkey R., Koser P. L., Johnson R. K., Livi G. P. Rapamycin sensitivity in Saccharomyces cerevisiae is mediated by a peptidyl-prolyl cis-trans isomerase related to human FK506-binding protein. Mol Cell Biol. 1991 Mar;11(3):1718–1723. doi: 10.1128/mcb.11.3.1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuchler K., Sterne R. E., Thorner J. Saccharomyces cerevisiae STE6 gene product: a novel pathway for protein export in eukaryotic cells. EMBO J. 1989 Dec 20;8(13):3973–3984. doi: 10.1002/j.1460-2075.1989.tb08580.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuchler K., Thorner J. Functional expression of human mdr1 in the yeast Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1992 Mar 15;89(6):2302–2306. doi: 10.1073/pnas.89.6.2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Loo T. W., Clarke D. M. Functional consequences of proline mutations in the predicted transmembrane domain of P-glycoprotein. J Biol Chem. 1993 Feb 15;268(5):3143–3149. [PubMed] [Google Scholar]
- McGrath J. P., Varshavsky A. The yeast STE6 gene encodes a homologue of the mammalian multidrug resistance P-glycoprotein. Nature. 1989 Aug 3;340(6232):400–404. doi: 10.1038/340400a0. [DOI] [PubMed] [Google Scholar]
- Michaelis S., Herskowitz I. The a-factor pheromone of Saccharomyces cerevisiae is essential for mating. Mol Cell Biol. 1988 Mar;8(3):1309–1318. doi: 10.1128/mcb.8.3.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno K., Furuhashi Y., Misawa T., Iwata M., Kawai M., Kikkawa F., Kano T., Tomoda Y. Modulation of multidrug resistance by immunosuppressive agents: cyclosporin analogues, FK506 and mizoribine. Anticancer Res. 1992 Jan-Feb;12(1):21–25. [PubMed] [Google Scholar]
- Raviv Y., Pollard H. B., Bruggemann E. P., Pastan I., Gottesman M. M. Photosensitized labeling of a functional multidrug transporter in living drug-resistant tumor cells. J Biol Chem. 1990 Mar 5;265(7):3975–3980. [PubMed] [Google Scholar]
- Raymond M., Gros P., Whiteway M., Thomas D. Y. Functional complementation of yeast ste6 by a mammalian multidrug resistance mdr gene. Science. 1992 Apr 10;256(5054):232–234. doi: 10.1126/science.1348873. [DOI] [PubMed] [Google Scholar]
- Riordan J. R., Rommens J. M., Kerem B., Alon N., Rozmahel R., Grzelczak Z., Zielenski J., Lok S., Plavsic N., Chou J. L. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science. 1989 Sep 8;245(4922):1066–1073. doi: 10.1126/science.2475911. [DOI] [PubMed] [Google Scholar]
- Saeki T., Shimabuku A. M., Azuma Y., Shibano Y., Komano T., Ueda K. Expression of human P-glycoprotein in yeast cells--effects of membrane component sterols on the activity of P-glycoprotein. Agric Biol Chem. 1991 Jul;55(7):1859–1865. [PubMed] [Google Scholar]
- Saeki T., Ueda K., Tanigawara Y., Hori R., Komano T. Human P-glycoprotein transports cyclosporin A and FK506. J Biol Chem. 1993 Mar 25;268(9):6077–6080. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber S. L. Chemistry and biology of the immunophilins and their immunosuppressive ligands. Science. 1991 Jan 18;251(4991):283–287. doi: 10.1126/science.1702904. [DOI] [PubMed] [Google Scholar]
- Sengstag C., Stirling C., Schekman R., Rine J. Genetic and biochemical evaluation of eucaryotic membrane protein topology: multiple transmembrane domains of Saccharomyces cerevisiae 3-hydroxy-3-methylglutaryl coenzyme A reductase. Mol Cell Biol. 1990 Feb;10(2):672–680. doi: 10.1128/mcb.10.2.672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skach W. R., Calayag M. C., Lingappa V. R. Evidence for an alternate model of human P-glycoprotein structure and biogenesis. J Biol Chem. 1993 Apr 5;268(10):6903–6908. [PubMed] [Google Scholar]
- Sonneveld P., Durie B. G., Lokhorst H. M., Marie J. P., Solbu G., Suciu S., Zittoun R., Löwenberg B., Nooter K. Modulation of multidrug-resistant multiple myeloma by cyclosporin. The Leukaemia Group of the EORTC and the HOVON. Lancet. 1992 Aug 1;340(8814):255–259. doi: 10.1016/0140-6736(92)92353-h. [DOI] [PubMed] [Google Scholar]
- Sprague G. F., Jr Assay of yeast mating reaction. Methods Enzymol. 1991;194:77–93. doi: 10.1016/0076-6879(91)94008-z. [DOI] [PubMed] [Google Scholar]
- Thomson A. W. FK-506 enters the clinic. Immunol Today. 1990 Feb;11(2):35–36. doi: 10.1016/0167-5699(90)90011-w. [DOI] [PubMed] [Google Scholar]
- Tropschug M., Barthelmess I. B., Neupert W. Sensitivity to cyclosporin A is mediated by cyclophilin in Neurospora crassa and Saccharomyces cerevisiae. Nature. 1989 Dec 21;342(6252):953–955. doi: 10.1038/342953a0. [DOI] [PubMed] [Google Scholar]
- Twentyman P. R. Cyclosporins as drug resistance modifiers. Biochem Pharmacol. 1992 Jan 9;43(1):109–117. doi: 10.1016/0006-2952(92)90668-9. [DOI] [PubMed] [Google Scholar]
- Ueda K., Okamura N., Hirai M., Tanigawara Y., Saeki T., Kioka N., Komano T., Hori R. Human P-glycoprotein transports cortisol, aldosterone, and dexamethasone, but not progesterone. J Biol Chem. 1992 Dec 5;267(34):24248–24252. [PubMed] [Google Scholar]
- Van der Bliek A. M., Baas F., Ten Houte de Lange T., Kooiman P. M., Van der Velde-Koerts T., Borst P. The human mdr3 gene encodes a novel P-glycoprotein homologue and gives rise to alternatively spliced mRNAs in liver. EMBO J. 1987 Nov;6(11):3325–3331. doi: 10.1002/j.1460-2075.1987.tb02653.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernet T., Dignard D., Thomas D. Y. A family of yeast expression vectors containing the phage f1 intergenic region. Gene. 1987;52(2-3):225–233. doi: 10.1016/0378-1119(87)90049-7. [DOI] [PubMed] [Google Scholar]
- Villalba J. M., Palmgren M. G., Berberián G. E., Ferguson C., Serrano R. Functional expression of plant plasma membrane H(+)-ATPase in yeast endoplasmic reticulum. J Biol Chem. 1992 Jun 15;267(17):12341–12349. [PubMed] [Google Scholar]
- Yusa K., Tsuruo T. Reversal mechanism of multidrug resistance by verapamil: direct binding of verapamil to P-glycoprotein on specific sites and transport of verapamil outward across the plasma membrane of K562/ADM cells. Cancer Res. 1989 Sep 15;49(18):5002–5006. [PubMed] [Google Scholar]
- Zamora J. M., Pearce H. L., Beck W. T. Physical-chemical properties shared by compounds that modulate multidrug resistance in human leukemic cells. Mol Pharmacol. 1988 Apr;33(4):454–462. [PubMed] [Google Scholar]
- Zhang J. T., Ling V. Study of membrane orientation and glycosylated extracellular loops of mouse P-glycoprotein by in vitro translation. J Biol Chem. 1991 Sep 25;266(27):18224–18232. [PubMed] [Google Scholar]