Abstract
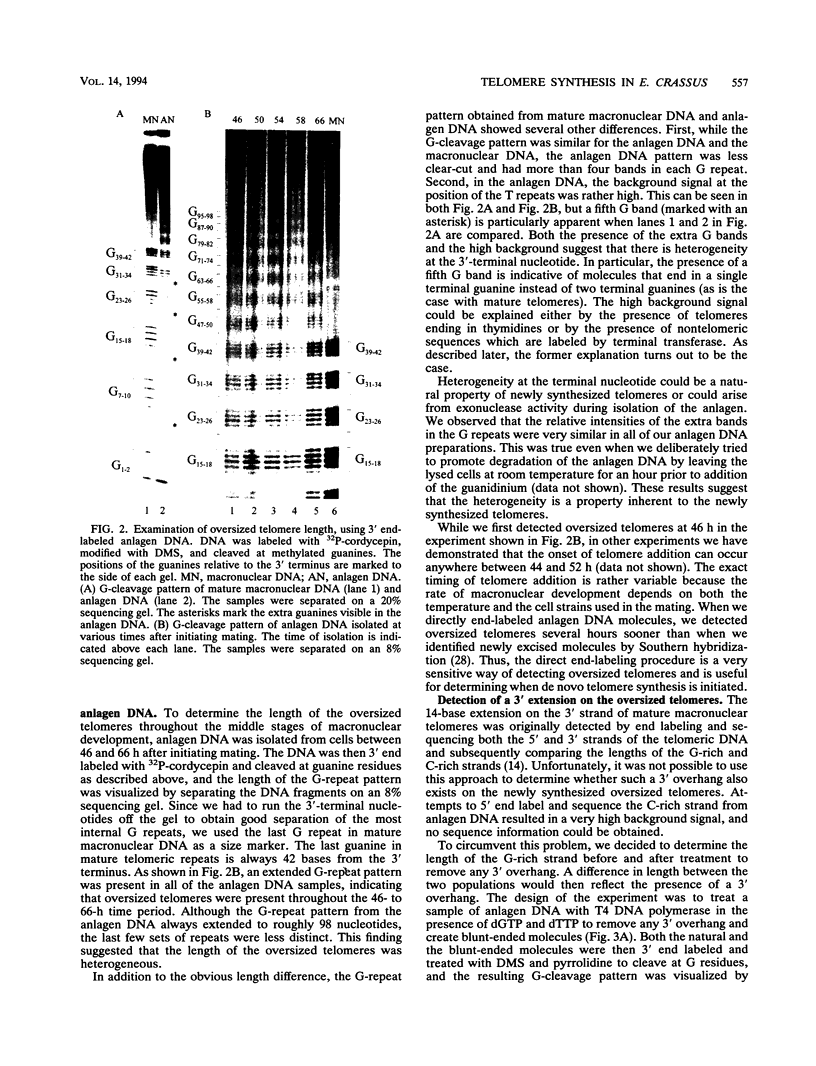
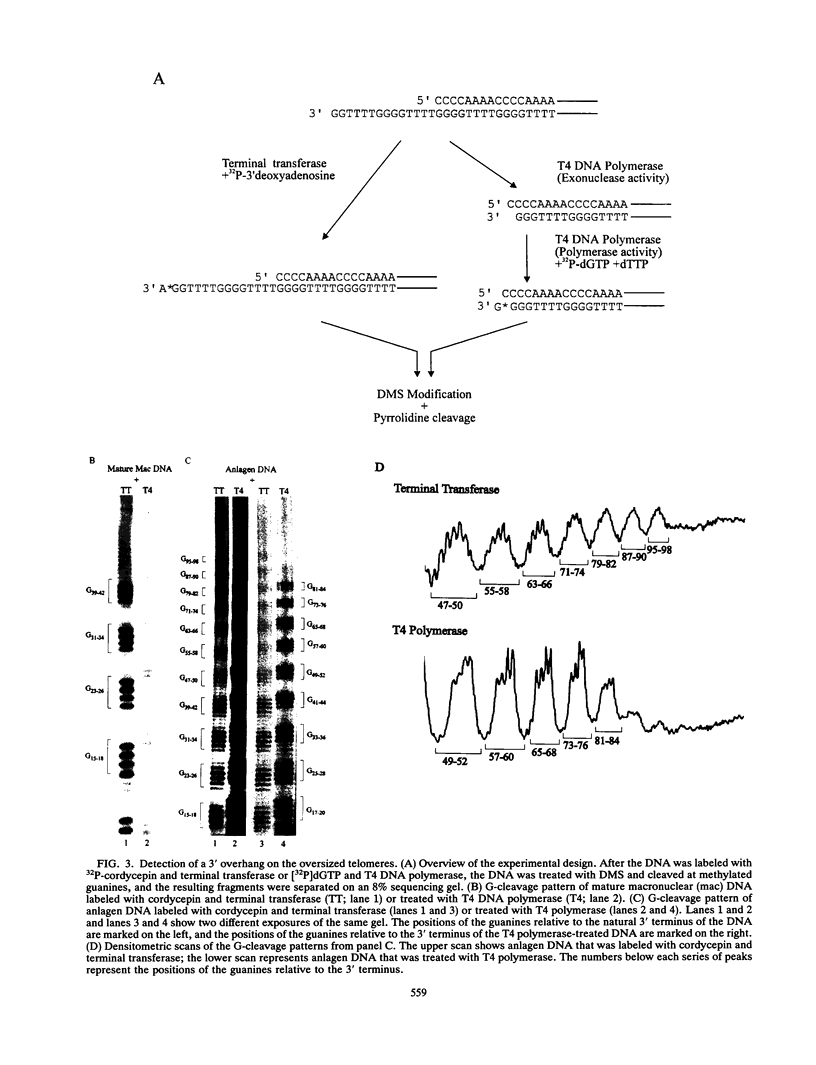
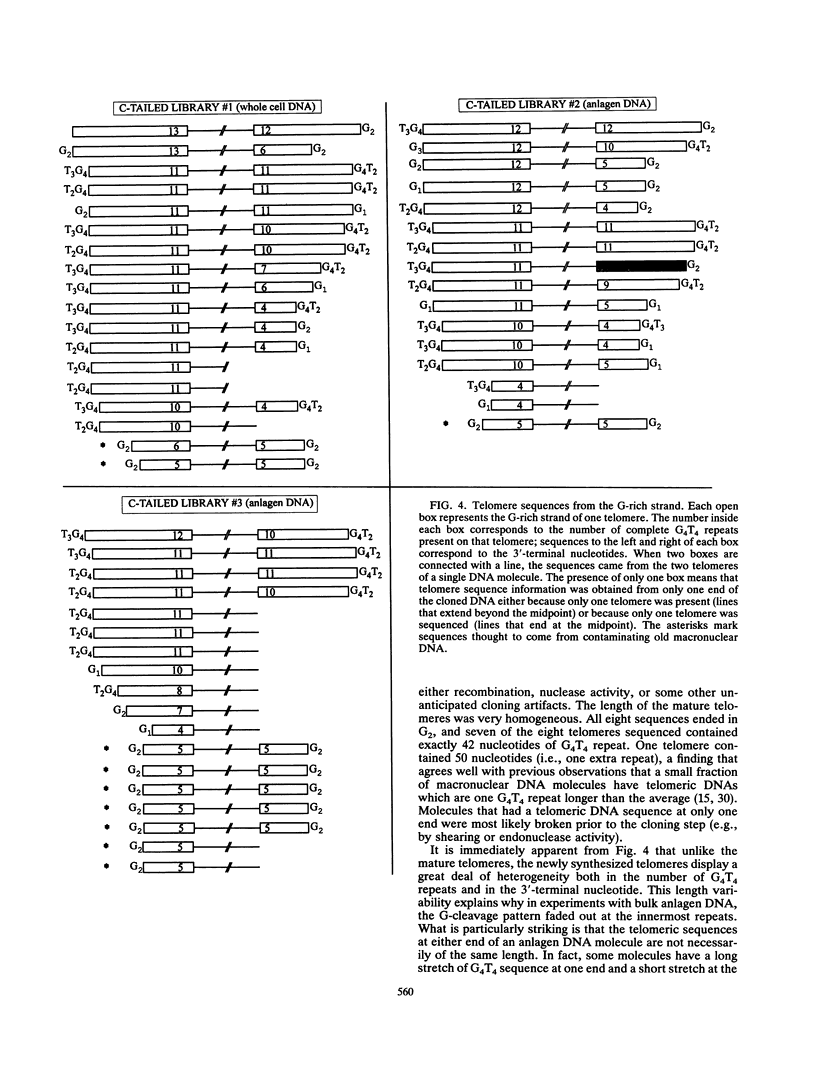
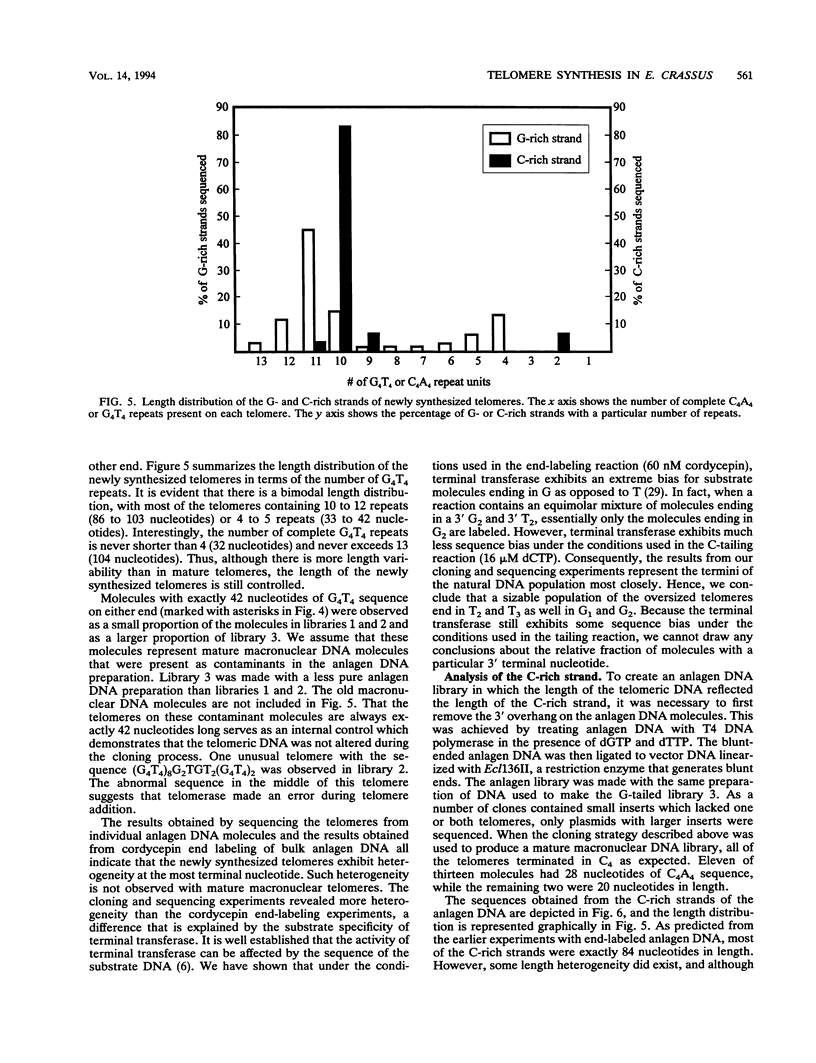
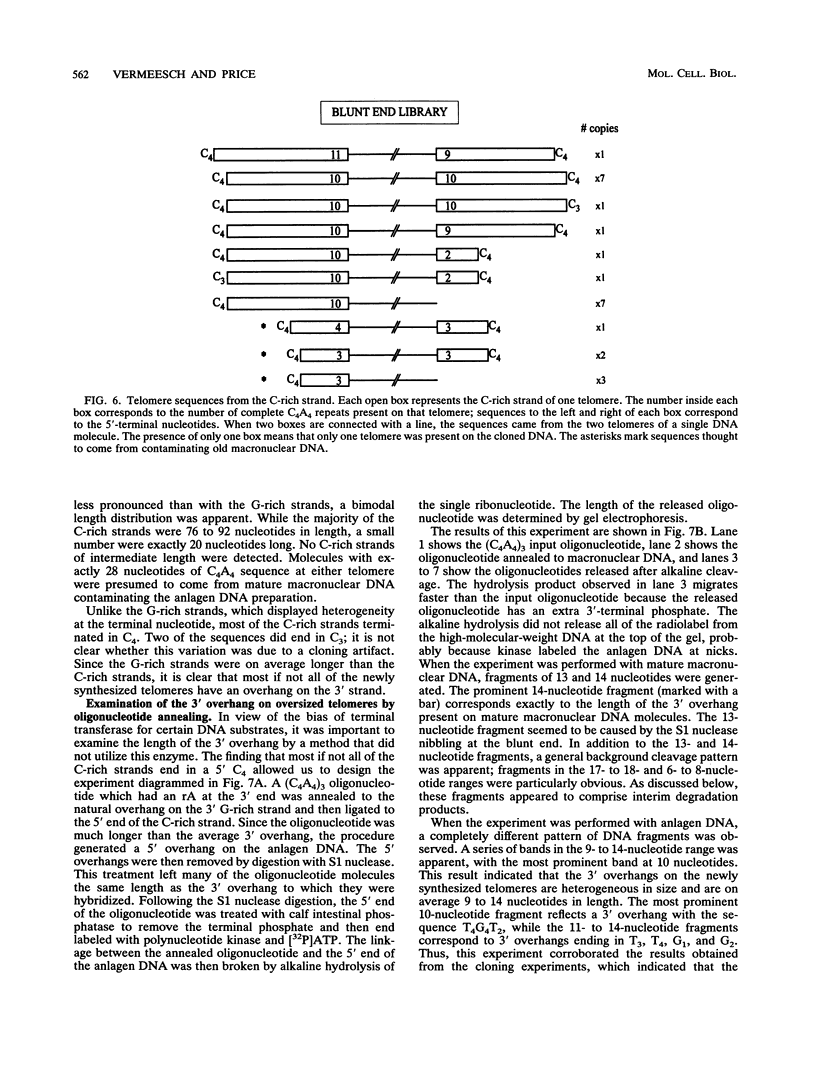
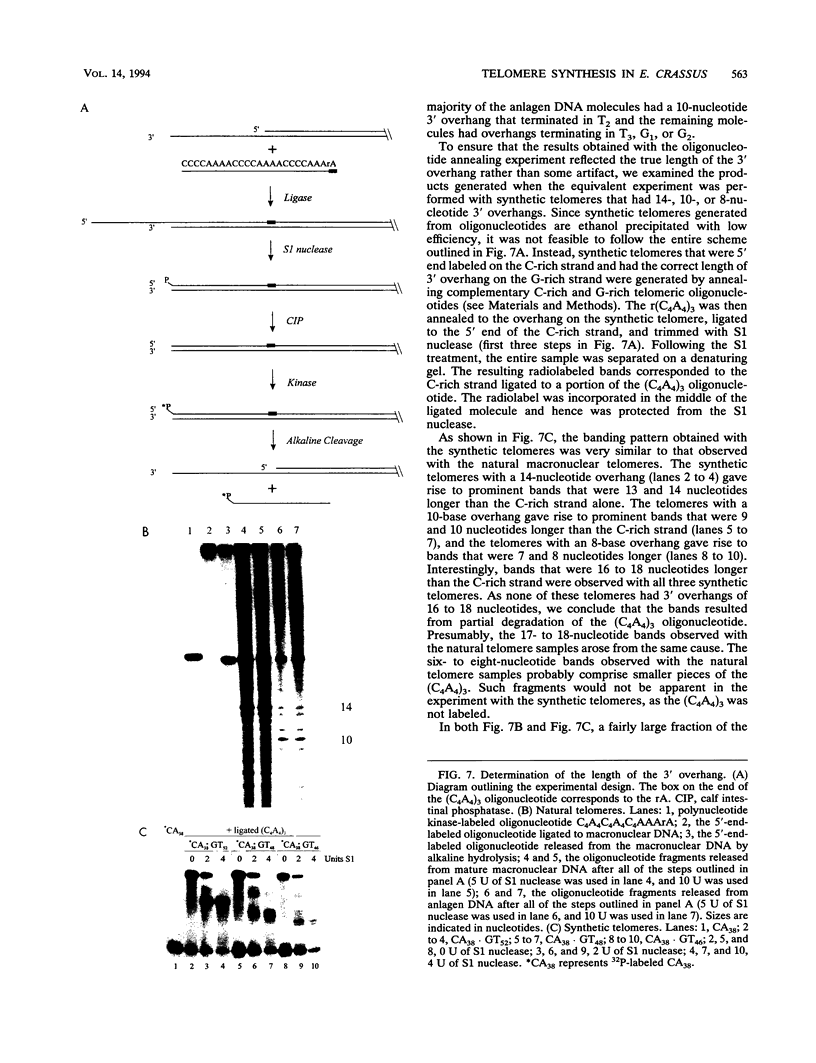
To learn more about the mechanism of de novo telomere synthesis, we have characterized the sequence and structure of newly synthesized telomeres from Euplotes crassus. E. crassus is a particularly useful organism for studying telomere synthesis because millions of telomeres are made in each cell at a well-defined time during the sexual stage of the life cycle. These newly synthesized telomeres are approximately 50 bp longer than mature macronuclear telomeres. We have investigated the structure of the newly synthesized telomeres and have found that they are much more heterogeneous in length than mature telomeres. Most of the heterogeneity is present on the G-rich strand, indicating that the length of this strand is rather loosely controlled. In contrast, the length of the C-rich strand is much less variable, suggesting that synthesis of this strand is the more precisely regulated step in telomere addition. The G-rich strand exhibits variability both in the total number of G4T4 repeats and in the identity of the terminal nucleotide. In most cases, the G-rich strnd extends beyond the C-rich strand to leave a 3' overhang. While the size of this overhang is variable, the median length is 10 nucleotides. This research provides the first detailed picture of a newly synthesized telomere and has allowed us to formulate a model to describe the various steps involved in de novo telomere synthesis.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmed S., Henderson E. Formation of novel hairpin structures by telomeric C-strand oligonucleotides. Nucleic Acids Res. 1992 Feb 11;20(3):507–511. doi: 10.1093/nar/20.3.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ammermann D. Morphology and development of the macronuclei of the ciliates Stylonychia mytilus and Euplotes aediculatus. Chromosoma. 1971;33(2):209–238. doi: 10.1007/BF00285634. [DOI] [PubMed] [Google Scholar]
- Biessmann H., Mason J. M. Genetics and molecular biology of telomeres. Adv Genet. 1992;30:185–249. doi: 10.1016/s0065-2660(08)60321-1. [DOI] [PubMed] [Google Scholar]
- Blackburn E. H. Telomerases. Annu Rev Biochem. 1992;61:113–129. doi: 10.1146/annurev.bi.61.070192.000553. [DOI] [PubMed] [Google Scholar]
- Eschenfeldt W. H., Puskas R. S., Berger S. L. Homopolymeric tailing. Methods Enzymol. 1987;152:337–342. doi: 10.1016/0076-6879(87)52040-7. [DOI] [PubMed] [Google Scholar]
- Gray J. T., Celander D. W., Price C. M., Cech T. R. Cloning and expression of genes for the Oxytricha telomere-binding protein: specific subunit interactions in the telomeric complex. Cell. 1991 Nov 15;67(4):807–814. doi: 10.1016/0092-8674(91)90075-a. [DOI] [PubMed] [Google Scholar]
- Greider C. W., Blackburn E. H. A telomeric sequence in the RNA of Tetrahymena telomerase required for telomere repeat synthesis. Nature. 1989 Jan 26;337(6205):331–337. doi: 10.1038/337331a0. [DOI] [PubMed] [Google Scholar]
- Henderson E. R., Blackburn E. H. An overhanging 3' terminus is a conserved feature of telomeres. Mol Cell Biol. 1989 Jan;9(1):345–348. doi: 10.1128/mcb.9.1.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahn C. L. The nuclear genomes of hypotrichous ciliates: maintaining the maximum and the minimum of information. J Protozool. 1991 May-Jun;38(3):252–258. doi: 10.1111/j.1550-7408.1991.tb04438.x. [DOI] [PubMed] [Google Scholar]
- Kaguni L. S., Lehman I. R. Eukaryotic DNA polymerase-primase: structure, mechanism and function. Biochim Biophys Acta. 1988 Jul 13;950(2):87–101. doi: 10.1016/0167-4781(88)90001-2. [DOI] [PubMed] [Google Scholar]
- Klobutcher L. A., Swanton M. T., Donini P., Prescott D. M. All gene-sized DNA molecules in four species of hypotrichs have the same terminal sequence and an unusual 3' terminus. Proc Natl Acad Sci U S A. 1981 May;78(5):3015–3019. doi: 10.1073/pnas.78.5.3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klobutcher L. A., Turner L. R., Peralta M. E. Sequence of a Euplotes crassus macronuclear DNA molecule encoding a protein with homology to a rat form-I phosphoinositide-specific phospholipase C. J Protozool. 1991 Jul-Aug;38(4):425–427. doi: 10.1111/j.1550-7408.1991.tb01381.x. [DOI] [PubMed] [Google Scholar]
- Price C. M., Skopp R., Krueger J., Williams D. DNA recognition and binding by the Euplotes telomere protein. Biochemistry. 1992 Nov 10;31(44):10835–10843. doi: 10.1021/bi00159a026. [DOI] [PubMed] [Google Scholar]
- Price C. M. Telomere structure in Euplotes crassus: characterization of DNA-protein interactions and isolation of a telomere-binding protein. Mol Cell Biol. 1990 Jul;10(7):3421–3431. doi: 10.1128/mcb.10.7.3421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth M., Lin M., Prescott D. M. Large scale synchronous mating and the study of macronuclear development in Euplotes crassus. J Cell Biol. 1985 Jul;101(1):79–84. doi: 10.1083/jcb.101.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheen J. Y., Seed B. Electrolyte gradient gels for DNA sequencing. Biotechniques. 1988 Nov-Dec;6(10):942–944. [PubMed] [Google Scholar]
- Shippen-Lentz D., Blackburn E. H. Functional evidence for an RNA template in telomerase. Science. 1990 Feb 2;247(4942):546–552. doi: 10.1126/science.1689074. [DOI] [PubMed] [Google Scholar]
- Shippen-Lentz D., Blackburn E. H. Telomere terminal transferase activity from Euplotes crassus adds large numbers of TTTTGGGG repeats onto telomeric primers. Mol Cell Biol. 1989 Jun;9(6):2761–2764. doi: 10.1128/mcb.9.6.2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tausta S. L., Klobutcher L. A. Internal eliminated sequences are removed prior to chromosome fragmentation during development in Euplotes crassus. Nucleic Acids Res. 1990 Feb 25;18(4):845–853. doi: 10.1093/nar/18.4.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Skopp R., Scofield M., Price C. Euplotes crassus has genes encoding telomere-binding proteins and telomere-binding protein homologs. Nucleic Acids Res. 1992 Dec 25;20(24):6621–6629. doi: 10.1093/nar/20.24.6621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellinger R. J., Wolf A. J., Zakian V. A. Origin activation and formation of single-strand TG1-3 tails occur sequentially in late S phase on a yeast linear plasmid. Mol Cell Biol. 1993 Jul;13(7):4057–4065. doi: 10.1128/mcb.13.7.4057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellinger R. J., Wolf A. J., Zakian V. A. Saccharomyces telomeres acquire single-strand TG1-3 tails late in S phase. Cell. 1993 Jan 15;72(1):51–60. doi: 10.1016/0092-8674(93)90049-v. [DOI] [PubMed] [Google Scholar]
- Wilkie A. O., Lamb J., Harris P. C., Finney R. D., Higgs D. R. A truncated human chromosome 16 associated with alpha thalassaemia is stabilized by addition of telomeric repeat (TTAGGG)n. Nature. 1990 Aug 30;346(6287):868–871. doi: 10.1038/346868a0. [DOI] [PubMed] [Google Scholar]
- Williamson J. R., Raghuraman M. K., Cech T. R. Monovalent cation-induced structure of telomeric DNA: the G-quartet model. Cell. 1989 Dec 1;59(5):871–880. doi: 10.1016/0092-8674(89)90610-7. [DOI] [PubMed] [Google Scholar]
- Yu G. L., Blackburn E. H. Developmentally programmed healing of chromosomes by telomerase in Tetrahymena. Cell. 1991 Nov 15;67(4):823–832. doi: 10.1016/0092-8674(91)90077-c. [DOI] [PubMed] [Google Scholar]
- Yu G. L., Bradley J. D., Attardi L. D., Blackburn E. H. In vivo alteration of telomere sequences and senescence caused by mutated Tetrahymena telomerase RNAs. Nature. 1990 Mar 8;344(6262):126–132. doi: 10.1038/344126a0. [DOI] [PubMed] [Google Scholar]
- Zahler A. M., Prescott D. M. DNA primase and the replication of the telomeres in Oxytricha nova. Nucleic Acids Res. 1989 Aug 11;17(15):6299–6317. doi: 10.1093/nar/17.15.6299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakian V. A. Structure and function of telomeres. Annu Rev Genet. 1989;23:579–604. doi: 10.1146/annurev.ge.23.120189.003051. [DOI] [PubMed] [Google Scholar]