Abstract
Retinal and choroidal vascular diseases constitute the most common causes of moderate and severe vision loss in developed countries. They can be divided into retinal vascular diseases, in which there is leakage and/or neovascularization (NV) from retinal vessels, and subretinal NV, in which new vessels grow into the normally avascular outer retina and subretinal space. The first category of diseases includes diabetic retinopathy, retinal vein occlusions, and retinopathy of prematurity and the second category includes neovascular age-related macular degeneration (AMD), ocular histoplasmosis, pathologic myopia, and other related diseases. Retinal hypoxia is a key feature of the first category of diseases resulting in elevated levels of hypoxia-inducible factor-1 (HIF-1) which stimulates expression of vascular endothelial growth factor (VEGF), platelet-derived growth factor-B (PDGF-B), placental growth factor, stromal-derived growth factor-1 and their receptors as well as other hypoxia-regulated gene products such as angiopoietin-2. Although hypoxia has not been demonstrated as part of the second category of diseases, HIF-1 is elevated and thus the same group of hypoxia-regulated gene products plays a role. Clinical trials have shown that VEGF antagonists provide major benefits for patients with subretinal NV due to AMD and even greater benefits are seen by combining antagonists of VEGF and PDGF-B. It is likely that addition of antagonists of other agents listed above will be tested in the future. Other appealing strategies are to directly target HIF-1 or to use gene transfer to express endogenous or engineered anti-angiogenic proteins. While substantial progress has been made, the future looks even brighter for patients with retinal and choroidal vascular diseases.
Keywords: Angiogenesis, age-related macular degeneration, diabetic retinopathy, hypoxia-inducible factor-1, vascular endothelial growth factor, platelet-derived growth factor
Introduction
The retina is supplied by both the retinal and choroidal vasculature
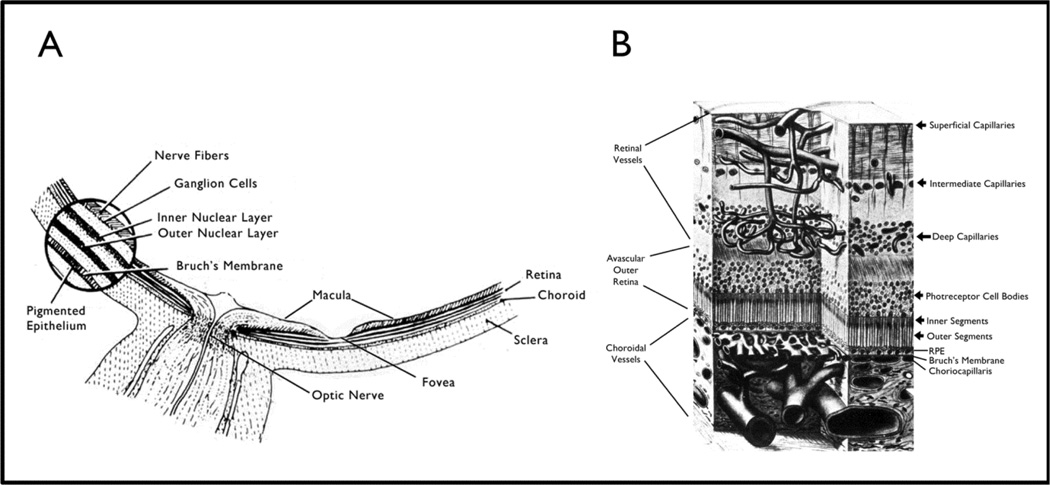
The retina consists of a series of neurons that transduce images into neuronal signals that are processed and sent to the visual cortex for decoding. The photoreceptors which capture light and initiate neural transmission are located at the back edge of the retina so that light must travel through all the other layers before it is captured (Figure 1A and B). Blood vessels absorb light and cast a shadow which would cause blank spots in images if the vessels were located immediately in front of photoreceptors. Therefore, the outer retina is avascular and retinal vessels supply only the inner retina; they are located far enough in front of photoreceptor outer segments that light is able to get around them. The retinal vessels enter at the optic nerve head, travel along the surface of the retina and send penetrating branches that form the intermediate and deep capillary beds that supply the inner two thirds of the retina (Figure 1B). The photoreceptors obtain oxygen and nutrients from choroidal vessels which are separated from them by the retinal pigmented epithelium (RPE).
Figure 1. Location of the retina within the eye (A) and retinal blood vessels within the retina (B).
(A) Schematic cross-section of the posterior part of the eye around the optic nerve shows the macula which is located temporal to the optic nerve and is responsible for our best vision. The foveal pit or depression is located in the center of the macula and is the area needed for the very best vision. The round blow up on the left shows that the multilayered retina sits on the retinal pigmented epithelium (RPE) which sits on Bruch’s membrane. The central retinal artery enters through the optic nerve, sends numerous branches along the surface of the retina to the peripheral edges of the retina. The arterioles enter capillaries which enter venules and then veins that run along the surface of the retina and enter the central retinal vein that exits through the optic nerve.
(B) A further blow up of the retina shows that the retinal arteries branch to form the superficial capillary bed near the surface of the retina and send penetrating branches to form the intermediate and deep capillaries. The outer third of the retina which consists of the photoreceptor outer and inner segments and cells bodies is avascular. It receives oxygen and nutrients from the choroidal circulation. Large choroidal vessels branch and become progressively smaller until they form the choriocapillaris which is fenestrated and allows plasma to pool along Bruch’s membrane. The RPE, which has barrier characteristics prevents fluid from entering the outer retina but allows oxygen and nutrients to enter.
Any disturbance in the concentration of electrolytes in the extracellular compartment of the retina disrupts neural transmission and degrades vision. The blood-retinal barrier (BRB) rigorously controls the entry of fluid and electrolytes into the extracellular space maintaining an appropriate milieu. There are two components of the BRB, the inner BRB constituted by retinal vascular endothelium, which has tight junctions and other specialized barrier characteristics, and the outer BRB constituted by the RPE, which also has tight junctions and other barrier properties. The RPE sits on Bruch’s membrane which separates it from the capillaries of choroidal vessels (choriocapillaris). The choriocapillaris is fenestrated allowing pooling of plasma along the basal surface of the RPE which facilitates diffusion and transport to the photoreceptors; the RPE barrier prevents excess fluid from entering the subretinal space and outer retina.
Retinal neovascularization (NV)
Wound repair in skin and many other tissues involves neovascularization (NV), sprouting of new vessels from pre-existent vessels, to repair or replace damaged vessels. In the retina, NV is not productive and rather than ameliorating the underlying problem, it exacerbates it. New vessels originating from retinal vessels may initially grow within the retina and clinicians call this intraretinal microvascular abnormalities (IRMA), but over time they grow to the retinal surface and into the vitreous at which point clinicians designate the new vessels as retinal NV. The NV adheres to the inner surface of the retina and outer surface of the vitreous. Unlike normal retinal vessels, IRMA and NV are deficient in tight junctions and hence leak plasma into surrounding tissue including the vitreous (Figure 2A). Plasma causes the vitreous gel to degenerate, contract, and eventually collapse which pulls on the retina. Since retinal NV is adherent to both retina and vitreous, as the vitreous contracts the NV may be sheared resulting in vitreous hemorrhage or the NV may remain intact and pull the retina with the vitreous resulting in retinal elevation referred to as traction retinal detachment. When traction retinal detachment involves the macula, which is responsible for reading and driving vision, severe visual loss occurs.
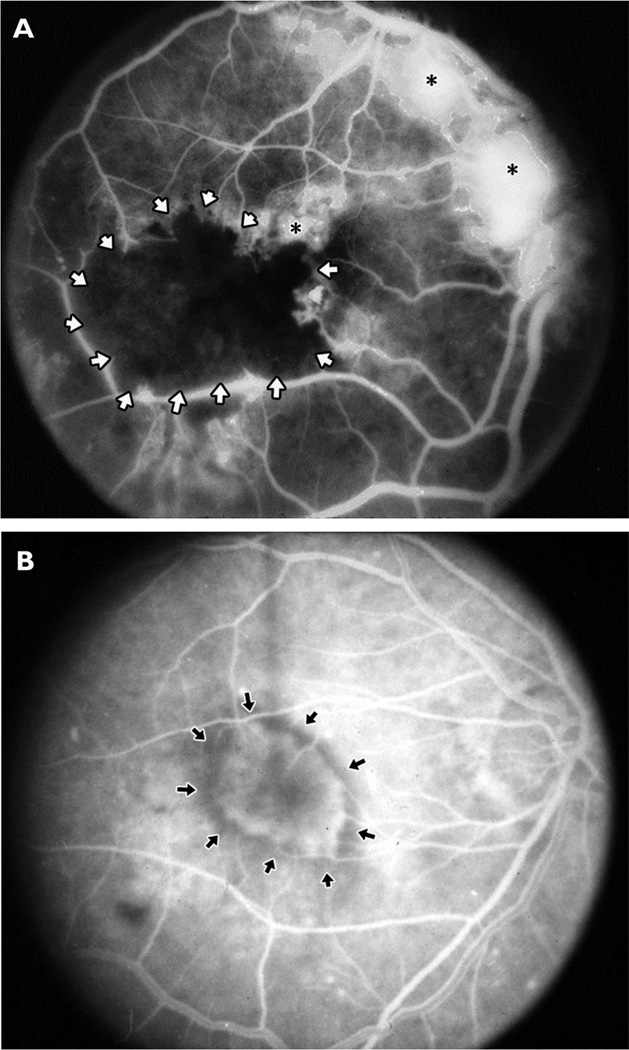
Figure 2. Fluorescein angiograms from patients with proliferative diabetic retinopathy, an ischemic retinopathy (A), or subretinal neovascularization (B).
Sodium fluorescein was injected intravenously and then a fundus camera with filters that block extraneous wavelengths of light was used to record the image caused by emission of fluorescence from the retina. The white dye should be confined within retinal vessels, which progressively branch to form capillaries.
(A) In a patient with proliferative diabetic retinopathy, the major retinal vessels branch into smaller vessels that extend into the macula. The resolution is not sufficient to see individual capillaries; they merge together and appear as gray areas between large vessels. There is a black area (surrounded by white arrows) of nonperfused retina in which all of the capillaries and larger vessels are closed due to damage from diabetes. There is retinal neovascularization (NV) on the surface of the retina adjacent to the nonperfused retina, at the optic disc, and above the optic disc (asterisks). The individual new vessels cannot be seen because they have leaked dye into the extracellular space causing confluent white patches.
(B) In a patient with choroidal NV due to age-related macular degeneration, major retinal vessels are seen extending from the optic nerve (far right of image) in an arc around the macula which is in the center with many branches from the arcade vessels extending into the macula. In the center of the macula an oval hyperfluorescent area is seen (arrows); it is beneath the retina and the retinal vessels pass over it. This is an area of choroidal NV which is a convoluted network of vessels like those shown histologically in Figure 3(D) viewed through the retina. Individual vessels are not seen because the vessels leak dye into the extracellular space and therefore the network of new vessels are seen as a hazy area of hyperfluorescence with a fairly distinct border.
The group of diseases in which retinal NV occurs including, diabetic retinopathy, retinopathy of prematurity, and retinal vein occlusions, a common feature is that the underlying disease process damages retinal vessels, causes them to close, and results in retinal ischemia (Figure 2A). Hence these diseases are referred to as ischemic retinopathies. In 1948 it was postulated that ischemic retina releases an angiogenic factor responsible for retinal NV [1] and since then considerable research has been devoted to identifying this factor. Initially most attention focused on fibroblast growth factor-1 (FGF-1) and FGF-2, because they are present in high levels in the retina and have angiogenic activity [2]. Attention was also given to vascular endothelial growth factor (VEGF) when it was demonstrated that it is increased in ischemic tissue [3, 4] and sampling of intraocular fluid from patients with ischemic retinopathies demonstrated high levels of VEGF [5].
The development of animal models that mimic key aspects of a disease provides invaluable tools for gaining insights into the disease and ultimately developing treatments. The observation that high levels of inspired oxygen suppresses retinal vascular development resulting in areas of nonperfused retina and retinal NV helped to elucidate the pathogenesis of retinopathy of prematurity and also led to the development of models relevant to all ischemic retinopathies [6–8]. The mouse model of oxygen-induced ischemic retinopathy has proven most useful [9]. The retinal vasculature in mouse retina (Figure 3A) is similar to that seen in humans (Figure 1); large vessels run along the surface and branch to form the superficial capillary bed and also send penetrating branches that form the intermediate and deep capillary beds. In mice with oxygen-induced ischemic retinopathy, the retinal vessels are dilated and there are tufts of NV on the surface of the retina (Figure 3B). Demonstration that a specific VEGF antagonist suppressed retinal NV in this model provided the first clear demonstration that VEGF stimulates retinal NV [10]. Further work demonstrated that VEGF also drives normal retinal vascular development and high inspired oxygen during retinal vascular development down-regulates VEGF, halts vascularization of the retina, causes newly formed vessels to regress, and results in areas of nonperfused retina [11, 12]. Return to room air results in hypoxia in the nonperfused areas of retina which then produce high levels of VEGF causing retinal NV [10, 13].
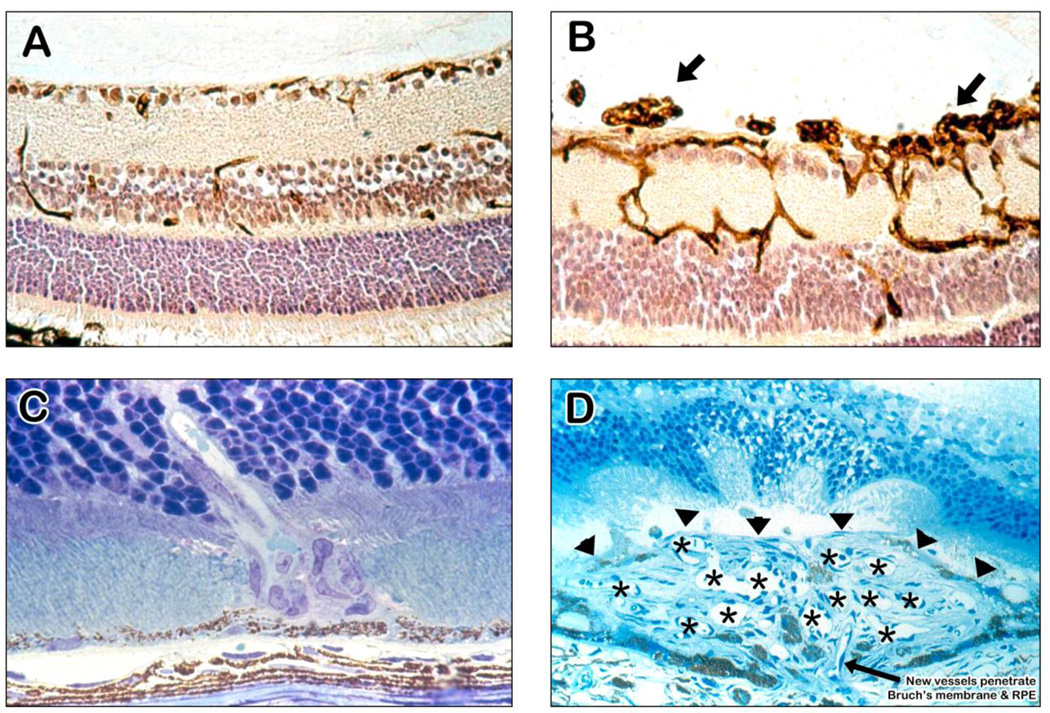
Figure 3. Ocular sections from mouse models of ocular neovascularization (NV).
(A) A normal mouse retina histochemically stained with a lectin that selectively stains vascular cells and counter-stained to show other retinal cells illustrates the 3 capillary beds of the retina with some penetrating vessels showing connections or part of connections between the beds.
(B) A retina from a mouse with oxygen-induced ischemic retinopathy shows dilation of the vessels within the retina with NV on the surface of the retina (arrows).
(C) A retina from a rho/VEGF transgenic mouse, a model of retinal angiomatous proliferation, shows a vessel extending from the deep capillary bed of the retina through the photoreceptors into the subretinal space.
(D) A retina from a mouse with choroidal NV after laser-induced rupture of Bruch’s membrane. The arrow shows a vessel extending from the choroid through the rupture in Bruch’s membrane into the subretinal space where it connects to a large convoluted network of new vessels, many of which are cut in cross section to show their lumens (astericks). The superior margin of the choroidal NV that borders the photoreceptors is shown by the arrowheads.
The identification of hypoxia-inducible factor-1 (HIF-1) as a key transcription factor that mediates increased expression of hypoxia-regulated genes [14–16] helped to fill in more detail regarding the pathogenesis of retinal NV. HIF-1 has two subunits, HIF-1α expression of which is increased in hypoxic tissue, and HIF-1β, which is constitutively expressed. In the mouse model of ischemic retinopathy, HIF-1α levels are increased and show temporal and spatial correlation with excess VEGF [17]. Gene transfer of a constitutively active form of HIF-1 induces retinal NV in the absence of retinal ischemia [18]. Implication of HIF-1 focused attention on other HIF-1-regulated genes, including placental growth factor (PlGF), platelet-derived growth factor-B (PDGF-B) and stromal derived growth factor-1 (SDF-1). PlGF is a member of the VEGF gene family that binds to VEGFR1 and like VEGF promotes recruitment of bone marrow-derived cells and stimulates retinal NV [19]. Retina-specific expression of PDGF-B in transgenic mice results in severe NV and retinal detachment, which is typical of ischemic retinopathies [20, 21]. SDF-1 is expressed in ischemic retina and promotes recruitment of bone marrow-derived cells which contribute to retinal NV, because specific antagonists of CXCR4, the receptor for SDF-1, reduce macrophage influx and retinal NV in ischemic retina [22].
As NV sprouts develop, the endothelial cells differentiate into tip and stalk cells and tip cells proliferate, extend filopodia, and migrate along VEGF gradients. The process is regulated by hypoxic induction of Notch signaling [23] which suppresses or stimulates tip cell formation through opposing actions of delta-like ligand 4 (Dll4) and Jagged1 [24–26]. Growth and guidance of tip cells is regulated by several signaling molecules that also participate in neuronal growth cone guidance [27]. Thus, retinal NV occurs in diseases in which the underlying disease process damages retinal vessels causing areas of vessel closure and retinal ischemia leading to increased levels of HIF-1 which stimulate expression of a group of hypoxia-regulated genes that together stimulate the growth of new vessels.
Subretinal NV
Subretinal NV refers to new vessels growing beneath the retina in the subretinal space regardless of the location from which the vessels originated (Figure 2B). There are two types of subretinal NV based upon origin of the vessels: (1) retinal angiomatous proliferation (RAP) which originates from the deep capillary bed of the retina and grows through the photoreceptor layer to reach the subretinal space (Figure 3C) and (2) choroidal NV which sprouts from choroidal vessels and extends through Bruch’s membrane and the RPE to reach the subretinal space (Figure 3D) [28]. Both types of subretinal NV occur in patients with AMD and they have similar consequences with regard to vision loss. Transgenic mice in which the rhodopsin promoter drives expression of VEGF in photoreceptors (rho/VEGF mice) develop RAP [29, 30]. Very low density lipoprotein receptor (Vldlr) knockout mice also develop RAP and they too have increased expression of VEGF in photoreceptors [31, 32]. Thus, VEGF is an important stimulus for RAP. In contrast, rho/FGF2 transgenic mice do not develop RAP and do not have a spontaneous phenotype [33].
A shared feature of diseases in which choroidal NV occurs is the presence of defects or deposits in Bruch’s membrane and abnormal RPE. Rupture of Bruch’s membrane by laser photocoagulation in monkeys results in choroidal NV [34]. This model was adapted to mice to take advantage of mouse genetics [35]. After laser-induced rupture of Bruch’s membrane in mice there is transient increase in VEGF expression in the retina [36] and if Bruch’s membrane is ruptured in rho/VEGF mice that have sustained increased expression of VEGF in photoreceptors, very severe choroidal NV occurs, much greater than that seen at Bruch’s membrane rupture sites in wild type mice [33]. This suggests that VEGF is a critical stimulus for choroidal NV, which was confirmed because it is suppressed by VEGF antagonists [37]. In the monkey model of laser-induced rupture of Bruch’s membrane, intraocular injection of ranibizumab, an antibody fragment that binds primate VEGF, suppressed choroidal NV [38]. In contrast, FGF-2 does not play a critical role, because FGF-2 knockout mice showed no difference from wild type mice in the amount of choroidal NV at Bruch’s membrane rupture sites [35]. Thus, both RAP and choroidal NV are associated with increased expression of VEGF, but unlike RAP, choroidal NV requires some type of perturbation of Bruch’s membrane and/or the RPE. The perturbation is not limited to rupture with a laser, because choroidal NV also occurs after subretinal injection of a viral vector containing a Vegf expression construct which causes inflammation in the subretinal space as well as increased expression of VEGF in the retina [39]. RPE cells are polarized and normally secrete VEGF from their basal surface toward the choriocapillaris and transgenic over-expression of VEGF in RPE is not sufficient to cause choroidal NV, unless there is inflammation in the subretinal space which experimentally can be produced by subretinal injection of an empty adenoviral vector [40].
Unlike retinal NV in which retinal ischemia is the clear cause of increased production of VEGF, there is no clear evidence of retinal or choroidal ischemia associated with subretinal NV. However, HIF-1 is still important, because mice that lack a hypoxia response element in the Vegf promoter develop significantly less NV at Bruch’s membrane rupture sites than wild type mice [41]. Furthermore, other hypoxia regulated gene products including PDGF-B and SDF-1 have been implicated in choroidal NV similar to the situation in retinal NV [22, 42]. Digoxin inhibits the transcriptional activity of HIF-1 [43] and strongly suppresses retinal and choroidal NV [44]. Oxidative stress is increased in RPE and photoreceptors in AMD and this may be the cause of increased levels of HIF-1, because mitochondrial reactive oxygen species stabilize HIF-1 by reducing the activity of prolyl hydroxylases [45–47]. This is consistent with the observations that oxidative stress exacerbates choroidal NV [48] and that antioxidant vitamins and zinc reduce the incidence of choroidal NV in patients with AMD [49]. Thus, while there are substantial differences in the pathogenesis of retinal and subretinal NV, there is also considerable overlap in the vasoactive mediators that participate.
Bone marrow-derived cells
HIF-1-induced upregulation of VEGF and SDF-1 stimulates recruitment and retention of bone marrow-derived cells into ischemic or injured tissue [50, 51]. One type of cell that is recruited is endothelial progenitor cells [52], but the manner in which they participate is controversial. Endothelial progenitor cells isolated from adult mouse bone marrow by flow cytometry and injected into the vitreous cavity were incorporated into developing retinal vessels and the retinal vessels of rd1 mice with retinal degeneration [53]. In mice treated with whole body irradiation followed by bone marrow transplantation of GFP-expressing cells, substantial numbers of GFP-positive cells were incorporated into retinal and choroidal NV [54, 55]. These studies suggest that after intraocular injection of a bolus of progenitor cells or after whole body irradiation which limits proliferation of endogenous endothelial cells, a substantial proportion of cells making up new vessels in the eye are derived from endothelial progenitor cells; however, in non-irradiated animals, it is not clear to what extent ocular NV is derived from endothelial progenitor cells versus proliferation and migration of endothelial cells from pre-existent ocular vessels. Regardless, it is quite clear that bone marrow-derived cells, including endothelial progenitor cells, monocytes/macrophages, and other leukocytes, are recruited into ischemic retina or damaged outer retina and RPE and contribute to retinal and subretinal NV through paracrine stimulation. The role of macrophages is complex because there are multiple macrophage phenotypes, some that stimulate NV and some that suppresses it or cause vascular regression [56–60]. After entering the retina, macrophages home to areas of NV and surround sprouts [61]. This close association allows stimulation through soluble factors, such as VEGF [51, 60], as well as Notch and Wnt signaling which requires cell-cell interaction and mediates both stimulation and suppression of NV [62, 63]. Hypoxia, likely through stabilization of HIF-1, may promote a pro-angiogenic macrophage phenotype [64], but it has been suggested that senescence or chronic viral infection may also contribute [60, 65]. It appears that under most circumstances, other than regression of the hyaloid vasculature in the embryonic or neonatal eye [56, 57], pro-angiogenic macrophages predominate, because interventions that reduce total numbers of macrophages in the circulation [66] or suppress macrophage influx into the eye [22, 67–70] suppress retinal or subretinal NV. Thus, VEGF antagonists reduce influx of bone-marrow derived cells into ischemic or damaged retina which likely contributes to the therapeutic activity of those antagonists, but there are other ways to more profoundly reduce bone marrow-derived cells from the retina and it must be determined if those approaches add benefit to VEGF antagonists and/or provide an alternative therapeutic approach that is safer.
The Tie2 pathway
In addition to input from soluble factors such as VEGF and PDGF-B, endothelial cells respond to signals from surrounding cells and extracellular matrix (ECM) that influence vascular maintenance and permeability. One such signaling pathway is regulated by Tie2, a receptor tyrosine kinase located predominately on vascular endothelial cells that binds the angiopoietin (Angpt) family of secreted proteins [71–73]. Angiopoietin (Angpt) 1 is an agonist that binds Tie2 and stimulates its phosphorylation, while Angpt2 acts as an antagonist under most circumstances. Angpt 2 plays an important role in retinal vascular development; it is expressed on the surface of the retina between P0 and P7 when the superficial retinal vessels are developing and after P7 expression shifts to the regions of the developing intermediate and deep capillary beds, [74, 75] and retinal vascular development fails to occur in Angpt2 knockout mice [75, 76]. In mice with oxygen-induced ischemic retinopathy, there is ectopic expression of Angpt2 at the retinal surface in association with sprouting of new vessels [75]. Expression of Angpt2 is maintained in adult retina only in horizontal cells in the region of the deep capillary bed and over-expression of VEGF in the retina results in NV originating only from the deep capillary bed [29]; however, co-expression of VEGF and Angpt2 at the surface of the retina results in NV originating from superficial capillaries [77]. Likewise expression of a constitutively active form of HIF-1 at the retinal surface, which increases expression of both VEGF and Angpt2, also causes NV to sprout from superficial vessels [18]. Thus, Angpt2 enables VEGF-induced retinal vascular development and also enables VEGF-stimulated pathologic vessel sprouting.
Double transgenic mice with doxycycline-inducible expression of Angpt2 in photoreceptors (Tet/opsin/ang2 mice) have helped to further clarify activities of Angpt2 in the retina [36]. In mice with ischemic retinopathy, induction of Angpt2 expression between P12 and P17 when VEGF levels are high markedly increased retinal NV, while induction of Angpt2 expression after P21 when VEGF levels are low caused rapid regression of retinal NV. Similarly Angpt2 stimulates choroidal NV when VEGF is high and causes rapid regression when VEGF is low. In contrast, double transgenic mice with doxycycline-inducible expression of Angpt1 (Tet/opsin/ang1 mice) demonstrated that Angpt1 is not context-dependent and always suppresses retinal or subretinal NV [78, 79]. These observations suggest that a model developed to explain effects of Tie2 and the angiopoietins in the ovary also applies to their actions in the eye [73]: stimulation of Tie2 by Angpt1 acts to stabilize retinal or choroidal vessels and reduces their ability to sprout new vessels in the presence of VEGF, whereas Angpt2 promotes dephosphorylation of Tie2, destabilizing vessels and enabling VEGF to stimulate sprouting of NV. After sprouting of new vessels their continued growth is greater in the presence of both Angpt2 and VEGF than with just VEGF alone, and if VEGF levels decline in the presence of high Angpt2, the new vessels rapidly regress.
The role of extracellular matrix and surrounding cells
At least part of the role of Tie2 seems to be to modulate signaling between vascular endothelial cells and surrounding cells and ECM, which occurs primarily through integrins. Tie2 stimulation by Angpt1 potentiates survival and maintenance signals from surrounding cells and ECM which maintain endothelial cells in a quiescent state. Inhibition of Tie2 signaling by Angpt2 interrupts survival and maintenance signals from surrounding cells and ECM, thereby making endothelial cells more responsive and more dependent upon soluble signals such as VEGF. Hypoxia, injury, and VEGF also cause endothelial cells to change their integrin expression: endothelial cells participating in NV express high levels of α5β1, αvβ3, and αvβ5, while these are undetectable in endothelial cells of quiescent ocular vessels [80–83]. These integrins provide survival signals from the ECM to endothelial cells of new vessels and ligands that bind to these integrins induce apoptosis of endothelial cells participating in NV, causing regression of NV with no effect on normal vessels [83].
Endogenous antiangiogenic proteins
Growth of new vessels requires proteolytic degradation of ECM, which liberates fragments with antiangiogenic activity such as the noncollagenous (NC1) domains of the basement membrane collagens XVIII (endostatin) [84], XV (restin) [85] or IV (canstatin) [86–88]. In many tissues, these antiangiogenic fragments provide negative feedback to terminate angiogenesis, but these control mechanisms are not robust in the eye, because retinal and subretinal NV rarely stop or regress spontaneously. However, intraocular injection of recombinant NC1 domain of collagen IV [89] or gene transfer of endostatin [90, 91] causes regression of retinal or subretinal NV. Since the eye is a relatively isolated compartment it is well-suited for gene transfer and this approach has been used in mouse models of retinal and choroidal NV to confirm the antiangiogenic activity of several proteins in addition to endostatin, including pigment epithelium-derived factor (PEDF), sVEGFR1, angiostatin, and vasohibin [92–96]. Gene transfer provides an appealing approach to provide long-term suppression of ocular NV and is currently being tested in clinical trials (see below).
Clinical trials in neovascular AMD
The mounting evidence from preclinical studies implicating VEGF in ocular NV led to the development of ranibizumab, a 48 kD antibody fragment that binds all isoforms of VEGF-A (Genentech, Inc., South San Francisco, CA) [97]. Genentech had previously developed a full-length anti-VEGF monoclonal antibody (bevacizumab) for systemic treatment of tumors, but it was felt that the smaller size of ranibizumab would be advantageous for intraocular delivery to enhance penetration through the retina to the choroid and speed clearance from the circulation once it exited the eye. In addition, ranibizumab was affinity matured and is 5 to 20-fold more potent than bevacizumab on a molar basis. The intraocular half-life of ranibizumab in monkeys was 3 days and serum levels were about 1000-fold lower than intraocular levels [98]. In patients with neovascular AMD who had reduced vision from intraretinal and subretinal fluid that had leaked from choroidal NV, monthly intraocular injections of ranibizumab for 1 year caused a marked reduction in leakage with resolution of fluid in most patients and substantial improvement in visual acuity [99, 100]. Ranibizumab injections suppress leakage and stop growth of choroidal NV, but usually do not cause regression of NV and when treatment is stopped, leakage and growth usually recur; however, with continued treatment many patients have maintained visual benefits many years.
Aflibercept is a recombinant protein consisting of the binding domains of VEGFR1 and VEGFR2 fused with an Fc fragment and it strongly suppressed subretinal NV in mice [101] and showed similar benefit as ranibizumab in patients with neovascular AMD [102]. Off label use of bevacizumab in patients with neovascular AMD has also shown similar benefit to that seen with ranibizumab [103]. Thus, multiple VEGF antagonists have shown strong efficacy in patients with neovascular AMD confirming that VEGF plays a major role in the pathogenesis of subretinal NV and demonstrating the predictive value of the mouse models of subretinal NV.
Recent studies in patients with neovascular AMD demonstrated that monthly injections of an aptamer that specifically binds PDGF-B combined with ranibizumab provided significantly better visual improvement and more frequent regression of NV than injections of ranibizumab alone [104]. Thus PDGF-B is a second validated target that was also predicted in preclinical studies. Intraocular injection of an adenoviral vector expressing PEDF showed good safety and evidence of biologic activity confirming preclinical studies in mice and providing proof of concept for gene transfer of anti-angiogenic proteins as a therapeutic approach in neovascular AMD [105]. This has led to clinical trials utilizing long-term expression vectors, one in which adeno-associated viral vector 2 (AAV2) is used to express a VEGF binding protein (modified sFlt1) and one in which a lentiviral vector is used to express endostatin and angiostatin [106, 107].
Conclusions
Many aspects of the molecular pathogenesis of retinal (Figure 4) and subretinal NV (Figure 5) have been elucidated. In both, there is increased HIF-1 activity causing increased production of several hypoxia-regulated vasoactive gene products. Studies in animal models predicted that VEGF plays a major role and this has been validated in clinical trials. VEGF antagonists provide major benefits in patients with neovascular AMD and other types of ocular NV. A second validated target is PDGF-B and combination therapy with VEGF and PDGF-B antagonists is in late stage clinical testing. In the future, antagonists of other hypoxia-regulated gene products may be added to anti-VEGF and anti-PDGF-B agents or drugs that directly target HIF-1 may be used to achieve “single agent combination therapy”. Ocular gene delivery to provide sustained expression of anti-angiogenic proteins is also being tested and holds considerable promise. Since 2006, the treatment of blinding vascular diseases of the eye has been tremendously improved and it is likely that substantial further improvement will occur in the near future.
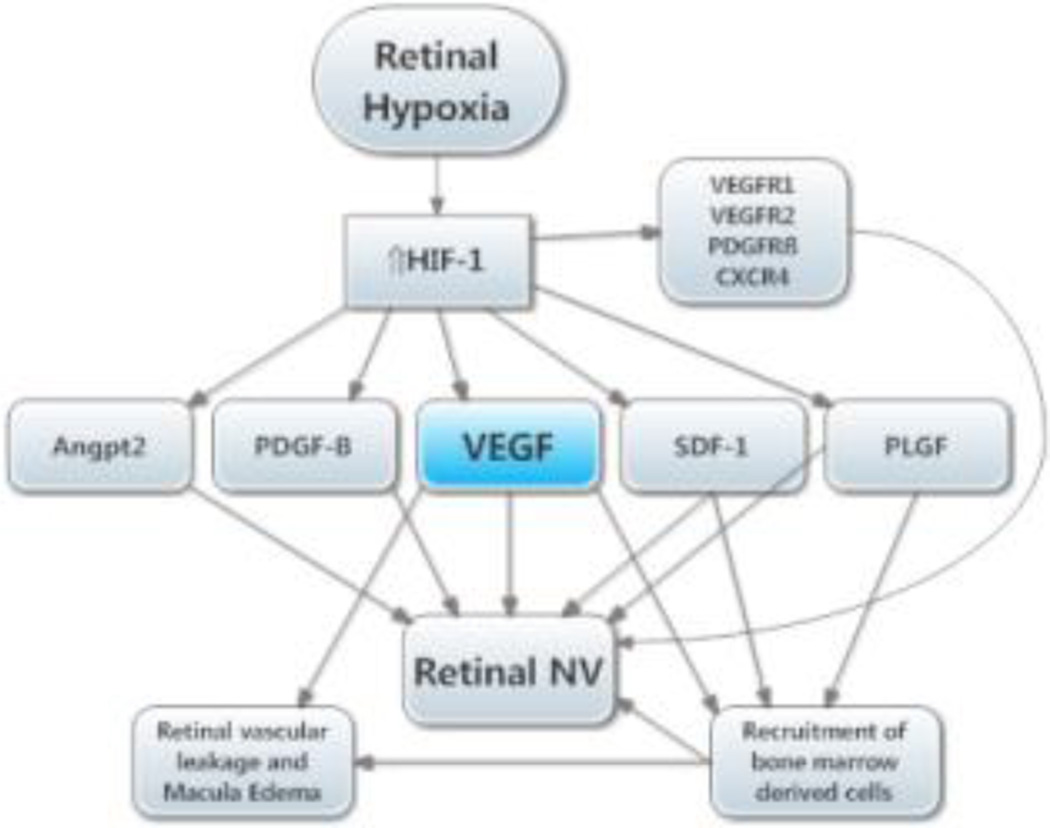
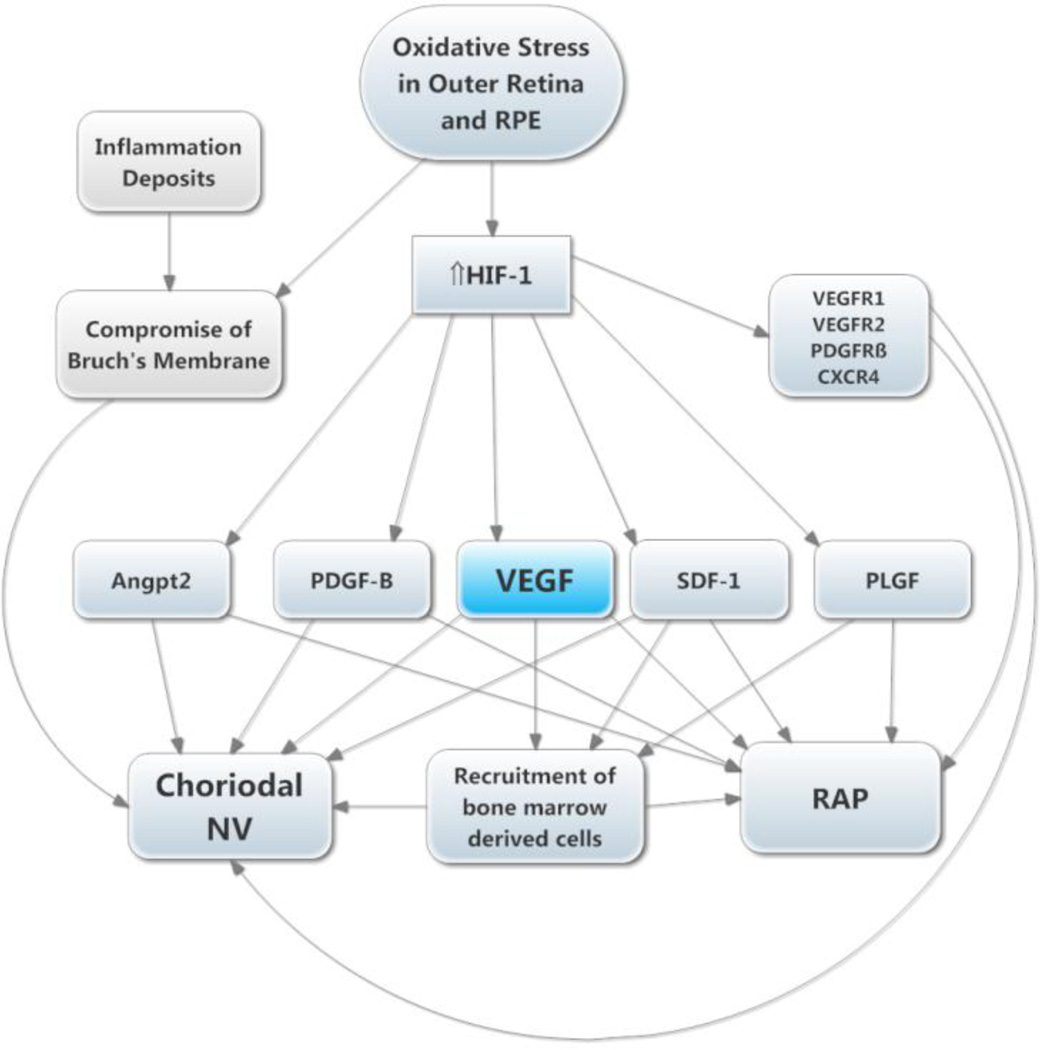
Figure 4. Molecular pathogenesis of retinal neovascularization (NV).
This simplified version of the molecular pathogenesis of retinal NV that illustrates several important molecular signals. It highlights soluble mediators and omits cell-cell and cell-matrix signaling. Retinal NV occurs in diabetic retinopathy and other ischemic retinopathies. The underlying disease process (e.g. high glucose in diabetic retinopathy) damages retinal vessels causing vessel closure and retinal ischemia, which results in elevated HIF-1 levels. HIF-1 upregulates several vasoactive gene products including vascular endothelial growth factor (VEGF), platelet-derived growth factor (PDGF-B), placental growth factor (PLGF), stromal-derived growth factor (SDF-1), and their receptors, and angiopoietin 2 (Angpt2). VEGF causes vascular leakage and in combination with Angpt2 causes sprouting of new vessels. VEGF, SDF-1, and PLGF recruit bone marrow-derived cells which provide paracrine stimulation. PDGF-B recruits pericytes which also provide paracrine stimulation.
Figure 5. Molecular pathogenesis of subretinal neovascularization (NV).
This schematic highlights several important molecular signals involved in subretinal NV. Oxidative stress in the retinal pigmented epithelium (RPE) and photoreceptors causes increased levels of HIF-1, which upregulates vasoactive gene products as described above. Retinal angiomatous proliferation (RAP) occurs if VEGF levels in photoreceptors are sufficiently high to cause an adequate gradient that reaches to the deep capillary bed of the retina. Choroidal NV occurs if there is elevation of VEGF and Angpt2 combined with perturbation of Bruch’s membrane and the RPE. The other HIF-1-responsive gene products fuel the process similar to the situation in retinal NV.
Acknowledgments
PAC is a consultant for Genentech, Regeneron, Allergan, and Aerpio for which his employer, the Johns Hopkins University, receives remuneration. PAC receives research funding for clinical trials from Genentech, Regeneron, Allergan, Aerpio, Genzyme, and Oxford BioMedica.
Footnotes
Disclosure: The author is consultant for Genentech, Regeneron, Allergan, and Aerpio for which Johns Hopkins University receives remuneration and receives research support for clinical trials from the above, Genzyme, and Oxford BioMedica.
References
- 1.Michaelson I. The mode of development of the vascular system of the retina with some observations on its significance for certain retinal diseases. Trans Ophthalmol Soc UK. 1948;68:137–180. [Google Scholar]
- 2.Baird A, Esch F, Gospodarowicz D, Guillemin R. Retina- and eye-derived endothelial cell growth factors: partial molecular chariacterization and identity with acidic and basic fibroblast growth factors. Biochemistry. 1985;24:7855–7860. doi: 10.1021/bi00348a001. [DOI] [PubMed] [Google Scholar]
- 3.Shweiki D, Itin A, Soffer D, Keshet E. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature. 1992;359:843–845. doi: 10.1038/359843a0. [DOI] [PubMed] [Google Scholar]
- 4.Plate KH, Breier G, Welch HA, Risau W. Vascular endothelial growth factor is a potential tumor angiogenesis factor in human gliomas in vivo. Nature. 1992;359:845–848. doi: 10.1038/359845a0. [DOI] [PubMed] [Google Scholar]
- 5.Aiello LP, Avery RL, Arrigg PG, Keyt BA, Jampel HD, Shah ST, Pasquale LR, Thieme H, Iwamoto MA, Park JE, et al. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med. 1994;331:1480–1487. doi: 10.1056/NEJM199412013312203. [DOI] [PubMed] [Google Scholar]
- 6.Patz A. The role of oxygen in retrolental fibroplasia. Trans Am Opthalmol Soc. 1968;66:940–985. [PMC free article] [PubMed] [Google Scholar]
- 7.Patz A. Current concepts of the effect of oxygen on the developing retina. Curr Eye Res. 1984;3:159–163. doi: 10.3109/02713688408997197. [DOI] [PubMed] [Google Scholar]
- 8.Patz A, Eastham A, Higgenbotham DH, Kleh T. Oxygen studies in retrolental figroplasia: Production of the microscopic changes of retrolental fibroplasia in experimental animals. Am J Ophthalmol. 1953;36:1511–1522. [PubMed] [Google Scholar]
- 9.Smith LEH, Wesolowski E, McLellan A, Kostyk SK, D'Amato R, Sullivan R, D'Amore PA. Oxygen-induced retinopathy in the mouse. Invest Ophthalmol Vis Sci. 1994;35:101–111. [PubMed] [Google Scholar]
- 10.Aiello LP, Pierce EA, Foley ED, Takagi H, Chen H, Riddle L, Ferrara N, King GL, Smith LEH. Suppression of retinal neovascularization in vivo by inhibition of vascular endothelial growth factor (VEGF) using soluble VEGF-receptor chimeric proteins. Proc Natl Acad Sci USA. 1995;92:10457–10461. doi: 10.1073/pnas.92.23.10457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alon T, Hemo I, Itin A, Pe'er J, Stone J, Keshet E. Vascular endothelial growth factor acts as a survival factor for newly formed retinal vessels and has implications for retinopathy of prematurity. Nature Med. 1995;1:1024–1028. doi: 10.1038/nm1095-1024. [DOI] [PubMed] [Google Scholar]
- 12.Pierce EA, Foley ED, Smith LE. Regulation of vascular endothelial growth factor by oxygen in a model of retinopathy of prematurity. Arch Ophthalmol. 1996;114:1219–1228. doi: 10.1001/archopht.1996.01100140419009. [DOI] [PubMed] [Google Scholar]
- 13.Pierce EA, Avery RL, Foley ED, Aiello LP, Smith LEH. Vascular endothelial growth factor/vascular permeability factor expression in a mouse model of retinal neovascularization. Proc Natl Acad Sci USA. 1995;92:905–909. doi: 10.1073/pnas.92.3.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang GL, Semenza GL. General involvement of hypoxia-inducible factor 1 in transcriptional response to hypoxia. Proc Natl Acad Sci USA. 1993;90:4304–4308. doi: 10.1073/pnas.90.9.4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang GL, Jiang B-H, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci USA. 1995;92:5510–5514. doi: 10.1073/pnas.92.12.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Semenza GL. HIF-1: mediator of physiological and pathophysiological responses to hypoxia. J Appl Physiol. 2000;88:1474–1480. doi: 10.1152/jappl.2000.88.4.1474. [DOI] [PubMed] [Google Scholar]
- 17.Ozaki H, Yu A, Della N, Ozaki K, Luna JD, Yamada H, Hackett SF, Okamoto N, Zack DJ, Semenza GL, Campochiaro PA. Hypoxia inducible factor-1α is increased in ischemic retina: temporal and spatial correlation with VEGF expression. Invest Ophthalmol Vis Sci. 1999;40:182–189. [PubMed] [Google Scholar]
- 18.Kelly BD, Hackett SF, Hirota K, Oshima Y, Cai Z, Berg-Dixon S, Rowan A, Yan Z, Campochiaro PA, Semenza GL. Cell type-specific regulation of angiogenic growth factor gene expression and induction of angiogenesis in nonischemic tissue by a constitutively active form of hypoxia-inducible factor 1. Circ Res. 2003;93:1074–1081. doi: 10.1161/01.RES.0000102937.50486.1B. [DOI] [PubMed] [Google Scholar]
- 19.Carmeliet P, Moons L, Luttun A, Vincenti V, Compernolle V, De Mol M, Wu Y, Bono F, Devy L, Beck H, et al. Synergism between vascular endothelial growth factor and placental growth factor contributes to angiogenesis and plasma extravasation in pathological conditions. Nat Med. 2001;7:575–583. doi: 10.1038/87904. [DOI] [PubMed] [Google Scholar]
- 20.Seo M-S, Okamoto N, Vinores MA, Vinores SA, Hackett SF, Yamada H, Yamada E, Derevjanik NL, LaRochelle W, Zack DJ, Campochiaro PA. Photoreceptor-specific expression of PDGF-B results in traction retinal detachment. Am J Pathol. 2000;157:995–1005. doi: 10.1016/S0002-9440(10)64612-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mori K, Gehlbach P, Ando A, Dyer G, Lipinsky E, Chaudhry AG, Hackett SF, Campochiaro PA. Retina-specific expression of PDGF-B versus PDGF-A: vascular versus nonvascular proliferative retinopathy. Invest Ophthalmol Vis Sci. 2002;43:2001–2006. [PubMed] [Google Scholar]
- 22.Lima e Silva R, Shen J, Hackett SF, Kachi S, Akiyama H, Kiuchi K, Yokoi K, Hatara C, McLauer T, Aslam S, et al. The SDF-1/CXCR4 ligand/receptor pair is an important contributor to several types of ocular neovascularization. FASEB J. 2007;21:3219–3230. doi: 10.1096/fj.06-7359com. [DOI] [PubMed] [Google Scholar]
- 23.Diez H, Fischer A, Winkler A, Hu C-J, Hatzopoulos AK, Breier G, Gessler M. Hypoxia-mediated activation of Dll4-Notch-Hey2 signaling in endothelial progenitor cells and adoption of arterial cell fate. Exp Cell Res. 2007;313:1–9. doi: 10.1016/j.yexcr.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 24.Hofmann JJ, Iruela-Arispe ML. Notch expression pattens in the retina: An eye on receptor-ligand distrubution during angiogenesis. Gene Expr Patterns. 2007;7:461–470. doi: 10.1016/j.modgep.2006.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hellstrom M, Phing LK, Hofmman JJ, Wallgard E, Coultas L, Lindblom P, Alva J, Nilsson AK, Karlsson L, Gaiano N, et al. Dll4 signalling through Notch 1 regulates formation of tip cells during angiogenesis. Nature. 2007;15:776–780. doi: 10.1038/nature05571. [DOI] [PubMed] [Google Scholar]
- 26.Benedito R, Roca C, Sorensen I, Adams s, Gossler A, Fruttiger M, Adams RH. The notch ligands Dll4 and Jagged1 have opposing effects on angiogenesis. Cell. 2009;137:1124–1135. doi: 10.1016/j.cell.2009.03.025. [DOI] [PubMed] [Google Scholar]
- 27.Carmeliet P, Tessier-Lavigne M. Common mechanisms of nerve and blood vessel wiring. Nature. 2005;436:193–200. doi: 10.1038/nature03875. [DOI] [PubMed] [Google Scholar]
- 28.Yannuzzi LA, Negrao S, Iida T, Carvalho C, Rodriguez-Coleman H, Slakter JS, Freund KB, Sorenson J, Orlock D, Borodoker N. Retinal angiomatous proliferation in age-related macular degeneration. Retina. 2001;21:416–434. doi: 10.1097/00006982-200110000-00003. [DOI] [PubMed] [Google Scholar]
- 29.Okamoto N, Tobe T, Hackett SF, Ozaki H, Vinores MA, LaRochelle W, Zack DJ, Campochiaro PA. Transgenic mice with increased expression of vascular endothelial growth factor in the retina: a new model of intraretinal and subretinal neovascularization. Am J Pathol. 1997;151:281–291. [PMC free article] [PubMed] [Google Scholar]
- 30.Tobe T, Okamoto N, Vinores MA, Derevjanik NL, Vinores SA, Zack DJ, Campochiaro PA. Evolution of neovascularization in mice with overexpression of vascular endothelial growth factor in photoreceptors. Invest Ophthalmol Vis Sci. 1998;39:180–188. [PubMed] [Google Scholar]
- 31.Heckenlively JR, Hawes NL, Friedlander M, Nusinowitz S, Hurd R, Davisson M, Chang B. Mouse model of subretinal neovascularization with choroidal anastomosis. Retina. 2003;23:518–522. doi: 10.1097/00006982-200308000-00012. [DOI] [PubMed] [Google Scholar]
- 32.Li C, Huang Z, Kingsley R, Zhou X, Li F, Parke DW, 2nd, Cao W. Biochemical alterations in the retinas of very low-density lipoprotein receptor knockout mice: an animal model of retinal angiomatous proliferation. Arch Ophthalmol. 2007;125:795–803. doi: 10.1001/archopht.125.6.795. [DOI] [PubMed] [Google Scholar]
- 33.Yamada H, Yamada E, Kwak N, Ando A, Suzuki A, Esumi N, Zack DJ, Campochiaro PA. Cell injury unmasks a latent proangiogenic phenotype in mice with increased expression of FGF2 in the retina. J Cell Physiol. 2000;185:135–142. doi: 10.1002/1097-4652(200010)185:1<135::AID-JCP13>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 34.Ryan SJ. Subretinal neovascularization: natural history of an experimental model. Arch Ophthalmol. 1982;100:1804–1809. doi: 10.1001/archopht.1982.01030040784015. [DOI] [PubMed] [Google Scholar]
- 35.Tobe T, Ortega S, Luna JD, Ozaki H, Okamoto N, Derevjanik NL, Vinores SA, Basilico C, Campochiaro PA. Targeted disruption of the FGF2 gene does not prevent choroidal neovascularization in a murine model. Am J Pathol. 1998;153:1641–1646. doi: 10.1016/S0002-9440(10)65753-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oshima Y, Oshima S, Nambu H, Kachi S, Takahashi K, Umeda N, Shen J, Dong A, Apte RS, Duh E, et al. Different effects of angiopoietin 2 in different vascular beds in the eye; new vessels are most sensitive. FASEB J. 2005;19:963–965. doi: 10.1096/fj.04-2209fje. [DOI] [PubMed] [Google Scholar]
- 37.Kwak N, Okamoto N, Wood JM, Campochiaro PA. VEGF is an important stimulator in a model of choroidal neovascularization. Invest Ophthalmol Vis Sci. 2000;41:3158–3164. [PubMed] [Google Scholar]
- 38.Kryzstolik MG, Afshari MA, Adamis AP, Gaudreault J, Gragoudas ES, Michaud NM, Li W, Connolly E, O'Neill CA, Miller JW. Prevention of experimental choroidal neovascularization with intravitreal anti-vascular endothelial growth factor antibody fragment. Arch Ophthalmol. 2002;120:338–346. doi: 10.1001/archopht.120.3.338. [DOI] [PubMed] [Google Scholar]
- 39.Baffi J, Byrnes G, Chan CC, Csaky KG. Choroidal neovascularization in the rat induced by adenovirus mediated expression of vascular endothelial growth factor. Invest Ophthalmol Vis Sci. 2000;41:3582–3589. [PubMed] [Google Scholar]
- 40.Oshima Y, Oshima S, Nambu H, Kachi S, Hackett SF, Melia M, Kaleko M, Connelly S, Esumi N, Zack DJ, Campochiaro PA. Increased expression of VEGF in retinal pigmented epithelial cells is not sufficient to cause choroidal neovascularization. J Cell Physiol. 2004;201:393–400. doi: 10.1002/jcp.20110. [DOI] [PubMed] [Google Scholar]
- 41.Vinores SA, Xiao WH, Aslam S, Shen J, Oshima Y, Nambu H, Liu H, Carmeliet P, Campochiaro PA. Implication of the hypoxia response element of the VEGF promoter in mouse models of retinal and choroidal neovascularization, but not retinal vascular development. J Cell Physiol. 2006;206:749–758. doi: 10.1002/jcp.20525. [DOI] [PubMed] [Google Scholar]
- 42.Jo N, Mailhos C, Ju M, Cheung E, Bradley J, Nishijima K, Robinson GS, Adamis AP, Shima DT. Inhibition of platelet-derived growth factor B signaling enhances the efficacy of anti-vascular endothelial growth factor therapy in multiple models of ocular neovascularization. Am J Pathol. 2006;168:2036–2053. doi: 10.2353/ajpath.2006.050588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang H, Qian DZ, Tan YS, Lee K, Gao P, Ren YR, Rey S, Hammers H, Chang D, Pili R, Dang CV, Liu JO, Semenza GL. Digoxin and other cardiac glycosides inhibit HIF-1 alpha sythesis and block tumor growth. Proc Natl Acad Sci USA. 2008;105:19579–19586. doi: 10.1073/pnas.0809763105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yoshida T, Zhang H, Iwase T, Shen J, Semenza G, Campochiaro PA. Digoxin inhibits retinal ischemia-induced HIF-1α expression and ocular neovascularization. FASEB J. 2010;24:1759–1767. doi: 10.1096/fj.09-145664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chandel NS, Maltepe E, Godwasser E, Mathieu CE, Simon MC, Schumacker PT. Mitochondrial reactive oxygen species trigger hypoxia-induced transcription. Proc Natl Acad Sci U S A. 1998;95:11715–11720. doi: 10.1073/pnas.95.20.11715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chandel NS, McClintock DS, Feliciano CE, Wood TM, Melendez JA, Rodriguez AM, Schumacker PT. Reactive oxygen species generated at mitochodrial complex III stabilize hypoxia-inducible factor-1 alpha during hypoxia. J Biol Chem. 2000;275:25130–21138. doi: 10.1074/jbc.M001914200. [DOI] [PubMed] [Google Scholar]
- 47.Lu H, Dalgard CL, Mohyeldin A, McGate T, Tait AS, Verma A. Reversible inactivation of HIF-1 prolyl hydroxylases allows cell metabolism to control basal HIF-1. J Biol Chem. 2005;280:41928–41939. doi: 10.1074/jbc.M508718200. [DOI] [PubMed] [Google Scholar]
- 48.Dong A, Xie B, Shen J, Yoshida T, Yokoi K, Hackett SF, Campochiaro PA. Oxidative stress promotes ocular neovascularization. J Cell Physiol. 2009;219:544–552. doi: 10.1002/jcp.21698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Age-Related Eye Disease Study Research Group. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss. Arch Ophthalmol. 2001;119:1417–1436. doi: 10.1001/archopht.119.10.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Barleon B, Sozzani S, Zhou D, Weich HA, Mantovani A, Marme D. Migration of human monocytes in response to vascular endothelial growth factor (VEGF) is mediated via the VEGF receptor flt-1. Blood. 1996;87:3336–3343. [PubMed] [Google Scholar]
- 51.Grunewald M, Avraham I, Dor Y, Bachar-Lustig E, Itin A, Yung S, Chimenti S, Landsman L, Abramaovitch R, Keshet E. VEGF-induced adult neovascularization: recruitment, retention, and role of accessory cells. Cell. 2006;124:175–189. doi: 10.1016/j.cell.2005.10.036. [DOI] [PubMed] [Google Scholar]
- 52.Takahashi T, Kalka C, Masuda H, Chen D, Silver M, Kearney M, Magner M, Isner JM, Asahara T. Ischemia- and cytokine-induced mobilzation of bone marrow-derived endothelial progenitor cells for neovascularization. Nat Med. 1999;5:434–438. doi: 10.1038/7434. [DOI] [PubMed] [Google Scholar]
- 53.Otani A, Kinder K, Ewait K, Otero FJ, Schimmel P, Friedlander M. Bone marrow-derived stem cells target retinal astrocytes and can promote or inhibit retinal angiogenesis. Nat Med. 2002;8:1004–1010. doi: 10.1038/nm744. [DOI] [PubMed] [Google Scholar]
- 54.Grant MB, May WS, Caballero S, Brown GA, Guthrie SM, Mamee RN, Byrne BJ, Vaught T, Spoerri PE, Peck AB, Scott EW. Adult hematopoietic stem cells provide functional hemangioblastic activity during retinal neovascularization. Nat Med. 2002;8:607–612. doi: 10.1038/nm0602-607. [DOI] [PubMed] [Google Scholar]
- 55.Sengupta N, Calballero S, Mames RN, Butler JM, Scott EW, Grant MB. The role of adult bone marrow-derived stem cells in choroidal neovascularization. Invest Ophthalmol Vis Sci. 2003;44:4908–4913. doi: 10.1167/iovs.03-0342. [DOI] [PubMed] [Google Scholar]
- 56.Lang RA, Bishop MJ. Macrophages are required for cell death and tissue remodeling in the developing mouse eye. Cell. 1993;74:453–462. doi: 10.1016/0092-8674(93)80047-i. [DOI] [PubMed] [Google Scholar]
- 57.Lobov IB, Rao S, Carroll TJ, Vallance JE, Ito M, Ondr JK, Kurup S, Galss DA, Patel MS, Shu W, Morrisey EE, McMahon AP, Karsenty G, Lang RA. WNT7b mediates macrophage-induced programmed cell death in patterning of the vasculature. Nature. 2005;437:417–421. doi: 10.1038/nature03928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Apte RS, Richter J, Herndon J, Ferguson TA. Macrophage inhibition of neovascularization in a murine model of age-related macular degeneration. PLoS Med. 2006;8:e310. doi: 10.1371/journal.pmed.0030310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lu P, Li L, LIu G, van Rooijen N, Mukaida N, Zhang X. Opposite roles of CCR2 and CX3CR1 macrophages in alkali-induced cornal neovascularization. Cornea. 2009;28:562–569. doi: 10.1097/ICO.0b013e3181930bcd. [DOI] [PubMed] [Google Scholar]
- 60.Cousins SW, Esponosa-Heidmann DG, Miller DM, Pereira-Simon S, Hernandez EP, Chien H, Meier-jewett C, Dix RD. Cytomegalovirus infection results in more severe experimental choroidal neovascularization. PLoS Pathog. 2012;8 doi: 10.1371/journal.ppat.1002671. e1002671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shen J, Xie B, Dong A, Swaim M, Hackett SF, Campochiaro PA. In vivo immunostaining demonstrates macrophages associate with growing and regressing vessels. Invest Ophthalmol Vis Sci. 2007;48:4335–4341. doi: 10.1167/iovs.07-0113. [DOI] [PubMed] [Google Scholar]
- 62.Outtz HH, Tattersall IW, Kofler NM, Steinbach N, Kitajewski J. Notch 1 controls macrophage recruitment and Notch signaling is activated at sites of endothelial cell anastomosis during reitnal angiogenesis in mice. Blood. 2011;118:3436–3439. doi: 10.1182/blood-2010-12-327015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stefater JAr, Lewkowich I, Rao S, Mariggi G, Carpenter AC, Burr AR, Fan J, Ajima R, Molkentin JD, Willimas BO, et al. Regulation of angiogenesis by a non-canonical Wnt-Flt1 pathway in myeloid cells. Nature. 2011;474:511–515. doi: 10.1038/nature10085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Knighton DR, Hunt TK, Scheuenstuhl H, Halliday BJ, Werb Z, Banda MJ. Oxygen tension regulates the expression of angiogenesis factor by macrophages. Science. 1983;221:1283–1285. doi: 10.1126/science.6612342. [DOI] [PubMed] [Google Scholar]
- 65.Kelly J, Khan AA, Yin J, Ferguson TA, Apte RS. Senescence regulates macrophage activation and angiogenic fate at site of tissue injury in mice. J Clin Invest. 2007;117:3421–3426. doi: 10.1172/JCI32430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Espinosa-Heidmann DG, Suner IJ, Hernandez E, Monroy D, Csaky KG, Cousins SW. Macrophage depletion diminishes lesion size and severity in experimental choroidal neovascularization. Invest Ophthalmol Vis Sci. 2003;44:3586–3592. doi: 10.1167/iovs.03-0038. [DOI] [PubMed] [Google Scholar]
- 67.Tsutsumi C, Sonoda KH, Egashira K, Qiao H, Hisatomi T, Nakao S, Ishibashi M, Charo IF, Sakamoto T, Murata T, Ishibashi T. The critical role of ocular-infiltrating macrophages in the development of choroidal neovascularization. J Leukoc Biol. 2003;74:25–32. doi: 10.1189/jlb.0902436. [DOI] [PubMed] [Google Scholar]
- 68.Noda K, She H, Nakazawa T, Hisatomi T, Nakao S, Almulki L, Zandi S, Miyahara S, Ito Y, Thomas KL, et al. Vascular adhesion protein-1 blockade suppresses choroidal neovascularization. FASEB J. 2008;22:2928–2935. doi: 10.1096/fj.07-105346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Xie B, Shen J, Dong A, Rashid A, Stoller G, Campochiaro PA. Blockade of sphingosine-1-phosphate reduces macrophage influx and retinal and choroidal neovascularization. J Cell Physiol. 2009;218:192–198. doi: 10.1002/jcp.21588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kubota Y, Takubo K, Shimizu T, Ohno H, Kishi K, Shibuya M, Saya H, Suda T. M-CSF inhibition selectively targets pathological angiogenesis and lymphangiogenesis. J Exp Med. 2009;206:1089–1102. doi: 10.1084/jem.20081605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sato TN, Tozawa Y, Deutsch U, Wolburg-Buchholz K, Fujiwara Y, Gendron-Maguire M, Gridley T, Wolburg H, Risau W, Qin Y. Distinct roles of the receptor tyrosine kinases Tie-1 and Tie-2 in blood vessel formation. Nature. 1995;376:70–74. doi: 10.1038/376070a0. [DOI] [PubMed] [Google Scholar]
- 72.Davis S, Aldrich TH, Jones P, Acheson A, Ryan TE, Bruno J, Radziejewski C, Maisonpierre PC, Yancopoulos GD. Isolation of angiopoietin-1, a ligand for the TIE2 receptor, by secretion-trap expression cloning. Cell. 1996;87:1161–1169. doi: 10.1016/s0092-8674(00)81812-7. [DOI] [PubMed] [Google Scholar]
- 73.Maisonpierre PC, Suri C, Jones PF, Bartunkova S, Wiegand SJ, Radziejewski C, Compton D, McClain J, Aldrich TH, Papadopoulos N, Daly TJ, Davis S, Sato TN, Yancopoulos GD. Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science. 1997;277:55–60. doi: 10.1126/science.277.5322.55. [DOI] [PubMed] [Google Scholar]
- 74.Hackett SF, Ozaki H, Strauss RW, Wahlin K, Suri C, Maisonpierre P, Yancopoulos G, Campochiaro PA. Angiopoietin 2 expression in the retina: upregulation during physiologic and pathologic neovascularization. J Cell Physiol. 2000;184:275–284. doi: 10.1002/1097-4652(200009)184:3<275::AID-JCP1>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 75.Hackett SF, Wiegand SJ, Yancopoulos G, Campochiaro P. Angiopoietin-2 plays an important role in retinal angiogenesis. J Cell Physiol. 2002;192:182–187. doi: 10.1002/jcp.10128. [DOI] [PubMed] [Google Scholar]
- 76.Gale NW, Thurston G, Hackett SF, Renard R, Wang Q, McClain J, Martin C, Witte C, Witte M, Jackson D, Suri C, Campochiaro PA, Wiegand SJ, Yancopoulos GD. Angiopoietin-2 is required for postnatal angiogenesis and lymphatic patterning, and only the latter role is rescued by angiopoietin-1. Devel Cell. 2002;3:411–423. doi: 10.1016/s1534-5807(02)00217-4. [DOI] [PubMed] [Google Scholar]
- 77.Oshima Y, Deering T, Oshima S, Nambu H, Reddy PS, Kaleko M, Connelly S, Hackett SF, Campochiaro PA. Angiopoietin-2 enhances retinal vessel sensitivity to vascular endothelial growth factor. J Cell Physiol. 2004;199:412–417. doi: 10.1002/jcp.10442. [DOI] [PubMed] [Google Scholar]
- 78.Nambu H, Nambu R, Oshima Y, Hackett SF, Wiegand SJ, Yancopoulos G, Zack DJ, Campochiaro PA. Angiopoietin 1 inhibits ocular neovascularization and breakdown of the blood-retinal barrier. Gene Ther. 2004;11:865–873. doi: 10.1038/sj.gt.3302230. [DOI] [PubMed] [Google Scholar]
- 79.Nambu H, Umeda N, Kachi S, Oshima Y, Nambu R, Campochiaro PA. Angiopoietin 1 prevents retinal detachment in an aggressive model of proliferative retinopathy, but has no effect on established neovascularization. J Cell Physiol. 2005;204:227–235. doi: 10.1002/jcp.20292. [DOI] [PubMed] [Google Scholar]
- 80.Friedlander M, Theesfeld CL, Sugita M, Fruttiger M, Thomas MA, Chang S, Cheresh DA. Involvement of integrins alpha-v beta-3 and alpha-v beta-5 in ocular neovascular diseases. Proc Natl Acad Sci USA. 1996;93:9764–9769. doi: 10.1073/pnas.93.18.9764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hammes H, Brownlee M, Jonczyk A, Sutter A, Preissner K. Subcutaneous injection of a cyclic peptide antagonist of vitronectin receptor-type integrins inhibits retinal neovascularization. Nat Med. 1996;2:529–533. doi: 10.1038/nm0596-529. [DOI] [PubMed] [Google Scholar]
- 82.Luna J, Tobe T, Mousa SA, Reilly TM, Campochiaro PA. Antagonists of integrin alpha-v beta-3 inhibit retinal neovascularization in a murine model. Lab Invest. 1996;75:563–573. [PubMed] [Google Scholar]
- 83.Umeda N, Kachi S, Akiyama H, Zahn G, Vossmeyer D, Stragies R, Campochiaro PA. Suppression and regression of choroidal neovascularization by systemic administration of an Alpha5 Beta1 integrin antagonist. Mol Pharmacol. 2006;69:1820–1828. doi: 10.1124/mol.105.020941. [DOI] [PubMed] [Google Scholar]
- 84.O'Reilly MS, Boehm T, Shing Y, Fukai N, Vasios G, Lane WS, Flynn E, Birknead JR, Olsen BR, Folkman J. Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell. 1997;88:277–285. doi: 10.1016/s0092-8674(00)81848-6. [DOI] [PubMed] [Google Scholar]
- 85.Ramchandran R, Dhanabal M, Volk R, Waterman MJ, Segal M, Lu H, Knebelmann B, Sukhatme VP. Antiangiogenic activity of restin, NC10 domain of human collagen XV: comparison to endostatin. Biochem Biophys Res Comm. 1999;255:735–739. doi: 10.1006/bbrc.1999.0248. [DOI] [PubMed] [Google Scholar]
- 86.Colorado PC, Torre A, Kamphaus G, Maeshima Y, Hopfer H, Takahashi K, Volk R, Zamborsky ED, Herman S, Sarkar PK, et al. Anti-angiogenic cues from vascular basement membrane collagen. Canc Res. 2000;60:2520–2526. [PubMed] [Google Scholar]
- 87.Kamphaus GD, Colorado PC, Panka DJ, Hopfer H, Ramchandran R, Torres A, Maeshima Y, Mier JW, Sukhatme VP, Kalluri R. Canstatin, a novel matrix-derived inhibitor of angiogenesis and tumor growth. J Biol Chem. 2000;275:1209–1215. doi: 10.1074/jbc.275.2.1209. [DOI] [PubMed] [Google Scholar]
- 88.Petitclerc C, Boutaud A, Prestayko A, Xu J, Sado Y, Ninomiya Y, Sarras MP, Jr, Hudson BG, Brooks PC. New functions for non-collagenous domains of human collagen type IV. Novel integrin ligands inhibiting angiogenesis and tumor growth in vivo. J Biol Chem. 2000;275:8051–8061. doi: 10.1074/jbc.275.11.8051. [DOI] [PubMed] [Google Scholar]
- 89.Lima e Silva R, Kachi S, Akiyama H, Shen J, Aslam S, Gong YY, Khu NH, Hatara MC, Boutaud A, Peterson R, Campochiaro PA. Recombinant non-collagenous domain of α2(IV) collagen causes involution of choroidal neovascularization by inducing apoptosis. J Cell Physiol. 2006;208:161–166. doi: 10.1002/jcp.20645. [DOI] [PubMed] [Google Scholar]
- 90.Mori K, Ando A, Gehlbach P, Nesbitt D, Takahashi K, Goldsteen D, Penn M, Chen CT, Melia M, Phipps S, et al. Inhibition of choroidal neovascularization by intravenous injection of adenoviral vectors expressing secretable endostatin. Am J Pathol. 2001;159:313–320. doi: 10.1016/S0002-9440(10)61697-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Takahashi K, Saishin Y, Saishin Y, Lima Silva R, Oshima Y, Oshima S, Melia M, Paszkiet B, Zerby D, Kadan MJ, et al. Intraocular expression of endostatin reduces VEGF-induced retinal vascular permeability, neovascularization, and retinal detachment. FASEB J. 2003;17:896–898. doi: 10.1096/fj.02-0824fje. [DOI] [PubMed] [Google Scholar]
- 92.Mori K, Duh E, Gehlbach P, Ando A, Takahashi K, Pearlman J, Mori K, Yang HS, Zack DJ, Ettyreddy D, Brough DE, Wei LL, Campochiaro PA. Pigment epithelium-derived factor inhibits retinal and choroidal neovascularization. J Cell Physiol. 2001;188:253–263. doi: 10.1002/jcp.1114. [DOI] [PubMed] [Google Scholar]
- 93.Lai C-C, Wu W-C, Chen S-L, Xiao X, Tsai T-C, Huan S-J, Chen T-L, Tsai RJ-F, Tsao Y-P. Suppression of choroidal neovascularization by adeno-associated virus vector expressing angiostatin. Invest Ophthalmol Vis Sci. 2001;42:2401–2407. [PubMed] [Google Scholar]
- 94.Lai C-M, Brankov M, Zaknich T, Lai YK-Y, Shen W-Y, Constable IJ, Kovesdi I, Rakoczy PE. Inhibition of angiogenesis by adenovirus-mediated sFlt-1 expression in a rat model of corneal neovascularization. Human Gene Ther. 2001;12:1299–1310. doi: 10.1089/104303401750270959. [DOI] [PubMed] [Google Scholar]
- 95.Lai YK, Shen WY, Brankov M, Lai CM, Constable IJ, Rakoczy PE. Potential long-term inhibition of ocular neovascularization by recombinant adeno-associated virus-mediated secretion gene therapy. Gene Ther. 2002;9:804–813. doi: 10.1038/sj.gt.3301695. [DOI] [PubMed] [Google Scholar]
- 96.Shen J, Yang XR, Xiao WH, Hackett SF, Sato Y, Campochiaro PA. Vasohibin is up-regulated by VEGF in the retina and suppresses VEGF receptor 2 and retinal neovascularization. FASEB J. 2006;20:723–725. doi: 10.1096/fj.05-5046fje. [DOI] [PubMed] [Google Scholar]
- 97.Ferrara N, Damico L, Shams N, Lowman H, Kim R. Development of ranibizumab, an anti-vascular endothelial growth factor antigen binding fragment, as therapy for neovascular age-related macular degeneration. Retina. 2006;26:859–870. doi: 10.1097/01.iae.0000242842.14624.e7. [DOI] [PubMed] [Google Scholar]
- 98.Gaudreault J, Fei D, Rusit J, Suboc P, Shiu V. Preclinical pharmacokinetics of ranibizumab (rhuFabV2) after a single intravitreal administration. Invest Ophthalmol Vis Sci. 2005;46:726–733. doi: 10.1167/iovs.04-0601. [DOI] [PubMed] [Google Scholar]
- 99.Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, Chung CY, Kim RY, Group MS. Ranibizumab for neovascular age-related macular degeneration. N Eng J Med. 2006;355:1419–1431. doi: 10.1056/NEJMoa054481. [DOI] [PubMed] [Google Scholar]
- 100.Brown DM, Kaiser PK, Michels M, Soubrane G, Heier JS, Kim RY, Sy JP, Schneider S, Group AS. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Eng J Med. 2006;355:1432–1444. doi: 10.1056/NEJMoa062655. [DOI] [PubMed] [Google Scholar]
- 101.Saishin Y, Saishin Y, Takahashi K, Lima Silva R, Hylton D, Rudge JJWS, Campochiaro PA. VEGF-TRAPR1R2 suppresses choroidal neovascularization and VEGF-induced breakdown of the blood-retinal barrier. J Cell Physiol. 2003;195:241–248. doi: 10.1002/jcp.10246. [DOI] [PubMed] [Google Scholar]
- 102.Heier JS, Brown DM, Chong V, Korobelnik JF, Kaiser PK, Nguyen QD, Kirchhof B, Ho A, Ogura Y, Yancopoulos GD, et al. Intravitreal Aflibercept (VEGF Trap-Eye) in wet age-related macular degeneration. Ophthalmology. 2012;119:2537–2548. doi: 10.1016/j.ophtha.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 103.Marin DF, Maguire MG, Ying GS, Grunwald JE, Fine SL, Jaffe GJ CATT Research Group. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Eng J Med. 2011;364:1897–1908. doi: 10.1056/NEJMoa1102673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Boyer DS Ophthotech Anti-PDGF in AMD Study Group. Combined inhibition of platelet-derived (PDGF) and vascular endothelial (VEGF) growth factors for the treatment of neovascular age-related macular degeneration (NV-AMD). Results of a phase 1 study. Invest Ophthalmol Vis Sci Online ARVO abstract. 2009;1260 [Google Scholar]
- 105.Campochiaro PA, Nguyen QD, Shah SM, Klein ML, Holz E, Frank RN, Saperstein DA, Gupta A, Stout JT, Macko J, DiBartolomeo R, Wei LL. Adenoviral vector-delivered pigment epithelium-derived factor for neovascular age-related macular degeneration: results of a phase I clinical trial. Hum Gene Ther. 2006;17:167–176. doi: 10.1089/hum.2006.17.167. [DOI] [PubMed] [Google Scholar]
- 106.Campochiaro PA. Gene transfer for neovascular age-related macular degeneration. Hum Gene Ther. 2011;22:523–529. doi: 10.1089/hum.2011.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Campochiaro PA. Gene tranfer for ocular neovascularization and macular edema. Gene Ther. 2012;19:121–126. doi: 10.1038/gt.2011.164. [DOI] [PubMed] [Google Scholar]