Abstract
The thick ascending limb of Henle's loop is a nephron segment that is vital to the formation of dilute and concentrated urine. This ability is accomplished by a consortium of functionally coupled proteins consisting of the apical Na+:K+:2Cl– co-transporter, the K+ channel, and basolateral Cl– channel that mediate electroneutral salt absorption. In thick ascending limbs, salt absorption is importantly regulated by the calcium-sensing receptor. Genetic or pharmacological disruption impairing the function of any of these proteins results in Bartter syndrome. The thick ascending limb is also an important site of Ca2+ and Mg2+ absorption. Calcium-sensing receptor activation inhibits cellular Ca2+ absorption induced by parathyroid hormone, as well as passive paracellular Ca2+ transport. The present review discusses these functions and their genetic and molecular regulation.
Keywords: Diuretics, Hypertension, Salt transport, Regulation, Phosphorylation, Isoforms
The function of the thick ascending limb of Henle's loop (TAL) is critical for salt absorption, for the regulation of divalent mineral cation, and acid–base metabolism. The TAL is also essential for the generation and maintenance of the countercurrent multiplication mechanism that allows the kidney to produce urine that can be more diluted or concentrated than plasma, a functional capacity that is essential for the survival of mammals that live on land, including human beings. Pioneering studies by Burg and Green [22] and Rocha and Kokko [128] were the first to suggest that chloride was absorbed by the TAL and that this process was inhibited by loop diuretics such as furosemide or bumetanide. Then, in the early 1980s, studies by Greger and co-workers [64, 65] and Hebert et al. [76, 77] established that the major salt transport pathway in apical membranes of TAL is an electroneutral Na+:K+:2Cl– co-transporter that is specifically inhibited by loop diuretics and activated by hormones acting through Gαs-coupled receptors such as vasopressin. Ten years later, work by Hebert and collaborators was crucial to define the molecular nature of ion transport mechanisms in TAL by isolating the complementary DNA (cDNA) encoding the renal-specific, apically expressed, bumetanide-sensitive Na+:K+:2Cl– co-transporter, NKCC2 [30]; the inward-rectifier potassium channel, ROMK [5]; and the basolateral calcium-sensing receptor, CaSR [18, 123]. In the present work, we review some of the knowledge that has become available during the last 15 years as a consequence of cloning NKCC2, ROMK, and CaSR.
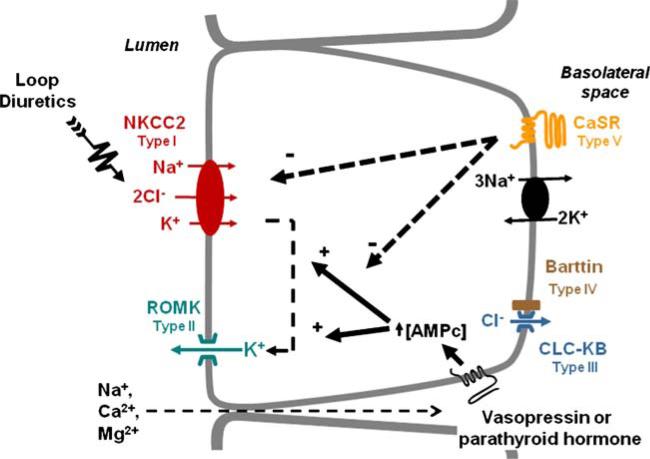
The molecular mechanisms of salt absorption by TAL are depicted in Fig. 1. As in many epithelia, the Na+:K+--ATPase, polarized to basolateral membranes, generates the gradient for sodium entry across apical membranes [66] in which most of the sodium movement occurs though NKCC2 [64, 65, 67, 68, 76]. Salt absorption by TAL, however, requires the simultaneous operation of several transport proteins (Fig. 1). Sodium and chloride ions traversing at the apical cell surface by way of NKCC2 leave the cell at the basolateral membrane through the Na+:K+-ATPase and Cl– channels (CLC-KB). These membrane proteins are composed of two subunits, one that mediate the transport function and another that is necessary to chaperon the protein to the plasma membrane [56, 88, 96, 122, 134, 135, 151]. The chaperon subunits are known as β-subunit for the Na+:K+-ATPase and Barttin for CLC-KB. Potassium ions entering across apical plasma membranes are returned to the tubular lumen via ROMK. The potassium concentration of glomerular ultrafiltrate (4 mEq/L) is much lower than that of sodium (145 mEq/L) or chloride (110 mEq/L). Without recirculation, the K+ concentration in the TAL lumen would be rapidly reduced, stopping the function of NKCC2. Thus, K+ recycling ensures that its concentration within the TAL lumen remains constant in order to allow proper function of NKCC2. Additionally, the lumen-positive voltage of TAL resulting from K+ recycling drives absorption of a second cation (Na+, Ca2+, Mg2+) through the paracellular pathway. Therefore, the coordinated function between NKCC2, ROMK, and CLC-KB, on the one hand, renders TAL epithelial cells thermodynamically more efficient because two cations are reabsorbed at the expense of ATP needed to pump one and, on the other hand, promotes the absorption not only of Na+ ions but also of divalent cations [141].
Fig. 1.
Molecular physiology of salt transport in TAL. Five genes are known to be the cause of Bartter syndrome type I to type V as stated. By acting in a Gαs-coupled receptor, vasopressin or parathyroid hormone increases cAMP production which in turns increases the activity of NCCC2 and ROMK, thereby augmenting salt reabsorption. In contrast, by acting in a Gαq-coupled receptor, extracellular Ca2+ inhibits, both NKCC2 and ROMK, decreasing salt reabsorption
Molecular physiology of NKCC2
NKCC2 belongs to solute carrier family 12 (SLC12; Human Genome Organization), the electrically silent, cation-coupled chloride co-transporter family [27, 54]. SLC12A1 located on human chromosome 15 encodes the Na+:K+:2Cl– co-transporter that is exclusively expressed on apical membranes of the TAL, whereas SLC12A2 located on human chromosome 5 encodes the Na+:K+:2Cl– co-transporter that is expressed on basolateral membranes of several epithelial cells and in many non-epithelial cells. The cDNA encoding these isoforms was simultaneously identified by two groups in 1994. Hebert and coworkers identified the apical renal isoform that was named BSC1 for bumetanide-sensitive co-transporter 1 [30] and then the basolateral isoform that was thus named BSC2 [35], while Forbush and coworkers first identified the basolateral isoform that was named NKCC1 [161] and then the renal-specific isoform, NKCC2 [111].
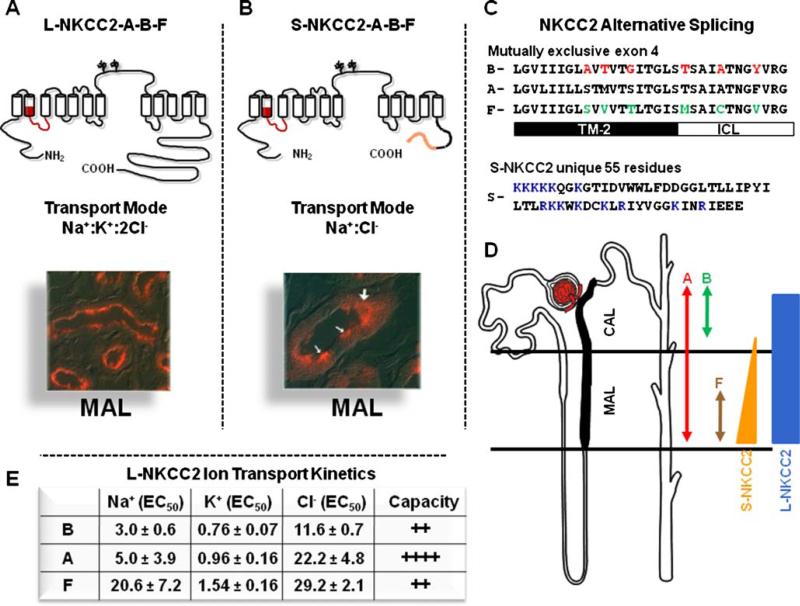
NKCC2 has been cloned and sequenced from human [136], rat [55], mouse [84, 100], rabbit [111], shark [53], and eel kidney [32]. It is a protein of 1,095 amino acid residues with a proposed topology featuring a central hydrophobic domain made up of 12 putative transmembrane (TM) spanning regions (Fig. 2a). A long hydrophilic loop connects TM segments 7 and 8 and contains two glycosylation sites. The hydrophobic domain is flanked by a short amino- and a long carboxyl-terminal domain that are located within the cell. Anti-NKCC2 polyclonal antibodies were used to demonstrate that NKCC2 is specifically expressed in the apical membrane of TAL (Fig. 2a) [38, 86]. NKCC2 is able to form dimmers, and it is likely that it functions as dimers [138]. NKCC2 is glycosylated at the two sites located in the long extracellular loop [109]. The absence of glycosylation is associated with decreased co-transporter activity and increased affinity for extracellular chloride [109]. Thus, the long glycosylated loop between TM 7 and 8 must be oriented toward the extracellular space.
Fig. 2.
Molecular physiology of the Na+:K+:2Cl– co-transporter, NKCC2. a Topology, transport mode, and immunolocalization of the long isoform of NKCC2 (L-NKCC2). There are 12 transmembrane spanning segments and a long hydrophilic loop between TM 7 and 8, with two glycosylation sites. Location of the mutually exclusive cassette exons is shown in red. b Topology, transport mode, and immunolocalization of the short isoform of NKCC2 (L-NKCC2). Location of the mutually exclusive cassette exons is shown in red and the unique 55 piece at the end is shown in orange. White arrows in the picture show positive cells. c Sequence and alignment of the alternative splicing of SLC12A1. The 31 residues of the three exons are shown. Switching the red or green residues between B and F isoforms is enough to switch their ion transport kinetics between each other. TM-2 transmembrane domain 2. ICL Interconnecting segment between TM2 and TM3. The 55 unique piece of S-NKCC2 is shown. Residues in blue are positively charged. d Distribution of L-NKCC2 and S-NKCC2, as well as exons A, B, and F along TAL, as stated. e Ion transport kinetics and capacity of transport for L-NKCC2 A, B, and F variants, as informed by Plata et al. [113]
Alternative spliced isoforms of NKCC2
The molecular and functional diversity of NKCC2 is enriched by the existence of at least six alternatively spliced variants that arise from the combination of two independent splicing mechanisms. The first is the existence of three mutually exclusive 96-bp cassette exon 4, denoted A, B, and F, that encode part of transmembrane 2 (TM2) and the interconnecting segment between TM2 and TM3 (Fig. 2a,c). These exons were originally described in rabbit [111] and later identified in human [136], mouse [100], and rat kidney [163]. Thus, NKCC2 can be expressed as NKCC2-A, NKCC2-B, or NKCC2-F.
Knowledge of the axial distribution of NKCC2 isoforms along TAL comes from molecular analysis using isoform-specific probes for Northern blots (Fig. 2d) [111] or in situ hybridization [84], as well as from isoform-specific polymerase chain reaction primers applied to single nephron segments [163]. Variant F is present only in the inner stripe of the outer medulla, that is, at the beginning of the medullary thick ascending limb (MAL), whereas variant B is present only toward the end of the cortical thick ascending limb (CAL) in the renal cortex where macula densa cells are located. Variant A is present in all TAL, suggesting that it is probably the default isoform, and that specific splicing mechanisms take place in MAL and CAL to ensure the formation of F and B isoforms, respectively. The A, B, and F variants exhibit different ion transport kinetics [61, 113] that explain the axial capacity of TAL for solute transport observed many years before [21]. The affinity for the three co-transported ions is B>A>F (Fig. 2e) in both mouse [113] and rabbit [61] NKCC2. Thus, the F isoform, located at the beginning of the MAL (Fig. 2d) where solutes are highly concentrated, is the low-affinity co-transporter, whereas the B-isoform, located at the end of CAL (Fig. 2d) where tubular fluid has been diluted, is the high-affinity isoform, thereby ensuring continuous salt absorption despite lower ion concentrations. The A variant, located throughout the TAL, exhibits an intermediary affinity and is the isoform with the greatest intrinsic transport capacity [113]. Experiments using chimeric constructs [52] or site-directed mutagenesis [60] of NKCC2 expressed in Xenopus laevis oocytes point to only six residues as responsible for kinetic differences between B and F isoforms (Fig. 2c).
A second splicing mechanism involves utilization of an alternative polyadenylation site in exon 16 that produces two distinct C terminal domains: a long isoform composed of 457 amino acid residues generating the NKCC2 protein of 1095 residues (L-NKCC2) and a truncated one of 129 residues producing a shorter NKCC2 isoform of 770 residues (S-NKCC2), which is lacking the last 329 residues of L-NKCC2 but contains 55 residues at the end, not present in L-NKCC2 (Fig. 2b). The expression S-NKCC2 at the protein level was corroborated using polyclonal antibodies raised against the unique 55-residue fragment [100]. Since this splicing mechanism is independent of A, B, and F exons, at least in mouse kidney, six isoforms are potentially present: L-NKCC2 A, B, and F and S-NKCC2 A, B, and F (Fig. 2a,b) [100]. S-NKCC2 exhibits functional properties of a co-transporter and as a regulator of L-NKCC2. As a co-transporter, S-NKCC2 functions as a K+-independent but bumetanide-sensitive Na+:Cl– co-transporter that is active only during cell swelling and is inhibited by cAMP [112]. Consistent with the size of S-NKCC2 and L-NKCC2 [100], Hass et al. [69] isolated 75- and 150-kDa proteins from mouse outer medulla membranes using a photosensitive bumetanide analogue [3H]BSTBA.
The S-NKCC2 isoform provides the molecular explanation for physiological observations conducted in rabbit and mouse TAL cells suggesting the presence of two operating modes of salt transport [41, 42, 141]. Under hypotonic conditions or in the absence of vasopressin (i.e., absence of cAMP), the furosemide-sensitive salt transporter behaves as a K+-independent, Na+:Cl– co-transporter (S-NKCC2). In contrast, when cells were exposed to hypertonicity or to vasopressin (i.e., cAMP) the furosemide-sensitive transport became K+-dependent, turning the transport mode to a Na+: K+:2Cl– co-transporter (L-NKCC2). It remains to be defined how the K+ transport ability is lost by the SNKCC2 variant, especially since it has been demonstrated in L-NKCC2 that information required for Na+, K+, and Cl– transport is located within the central hydrophobic domain [146]. Therefore, it is possible that the 55 amino acid residues play a role in reducing the K+ transport ability of S-NKCC2. Interestingly, there are many positively charged residues within the unique 55-residue segment of S-NKCC2 (Fig. 2c) that could function in preventing translocation of a positively charged ion such as K+.
The role of A and B isoforms in TAL physiology in vivo has been analyzed by elegant studies in which isoform-specific knockout mice were generated [24, 106]. Complete deletion of NKCC2 in mice resulted in a very severe salt-losing nephropathy, leading to early death of all pups in the first or second week of life [143]. In contrast, heterozygous deletion of one allele (NKCC2+/– mice) had no apparent consequences upon renal function [142]. Thus, it is not surprising that elimination of a single NKCC2 isoform, either A or B, had no appreciable effects upon salt and water balance [24, 106]. These studies, however, revealed that the A variant operates better under high perfusion flow rates, supporting previous observations that this isoform mediates high capacity transport [113]. In addition, in NKCC2-A–/– mice, the expected reduction in renin secretion by intravenous infusion of saline solution was not observed, whereas in NKCC2-B–/– mice, the renin secretion reduction was similar to wild-type mice, suggesting that because of its high capacity for Cl– transport, the A isoform may be required for the macula-densa-induced decrease in renin secretion during high flow rates. The double knockout A-B or null mice with specific deletions of F isoforms have not been generated.
Regulation of NKCC2
Modulation of NKCC2 activity by cAMP-generating hormones such as vasopressin, glucagon, isoproterenol, and parathyroid hormone is a fundamental mechanism regulating salt transport in TAL [77, 78]. Before NKCC2 cDNA was identified, the most studied of these hormones was vasopressin (Fig. 1). Experiments using isolated perfused tubules showed that vasopressin increases TAL NaCl absorption [70, 77, 131] by what appeared to involve trafficking of NKCC2 to apical plasma membrane. Surprisingly, however, since NKCC2 cDNA was identified, few studies have analyzed the regulation of NKCC2 by cAMP or vasopressin. As mentioned above, regulation of LNKCC2 is another function that has been suggested for the S-NKCC2 isoform. Although it is well known that the apical Na+:K+:2Cl– co-transporter in TAL is activated by vasopressin–cAMP, when expressed in oocytes, L-NKCC2 activity is not affected either by increasing cAMP or by inhibiting PKA activity. However, when oocytes were injected with both L-NKCC2 and S-NKCC2, the activity of L-NKCC2 was reduced and fully restored by adding cAMP [114]. Colchicine prevents the cAMP-induced increase of L-NKCC2 activity, suggesting that it is due to an increase in L-NKCC2 translocation to the plasma membrane [97]. Thus, it is possible that S-NKCC2 has a dominant negative effect upon L-NKCC2, which is released when cAMP is increased. The distribution of S-NKCC2 in TAL is consistent with its regulatory function (Fig. 2b). S-NKCC2 exhibits a predominant location in sub-apical membrane space; it is expressed in some, but not in all MAL cells, and is present in the medullary but not in the cortical portion of TAL [100]. In this regard, vasopressin has no effect upon salt absorption in the cortical portion of mouse TAL [76].
Compelling evidence indicates that less than 2% of NKCC2 in isolated TAL tubules is located at the apical membrane under basal conditions and that this percentage increased remarkably after exposing the tubules to cAMP [107]. This was also demonstrated in vivo in another study using polyclonal antibodies that recognize NKCC2 when phosphorylated at threonine residues 96 and 101 of the amino-terminal domain [58]. Treatment of mice with vasopressin increased phosphorylation of NKCC2 at these threonines and transporter translocation to the apical membrane. Another study showed that growth hormone also activates TAL salt absorption. Growth hormone, which acts through a tyrosine-kinase-associated receptor, induces phosphorylation of NKCC2 at the same threonines as vasopressin [37], thus suggesting that the same residues can be phosphorylated by a non-cAMP-dependent pathway. Finally, recent studies suggest that vasopressin-induced trafficking of NKCC2 is mediated by lipid rafts [160] and that NKCC2 is also regulated by direct interaction between the co-transporter and aldolase B [10].
Few studies have addressed acute regulation of NKCC2 by pathways other than cAMP-PKA. The metabolic sensing AMP kinase, AMPK, directly interacts with and phosphorylates NKCC2 at serine 126, resulting in activation of the co-transporter [47]. Such an action could be a coupling signal between the metabolic state of the cell and the activity of NKCC2. Cells shrinkage activates NKCC2 and is associated with phosphorylation of threonines 96 and 101 [59]. NKCC2 is a target of the recently discovered kinase family known as WNKs (with no lysine kinases) implicated in the genesis of hereditary hypertension [93]. WNK3 is expressed in TAL cells and is a positive regulator of NKCC2 [127]. In this study, it was observed that co-injection of Xenopus oocytes with NKCC2 and WNK3 increased the 86Rb+ uptake induced by NKCC2. The observed increase was greater than twofold over control-injected oocytes with NKCC2 complementary RNA (cRNA) alone. It was also observed in NKCC2 + WNK3 cRNA-injected oocytes that the presence of WNK3 promotes phosphorylation of threonines 96 and 101. Interestingly, elimination of WNK3 catalytic activity, by introducing the D294A mutation in WNK3 that is known to turn WNK3 into a catalytically inactive form, not only prevented the positive effect of the kinase upon NKCC2 but also turned WNK3 into a negative regulator [127]. Additionally, recent studies suggest that NKCC2 is activated by intracellular chloride depletion and that two type of kinases, WNK1/WNK3 and the STE-20 kinases SPAK/OSR1, are implicated [98, 117]. These studies suggest that intracellular chloride depletion activates WNK1/WNK3 that in turn interact with and activate SPAK/OSR1, which could be the kinase phosphorylating NKCC2.
Since different stimuli induce NKCC2 activation and phosphorylation of threonines 96 and 101, it is possible that these threonines are part of a common mechanism to activate NKCC2 rather than a specific cAMP-PKA phosphorylation site. Thus, the molecular mechanisms for vasopressin- or cAMP-induced activation of NKCC2 remain to be uncovered.
Salt transport in TAL can also be modulated by changing the level of NKCC2 gene expression. Vasopressin, for instance, induces a long-term increase of NaCl transport in TAL in isolated perfused tubule studies in Brattleboro rats [11] presumably due to upregulation of NKCC2 protein expression in TAL cells [90]. Similarly, consistent with the observation that arachidonic acid metabolites inhibits NKCC2 activity in rabbit MALs [40], increased NKCC2 expression induced by the cyclooxygenase inhibitors indomethacin or diclofenac was reversed by the prostaglandin E2 analog misoprostol [43]. Long-term expression of NKCC2 is also regulated by acid–base status. NKCC2 expression increases during acidosis [6, 7] by augmenting the stability of NKCC2 messenger RNA (mRNA) without affecting SLC12A1 transcription rate [87]. Under physiologic conditions, most of the ammonium produced by proximal tubules is reabsorbed by TAL to be later secreted in medullary collecting ducts and excreted into the urine [63, 91]. Thus, during acidosis in which production of ammonium by proximal tubules increases, NKCC2 expression increases as a compensatory mechanism to enhance ammonium absorption.
Bartter syndrome type I
In 1962, Frederick Bartter described a salt-losing nephropathy that featured hypokalemia, metabolic alkalosis, polyuria, and hypertrophy of the juxtaglomerular apparatus [9]. The clinical manifestation suggested that the affected segment of the nephron was the TAL. It is now known that Bartter syndrome is a heterogenous but nevertheless monogenic autosomal-recessive disorder that has been characterized at the molecular level, mostly in consanguineous families. Up to five genes have been shown to cause Bartter syndrome: Inactivating mutations of NKCC2, the apical K+ channel ROMK, the basolateral Cl– channel CLC-KB, and its chaperon subunit Barttin result in Bartter syndrome types I, II, III, and IV, respectively, whereas activating mutations of the calcium sensing receptor, CaSR, produce Bartter syndrome type V. That all these five genes when mutated produce the same disease strongly supports the molecular model of TAL salt absorption depicted in Fig. 1 in which simultaneous operation of all these proteins is required for proper function of this nephron segment. Types I and II Bartter syndrome are the more severe forms since the clinical picture is usually present during the antenatal period as excessive accumulation of amniotic fluid (polyhydramnios). After birth, most of the patients present with low blood pressure, metabolic alkalosis with hypokalemia, hyperreninemia, secondary aldosteronism, hypercalciuria, and nephrocalcinosis [132]. Consistent with reports of mutations in SLC12A1 as the cause of Bartter's syndrome type I, targeted disruption of this gene in mice produces a profound salt-wasting nephropathy with elevated mortality during the first 2 weeks of life [143].
Figure 3 shows the proposed secondary structure of NKCC2 to indicate the specific missense mutations and location within the protein that have been defined as the cause of Bartter syndrome type I. In addition, ten different small deletions (not shown in Fig. 3) or mutations producing frame shifts, and thus truncated proteins, have been described [2, 12, 51, 92, 136, 137, 148]. Mutations are distributed throughout the co-transporter. Analysis of the functional consequences of mutations G193R, A267S, G319R, A508T, Δ526N, and Y998X in Xenopus laevis oocytes resulted in severely reduced activity of proteins that were correctly routed to the plasma membrane, suggesting that intrinsic activity was diminished [137]. One boy with a mild phenotype was found to have a G224D mutation affecting only isoform B [147], thus producing an isoform-specific disease. More recently, two brothers with late-onset manifestations of Bartter syndrome type I were described [118]. Both were compound heterozygous, harboring a frame shift, D918fs, and a missense mutation, F177Y, that reduced NKCC2 activity by half compared with wild-type NKCC2. It was suggested that in these cases, the disease did not begin until the second decade of life due to the residual activity of F177Y.
Fig. 3.
Missense mutations informed for NKCC2. Mutations in blue were informed as harbored by patients with Bartter syndrome [2, 12, 51, 92, 136, 137, 148]. Mutations in red were informed in normal subjects of the Framingham Heart Study [85]
As discussed in the section on CaSR, Ca2+ directly regulates salt transport by TAL, and this occurs by interaction with the CaSR in the basolateral membrane. Activation of this sensor by Ca2+ decreases salt reabsorption by TAL, thus reducing the paracelluar calcium reabsorption. Consistent with this, two studies show that activating mutations of the CaSR results in a Bartter phenotype [149, 159]. Thus, mutations in the CaSR causing Bartter syndrome are the gain-of-function type. Watanabe et al. [159] described two unrelated patients with activating mutations in the CaSR (A843E and C131W) and a Bartter phenotype. In the second study, Vargas-Poussou et al. [149] described another pediatric patient with an L125P mutation in the CaSR that also exhibited a Bartter phenotype.
NKCC2 and arterial hypertension
The fact that mutations in SLC12A1 cause a monogenic disease that features arterial hypotension due to decreased NKCC2 activity indicates that this is a fundamental gene defending normal blood pressure, and thus, single nucleotide polymorphisms (SNPs) or mutations within SLC12A1 potentially could be implicated in heightening or diminishing the risk for developing arterial hypertension. In this regard, a recent study [85] analyzed the presence and distribution of rare independent SLC12A1 mutations encoding NKCC2, SLC12A3 encoding the thiazide-sensitive Na+:Cl– co-transporter NCC, and KCNJ1 encoding the apical ROMK potassium channel in 3125 subjects of the Framingham Heart Study who have been studied for 35 years with frequent periodic cardiovascular evaluation and for which DNA was available. Thirty different mutations were observed in 49 subjects. Most of them were missense mutations and were predicted to impair protein function. Of these, ten were mutations in SLC12A1 (Fig. 3), 15 in SLC12A3, and five in KCNJ1. The clinical and epidemiological analysis of this cohort of subjects revealed that carriers of these mutations were located within the lower deciles of blood pressure, with significantly lower systolic and diastolic blood pressures when compared with non-carriers. In addition, carriers of these mutations were significantly protected against the development of arterial hypertension, since prevalence of this disease was significantly lower at all ages when compared with non-carriers. The results of this study strongly suggest that in the open population, NKCC2 contributes to normal variation in blood pressure and some mutations are protective against hypertension. It remains to be determined if there are mutations or SNPs in SLC12A1 that increases the activity and/or expression levels of NKCC2, which could be implicated in enhancing the risk for hypertension.
CaSR regulation of NKCC2, ROMK, and TAL calcium absorption
The role of calcium in regulating renal tubular transport processes and metabolism had been investigated and described and was generally accepted phenomenologically, though no convincing explanation was advanced to clarify the mechanism of such regulation. The concept and existence of a calcium receptor was proposed and its features presciently predicted [15]. Several years later, Brown, Hebert, and their colleagues succeeded in the molecular cloning of the CaSR, the features of which were entirely consistent with its anticipated characteristics [19, 126]. A number of comprehensive reviews discuss the general physiology, pathophysiology, and pharmacology of the CaSR [16, 20, 25, 83]. Here, we focus on the role of the CaSR in modulating Na+, Ca2+, and K+ transport in TALs.
Molecular physiology of the CaSR
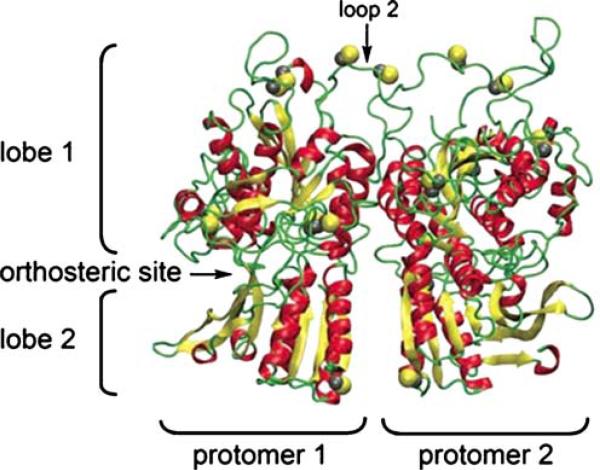
The CaSR is a member of the class C superfamily of G-protein-coupled membrane receptors (GPCR). Class C also includes pheromone and odorant receptors, to which the CaSR is most structurally related, as well as metabotropic glutamate and γ-aminobutyric (GABA) receptors [57]. The distinguishing structural feature of these GPCRs is that the ligand-binding sites are contained within the large (≈600 amino acid) extracellular receptor domain. The primary ligand-binding pocket, or orthosteric site,1 is formed by two prominent three-dimensional lobes that characterize class C receptors. This structure has been compared to a venus flytrap [29]. The CaSR constitutively forms homodimers, which is essential to its action [8, 108].
Except for two cysteines forming a putative disulfide bridge, class C receptors do not have any of the key features that characterize class A (rhodopsin, β-adrenergic) or class B (PTH, calcitonin, secretin) receptors [57].
Ligand bias
Although ionized calcium (Ca2+) is the cognate physiological CaSR ligand, its selectivity is not absolute. Mg2+, Sr2+, Ba2+, Cd2+, Co2+, Fe2+, Ni2+, Pb2+, Gd3+, La3+, Eu3+, Tb3+, and Yt3+ activate the CaSR [17, 73, 103]. A comprehensive pharmacological evaluation with concentrations for half-maximal activation for comparable activation or signaling parameters remains to be performed. Nonetheless, these inorganic di- and trivalent cations, along with polycations such as spermine, aminoglycosides (e.g., streptomycin, gentamicin, and neomycin), and polybasic amino acids (e.g., polylysine) are full agonists and are referred to as type I calcimimetics. Allosteric modulators that require the presence of Ca2+ or other full agonists that enhance the sensitivity of activation without altering the maximal response also regulate the CaSR and are designated type II calcimimetics. Allosteric modulators include organic polycations [NPS R467, NPS R568, and cinacalcet (AMG R073)] [74, 104] and L-amino acids [28]. The venus flytrap extracellular domain is required for amino acid binding [101] as it is for activation by cationic agonists Ca2+, Gd3+, and neomycin [72]. Though both amino acids and type II calcimimetics allosterically activate the CaSR, they do so through different mechanisms [164]. CaSR activity is also modulated by ionic strength [73] and by pH [121]. These actions could have significant regulatory effects especially for TALs, which are found in a high-ionic strength environment, and collecting tubules where urinary acidification proceeds.
The CaSR is abundantly expressed in the kidneys. CaSR mRNA transcripts are present essentially throughout the nephron, viz., glomerulus, proximal convoluted and straight tubules, medullary and cortical TALs, distal convoluted tubules, cortical and inner medullary collecting ducts [125]. CaSR protein expression is prominently found in proximal tubules, TALs, and cortical collecting tubules [124]. Notably, the membrane domain on polarized renal tubular epithelial cells on which the CaSR is found differs between nephron segments. In proximal tubules, the CaSR is expressed at the base of apical brush-border membranes. Expression decreases from S1 to S3 proximal tubule segments. By contrast, in TALs, the CaSR is found on basolateral cell membranes [23, 124]. In cortical and inner medullary collecting ducts, the CaSR is localized to apical plasma membranes [23, 124, 130]. The CaSR is expressed only in some of the type A intercalated cells of the cortical collecting duct [124]. The trafficking motifs responsible for directed membrane targeting of the CaSR have not been identified (Fig. 4).
Fig. 4.
Three-dimensional structure of the dimerized CaSR. α-Helices are shown in red, β-sheets are yellow, and loops and turns in green. Cysteines are shown in yellow. The putative orthosteric site in protomer 1 is labeled. The dimer interface runs along the vertical axis between the two protomers (from [80])
G proteins, CaSR signaling
G protein binding
Signaling by the CaSR is remarkably complex and incompletely defined. Studies of isolated parathyroid cells established that raising extracellular Ca2+ activated phospholipase C (PLC) with attendant inositol phosphate formation and transient elevations of intracellular Ca2+ and inhibited adenylyl cyclase with decreased cAMP accumulation [15, 79, 157]. Initial characterization of the cloned and heterologously expressed CaSR demonstrated that these properties were attributable to the receptor itself and not an epiphenomenon [19]. The CaSR is expressed by Madin–Darby canine kidney (MDCK) cells where it couples to Gαq/11 and Gαi2,3 [4]. These and similar findings prove that CaSR signaling is mediated by the G protein families Gq/11 and Gi, although Gq/11 is believed to be the more relevant signaling pathway. Recent evidence suggests that G protein signaling by the CaSR may change from Gq/11 to Gi following malignant transformation [94].
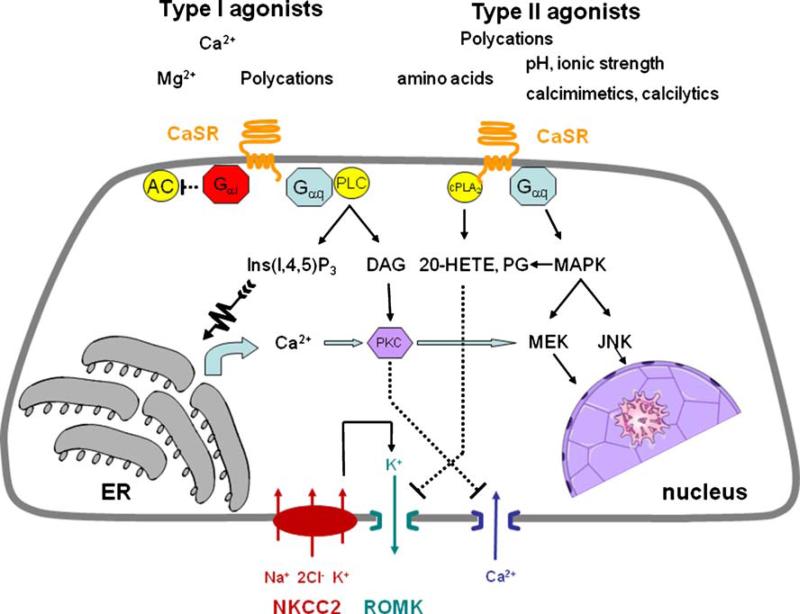
An overview of major CaSR signaling pathways is shown in Fig. 5. Calcium binding to the CaSR results in G-protein-dependent stimulation of PLC with attendant inositol trisphosphate (IP3) formation and rapid but transient release of Ca2+ from intracellular stores. In dispersed parathyroid cells, PLC activation and PTH secretion is inhibited by treatment with pertussis toxin [26, 45] as is CaSR activation in MAL cells [1], indicating that it is mediated by Gαi/o, whereas in MDCK cells [4], PLC activation is refractory to pertussis toxin, suggesting that Gαq/11 mediates PLC activation. Thus, different G proteins may mediate CaSR activation in distinct cell types where in some stimulation proceeds through a mechanism involving Gi as opposed to releasing Gβγ subunits from pertussis toxin-insensitive Gq/11 or through PLC-mediated increases in Ca2+, which then inhibits, respectively, Gβγ- or Ca2+-sensitive isoforms of adenylate cyclase.
Fig. 5.
CaSR signaling in TAL. Simplified model of CaSR by representative type I and type II agonists. The CaSR is located on basolateral cell membranes of TAL. Its activation inhibits cAMP formation mediated by Gαi and formation of lipid second messengers and prostanoids through phospholipase C (PLC) and cytoplasmic phospholipase A2 (cPLA2), respectively. Inhibition of Na+:K+:2Cl– co-transport on apical cell membranes is indirect and mediated by blockade of ROMK by 20-HETE or PGE2. CaSR activation inhibits both PTH-stimulated calcium absorption and passive paracellular calcium absorption (not shown). Further details are provided in the text
PLC activation is a direct consequence of CaSR occupancy and is mediated by Gq/11 [150]. Other CaSR signaling pathways including activation of Gi, phospholipase A2 (PLA2), phospholipase D (PLD), mitogen-activated protein kinase (MAPK), and phosphatidylinositol-3 and phosphatidylinositol-4 kinases (PI3K and PI4K, respectively) have been described but are less well characterized [19, 81, 89, 158]. Activation of PLD and Rho is mediated by Gα12/13 [82].
CaSR signaling in TAL
Elevated extracellular Ca2+ in MALs inhibits vasopressin-stimulated cAMP accumulation, whereas in CALs, raising extracellular Ca2+ suppresses PTH-stimulated cAMP formation while not impairing PTH-induced cAMP accumulation by proximal tubules. Takaichi and Kurokawa [144, 145] noted that in dissected nephron segments, increasing extracellular calcium from 1 to 5 mM inhibited cAMP production in response to PTH and calcitonin in cortical TALs of Henle, but not in proximal convoluted tubules.
Although CaSR signaling is primarily effected by PLC in most cell types, PLC activation in TAL does not appear to mediate increases of intracellular Ca2+, which has its origin in extracellular fluid rather than from release from cytoplasmic stores [34, 154]. Moreover, increases of intracellular Ca2+ reduce cyclic AMP formation by inhibiting the type 6 adenylate cyclase and increasing cAMP metabolism by activating a Ca2+-dependent phosphodiesterase as noted above.
An alternative mechanism involved in CaSR signaling in TAL involves Ca2+ modulation of phospholipase A2 (PLA2) activity. Ca2+ activation of PLA2 increases cytosolic arachidonic acid, which is rapidly metabolized by a P450 ω-hydroxylase (CYP4) to 20-hydroxyeicosatetraenoic acid (20-HETE) or by COX2 to prostaglandins [154]. Because P450 is more highly expressed than COX2, 20-HETE formation is normally favored. CaSR activation, however, increases TNFα stimulation of COX2 [44], which diverts arachidonic acid metabolism toward prostaglandin synthesis at the expense of 20-HETE.
Increasing extracellular calcium stimulates cyclooxygenase-2 (COX2) and PGE2 synthesis by MAL cells in a dose- and time-dependent manner [152, 153]. These effects, as discussed below, are important for the regulatory effect of CaSR activation on NaCl absorption.
Regulation of NKCC2, ROMK, and other proteins in TAL by the CaSR
CaSR activation has two prominent physiological actions to regulate electrolyte absorption in TALs. Moreover, mutations of the CaSR cause profound disruption of renal sodium and calcium ion homeostasis. As noted earlier, in the TAL, the CaSR is expressed on basolateral cell membranes. As such, it monitors extracellular calcium concentrations in the renal interstitium. Importantly, variations of interstitial calcium occur under normal conditions, to an extent permitting physiological regulation of CaSR modulated functions [102].
Activation of the TAL CaSR directly affects NaCl absorption mediated by NKCC2 and indirectly regulates NaCl absorption by modulating ROMK (renal outer medullary K+ channel; Kir 1.1) channels. Likewise, CaSR activation directly affects Ca2+ transport. Whether or not effects on Na+ transport indirectly affect Ca2+ is uncertain and controversial. The mechanisms of Na+, K+, and Ca2+ transport in TAL are outlined at the beginning of this review (cf. Fig. 1) and serve as a general background for understanding the regulatory influences of CaSR actions. It should be borne in mind that in addition to passive paracellular Ca2+ movement that occurs along the entire length of the TAL, hormone-regulated active cellular Ca2+ absorption occurs selectively in MAL, which is regulated by calcitonin, and in CAL, where it is governed by PTH [139, 140].
NKCC2 and CaSR
Luminal membrane Na+ entry in TALs is mediated mostly by the electroneutral, furosemide-sensitive Na+–K+–2Cl– co-transport, NKCC2 (SLC12A1). The observation that Ca2+ regulates TAL Na+ transport predates the molecular cloning of either NKCC2 or the CaSR. Calcium infusion increases both calcium and sodium excretion [95], in part due to inhibition of iso-osmotic solute absorption by proximal tubules [39]. However, urinary calcium excretion increases to a greater extent than does sodium excretion, suggesting both a specific inhibitory effect of hypercalcemia on calcium transport and, by inference, that this action occurs in distal tubules where calcium and sodium absorption is inversely related and can be dissociated [48]. Evidently, in intact animals, elevations of serum calcium are accompanied by suppression of PTH secretion [39], which decreases calcium absorption at hormone-sensitive sites in cortical TALs and distal convoluted tubules. However, hypercalcemia also directly inhibits calcium absorption by TALs of Henle's loop [119, 133].
Hypercalcemia suppresses PTH-stimulated cAMP formation specifically in TALs while not impairing PTH-induced cAMP accumulation by proximal tubules. Takaichi and Kurokawa [144] noted that in dissected nephron segments, increasing extracellular calcium from 1 to 5 mM inhibited cAMP production in response to PTH and calcitonin in cortical TALs of Henle, but not in proximal convoluted tubules. High ambient calcium also inhibited cAMP production stimulated by forskolin, indicating that a post-receptor mechanism additionally contributes to Ca2+ regulatory effects. These additional mechanisms are likely to be mediated by the type 6, Ca2+-inhibitable adenylyl cyclase and the Ca2+-activated phosphodiesterase.
Subsequent studies established that Ca2+ directly inhibits NaCl absorption in TALs [33] and that elevated peritubular calcium (the location of the CaSR), but not luminal calcium or magnesium, decreases the absorption of both Ca2+ and Mg2+ [120]. Contrary to these observations, Desfleurs et al. [36] found that elevating extracellular calcium stimulated cAMP formation but did not affect NaCl absorption or vasopressin-stimulated NaCl transport in mouse CAL. These results are difficult to interpret because of the stimulatory action of Ca2+ on cAMP given that the CaSR is not known to couple to Gαs. Indeed, CAL express the type 6 Ca2+-sensitive adenylyl cyclase, which is inhibited upon raising extracellular calcium [34]. Enhanced phosphodiesterase activity also contributes to diminished cAMP formation. The previous findings pointing to an action of vasopressin effects on CAL are at odds with the segment-specific actions of vasopressin on NaCl, which proceeds in MAL but not CAL of the mouse [75].
ROMK
ROMK22 (Kir1.1b), as noted above, mediates K+ ion recycling across apical plasma membranes to the luminal fluid of the TAL (Fig. 1). This action is largely responsible for generating the lumen-positive voltage that serves as a driving force for the cation-selective paracellular movement of Ca2+, Mg2+, and of additional Na+ absorption by the TAL.
CaSR regulation of ROMK
The regulation of TAL K+ channels by eicosanoids and P450 metabolites has been elegantly delineated [155]. As mentioned above, CaSR activation in TALs increases 20-HETE formation in the short term and prostaglandin synthesis after longer times [153]. 20-HETE potently inhibits NKCC2, ROMK, and the basolateral Na+/K+-ATPase and, by this means, disrupts NaCl absorption at multiple cellular sites and through independent mechanisms [3, 40, 156]. CaSR stimulation also induces TNFα expression, activating COX2, thereby generating PGE2 which contributes further to inhibition of NKCC2 [31, 152]. Notably, increasing extracellular Ca2+ reduces the activity of the 70-pS ROMK. The 30-pS ROMK per se is insensitive to 20-HETE or CaSR activation. Yet, 20-HETE and CaSR activation clearly inhibit NaCl absorption by TALs. It is possible that 20-HETE may regulate another subunit of the 70-pS K+ channel, thereby explaining how CaSR activation modulates apical K+ channels activity and, thereby, NKCC2-mediated Na+ uptake.
CaSR regulation of TAL Ca2+ absorption
An extensive literature testifies to the inhibitory effect of hypercalcemia on renal calcium absorption [110]. The physiological relevance of CaSR regulation of renal calcium transport is underscored by hyper- and hypocalcemic disorders resulting from CaSR mutations: Familial hypocalciuric hypercalcemia and neonatal severe hyper-parathyroidism are caused by inactivating CaSR mutations [115], whereas autosomal dominant hypocalcemia is caused by activating mutations [116].
Calcium absorption in the TAL proceeds by parallel routes and mechanisms. Passive calcium absorption occurs across the lateral intercellular space that forms the para-cellular pathway. Transport is proportional to the net electrical and chemical driving forces. PTH in the CAL and calcitonin in the MAL activates active transcellular calcium movement. Thus, in contrast to proximal tubules, where calcium absorption is entirely passive and parallels that of sodium, or distal tubules, where only active calcium absorption occurs and is inversely related to the rate and magnitude of sodium transport, the TAL represents a hybrid situation in which calcium and sodium movement may occur in parallel or may be dissociated depending on the prevailing hormonal status and other ambient conditions affecting mineral ion and salt homeostasis.
Hypercalcemia directly inhibits basal calcium absorption by TALs of Henle's loop [119, 133]. The participation of the CaSR in regulating basal and PTH-dependent Ca2+ transport was analyzed in single perfused in CAL [99]. CaSR activation suppressed passive paracellular calcium absorption, thereby confirming the findings obtained by Desfleurs et al. [36]. The effects of CaSR activation were specific for calcium absorption and had no effect on sodium transport. These findings are consistent with the finding that hypercalcemia exerts a profound inhibitory action on calcium absorption by TALs in thyroparathyroidectomized rats but only a minor reduction of sodium absorption [119]. Further studies determined that CaSR activation modulated PTH-dependent Ca2+ absorption. Gd3+, the prototype of a non-calcium type I CaSR agonist, was chosen for these experiments so as to maintain equal concentrations of calcium at both apical and basolateral surfaces, thereby avoiding a transepithelial calcium gradient that would alter passive calcium diffusion3. PTH increased Ca2+ absorption without an accompanying change of the transepithelial voltage. Gd3+ decreased Ca2+ absorption to control levels, again without altering the transepithelial voltage. The type II calcimimetic NPS R-467 exerted comparable actions to inhibit PTH-dependent Ca2+ transport. Thus, CaSR activation inhibited PTH-dependent Ca2+ transport. Moreover, these effects occurred in the absence of a change of electromotive driving force, i.e., the reduction of Ca2+ absorption was due to inhibition of active, transcellular Ca2+ absorption and not to a change in passive, paracellular calcium movement. The use of non-Ca2+ ligands to activate the CaSR were not due to blockade of ATP-permeable channels [129], Ca2+-sensitive K+ channels, the nonselective Ca2+-permeable cation channel, polycystin-2 [62], the Ca2+-selective Trp3 channel [105], or other non-selective cation channels [46, 162] or mechano-sensitive channels [71]. Indeed, the precise molecular target of PTH-dependent, CaSR-regulated Ca2+ absorption by CALs is not known. TRPV5, which mediates vitamin-D-dependent Ca2+ transport in distal tubules, is not expressed in TAL. Yet, as noted above, PTH clearly stimulates cellular calcium absorption by CAL.
CaSR activation with Gd3+ or NPS R-467 dissociated Na+ and Ca2+ movement in CAL insofar as it inhibited Ca2+ absorption without affecting Na+ transport or the transepithelial voltage [99]. Addition of bumetanide to the luminal fluid virtually abolished Na+ absorption and transepithelial voltage.
The mechanism by which CaSR activation inhibits PTH-stimulated Ca2+ absorption in CAL has not been examined. However, it is known that stimulation of Ca2+ transport by PTH in CAL cells requires parallel activation of both protein kinase A and of protein kinase C; interfering with either pathway is sufficient to inhibit Ca2+ movement [49, 50]. Based on these observations, we imagine that by activating Gi, cAMP formation is attenuated, thereby interfering with PTH action.
Acknowledgments
Original studies described here were supported by grants DK 54171 and DK 64635 from the National Institutes of Health to PAF and GG, respectively, CONACYT grant 59992 to GG, and by a grant from the Foundation Leducq for the Transatlantic Network on Hypertension—Renal Salt Handling in the Control of Blood Pressure (GG).
Footnotes
This review is dedicated to the memory of Dr. Steven C. Hebert. Dr. Hebert, our colleague and friend, was a leader in elucidating the mechanism and regulation of salt and water balance by the kidney. He embodied the foresight to anticipate many of the elements contributing to renal homeostasis, an infectious enthusiasm that inspired fellows and colleagues alike, and an open and welcoming collegiality that represents all the best traits of academia.
Binding to the same recognition site as an endogenous agonist.
TAL apical membranes express 30- and 70-pS channels, and high-conductance, Ca2+-activated maxi K+ channels. ROMK, the 30-pS channel is absent from apical membranes of ROMK-null mice. The 70-pS channel mediates 80% of the apical K+ conductance. Current thinking suggests that the 70-pS K+ channel is a heterotetramer that includes ROMK, which may be a pore-containing subunit of the 70-pS K+ channels. ROMK1 is expressed in cortical collecting ducts, whereas ROMK2 is present in TAL [13].
Such a problem may contribute to or account for the findings of Desfleurs, who found that increasing basolateral calcium inhibited net calcium absorption without altering Na, Cl, K, or Mg transport by single perfused mouse CALs, [36]. CaSR activation was achieved by increasing basolateral calcium. The CAL, however, is highly permeable to calcium and elevating calcium asymmetrically at the serosal surface increases calcium backflux and results in diminished net calcium absorption regardless of an effect on the CaSR [14]. Under these conditions, it is not possible to distinguish a non-specific effect of diminished driving force from a specific action that can be attributed to the CaSR.
Contributor Information
Gerardo Gamba, Molecular Physiology Unit, Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán, Mexico City, Mexico gamba@biomedicas.unam.mx; Instituto de Investigaciones Biomédicas, Universidad Nacional Autónoma de México, Tlalpan, 14000 Mexico City, Mexico.
Peter A. Friedman, Department of Pharmacology and Chemical Biology, University of Pittsburgh School of Medicine, Pittsburgh, PA 15261, USA paf10@pitt.edu
References
- 1.Abdullah HI, Pedraza PL, McGiff JC, Ferreri NR. CaR activation increases TNF production by mTAL cells via a Gi-dependent mechanism. Am J Physiol Renal Physiol. 2008;294:F345–F354. doi: 10.1152/ajprenal.00509.2006. [DOI] [PubMed] [Google Scholar]
- 2.Adachi M, Asakura Y, Sato Y, Tajima T, Nakajima T, Yamamoto T, Fujieda K. Novel SLC12A1 (NKCC2) mutations in two families with Bartter syndrome type 1. Endocr J. 2007;54:1003–1007. doi: 10.1507/endocrj.k06-204. [DOI] [PubMed] [Google Scholar]
- 3.Amlal H, Legoff C, Vernimmen C, Paillard M, Bichara M. Na+–K+ (NH4+)–2Cl− cotransport in medullary thick ascending limb: control by PKA, PKC, and 20-HETE. Am J Physiol. 1996;271:C455–C463. doi: 10.1152/ajpcell.1996.271.2.C455. [DOI] [PubMed] [Google Scholar]
- 4.Arthur JM, Collinsworth GP, Gettys TW, Quarles LD, Raymond JR. Specific coupling of a cation-sensing receptor to G protein a-subunits in MDCK cells. Am J Physiol. 1997;273:F129–F135. doi: 10.1152/ajprenal.1997.273.1.F129. [DOI] [PubMed] [Google Scholar]
- 5.Asano T, Takata K, Katagiri H, Ishihara H, Inukai K, Anai M, Hirano H, Yazaki Y, Oka Y. The role of N-glycosylation in the targeting and stability of GLUT1 glucose transporter. FEBS Lett. 1993;324:258–261. doi: 10.1016/0014-5793(93)80129-i. [DOI] [PubMed] [Google Scholar]
- 6.Attmane-Elakeb A, Mount DB, Sibella V, Vernimmen C, Hebert SC, Bichara M. Stimulation by in vivo and in vitro metabolic acidosis of expression of rBSC-1, the Na+–K+(NH4+)–2Cl− cotransporter of the rat medullary thick ascending limb. J Biol Chem. 1998;273:33681–33691. doi: 10.1074/jbc.273.50.33681. [DOI] [PubMed] [Google Scholar]
- 7.Attmane-Elakeb A, Sibella V, Vernimmen C, Belenfant X, Hebert SC, Bichara M. Regulation by glucocorticoids of expression and activity of rBSC1, the Na+–K+(NH4+)–2Cl− cotransporter of medullary thick ascending limb. J Biol Chem. 2000;275:33548–33553. doi: 10.1074/jbc.M006591200. [DOI] [PubMed] [Google Scholar]
- 8.Bai M, Trivedi S, Brown EM. Dimerization of the extracellular CaSR (CaR) on the cell surface of CaR-transfected HEK293 cells. J Biol Chem. 1998;273:23605–23610. doi: 10.1074/jbc.273.36.23605. [DOI] [PubMed] [Google Scholar]
- 9.Bartter FC, Pronove P, Gill JR, Jr., MacCardle RC. Hyperplasia of the juxtaglomerular complex with hyperaldosteronism and hypokalemic alkalosis. A new syndrome. 1962. J Am Soc Nephrol. 1998;9:516–528. doi: 10.1681/ASN.V93516. [DOI] [PubMed] [Google Scholar]
- 10.Benziane B, Demaratez S, Defontaine N, Zaarour N, Cheval L, Bourgeois S, Klein C, Froissart M, Blanchard A, Pailard M, Gamba G, Houillier P, Laghmani K. NKCC2 surface expression in mammalian cells: down-regulation by novel interaction with aldolase B. J Biol Chem. 2007;282:33817–33830. doi: 10.1074/jbc.M700195200. [DOI] [PubMed] [Google Scholar]
- 11.Besseghir K, Trimble ME, Stoner L. Action of ADH on isolated medullary thick ascending limb of the Brattleboro rat. Am J Physiol. 1986;251:F271–F277. doi: 10.1152/ajprenal.1986.251.2.F271. [DOI] [PubMed] [Google Scholar]
- 12.Bettinelli A, Ciarmatori S, Cesareo L, Tedeschi S, Ruffa G, Appiani AC, Rosini A, Grumieri G, Mercuri B, Sacco M, Leozappa G, Binda S, Cecconi M, Navone C, Curcio C, Syren ML, Casari G. Phenotypic variability in Bartter syndrome type I. PediatrNephrol. 2000;14:940–945. doi: 10.1007/pl00013418. [DOI] [PubMed] [Google Scholar]
- 13.Boim MA, Ho K, Shuck ME, Bienkowski MJ, Block JH, Slightom JL, Yang Y, Brenner BM, Hebert SC. ROMK inwardly rectifying ATP-sensitive K+ channel. II. Cloning and distribution of alternative forms. Am J Physiol. 1995;268:F1132–1140. doi: 10.1152/ajprenal.1995.268.6.F1132. [DOI] [PubMed] [Google Scholar]
- 14.Bourdeau JE, Burg MB. Voltage dependence of calcium transport in the thick ascending limb of Henle's loop. Am J Physiol. 1979;236:F357–F364. doi: 10.1152/ajprenal.1979.236.4.F357. [DOI] [PubMed] [Google Scholar]
- 15.Brown EM. Extracellular Ca2+ sensing, regulation of parathyroid cell function, and role of Ca2+ and other ions as extracellular (first) messengers. Physiol Rev. 1991;71:371–411. doi: 10.1152/physrev.1991.71.2.371. [DOI] [PubMed] [Google Scholar]
- 16.Brown EM. The CaSR: physiology, pathophysiology and CaR-based therapeutics. Subcell Biochem. 2007;45:139–167. doi: 10.1007/978-1-4020-6191-2_6. [DOI] [PubMed] [Google Scholar]
- 17.Brown EM, Fuleihan Ge-H, Chen CJ, Kifor O. A comparison of the effects of divalent and trivalent cations on parathyroid hormone release, 3′,5′-cyclic-adenosine monophosphate accumulation, and the levels of inositol phosphates in bovine parathyroid cells. Endocrinology. 1990;127:1064–1071. doi: 10.1210/endo-127-3-1064. [DOI] [PubMed] [Google Scholar]
- 18.Brown EM, Gamba G, Riccardi D, Lombardi M, Butters R, Kifor O, Sun A, Hediger MA, Hebert SC. Cloning, expression, and characterization of the bovine parathyroid Ca2+ sensing receptor (BOPCAR). J Am Soc Nephrol. 1993;4:704. doi: 10.1038/366575a0. [DOI] [PubMed] [Google Scholar]
- 19.Brown EM, Gamba G, Riccardi D, Lombardi M, Butters R, Kifor O, Sun A, Hediger MA, Lytton J, Hebert SC. Cloning and characterization of an extracellular Ca2+-sensing receptor from bovine parathyroid. Nature. 1993;366:575–580. doi: 10.1038/366575a0. [DOI] [PubMed] [Google Scholar]
- 20.Brown EM, MacLeod RJ. Extracellular calcium sensing and extracellular calcium signaling. Physiol Rev. 2001;81:239–297. doi: 10.1152/physrev.2001.81.1.239. [DOI] [PubMed] [Google Scholar]
- 21.Burg MB. Thick ascending limb of Henle's loop. Kidney Int. 1982;22:454–464. doi: 10.1038/ki.1982.198. [DOI] [PubMed] [Google Scholar]
- 22.Burg MB, Green N. Function of the thick ascending limb of Henle's loop. Am J Physiol. 1973;224:659–668. doi: 10.1152/ajplegacy.1973.224.3.659. [DOI] [PubMed] [Google Scholar]
- 23.Butters RR, Jr., Chattopadhyay N, Nielsen P, Smith CP, Mithal A, Kifor O, Bai M, Quinn S, Goldsmith P, Hurwitz S, Krapcho K, Busby J, Brown EM. Cloning and characterization of a CaSR from the hypercalcemic New Zealand white rabbit reveals unaltered responsiveness to extracellular calcium. J Bone Miner Res. 1997;12:568–579. doi: 10.1359/jbmr.1997.12.4.568. [DOI] [PubMed] [Google Scholar]
- 24.Castrop H, Lorenz JN, Hansen P, Friis U, Mizel D, Oppermann M, Jensen B, Briggs J, Skott O, Schnermann J. Contribution of the basolateral isoform of the Na,K,2Cl-cotransporter (NKCC1/BSC2) to renin secretion. Am J Physiol Renal Physiol. 2005;289:F1185–F1192. doi: 10.1152/ajprenal.00455.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chang W, Shoback D. Extracellular Ca2+-sensing receptors—an overview. Cell Calcium. 2004;35:183–196. doi: 10.1016/j.ceca.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 26.Chen CJ, Barnett JV, Congo DA, Brown EM. Divalent cations suppress 3′,5′-adenosine monophosphate accumulation by stimulating a pertussis toxin-sensitive guanine nucleotide-binding protein in cultured bovine parathyroid cells. Endocrinology. 1989;124:233–239. doi: 10.1210/endo-124-1-233. [DOI] [PubMed] [Google Scholar]
- 27.Cheng SX, Geibel JP, Hebert SC. Extracellular polyamines regulate fluid secretion in rat colonic crypts via the extracellular calcium-sensing receptor. Gastroenterology. 2004;126:148–158. doi: 10.1053/j.gastro.2003.10.064. [DOI] [PubMed] [Google Scholar]
- 28.Conigrave AD, Quinn SJ, Brown EM. L-amino acid sensing by the extracellular Ca2+-sensing receptor. Proc Natl Acad Sci U S A. 2000;97:4814–4819. doi: 10.1073/pnas.97.9.4814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Conklin BR, Bourne HR. Homeostatic signals. Marriage of the flytrap and the serpent. Nature. 1994;367:22. doi: 10.1038/367022a0. [DOI] [PubMed] [Google Scholar]
- 30.Contreras AM, Ramirez M, Cueva L, Alvarez S, de Loza R, Gamba G. Low serum albumin and the increased risk of amikacin nephrotoxicity. Rev Invest Clin. 1994;46:37–43. [PubMed] [Google Scholar]
- 31.Culpepper RM, Andreoli TE. PGE2, forskolin, and cholera toxin interactions in modulating NaCl transport in mouse mTALH. Am J Physiol Renal Physiol. 1984;247:F784–792. doi: 10.1152/ajprenal.1984.247.5.F784. [DOI] [PubMed] [Google Scholar]
- 32.Cutler CP, Cramb G. Differential expression of absorptive cation-chloride-cotransporters in the intestinal and renal tissues of the European eel (Anguilla anguilla). Comp Biochem Physiol B Biochem Mol Biol. 2008;149:63–73. doi: 10.1016/j.cbpb.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 33.de Jesus Ferreira MC, Bailly C. Extracellular Ca2+ decreases chloride reabsorption in rat CTAL by inhibiting cAMP pathway. Am J Physiol Renal Physiol. 1998;275:F198–F203. doi: 10.1152/ajprenal.1998.275.2.F198. [DOI] [PubMed] [Google Scholar]
- 34.de Jesus Ferreira MC, Helies-Toussaint C, Imbert-Teboul M, Bailly C, Verbavatz JM, Bellanger AC, Chabardes D. Co-expression of a Ca2+-inhibitable adenylyl cyclase and of a Ca2+-sensing receptor in the cortical thick ascending limb cell of the rat kidney. Inhibition of hormone-dependent cAMP accumulation by extracellular Ca2+. J Biol Chem. 1998;273:15192–15202. doi: 10.1074/jbc.273.24.15192. [DOI] [PubMed] [Google Scholar]
- 35.Delpire E, Rauchman MI, Beier DR, Hebert SC, Gullans SR. Molecular cloning and chromosome localization of a putative basolateral Na+–K+–2Cl− cotransporter from mouse inner medullary collecting duct (mIMCD-3) cells. J Biol Chem. 1994;269:25677–25683. [PubMed] [Google Scholar]
- 36.Desfleurs E, Wittner M, Simeone S, Pajaud S, Moine G, Rajerison R, Di Stefano A. CaSR: regulation of electrolyte transport in the thick ascending limb of Henle's loop. Kidney Blood Press Res. 1998;21:401–412. doi: 10.1159/000025892. [DOI] [PubMed] [Google Scholar]
- 37.Dimke H, Flyvbjerg A, Bourgeois S, Thomsen K, Frokiaer J, Houillier P, Nielsen S, Frische S. Acute growth hormone administration induces antidiuretic and antinatriuretic effects and increases phosphorylation of NKCC2. Am J Physiol Renal Physiol. 2007;292:F723–F735. doi: 10.1152/ajprenal.00276.2006. [DOI] [PubMed] [Google Scholar]
- 38.Ecelbarger CA, Terris J, Hoyer JR, Nielsen A, Wade JB, Knepper MA. Localization and regulation of the rat renal Na+–K+–2Cl− cotransporter, BSC1. Am J Physiol Renal Physiol. 1996;271:F619–F628. doi: 10.1152/ajprenal.1996.271.3.F619. [DOI] [PubMed] [Google Scholar]
- 39.Edwards BR, Sutton RAL, Dirks JH. Effect of calcium infusion on renal tubular reabsorption in the dog. Am J Physiol. 1974;227:13–18. doi: 10.1152/ajplegacy.1974.227.1.13. [DOI] [PubMed] [Google Scholar]
- 40.Escalante B, Erlij D, Falck JR, McGiff JC. Effect of cytochrome P450 arachidonate metabolites on ion transport in rabbit kidney loop of Henle. Science. 1991;251:799–802. doi: 10.1126/science.1846705. [DOI] [PubMed] [Google Scholar]
- 41.Eveloff J, Calamia J. Effect of osmolarity on cation fluxes in medullary thick ascending limb cells. Am J Physiol Renal Physiol. 1986;250:F176–F180. doi: 10.1152/ajprenal.1986.250.1.F176. [DOI] [PubMed] [Google Scholar]
- 42.Eveloff J, Warnock DK. Activation of ion transport system during cell volume regulation. Am J Physiol Renal Physiol. 1987;252:F1–F10. doi: 10.1152/ajprenal.1987.252.1.F1. [DOI] [PubMed] [Google Scholar]
- 43.Fernandez-Llama P, Ecelbarger CA, Ware JA, Andrews P, Lee AJ, Turner R, Nielsen S, Knepper MA. Cyclooxygenase inhibitors increase Na–K–2Cl cotransporter abundance in thick ascending limb of Henle's loop. Am J Physiol. 1999;277:F219–F226. doi: 10.1152/ajprenal.1999.277.2.F219. [DOI] [PubMed] [Google Scholar]
- 44.Ferreri NR, McGiff JC, Vio CP, Carroll MA. TNFα regulates renal COX-2 in the rat thick ascending limb (TAL). Thromb Res. 2003;110:277–280. doi: 10.1016/s0049-3848(03)00381-5. [DOI] [PubMed] [Google Scholar]
- 45.Fitzpatrick LA, Brandi ML, Aurbach GD. Calcium-controlled secretion is effected through a guanine nucleotide regulatory protein in parathyroid cells. Endocrinology. 1986;119:2700–2703. doi: 10.1210/endo-119-6-2700. [DOI] [PubMed] [Google Scholar]
- 46.Formenti A, De Simoni A, Arrigoni E, Martina M. Changes in extracellular Ca2+ can affect the pattern of discharge in rat thalamic neurons. J Physiol. 2001;535:33–45. doi: 10.1111/j.1469-7793.2001.00033.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fraser SA, Gimenez I, Cook N, Jennings I, Katerelos M, Katsis F, Levidiotis V, Kemp BE, Power DA. Regulation of the renal-specific Na+–K+–2Cl− co-transporter NKCC2 by AMP-activated protein kinase (AMPK). Biochem J. 2007;405:85–93. doi: 10.1042/BJ20061850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Friedman PA. Codependence of renal calcium and sodium transport. Annu Rev Physiol. 1998;60:179–197. doi: 10.1146/annurev.physiol.60.1.179. [DOI] [PubMed] [Google Scholar]
- 49.Friedman PA, Gesek FA. Calcium transport in renal epithelial cells. Am J Physiol. 1993;264:F181–F198. doi: 10.1152/ajprenal.1993.264.2.F181. [DOI] [PubMed] [Google Scholar]
- 50.Friedman PA, Gesek FA, Coutermarsh BA, Kennedy SM. PKA and PKC activation is required for PTH-stimulated calcium uptake by distal convoluted tubule cells. J Am Soc Nephrol. 1994;5:715. [Google Scholar]
- 51.Fukuyama S, Okudaira S, Yamazato S, Yamazato M, Ohta T. Analysis of renal tubular electrolyte transporter genes in seven patients with hypokalemic metabolic alkalosis. Kidney Int. 2003;64:808–816. doi: 10.1046/j.1523-1755.2003.00163.x. [DOI] [PubMed] [Google Scholar]
- 52.Gagnon E, Bergeron MJ, Brunet GM, Daigle ND, Simard CF, Isenring P. Molecular mechanisms of Cl transport by the renal Na–K–Cl cotransporter: identification of an intracellular locus that may form part of a high affinity Cl-binding site. J Biol Chem. 2003;279:5648–5654. doi: 10.1074/jbc.M311218200. [DOI] [PubMed] [Google Scholar]
- 53.Gagnon E, Forbush B, Flemmer AW, Gimenez I, Caron L, Isenring P. Functional and molecular characterization of the shark renal Na–K–Cl cotransporter: novel aspects. Am J Physiol Renal Physiol. 2002;283:F1046–F1055. doi: 10.1152/ajprenal.00107.2002. [DOI] [PubMed] [Google Scholar]
- 54.Gamba G. Molecular physiology and pathophysiology of the electroneutral cation-chloride cotransporters. Physiol Rev. 2005;85:423–493. doi: 10.1152/physrev.00011.2004. [DOI] [PubMed] [Google Scholar]
- 55.Gamba G, Miyanoshita A, Lombardi M, Lytton J, Lee WS, Hediger MA, Hebert SC. Molecular cloning, primary structure and characterization of two members of the mammalian electroneutral sodium–(potassium)–chloride cotransporter family expressed in kidney. J Biol Chem. 1994;269:17713–17722. [PubMed] [Google Scholar]
- 56.Geering K, Theulaz F, Verrey M, Hauptle T, Rossier BC. A role for the β-subunit in the expression of functional Na+–K+--ATPase in Xenopus oocytes. Am J Physiol Cell Physiol. 1989;257:C851–C858. doi: 10.1152/ajpcell.1989.257.5.C851. [DOI] [PubMed] [Google Scholar]
- 57.Gether U. Uncovering molecular mechanisms involved in activation of G protein-coupled receptors. Endocrine Rev. 2000;21:90–113. doi: 10.1210/edrv.21.1.0390. [DOI] [PubMed] [Google Scholar]
- 58.Gimenez I, Forbush B. Short-term stimulation of the renal Na–K–Cl cotransporter (NKCC2) by vasopressin involves phosphorylation and membrane translocation of the protein. J Biol Chem. 2003;278:26946–26951. doi: 10.1074/jbc.M303435200. [DOI] [PubMed] [Google Scholar]
- 59.Gimenez I, Forbush B. Regulatory phosphorylation sites in the N-terminus of the renal Na–K–Cl cotransporter (NKCC2). Am J Physiol Renal Physiol. 2005;289:F1341–F1345. doi: 10.1152/ajprenal.00214.2005. [DOI] [PubMed] [Google Scholar]
- 60.Gimenez I, Forbush B. The residues determining differences in ion affinities among the alternative splice variants F, A, and B of the mammalian Renal Na–K–Cl cotransporter (NKCC2). J Biol Chem. 2007;282:6540–6547. doi: 10.1074/jbc.M610780200. [DOI] [PubMed] [Google Scholar]
- 61.Gimenez I, Isenring P, Forbush B., III Spatially distributed alternative splice variants of the renal Na–K–Cl cotransporter exhibit dramatically different affinities for the transported ions. J Biol Chem. 2002;277:8767–8770. doi: 10.1074/jbc.C200021200. [DOI] [PubMed] [Google Scholar]
- 62.Gonzalez-Perret S, Kim K, Ibarra C, Damiano AE, Zotta E, Batelli M, Harris PC, Reisin IL, Arnaout MA, Cantiello HF. Polycystin-2, the protein mutated in autosomal dominant polycystic kidney disease (ADPKD), is a Ca2+-permeable nonselective cation channel. Proc Natl Acad Sci U S A. 2001;98:1182–1187. doi: 10.1073/pnas.98.3.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Good DW. Ammonium transport by the thick ascending limb of Henle's loop. Annu Rev Physiol. 1994;56:623–647. doi: 10.1146/annurev.ph.56.030194.003203. [DOI] [PubMed] [Google Scholar]
- 64.Greger R. Chloride reabsorption in the rabbit cortical thick ascending limb of the loop of Henle. A sodium dependent process. Pflugers Arch. 1981;390:38–43. doi: 10.1007/BF00582708. [DOI] [PubMed] [Google Scholar]
- 65.Greger R, Schlatter E. Presence of luminal K+, a prerequisite for active NaCl transport in the cortical thick ascending limb of Henle's loop of rabbit kidney. Pflugers Arch. 1981;392:92–94. doi: 10.1007/BF00584588. [DOI] [PubMed] [Google Scholar]
- 66.Greger R, Schlatter E. Properties of the basolateral membrane of the cortical thick ascending limb of Henle's loop of rabbit kidney. A model for secondary active chloride transport. Pflugers Arch. 1983;396:325–334. doi: 10.1007/BF01063938. [DOI] [PubMed] [Google Scholar]
- 67.Greger R, Schlatter E. Properties of the lumen membrane of the cortical thick ascending limb of Henle's loop of rabbit kidney. Pflugers Arch. 1983;396:315–324. doi: 10.1007/BF01063937. [DOI] [PubMed] [Google Scholar]
- 68.Greger R, Schlatter E, Lang F. Evidence for electroneutral sodium chloride cotransport in the cortical thick ascending limb of Henle's loop of rabbit kidney. Pflugers Arch. 1983;396:308–314. doi: 10.1007/BF01063936. [DOI] [PubMed] [Google Scholar]
- 69.Haas M, Dunham PB, Forbush IB. [3H]Bumetanide binding to mouse kidney membranes: identification of corresponding membrane proteins. Am J Physiol Cell Physiol. 1991;260:C791–C804. doi: 10.1152/ajpcell.1991.260.4.C791. [DOI] [PubMed] [Google Scholar]
- 70.Hall DA, Varney DM. Effect of vasopressin on electrical potential difference and chloride transport in mouse medullary thick ascending limb of Henle's loop. J Clin Invest. 1980;66:792–802. doi: 10.1172/JCI109917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hamill OP, McBride DW., Jr The pharmacology of mechanogated membrane ion channels. Pharmacol Rev. 1996;48:231–252. [PubMed] [Google Scholar]
- 72.Hammerland LG, Krapcho KJ, Garrett JE, Alasti N, Hung BCP, Simin RT, Levinthal C, Nemeth EF, Fuller FH. Domains determining ligand specificity for Ca2+ receptors. Mol Pharmacol. 1999;55:642–648. [PubMed] [Google Scholar]
- 73.Handlogten ME, Shiraishi N, Awata H, Huang C, Miller RT. Extracellular Ca2+-sensing receptor is a promiscuous divalent cation sensor that responds to lead. Am J Physiol Renal Physiol. 2000;279:F1083–F1091. doi: 10.1152/ajprenal.2000.279.6.F1083. [DOI] [PubMed] [Google Scholar]
- 74.Harrington PE, Fotsch C. Calcium sensing receptor activators: calcimimetics. Curr Med Chem. 2007;14:3027–3034. doi: 10.2174/092986707782794096. [DOI] [PubMed] [Google Scholar]
- 75.Hebert SC, Culpepper RM, Andreoli TE. NaCl transport in mouse medullary thick ascending limbs. I. Functional nephron heterogeneity and ADH-stimulated NaCl cotransport. Am J Physiol. 1981;241:F412–F431. doi: 10.1152/ajprenal.1981.241.4.F412. [DOI] [PubMed] [Google Scholar]
- 76.Hebert SC, Culpepper RM, Andreoli TE. NaCl transport in mouse medullary thick ascending limbs. I. Functional nephron heterogeneity and ADH-stimulated NaCl cotransport. Am J Physiol Renal Physiol. 1981;241:F412–F431. doi: 10.1152/ajprenal.1981.241.4.F412. [DOI] [PubMed] [Google Scholar]
- 77.Hebert SC, Culpepper RM, Andreoli TE. NaCl transport in mouse medullary thick ascending limbs. II. ADH enhancement of transcellular NaCl cotransport; origin of transepithelial voltage. Am J Physiol Renal Physiol. 1981;241:F432–F442. doi: 10.1152/ajprenal.1981.241.4.F432. [DOI] [PubMed] [Google Scholar]
- 78.Hebert SC, Culpepper RM, Andreoli TE. NaCl transport in mouse medullary thick ascending limbs. III. Modulation of ADH effect by peritubular osmolality. Am J Physiol Renal Physiol. 1981;241:F443–F451. doi: 10.1152/ajprenal.1981.241.4.F443. [DOI] [PubMed] [Google Scholar]
- 79.Hofer AM, Brown EM. Extracellular calcium sensing and signalling. Nat Rev Mol Cell Biol. 2003;4:530–538. doi: 10.1038/nrm1154. [DOI] [PubMed] [Google Scholar]
- 80.Hu J, Spiegel AM. Structure and function of the human calcium-sensing receptor: insights from natural and engineered mutations and allosteric modulators. J Cell Mol Med. 2007;11:908–922. doi: 10.1111/j.1582-4934.2007.00096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Huang C, Handlogten ME, Miller RT. Parallel activation of phosphatidylinositol 4-kinase and phospholipase C by the extracellular CaSR. J Biol Chem. 2002;277:20293–20300. doi: 10.1074/jbc.M200831200. [DOI] [PubMed] [Google Scholar]
- 82.Huang C, Hujer KM, Wu Z, Miller RT. The Ca2+-sensing receptor couples to Gα12/13 to activate phospholipase D in Madin–Darby canine kidney cells. Am J Physiol Cell Physiol. 2004;286:C22–C30. doi: 10.1152/ajpcell.00229.2003. [DOI] [PubMed] [Google Scholar]
- 83.Huang C, Miller RT. Regulation of renal ion transport by the CaSR: an update. Curr Opin Nephrol Hypertens. 2007;16:437–443. doi: 10.1097/MNH.0b013e3282b974a6. [DOI] [PubMed] [Google Scholar]
- 84.Igarashi P, Vanden Heuver GB, Payne JA, Forbush IB. Cloning, embryonic expression, and alternative splicing of a murine kidney-specific Na–K–Cl cotransporter. Am J Physiol Renal Physiol. 1995;269:F406–F418. doi: 10.1152/ajprenal.1995.269.3.F405. [DOI] [PubMed] [Google Scholar]
- 85.Ji W, Foo JN, O'Roak BJ, Zhao H, Larson MG, Simon DB, Newton-Cheh C, State MW, Levy D, Lifton RP. Rare independent mutations in renal salt handling genes contribute to blood pressure variation. Nat Genet. 2008;40:592–599. doi: 10.1038/ng.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kaplan MR, Plotkin MD, Lee WS, Xu ZC, Lytton J, Hebert SC. Apical localization of the Na–K–Cl cotransporter, rBSC1, on rat thick ascending limbs. Kidney Int. 1996;49:40–47. doi: 10.1038/ki.1996.6. [DOI] [PubMed] [Google Scholar]
- 87.Karim Z, Attmane-Elakeb A, Sibella V, Bichara M. Acid pH increases the stability of BSC1/NKCC2 mRNA in the medullary thick ascending limb. J Am Soc Nephrol. 2003;14:2229–2236. doi: 10.1097/01.asn.0000085023.73801.4a. [DOI] [PubMed] [Google Scholar]
- 88.Kawakami K, Noguchi S, Noda M, Takahashi H, Ohta T, Kawamura M, Nojima H, Nagano K, Hirose T, Inayama S, Hayashida H, Miyata T, Numa S. Primary structure of the α-subunit of Torpedo californica (Na+ + K+)ATPase deduced from cDNA sequence. Nature. 1985;316:733–736. doi: 10.1038/316733a0. [DOI] [PubMed] [Google Scholar]
- 89.Kifor O, Diaz R, Butters R, Brown EM. The Ca2+-sensing receptor (CaR) activates phospholipases C, A2, and D in bovine parathyroid and CaR-transfected, human embryonic kidney (HEK293) cells. J Bone Miner Res. 1997;12:715–725. doi: 10.1359/jbmr.1997.12.5.715. [DOI] [PubMed] [Google Scholar]
- 90.Kim GH, Ecelbarger CA, Mitchell C, Packer RK, Wade JB, Knepper MA. Vasopressin increases Na–K–2Cl cotransporter expression in thick ascending limb of Henle's loop. Am J Physiol Renal Physiol. 1999;276:F96–F103. doi: 10.1152/ajprenal.1999.276.1.F96. [DOI] [PubMed] [Google Scholar]
- 91.Knepper MA, Packer R, Good DW. Ammonium transport in the kidney. Physiol Rev. 1989;69:179–249. doi: 10.1152/physrev.1989.69.1.179. [DOI] [PubMed] [Google Scholar]
- 92.Kurtz CL, Karolyi L, Seyberth HW, Koch MC, Vargas R, Feldmann D, Vollmer M, Knoers NV, Madrigal G, Guay-Woodford LM. A common NKCC2 mutation in Costa Rican Bartter's syndrome patients: evidence for a founder effect. J Am Soc Nephrol. 1997;8:1706–1711. doi: 10.1681/ASN.V8111706. [DOI] [PubMed] [Google Scholar]
- 93.Lalioti MD, Zhang J, Volkman HM, Kahle KT, Hoffmann KE, Toka HR, Nelson-Williams C, Ellison DH, Flavell R, Booth CJ, Lu Y, Geller DS, Lifton RP. Wnk4 controls blood pressure and potassium homeostasis via regulation of mass and activity of the distal convoluted tubule. Nat Genet. 2006;38:1124–1132. doi: 10.1038/ng1877. [DOI] [PubMed] [Google Scholar]
- 94.Mamillapalli R, VanHouten J, Zawalich W, Wysolmerski J. Switching of G-protein usage by the calcium-sensing receptor reverses its effect on parathyroid hormone-related protein secretion in normal versus malignant breast cells. J Biol Chem. 2008;283:24435–24447. doi: 10.1074/jbc.M801738200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Massry SG, Coburn JW, Chapman LW, Kleeman CR. Role of serum Ca, parathyroid hormone, and NaCl infusion on renal Ca and Na clearances. Am J Physiol. 1968;214:1403–1409. doi: 10.1152/ajplegacy.1968.214.6.1403. [DOI] [PubMed] [Google Scholar]
- 96.McDonough AA, Geering K, Farley RA. The sodium pump needs its β subunit. FASEB J. 1990;4:1598–1605. doi: 10.1096/fasebj.4.6.2156741. [DOI] [PubMed] [Google Scholar]
- 97.Meade P, Hoover RS, Plata C, Vazquez N, Bobadilla NA, Gamba G, Hebert SC. cAMP-dependent activation of the renal-specific Na+–K+–2Cl− cotransporter is mediated by regulation of cotransporter trafficking. Am J Physiol Renal Physiol. 2003;284:F1145–F1154. doi: 10.1152/ajprenal.00421.2002. [DOI] [PubMed] [Google Scholar]
- 98.Moriguchi T, Urushiyama S, Hisamoto N, Iemura S, Uchida S, Natsume T, Matsumoto K, Shibuya H. WNK1 regulates phosphorylation of cation-chloride-coupled cotransporters via the STE20-related kinases, SPAK and OSR1. J Biol Chem. 2005;280:42685–42693. doi: 10.1074/jbc.M510042200. [DOI] [PubMed] [Google Scholar]
- 99.Motoyama HI, Friedman PA. CaSR regulation of PTH-dependent calcium absorption by mouse cortical ascending limbs. Am J Physiol Renal Physiol. 2002;283:F399–F406. doi: 10.1152/ajprenal.00346.2001. [DOI] [PubMed] [Google Scholar]
- 100.Mount DB, Baekgard A, Hall AE, Plata C, Xu J, Beier DR, Gamba G, Hebert SC. Isoforms of the Na–K–2Cl transporter in murine TAL I. Molecular characterization and intrarenal localization. Am J Physiol Renal Physiol. 1999;276:F347–F358. doi: 10.1152/ajprenal.1999.276.3.F347. [DOI] [PubMed] [Google Scholar]
- 101.Mun HC, Franks AH, Culverston EL, Krapcho K, Nemeth EF, Conigrave AD. The venus fly trap domain of the extracellular Ca2+-sensing receptor is required for l-amino acid sensing. J Biol Chem. 2004;279:51739–51744. doi: 10.1074/jbc.M406164/200. [DOI] [PubMed] [Google Scholar]
- 102.Mupanomunda MM, Tian B, Ishioka N, Bukoski RD. Renal interstitial Ca2+. Am J Physiol Renal Physiol. 2000;278:F644–F669. doi: 10.1152/ajprenal.2000.278.4.F644. [DOI] [PubMed] [Google Scholar]
- 103.Nemeth EF, Fox J. Calcimimetic compounds: a direct approach to controlling plasma levels of parathyroid hormone in hyperparathyroidism. Trends Endocrinol Metab. 1999;10:66–71. doi: 10.1016/s1043-2760(98)00119-2. [DOI] [PubMed] [Google Scholar]
- 104.Nemeth EF, Heaton WH, Miller M, Fox J, Balandrin MF, Van Wagenen BC, Colloton M, Karbon W, Scherrer J, Shatzen E, Rishton G, Scully S, Qi M, Harris R, Lacey D, Martin D. Pharmacodynamics of the type II calcimimetic compound cinacalcet HCl. J Pharmacol Toxicol Methods. 2004;308:627–635. doi: 10.1124/jpet.103.057273. [DOI] [PubMed] [Google Scholar]
- 105.Ohki G, Miyoshi T, Murata M, Ishibashi K, Imai M, Suzuki M. A calcium-activated cation current by an alternatively spliced form of Trp3 in the heart. J Biol Chem. 2000;275:39055–39060. doi: 10.1074/jbc.M003606200. [DOI] [PubMed] [Google Scholar]
- 106.Oppermann M, Mizel D, Kim SM, Chen L, Faulhaber-Walter R, Huang Y, Li C, Deng C, Briggs J, Schnermann J, Castrop H. Renal function in mice with targeted disruption of the A isoform of the Na–K–2Cl co-transporter. J Am Soc Nephrol. 2007;18:440–448. doi: 10.1681/ASN.2006091070. [DOI] [PubMed] [Google Scholar]
- 107.Ortiz PA. cAMP increases surface expression of NKCC2 in rat thick ascending limbs: role of VAMP. Am J Physiol Renal Physiol. 2006;290:F608–F616. doi: 10.1152/ajprenal.00248.2005. [DOI] [PubMed] [Google Scholar]
- 108.Pace AJ, Gama L, Breitwieser GE. Dimerization of the CaSR occurs within the extracellular domain and is eliminated by Cys→Ser mutations at Cys101 and Cys236. J Biol Chem. 1999;274:11629–11634. doi: 10.1074/jbc.274.17.11629. [DOI] [PubMed] [Google Scholar]
- 109.Paredes A, Plata C, Rivera M, Moreno E, Vazquez N, Munoz-Clares R, Hebert SC, Gamba G. Activity of the renal Na+: K+:2Cl− cotransporter is reduced by mutagenesis of N-glycosylation sites: role for protein surface charge in Cl− transport. Am J Physiol Renal Physiol. 2006;290:F1094–F1102. doi: 10.1152/ajprenal.00071.2005. [DOI] [PubMed] [Google Scholar]
- 110.Parfitt AM, Kleerekoper M. Clinical disorders of calcium, phosphorous, and magnesium metabolism. In: Maxwell MH, Kleeman CR, editors. Clinical disorders of fluid and electrolyte metabolism. McGraw-Hill Book Company; New York: 1980. pp. 947–1151. [Google Scholar]
- 111.Payne JA, Forbush IB. Alternatively spliced isoforms of the putative renal Na–K–Cl cotransporter are differentially distributed within the rabbit kidney. Proc Natl Acad Sci U S A. 1994;91:4544–4548. doi: 10.1073/pnas.91.10.4544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Plata C, Meade P, Hall AE, Welch RC, Vazquez N, Hebert SC, Gamba G. Alternatively spliced isoform of the apical Na–K–Cl cotransporter gene encodes a furosemide sensitive Na–Cl cotransporter. Am J Physiol Renal Physiol. 2001;280:F574–F582. doi: 10.1152/ajprenal.2001.280.4.F574. [DOI] [PubMed] [Google Scholar]
- 113.Plata C, Meade P, Vazquez N, Hebert SC, Gamba G. Functional properties of the apical Na+–K+–2Cl− cotransporter isoforms. J Biol Chem. 2002;277:11004–11012. doi: 10.1074/jbc.M110442200. [DOI] [PubMed] [Google Scholar]
- 114.Plata C, Mount DB, Rubio V, Hebert SC, Gamba G. Isoforms of the Na–K–2Cl cotransporter in murine TAL. II. Functional characterization and activation by cAMP. Am J Physiol Renal Physiol. 1999;276:F359–F366. doi: 10.1152/ajprenal.1999.276.3.F359. [DOI] [PubMed] [Google Scholar]
- 115.Pollak MR, Brown EM, Chou Y-HW, Hebert SC, Marx SJ, Steinmann B, Levi T, Seidman CE, Seidman JG. Mutations in the human Ca2+-sensing receptor gene cause familial hypocalciuric hypercalcemia and neonatal severe hyper-parathyroidism. Cell. 1993;75:1297–1303. doi: 10.1016/0092-8674(93)90617-y. [DOI] [PubMed] [Google Scholar]
- 116.Pollak MR, Seidman CE, Brown EM. Three inherited disorders of calcium sensing. Medicine. 1996;75:115–123. doi: 10.1097/00005792-199605000-00001. [DOI] [PubMed] [Google Scholar]
- 117.Ponce-Coria J, San Cristobal P, Kahle KT, Vazquez N, Pacheco-Alvarez D, De Los HP, Juarez P, Munoz E, Michel G, Bobadilla NA, Gimenez I, Lifton RP, Hebert SC, Gamba G. Regulation of NKCC2 by a chloride-sensing mechanism involving the WNK3 and SPAK kinases. Proc Natl Acad Sci U S A. 2008;105:8458–8463. doi: 10.1073/pnas.0802966105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Pressler CA, Heinzinger J, Jeck N, Waldegger P, Pechmann U, Reinalter S, Konrad M, Beetz R, Seyberth HW, Waldegger S. Late-onset manifestation of antenatal Bartter syndrome as a result of residual function of the mutated renal Na+–K+–2Cl− co-transporter. J Am Soc Nephrol. 2006;17:2136–2142. doi: 10.1681/ASN.2005101071. [DOI] [PubMed] [Google Scholar]
- 119.Quamme GA. Effect of hypercalcemia on renal tubular handling of calcium and magnesium. Can J Physiol Pharmacol. 1982;60:1275–1280. doi: 10.1139/y82-187. [DOI] [PubMed] [Google Scholar]
- 120.Quamme GA. Control of magnesium transport in the thick ascending limb. Am J Physiol. 1989;256:F197–F210. doi: 10.1152/ajprenal.1989.256.2.F197. [DOI] [PubMed] [Google Scholar]
- 121.Quinn SJ, Bai M, Brown EM. pH Sensing by the calcium-sensing receptor. J Biol Chem. 2004;279:37241–37249. doi: 10.1074/jbc.M404520200. [DOI] [PubMed] [Google Scholar]
- 122.Reeves WB, Winters CJ, Andreoli TE. Chloride channels in the loop of Henle. Annu Rev Physiol. 2001;63:631–645. doi: 10.1146/annurev.physiol.63.1.631. [DOI] [PubMed] [Google Scholar]
- 123.Riccardi D, Hall AE, Chattopadhyay N, Xu JZ, Brown EM, Hebert SC. Localization of the extracellular Ca2+/polyvalent cation-sensing protein in rat kidney. Am J Physiol Renal Physiol. 1998;274:F611–F622. doi: 10.1152/ajprenal.1998.274.3.F611. [DOI] [PubMed] [Google Scholar]
- 124.Riccardi D, Hall AE, Chattopadhyay N, Xu JZ, Brown EM, Hebert SC. Localization of the extracellular Ca2+ polyvalent cation-sensing protein in rat kidney. Am J Physiol Renal Physiol. 1998;274:F611–F622. doi: 10.1152/ajprenal.1998.274.3.F611. [DOI] [PubMed] [Google Scholar]
- 125.Riccardi D, Lee WS, Lee K, Segre GV, Brown EM, Hebert SC. Localization of the extracellular Ca2+-sensing receptor and PTH/PTHrP receptor in rat kidney. Am J Physiol. 1996;271:F951–F956. doi: 10.1152/ajprenal.1996.271.4.F951. [DOI] [PubMed] [Google Scholar]
- 126.Riccardi D, Park J, Lee W-S, Gamba G, Brown EM, Hebert SC. Cloning and functional expression of a rat kidney extracellular calcium/polyvalent cation-sensing receptor. Proc Natl Acad Sci USA. 1995;92:131–135. doi: 10.1073/pnas.92.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Rinehart J, Kahle KT, De Los HP, Vazquez N, Meade P, Wilson FH, Hebert SC, Gimenez I, Gamba G, Lifton RP. WNK3 kinase is a positive regulator of NKCC2 and NCC, renal cation-Cl − cotransporters required for normal blood pressure homeostasis. Proc Natl Acad Sci U S A. 2005;102:16777–16782. doi: 10.1073/pnas.0508303102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Rocha AS, Kokko JP. Sodium chloride and water transport in the medullary thick ascending limb of Henle. Evidence for active chloride transport. J Clin Invest. 1973;52:612–623. doi: 10.1172/JCI107223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Roman RM, Feranchak AP, Davison AK, Schwiebert EM, Fitz JG. Evidence for Gd3+ inhibition of membrane ATP permeability and purinergic signaling. Am J Physiol. 1999;277:G1222–G1230. doi: 10.1152/ajpgi.1999.277.6.G1222. [DOI] [PubMed] [Google Scholar]
- 130.Sands JM, Naruse M, Baum M, Jo I, Hebert SC, Brown EM, Harris HW. Apical extracellular calcium/polyvalent cation-sensing receptor regulates vasopressin-elicited water permeability in rat kidney inner medullary collecting duct. J Clin Invest. 1997;99:1399–1405. doi: 10.1172/JCI119299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sasaki S, Imai M. Effects of vasopressin on water and NaCl transport across the in vitro perfused medullary thick ascending limb of Henle's loop of mouse, rat, and rabbit kidneys. Pflugers Arch. 1980;383:215–221. doi: 10.1007/BF00587521. [DOI] [PubMed] [Google Scholar]
- 132.Shaer AJ. Inherited primary renal tubular hypokalemic alkalosis: a review of Gitelman and Bartter syndromes. Am J Med Sci. 2001;322:316–332. doi: 10.1097/00000441-200112000-00004. [DOI] [PubMed] [Google Scholar]
- 133.Shareghi GR, Agus ZS. Magnesium transport in the cortical thick ascending limb of Henle's loop of the rabbit. J Clin Invest. 1982;69:759–769. doi: 10.1172/JCI110514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Shull GE, Lane LK, Lingrel JB. Amino-acid sequence of the β-subunit of the (Na+ + K+)ATPase deduced from a cDNA. Nature. 1986;321:429–431. doi: 10.1038/321429a0. [DOI] [PubMed] [Google Scholar]
- 135.Shull GE, Schwartz A, Lingrel JB. Amino-acid sequence of the catalytic subunit of the (Na+K+)ATPase deduced from a complementary DNA. Nature. 1985;316:691–695. doi: 10.1038/316691a0. [DOI] [PubMed] [Google Scholar]
- 136.Simon DB, Karet FE, Hamdan JM, Di Pietro A, Sanjad SA, Lifton RP. Bartter's syndrome, hypokalaemic alkalosis with hypercalciuria, is caused by mutations in the Na–K–2Cl cotransporter NKCC2. Nat Genet. 1996;13:183–188. doi: 10.1038/ng0696-183. [DOI] [PubMed] [Google Scholar]
- 137.Starremans PG, Kersten FF, Knoers NV, van den Heuvel LP, Bindels RJ. Mutations in the human Na–K–2Cl cotransporter (NKCC2) identified in Bartter syndrome type I consistently result in nonfunctional transporters. J Am Soc Nephrol. 2003;14:1419–1426. doi: 10.1097/01.asn.0000064948.39199.a0. [DOI] [PubMed] [Google Scholar]
- 138.Starremans PG, Kersten FF, van den Heuvel LP, Knoers NV, Bindels RJ. Dimeric architecture of the human bumetanide-sensitive Na–K–Cl Co-transporter. J Am Soc Nephrol. 2003;14:3039–3046. doi: 10.1097/01.asn.0000097370.29737.5b. [DOI] [PubMed] [Google Scholar]
- 139.Suki WN, Rouse D. Hormonal regulation of calcium transport in thick ascending limb renal tubules. Am J Physiol. 1981;241:F171–F174. doi: 10.1152/ajprenal.1981.241.2.F171. [DOI] [PubMed] [Google Scholar]
- 140.Suki WN, Rouse D, Ng RCK, Kokko JP. Calcium transport in the thick ascending limb of Henle. Heterogeneity of function in the medullary and cortical segments. J Clin Invest. 1980;66:1004–1009. doi: 10.1172/JCI109928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Sun A, Grossman EB, Lombardi M, Hebert SC. Vasopressin alters the mechanism of apical Cl− entry from Na+:Cl− to Na+:K+:2Cl− cotransport in mouse medullary thick ascending limb. J Membr Biol. 1991;120:83–94. doi: 10.1007/BF01868594. [DOI] [PubMed] [Google Scholar]
- 142.Takahashi N, Brooks HL, Wade JB, Liu W, Kondo Y, Ito S, Knepper MA, Smithies O. Posttranscriptional compensation for heterozygous disruption of the kidney-specific NaK2Cl cotransporter gene. J Am Soc Nephrol. 2002;13:604–610. doi: 10.1681/ASN.V133604. [DOI] [PubMed] [Google Scholar]
- 143.Takahashi N, Chernavvsky DR, Gomez RA, Igarashi P, Gitelman HJ, Smithies O. Uncompensated polyuria in a mouse model of Bartter's syndrome. Proc Natl Acad Sci U S A. 2000;97:5434–5439. doi: 10.1073/pnas.090091297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Takaichi K, Kurokawa K. High Ca2+ inhibits peptide hormone-dependent cAMP production specifically in thick ascending limbs of Henle. Miner Electrolyte Metab. 1986;12:342–346. [PubMed] [Google Scholar]
- 145.Takaichi K, Kurokawa K. Inhibitory guanosine triphosphate-binding protein-mediated regulation of vasopressin action in isolated single medullary tubules of mouse kidney. J Clin Invest. 1988;82:1437–1444. doi: 10.1172/JCI113749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Tovar-Palacio C, Bobadilla NA, Cortes P, Plata C, De Los HP, Vazquez N, Gamba G. Ion and diuretic specificity of chimeric proteins between apical Na+:K+:2Cl− and Na+:Cl− cotransporters. Am J Physiol Renal Physiol. 2004;287:F570–F577. doi: 10.1152/ajprenal.00124.2004. [DOI] [PubMed] [Google Scholar]
- 147.Vargas-Poussou R, Feldman D, Vollmer M, Konrad M, Kelly L, Van der Heuvel LPWJ, Tebouri L, Brandis M, Karolyi L, Hebert SC, Lemmink HH, Deschnes G, Hildebrandt F, Seyberth HW, Guay-Woodford LM, Knoers NVAM, Antignac C. Novel molecular variants of the Na–K–2Cl cotransporter gene are responsible for antenatal Bartter syndrome. Am J Hum Genet. 1998;62:1332–1340. doi: 10.1086/301872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Vargas-Poussou R, Feldmann D, Vollmer M, Konrad M, Kelly L, Van den Heuvel LPWJ, Tebourbi L, Brandis M, Karolyi L, Hebert SC, Lemmink HH, Deschênes G, Hildebrandt F, Seyberth HW, Guay-Woodford LM, Knoers NVAM, Antignac C. Novel molecular variants of the Na–K–2Cl cotransporter gene are responsible for antenatal Bartter syndrome. Am J Hum Genet. 1998;62:1332–1340. doi: 10.1086/301872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Vargas-Poussou R, Huang C, Hulin P, Houillier P, Jeunemaitre X, Paillard M, Planelles G, Dechaux M, Miller RT, Antignac C. Functional characterization of a calcium-sensing receptor mutation in severe autosomal dominant hypocalcemia with a Bartter-like syndrome. J Am Soc Nephrol. 2002;13:2259–2266. doi: 10.1097/01.asn.0000025781.16723.68. [DOI] [PubMed] [Google Scholar]
- 150.Varrault A, Pena MSR, Goldsmith PK, Mithal A, Brown EM, Spiegel AM. Expression of G protein a-subunits in bovine parathyroid. Endocrinology. 1995;136:4390–4396. doi: 10.1210/endo.136.10.7664659. [DOI] [PubMed] [Google Scholar]
- 151.Waldegger S, Jeck N, Barth P, Peters M, Vitzthum H, Wolf K, Kurtz A, Konrad M, Seyberth HW. Barttin increases surface expression and changes current properties of ClC–K channels. Pflugers Arch. 2002;444:411–418. doi: 10.1007/s00424-002-0819-8. [DOI] [PubMed] [Google Scholar]
- 152.Wang D, An SJ, Wang WH, McGiff JC, Ferreri NR. CaR-mediated COX-2 expression in primary cultured mTAL cells. Am J Physiol Renal Physiol. 2001;281:F658–F664. doi: 10.1152/ajprenal.2001.281.4.F658. [DOI] [PubMed] [Google Scholar]
- 153.Wang D, McGiff JC, Ferreri NR. Regulation of cyclooxygenase isoforms in the renal thick ascending limb: effects of extracellular calcium. J Physiol Pharmacol. 2000;51:587–595. [PubMed] [Google Scholar]
- 154.Wang W, Lu M, Balazy M, Hebert SC. Phospholipase A2 is involved in mediating the effect of extracellular Ca2+ on apical K+ channels in rat TAL. Am J Physiol. 1997;273:F421–F429. doi: 10.1152/ajprenal.1997.273.3.F421. [DOI] [PubMed] [Google Scholar]
- 155.Wang WH. Regulation of ROMK (Kir1.1) channels: new mechanisms and aspects. Am J Physiol Renal Physiol. 2006;290:F14–F19. doi: 10.1152/ajprenal.00093.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Wang WH, Lu M, Hebert SC. Cytochrome P-450 metabolites mediate extracellular Ca2+-induced inhibition of apical K+ channels in the TAL. Am J Physiol. 1996;271:C103–C111. doi: 10.1152/ajpcell.1996.271.1.C103. [DOI] [PubMed] [Google Scholar]
- 157.Ward DT. Calcium receptor-mediated intracellular signalling. Cell Calcium. 2004;35:217–228. doi: 10.1016/j.ceca.2003.10.017. [DOI] [PubMed] [Google Scholar]
- 158.Ward DT, McLarnon SJ, Riccardi D. Aminoglycosides increase intracellular calcium levels and ERK activity in proximal tubular OK cells expressing the extracellular CaSR. J Am Soc Nephrol. 2002;13:1481–1489. doi: 10.1097/01.asn.0000015623.73739.b8. [DOI] [PubMed] [Google Scholar]
- 159.Watanabe S, Fukumoto S, Chang H, Takeuchi Y, Hasegawa Y, Okazaki R, Chikatsu N, Fujita T. Association between activating mutations of calcium-sensing receptor and Bartter's syndrome. Lancet. 2002;360:692–694. doi: 10.1016/S0140-6736(02)09842-2. [DOI] [PubMed] [Google Scholar]
- 160.Welker P, Boehlick A, Mutig K, Salanova M, Kahl T, Schlueter H, Blottner D, Ponce-Coria J, Gamba G, Bachmann S. Renal Na–K–Cl cotransporter activity and vasopressin-induced trafficking are lipid raft-dependent. Am J Physiol Renal Physiol. 2008;295:F978–F802. doi: 10.1152/ajprenal.90227.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Xu JC, Lytle C, Zhu TT, Payne JA, Benz E, Jr, Forbush IB. Molecular cloning and functional expression of the bumetanide-sensitive Na–K–Cl cotransporter. Proc Natl Acad Sci U S A. 1994;91:2201–2205. doi: 10.1073/pnas.91.6.2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Yamaguchi T, Ye C, Chattopadhyay N, Sanders JL, Vassilev PM, Brown EM. Enhanced expression of extracellular calcium sensing receptor in monocyte-differentiated versus undifferentiated HL-60 cells: potential role in regulation of a nonselective cation channel. Calcif Tissue Int. 2000;66:375–382. doi: 10.1007/s002230010076. [DOI] [PubMed] [Google Scholar]
- 163.Yang T, Huang YG, Singh I, Schnermann J, Briggs JP. Localization of bumetanide- and thiazide-sensitive Na–K–Cl cotransporters along the rat nephron. Am J Physiol Renal Physiol. 1996;271:F931–F939. doi: 10.1152/ajprenal.1996.271.4.F931. [DOI] [PubMed] [Google Scholar]
- 164.Zhang Z, Jiang Y, Quinn SJ, Krapcho K, Nemeth EF, Bai M. l-Phenylalanine and NPS R-467 synergistically potentiate the function of the extracellular calcium-sensing receptor through distinct sites. J Biol Chem. 2002;277:33736–33741. doi: 10.1074/jbc.M200978200. [DOI] [PubMed] [Google Scholar]