Abstract
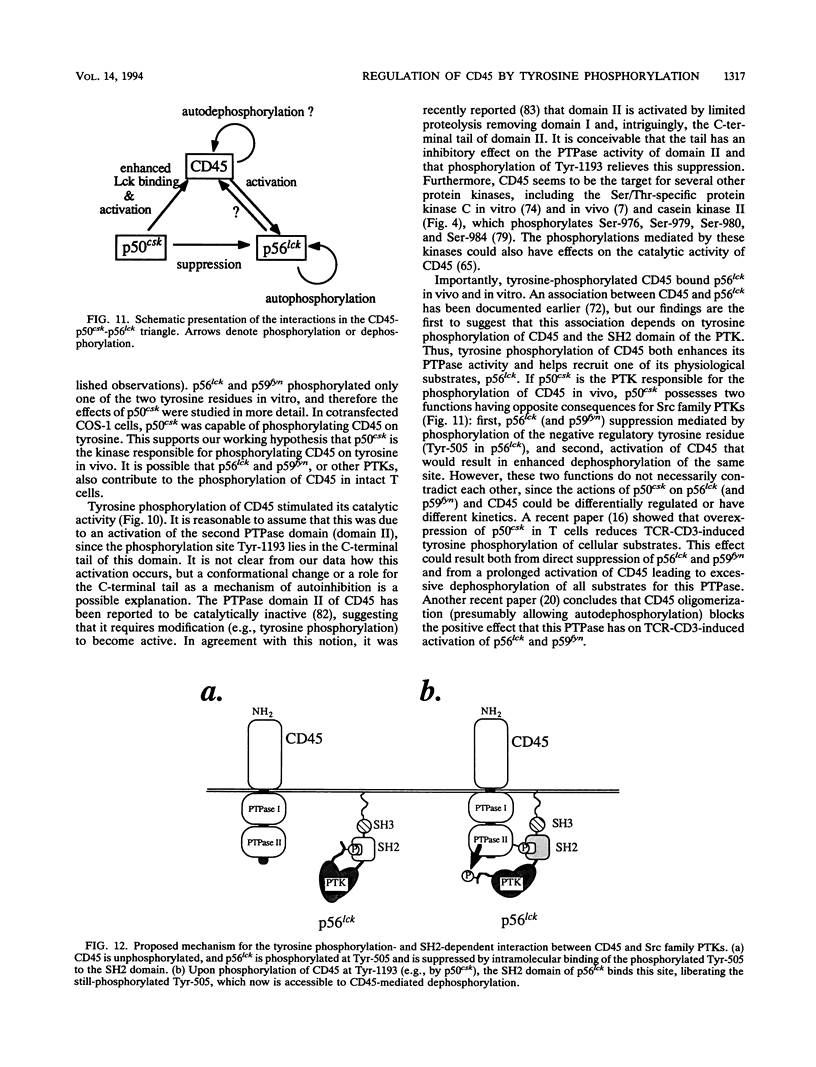
Src family protein tyrosine kinases (PTKs) play an essential role in antigen receptor-initiated lymphocyte activation. Their activity is largely regulated by a negative regulatory tyrosine which is a substrate for the activating action of the CD45 phosphotyrosine phosphatase (PTPase) or, conversely, the suppressing action of the cytosolic p50csk PTK. Here we report that CD45 was phosphorylated by p50csk on two tyrosine residues, one of them identified as Tyr-1193. This residue was not phosphorylated by T-cell PTKs p56lck and p59fyn. Tyr-1193 was phosphorylated in intact T cells, and phosphorylation increased upon treatment with PTPase inhibitors, indicating that this tyrosine is a target for a constitutively active PTK. Cotransfection of CD45 and csk into COS-1 cells caused tyrosine phosphorylation of CD45 in the intact cells. Tyrosine-phosphorylated CD45 bound p56lck through the SH2 domain of the kinase. Finally, p50csk-mediated phosphorylation of CD45 caused a severalfold increase in its PTPase activity. Our results show that direct tyrosine phosphorylation of CD45 can affect its activity and association with Src family PTKs and that this phosphorylation could be mediated by p50csk. If this is also true in the intact cells, it adds a new dimension to the physiological function of p50csk in T lymphocytes.
Full text
PDF












Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham N., Miceli M. C., Parnes J. R., Veillette A. Enhancement of T-cell responsiveness by the lymphocyte-specific tyrosine protein kinase p56lck. Nature. 1991 Mar 7;350(6313):62–66. doi: 10.1038/350062a0. [DOI] [PubMed] [Google Scholar]
- Amrein K. E., Flint N., Panholzer B., Burn P. Ras GTPase-activating protein: a substrate and a potential binding protein of the protein-tyrosine kinase p56lck. Proc Natl Acad Sci U S A. 1992 Apr 15;89(8):3343–3346. doi: 10.1073/pnas.89.8.3343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amrein K. E., Panholzer B., Flint N. A., Bannwarth W., Burn P. The Src homology 2 domain of the protein-tyrosine kinase p56lck mediates both intermolecular and intramolecular interactions. Proc Natl Acad Sci U S A. 1993 Nov 1;90(21):10285–10289. doi: 10.1073/pnas.90.21.10285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amrein K. E., Sefton B. M. Mutation of a site of tyrosine phosphorylation in the lymphocyte-specific tyrosine protein kinase, p56lck, reveals its oncogenic potential in fibroblasts. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4247–4251. doi: 10.1073/pnas.85.12.4247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson L. C., Karhi K. K., Gahmberg C. G., Rodt H. Molecular identification of T cell-specific antigens on human T lymphocytes and thymocytes. Eur J Immunol. 1980 May;10(5):359–362. doi: 10.1002/eji.1830100508. [DOI] [PubMed] [Google Scholar]
- Appleby M. W., Gross J. A., Cooke M. P., Levin S. D., Qian X., Perlmutter R. M. Defective T cell receptor signaling in mice lacking the thymic isoform of p59fyn. Cell. 1992 Sep 4;70(5):751–763. doi: 10.1016/0092-8674(92)90309-z. [DOI] [PubMed] [Google Scholar]
- Autero M., Gahmberg C. G. Phorbol diesters increase the phosphorylation of the leukocyte common antigen CD45 in human T cells. Eur J Immunol. 1987 Oct;17(10):1503–1506. doi: 10.1002/eji.1830171018. [DOI] [PubMed] [Google Scholar]
- Bergman M., Mustelin T., Oetken C., Partanen J., Flint N. A., Amrein K. E., Autero M., Burn P., Alitalo K. The human p50csk tyrosine kinase phosphorylates p56lck at Tyr-505 and down regulates its catalytic activity. EMBO J. 1992 Aug;11(8):2919–2924. doi: 10.1002/j.1460-2075.1992.tb05361.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bougeret C., Rothhut B., Jullien P., Fischer S., Benarous R. Recombinant Csk expressed in Escherichia coli is autophosphorylated on tyrosine residue(s). Oncogene. 1993 May;8(5):1241–1247. [PubMed] [Google Scholar]
- Cahir McFarland E. D., Hurley T. R., Pingel J. T., Sefton B. M., Shaw A., Thomas M. L. Correlation between Src family member regulation by the protein-tyrosine-phosphatase CD45 and transmembrane signaling through the T-cell receptor. Proc Natl Acad Sci U S A. 1993 Feb 15;90(4):1402–1406. doi: 10.1073/pnas.90.4.1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantley L. C., Auger K. R., Carpenter C., Duckworth B., Graziani A., Kapeller R., Soltoff S. Oncogenes and signal transduction. Cell. 1991 Jan 25;64(2):281–302. doi: 10.1016/0092-8674(91)90639-g. [DOI] [PubMed] [Google Scholar]
- Chalupny N. J., Ledbetter J. A., Kavathas P. Association of CD8 with p56lck is required for early T cell signalling events. EMBO J. 1991 May;10(5):1201–1207. doi: 10.1002/j.1460-2075.1991.tb08061.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan A. C., Irving B. A., Fraser J. D., Weiss A. The zeta chain is associated with a tyrosine kinase and upon T-cell antigen receptor stimulation associates with ZAP-70, a 70-kDa tyrosine phosphoprotein. Proc Natl Acad Sci U S A. 1991 Oct 15;88(20):9166–9170. doi: 10.1073/pnas.88.20.9166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan A. C., Iwashima M., Turck C. W., Weiss A. ZAP-70: a 70 kd protein-tyrosine kinase that associates with the TCR zeta chain. Cell. 1992 Nov 13;71(4):649–662. doi: 10.1016/0092-8674(92)90598-7. [DOI] [PubMed] [Google Scholar]
- Charbonneau H., Tonks N. K., Walsh K. A., Fischer E. H. The leukocyte common antigen (CD45): a putative receptor-linked protein tyrosine phosphatase. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7182–7186. doi: 10.1073/pnas.85.19.7182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow L. M., Fournel M., Davidson D., Veillette A. Negative regulation of T-cell receptor signalling by tyrosine protein kinase p50csk. Nature. 1993 Sep 9;365(6442):156–160. doi: 10.1038/365156a0. [DOI] [PubMed] [Google Scholar]
- Cooke M. P., Abraham K. M., Forbush K. A., Perlmutter R. M. Regulation of T cell receptor signaling by a src family protein-tyrosine kinase (p59fyn). Cell. 1991 Apr 19;65(2):281–291. doi: 10.1016/0092-8674(91)90162-r. [DOI] [PubMed] [Google Scholar]
- Danielian S., Alcover A., Polissard L., Stefanescu M., Acuto O., Fischer S., Fagard R. Both T cell receptor (TcR)-CD3 complex and CD2 increase the tyrosine kinase activity of p56lck. CD2 can mediate TcR-CD3-independent and CD45-dependent activation of p56lck. Eur J Immunol. 1992 Nov;22(11):2915–2921. doi: 10.1002/eji.1830221124. [DOI] [PubMed] [Google Scholar]
- Desai D. M., Sap J., Schlessinger J., Weiss A. Ligand-mediated negative regulation of a chimeric transmembrane receptor tyrosine phosphatase. Cell. 1993 May 7;73(3):541–554. doi: 10.1016/0092-8674(93)90141-c. [DOI] [PubMed] [Google Scholar]
- Eck M. J., Shoelson S. E., Harrison S. C. Recognition of a high-affinity phosphotyrosyl peptide by the Src homology-2 domain of p56lck. Nature. 1993 Mar 4;362(6415):87–91. doi: 10.1038/362087a0. [DOI] [PubMed] [Google Scholar]
- Feng G. S., Hui C. C., Pawson T. SH2-containing phosphotyrosine phosphatase as a target of protein-tyrosine kinases. Science. 1993 Mar 12;259(5101):1607–1611. doi: 10.1126/science.8096088. [DOI] [PubMed] [Google Scholar]
- Gahmberg C. G., Andersson L. C. Selective radioactive labeling of cell surface sialoglycoproteins by periodate-tritiated borohydride. J Biol Chem. 1977 Aug 25;252(16):5888–5894. [PubMed] [Google Scholar]
- Gahmberg C. G., Häyry P., Andersson L. C. Characterization of surface glycoproteins of mouse lymphoid cells. J Cell Biol. 1976 Mar;68(3):642–653. doi: 10.1083/jcb.68.3.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Morales P., Minami Y., Luong E., Klausner R. D., Samelson L. E. Tyrosine phosphorylation in T cells is regulated by phosphatase activity: studies with phenylarsine oxide. Proc Natl Acad Sci U S A. 1990 Dec;87(23):9255–9259. doi: 10.1073/pnas.87.23.9255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gassmann M., Amrein K. E., Burn P. CD4:p56lck association studied in vivo using antibody-induced capping and double indirect immunofluorescence microscopy. J Recept Res. 1993;13(1-4):711–724. doi: 10.3109/10799899309073688. [DOI] [PubMed] [Google Scholar]
- Gassmann M., Guttinger M., Amrein K. E., Burn P. Protein tyrosine kinase p59fyn is associated with the T cell receptor-CD3 complex in functional human lymphocytes. Eur J Immunol. 1992 Jan;22(1):283–286. doi: 10.1002/eji.1830220142. [DOI] [PubMed] [Google Scholar]
- Glaichenhaus N., Shastri N., Littman D. R., Turner J. M. Requirement for association of p56lck with CD4 in antigen-specific signal transduction in T cells. Cell. 1991 Feb 8;64(3):511–520. doi: 10.1016/0092-8674(91)90235-q. [DOI] [PubMed] [Google Scholar]
- Guttinger M., Gassmann M., Amrein K. E., Burn P. CD45 phosphotyrosine phosphatase and p56lck protein tyrosine kinase: a functional complex crucial in T cell signal transduction. Int Immunol. 1992 Nov;4(11):1325–1330. doi: 10.1093/intimm/4.11.1325. [DOI] [PubMed] [Google Scholar]
- Hsi E. D., Siegel J. N., Minami Y., Luong E. T., Klausner R. D., Samelson L. E. T cell activation induces rapid tyrosine phosphorylation of a limited number of cellular substrates. J Biol Chem. 1989 Jun 25;264(18):10836–10842. [PubMed] [Google Scholar]
- Hunter T., Cooper J. A. Protein-tyrosine kinases. Annu Rev Biochem. 1985;54:897–930. doi: 10.1146/annurev.bi.54.070185.004341. [DOI] [PubMed] [Google Scholar]
- Hunter T. Protein-tyrosine phosphatases: the other side of the coin. Cell. 1989 Sep 22;58(6):1013–1016. doi: 10.1016/0092-8674(89)90496-0. [DOI] [PubMed] [Google Scholar]
- Hunter T., Sefton B. M. Transforming gene product of Rous sarcoma virus phosphorylates tyrosine. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1311–1315. doi: 10.1073/pnas.77.3.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley T. R., Hyman R., Sefton B. M. Differential effects of expression of the CD45 tyrosine protein phosphatase on the tyrosine phosphorylation of the lck, fyn, and c-src tyrosine protein kinases. Mol Cell Biol. 1993 Mar;13(3):1651–1656. doi: 10.1128/mcb.13.3.1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julius M. H., Simpson E., Herzenberg L. A. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973 Oct;3(10):645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- June C. H., Fletcher M. C., Ledbetter J. A., Samelson L. E. Increases in tyrosine phosphorylation are detectable before phospholipase C activation after T cell receptor stimulation. J Immunol. 1990 Mar 1;144(5):1591–1599. [PubMed] [Google Scholar]
- June C. H., Fletcher M. C., Ledbetter J. A., Schieven G. L., Siegel J. N., Phillips A. F., Samelson L. E. Inhibition of tyrosine phosphorylation prevents T-cell receptor-mediated signal transduction. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7722–7726. doi: 10.1073/pnas.87.19.7722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justement L. B., Campbell K. S., Chien N. C., Cambier J. C. Regulation of B cell antigen receptor signal transduction and phosphorylation by CD45. Science. 1991 Jun 28;252(5014):1839–1842. doi: 10.1126/science.1648262. [DOI] [PubMed] [Google Scholar]
- Karnitz L., Sutor S. L., Torigoe T., Reed J. C., Bell M. P., McKean D. J., Leibson P. J., Abraham R. T. Effects of p56lck deficiency on the growth and cytolytic effector function of an interleukin-2-dependent cytotoxic T-cell line. Mol Cell Biol. 1992 Oct;12(10):4521–4530. doi: 10.1128/mcb.12.10.4521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami T., Kawakami Y., Aaronson S. A., Robbins K. C. Acquisition of transforming properties by FYN, a normal SRC-related human gene. Proc Natl Acad Sci U S A. 1988 Jun;85(11):3870–3874. doi: 10.1073/pnas.85.11.3870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiener P. A., Mittler R. S. CD45-protein tyrosine phosphatase cross-linking inhibits T cell receptor CD3-mediated activation in human T cells. J Immunol. 1989 Jul 1;143(1):23–28. [PubMed] [Google Scholar]
- Klausner R. D., Samelson L. E. T cell antigen receptor activation pathways: the tyrosine kinase connection. Cell. 1991 Mar 8;64(5):875–878. doi: 10.1016/0092-8674(91)90310-u. [DOI] [PubMed] [Google Scholar]
- Koch C. A., Anderson D., Moran M. F., Ellis C., Pawson T. SH2 and SH3 domains: elements that control interactions of cytoplasmic signaling proteins. Science. 1991 May 3;252(5006):668–674. doi: 10.1126/science.1708916. [DOI] [PubMed] [Google Scholar]
- Koretzky G. A., Kohmetscher M. A., Kadleck T., Weiss A. Restoration of T cell receptor-mediated signal transduction by transfection of CD45 cDNA into a CD45-deficient variant of the Jurkat T cell line. J Immunol. 1992 Aug 15;149(4):1138–1142. [PubMed] [Google Scholar]
- Koretzky G. A., Picus J., Schultz T., Weiss A. Tyrosine phosphatase CD45 is required for T-cell antigen receptor and CD2-mediated activation of a protein tyrosine kinase and interleukin 2 production. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2037–2041. doi: 10.1073/pnas.88.6.2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koretzky G. A., Picus J., Thomas M. L., Weiss A. Tyrosine phosphatase CD45 is essential for coupling T-cell antigen receptor to the phosphatidyl inositol pathway. Nature. 1990 Jul 5;346(6279):66–68. doi: 10.1038/346066a0. [DOI] [PubMed] [Google Scholar]
- Krueger N. X., Streuli M., Saito H. Structural diversity and evolution of human receptor-like protein tyrosine phosphatases. EMBO J. 1990 Oct;9(10):3241–3252. doi: 10.1002/j.1460-2075.1990.tb07523.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledbetter J. A., Tonks N. K., Fischer E. H., Clark E. A. CD45 regulates signal transduction and lymphocyte activation by specific association with receptor molecules on T or B cells. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8628–8632. doi: 10.1073/pnas.85.22.8628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Brodeur S. R., Gish G., Songyang Z., Cantley L. C., Laudano A. P., Pawson T. Regulation of c-Src tyrosine kinase activity by the Src SH2 domain. Oncogene. 1993 May;8(5):1119–1126. [PubMed] [Google Scholar]
- Luo K., Hurley T. R., Sefton B. M. Transfer of proteins to membranes facilitates both cyanogen bromide cleavage and two-dimensional proteolytic mapping. Oncogene. 1990 Jun;5(6):921–923. [PubMed] [Google Scholar]
- Luo K., Sefton B. M. Activated lck tyrosine protein kinase stimulates antigen-independent interleukin-2 production in T cells. Mol Cell Biol. 1992 Oct;12(10):4724–4732. doi: 10.1128/mcb.12.10.4724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marth J. D., Cooper J. A., King C. S., Ziegler S. F., Tinker D. A., Overell R. W., Krebs E. G., Perlmutter R. M. Neoplastic transformation induced by an activated lymphocyte-specific protein tyrosine kinase (pp56lck). Mol Cell Biol. 1988 Feb;8(2):540–550. doi: 10.1128/mcb.8.2.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews R. J., Bowne D. B., Flores E., Thomas M. L. Characterization of hematopoietic intracellular protein tyrosine phosphatases: description of a phosphatase containing an SH2 domain and another enriched in proline-, glutamic acid-, serine-, and threonine-rich sequences. Mol Cell Biol. 1992 May;12(5):2396–2405. doi: 10.1128/mcb.12.5.2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina T. J., Kishihara K., Siderovski D. P., van Ewijk W., Narendran A., Timms E., Wakeham A., Paige C. J., Hartmann K. U., Veillette A. Profound block in thymocyte development in mice lacking p56lck. Nature. 1992 May 14;357(6374):161–164. doi: 10.1038/357161a0. [DOI] [PubMed] [Google Scholar]
- Mustelin T., Altman A. Dephosphorylation and activation of the T cell tyrosine kinase pp56lck by the leukocyte common antigen (CD45). Oncogene. 1990 Jun;5(6):809–813. [PubMed] [Google Scholar]
- Mustelin T., Burn P. Regulation of src family tyrosine kinases in lymphocytes. Trends Biochem Sci. 1993 Jun;18(6):215–220. doi: 10.1016/0968-0004(93)90192-p. [DOI] [PubMed] [Google Scholar]
- Mustelin T., Coggeshall K. M., Altman A. Rapid activation of the T-cell tyrosine protein kinase pp56lck by the CD45 phosphotyrosine phosphatase. Proc Natl Acad Sci U S A. 1989 Aug;86(16):6302–6306. doi: 10.1073/pnas.86.16.6302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustelin T., Coggeshall K. M., Isakov N., Altman A. T cell antigen receptor-mediated activation of phospholipase C requires tyrosine phosphorylation. Science. 1990 Mar 30;247(4950):1584–1587. doi: 10.1126/science.2138816. [DOI] [PubMed] [Google Scholar]
- Mustelin T., Pessa-Morikawa T., Autero M., Gassmann M., Andersson L. C., Gahmberg C. G., Burn P. Regulation of the p59fyn protein tyrosine kinase by the CD45 phosphotyrosine phosphatase. Eur J Immunol. 1992 May;22(5):1173–1178. doi: 10.1002/eji.1830220510. [DOI] [PubMed] [Google Scholar]
- Nada S., Okada M., MacAuley A., Cooper J. A., Nakagawa H. Cloning of a complementary DNA for a protein-tyrosine kinase that specifically phosphorylates a negative regulatory site of p60c-src. Nature. 1991 May 2;351(6321):69–72. doi: 10.1038/351069a0. [DOI] [PubMed] [Google Scholar]
- O'Shea J. J., McVicar D. W., Bailey T. L., Burns C., Smyth M. J. Activation of human peripheral blood T lymphocytes by pharmacological induction of protein-tyrosine phosphorylation. Proc Natl Acad Sci U S A. 1992 Nov 1;89(21):10306–10310. doi: 10.1073/pnas.89.21.10306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oetken C., von Willebrand M., Autero M., Ruutu T., Andersson L. C., Mustelin T. Phenylarsine oxide augments tyrosine phosphorylation in hematopoietic cells. Eur J Haematol. 1992 Oct;49(4):208–214. doi: 10.1111/j.1600-0609.1992.tb00048.x. [DOI] [PubMed] [Google Scholar]
- Okada M., Nada S., Yamanashi Y., Yamamoto T., Nakagawa H. CSK: a protein-tyrosine kinase involved in regulation of src family kinases. J Biol Chem. 1991 Dec 25;266(36):24249–24252. [PubMed] [Google Scholar]
- Ostergaard H. L., Shackelford D. A., Hurley T. R., Johnson P., Hyman R., Sefton B. M., Trowbridge I. S. Expression of CD45 alters phosphorylation of the lck-encoded tyrosine protein kinase in murine lymphoma T-cell lines. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8959–8963. doi: 10.1073/pnas.86.22.8959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostergaard H. L., Trowbridge I. S. Coclustering CD45 with CD4 or CD8 alters the phosphorylation and kinase activity of p56lck. J Exp Med. 1990 Jul 1;172(1):347–350. doi: 10.1084/jem.172.1.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostergaard H. L., Trowbridge I. S. Negative regulation of CD45 protein tyrosine phosphatase activity by ionomycin in T cells. Science. 1991 Sep 20;253(5026):1423–1425. doi: 10.1126/science.1654595. [DOI] [PubMed] [Google Scholar]
- Partanen J., Armstrong E., Bergman M., Mäkelä T. P., Hirvonen H., Huebner K., Alitalo K. cyl encodes a putative cytoplasmic tyrosine kinase lacking the conserved tyrosine autophosphorylation site (Y416src). Oncogene. 1991 Nov;6(11):2013–2018. [PubMed] [Google Scholar]
- Pingel J. T., Thomas M. L. Evidence that the leukocyte-common antigen is required for antigen-induced T lymphocyte proliferation. Cell. 1989 Sep 22;58(6):1055–1065. doi: 10.1016/0092-8674(89)90504-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roussel R. R., Brodeur S. R., Shalloway D., Laudano A. P. Selective binding of activated pp60c-src by an immobilized synthetic phosphopeptide modeled on the carboxyl terminus of pp60c-src. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10696–10700. doi: 10.1073/pnas.88.23.10696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samelson L. E., Davidson W. F., Morse H. C., 3rd, Klausner R. D. Abnormal tyrosine phosphorylation on T-cell receptor in lymphoproliferative disorders. Nature. 1986 Dec 18;324(6098):674–676. doi: 10.1038/324674a0. [DOI] [PubMed] [Google Scholar]
- Samelson L. E., Phillips A. F., Luong E. T., Klausner R. D. Association of the fyn protein-tyrosine kinase with the T-cell antigen receptor. Proc Natl Acad Sci U S A. 1990 Jun;87(11):4358–4362. doi: 10.1073/pnas.87.11.4358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schraven B., Kirchgessner H., Gaber B., Samstag Y., Meuer S. A functional complex is formed in human T lymphocytes between the protein tyrosine phosphatase CD45, the protein tyrosine kinase p56lck and pp32, a possible common substrate. Eur J Immunol. 1991 Oct;21(10):2469–2477. doi: 10.1002/eji.1830211025. [DOI] [PubMed] [Google Scholar]
- Secrist J. P., Burns L. A., Karnitz L., Koretzky G. A., Abraham R. T. Stimulatory effects of the protein tyrosine phosphatase inhibitor, pervanadate, on T-cell activation events. J Biol Chem. 1993 Mar 15;268(8):5886–5893. [PubMed] [Google Scholar]
- Shackelford D. A., Trowbridge I. S. Identification of lymphocyte integral membrane proteins as substrates for protein kinase C. Phosphorylation of the interleukin-2 receptor, class I HLA antigens, and T200 glycoprotein. J Biol Chem. 1986 Jun 25;261(18):8334–8341. [PubMed] [Google Scholar]
- Shiroo M., Goff L., Biffen M., Shivnan E., Alexander D. CD45 tyrosine phosphatase-activated p59fyn couples the T cell antigen receptor to pathways of diacylglycerol production, protein kinase C activation and calcium influx. EMBO J. 1992 Dec;11(13):4887–4897. doi: 10.1002/j.1460-2075.1992.tb05595.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieh M., Bolen J. B., Weiss A. CD45 specifically modulates binding of Lck to a phosphopeptide encompassing the negative regulatory tyrosine of Lck. EMBO J. 1993 Jan;12(1):315–321. doi: 10.1002/j.1460-2075.1993.tb05659.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Songyang Z., Shoelson S. E., Chaudhuri M., Gish G., Pawson T., Haser W. G., King F., Roberts T., Ratnofsky S., Lechleider R. J. SH2 domains recognize specific phosphopeptide sequences. Cell. 1993 Mar 12;72(5):767–778. doi: 10.1016/0092-8674(93)90404-e. [DOI] [PubMed] [Google Scholar]
- Stein P. L., Lee H. M., Rich S., Soriano P. pp59fyn mutant mice display differential signaling in thymocytes and peripheral T cells. Cell. 1992 Sep 4;70(5):741–750. doi: 10.1016/0092-8674(92)90308-y. [DOI] [PubMed] [Google Scholar]
- Stover D. R., Charbonneau H., Tonks N. K., Walsh K. A. Protein-tyrosine-phosphatase CD45 is phosphorylated transiently on tyrosine upon activation of Jurkat T cells. Proc Natl Acad Sci U S A. 1991 Sep 1;88(17):7704–7707. doi: 10.1073/pnas.88.17.7704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straus D. B., Weiss A. Genetic evidence for the involvement of the lck tyrosine kinase in signal transduction through the T cell antigen receptor. Cell. 1992 Aug 21;70(4):585–593. doi: 10.1016/0092-8674(92)90428-f. [DOI] [PubMed] [Google Scholar]
- Streuli M., Hall L. R., Saga Y., Schlossman S. F., Saito H. Differential usage of three exons generates at least five different mRNAs encoding human leukocyte common antigens. J Exp Med. 1987 Nov 1;166(5):1548–1566. doi: 10.1084/jem.166.5.1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streuli M., Krueger N. X., Tsai A. Y., Saito H. A family of receptor-linked protein tyrosine phosphatases in humans and Drosophila. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8698–8702. doi: 10.1073/pnas.86.22.8698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan X., Stover D. R., Walsh K. A. Demonstration of protein tyrosine phosphatase activity in the second of two homologous domains of CD45. J Biol Chem. 1993 Apr 5;268(10):6835–6838. [PubMed] [Google Scholar]
- Thomas M. L. The leukocyte common antigen family. Annu Rev Immunol. 1989;7:339–369. doi: 10.1146/annurev.iy.07.040189.002011. [DOI] [PubMed] [Google Scholar]
- Thompson P. A., Gutkind J. S., Robbins K. C., Ledbetter J. A., Bolen J. B. Identification of distinct populations of PI-3 kinase activity following T-cell activation. Oncogene. 1992 Apr;7(4):719–725. [PubMed] [Google Scholar]
- Tonks N. K., Charbonneau H., Diltz C. D., Fischer E. H., Walsh K. A. Demonstration that the leukocyte common antigen CD45 is a protein tyrosine phosphatase. Biochemistry. 1988 Nov 29;27(24):8695–8701. doi: 10.1021/bi00424a001. [DOI] [PubMed] [Google Scholar]
- Trowbridge I. S. CD45. A prototype for transmembrane protein tyrosine phosphatases. J Biol Chem. 1991 Dec 15;266(35):23517–23520. [PubMed] [Google Scholar]
- Trowbridge I. S., Ralph P., Bevan M. J. Differences in the surface proteins of mouse B and T cells. Proc Natl Acad Sci U S A. 1975 Jan;72(1):157–161. doi: 10.1073/pnas.72.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsygankov A. Y., Bröker B. M., Fargnoli J., Ledbetter J. A., Bolen J. B. Activation of tyrosine kinase p60fyn following T cell antigen receptor cross-linking. J Biol Chem. 1992 Sep 15;267(26):18259–18262. [PubMed] [Google Scholar]
- Turka L. A., Kanner S. B., Schieven G. L., Thompson C. B., Ledbetter J. A. CD45 modulates T cell receptor/CD3-induced activation of human thymocytes via regulation of tyrosine phosphorylation. Eur J Immunol. 1992 Feb;22(2):551–557. doi: 10.1002/eji.1830220238. [DOI] [PubMed] [Google Scholar]
- Ullrich A., Schlessinger J. Signal transduction by receptors with tyrosine kinase activity. Cell. 1990 Apr 20;61(2):203–212. doi: 10.1016/0092-8674(90)90801-k. [DOI] [PubMed] [Google Scholar]
- Vogel W., Lammers R., Huang J., Ullrich A. Activation of a phosphotyrosine phosphatase by tyrosine phosphorylation. Science. 1993 Mar 12;259(5101):1611–1614. doi: 10.1126/science.7681217. [DOI] [PubMed] [Google Scholar]
- Volarević S., Niklinska B. B., Burns C. M., Yamada H., June C. H., Dumont F. J., Ashwell J. D. The CD45 tyrosine phosphatase regulates phosphotyrosine homeostasis and its loss reveals a novel pattern of late T cell receptor-induced Ca2+ oscillations. J Exp Med. 1992 Sep 1;176(3):835–844. doi: 10.1084/jem.176.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber J. R., Bell G. M., Han M. Y., Pawson T., Imboden J. B. Association of the tyrosine kinase LCK with phospholipase C-gamma 1 after stimulation of the T cell antigen receptor. J Exp Med. 1992 Aug 1;176(2):373–379. doi: 10.1084/jem.176.2.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada N., Kawakami Y., Kimura H., Fukamachi H., Baier G., Altman A., Kato T., Inagaki Y., Kawakami T. Structure and expression of novel protein-tyrosine kinases, Emb and Emt, in hematopoietic cells. Biochem Biophys Res Commun. 1993 Apr 15;192(1):231–240. doi: 10.1006/bbrc.1993.1404. [DOI] [PubMed] [Google Scholar]
- Yang Q., Tonks N. K. Isolation of a cDNA clone encoding a human protein-tyrosine phosphatase with homology to the cytoskeletal-associated proteins band 4.1, ezrin, and talin. Proc Natl Acad Sci U S A. 1991 Jul 15;88(14):5949–5953. doi: 10.1073/pnas.88.14.5949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng X. M., Wang Y., Pallen C. J. Cell transformation and activation of pp60c-src by overexpression of a protein tyrosine phosphatase. Nature. 1992 Sep 24;359(6393):336–339. doi: 10.1038/359336a0. [DOI] [PubMed] [Google Scholar]
- da Silva A. J., Yamamoto M., Zalvan C. H., Rudd C. E. Engagement of the TcR/CD3 complex stimulates p59fyn(T) activity: detection of associated proteins at 72 and 120-130 kD. Mol Immunol. 1992 Dec;29(12):1417–1425. doi: 10.1016/0161-5890(92)90215-j. [DOI] [PubMed] [Google Scholar]












