Abstract
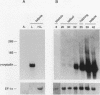
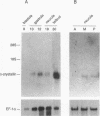
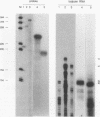
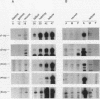
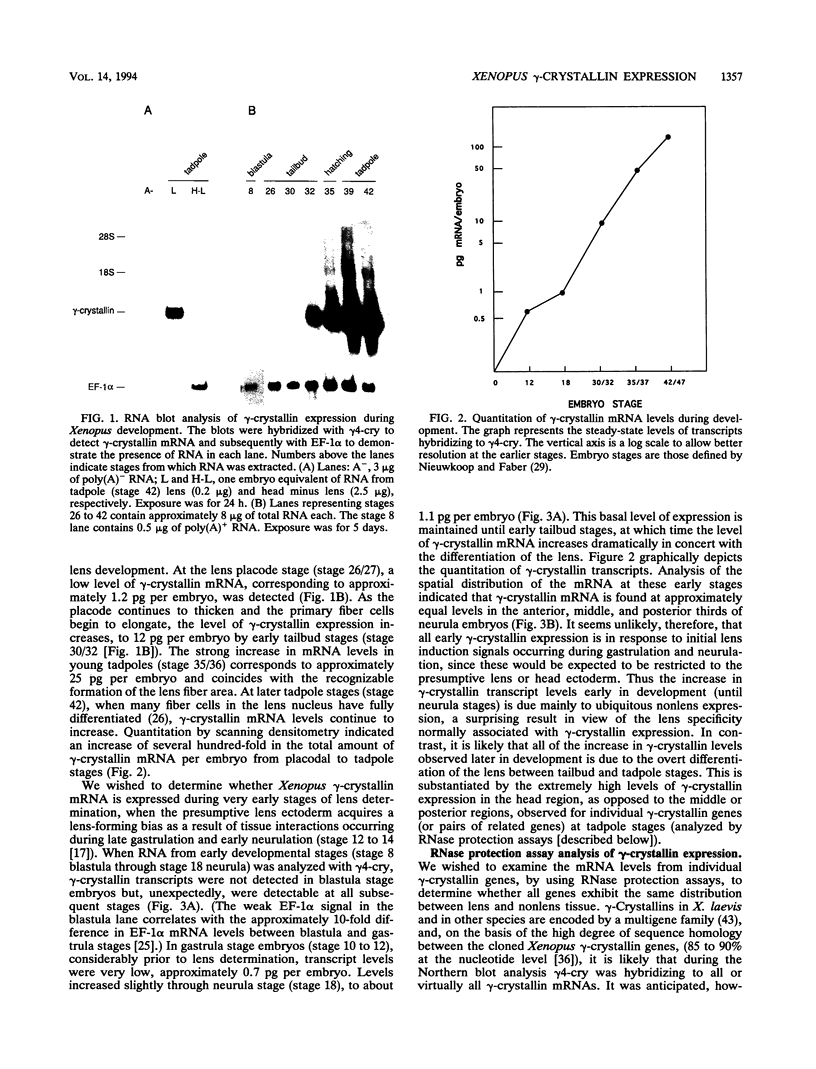
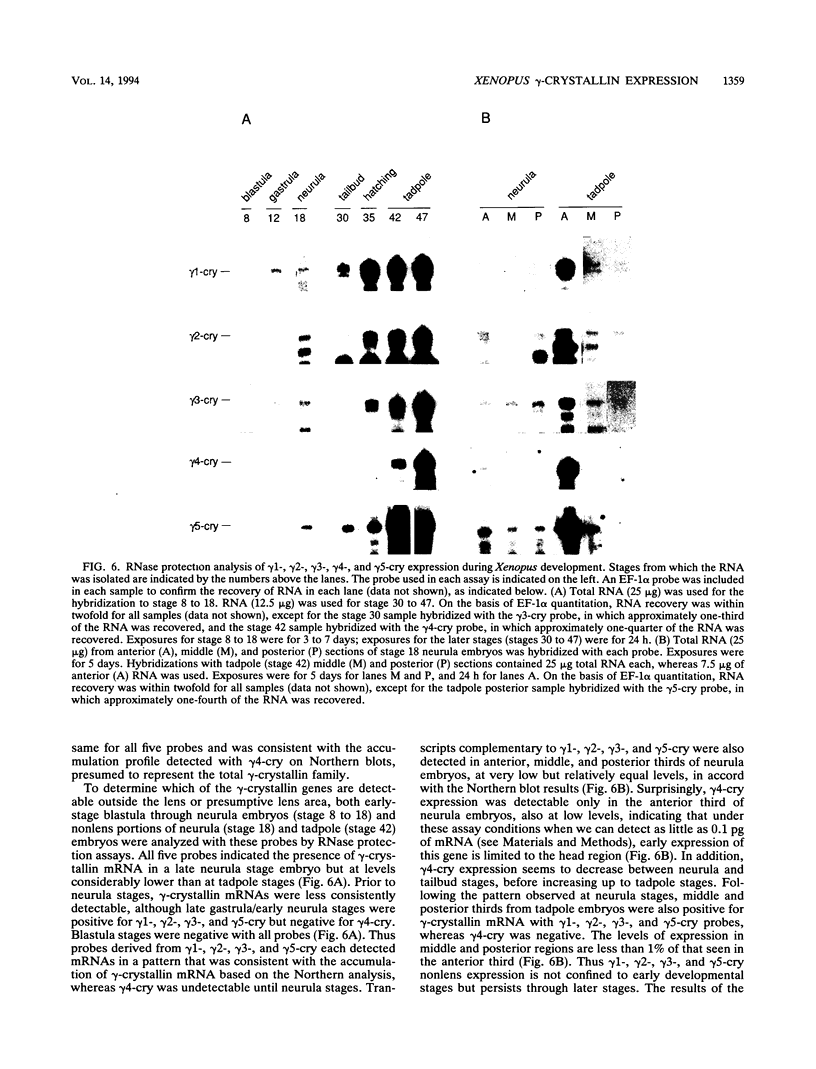
Crystallins, the major gene products of the lens, accumulate to high levels during the differentiation of the vertebrate lens. Although crystallins were traditionally thought to be lens specific, it has recently been shown that some are also expressed at very low levels in nonlens tissues. We have examined the embryonic expression pattern of gamma-crystallins, the most abundant crystallins of the embryonic lens in Xenopus laevis. The expression profile of five Xenopus gamma-crystallin genes mirrors the pattern of lens differentiation in X. laevis, exhibiting on average a 100-fold increase between tailbud and tadpole stages. Four of these genes are also ubiquitously expressed outside the lens at a very low level, the first demonstration of nonlens expression of any gamma-crystallin gene; expression of the remaining gene was not detected outside the head region, thus suggesting that there may be two classes of gamma-crystallin genes in X. laevis. Predictions regarding control mechanisms responsible for this dual mode of expression are discussed. This study raises the question of whether any crystallin, on stringent examination, will be found exclusively in the lens.
Full text
PDF



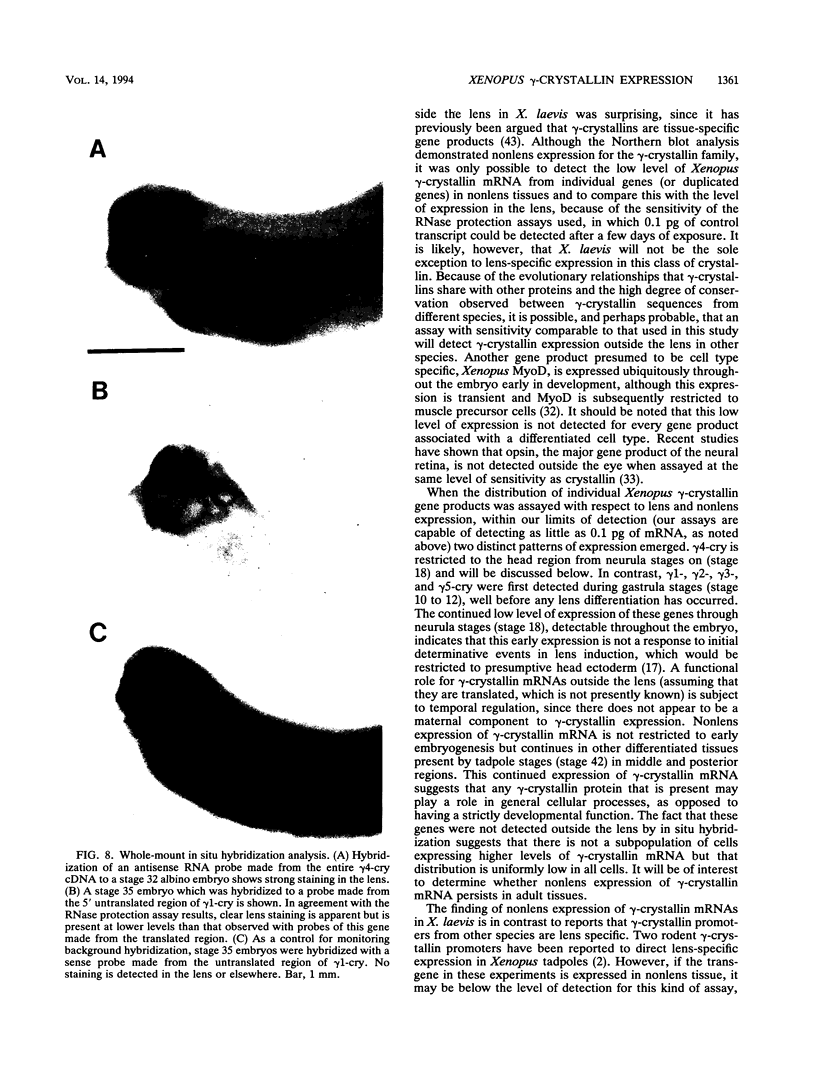


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agata K., Yasuda K., Okada T. S. Gene coding for a lens-specific protein, delta-crystallin, is transcribed in nonlens tissues of chicken embryos. Dev Biol. 1983 Nov;100(1):222–226. doi: 10.1016/0012-1606(83)90214-2. [DOI] [PubMed] [Google Scholar]
- Brakenhoff R. H., Ruuls R. C., Jacobs E. H., Schoenmakers J. G., Lubsen N. H. Transgenic Xenopus laevis tadpoles: a transient in vivo model system for the manipulation of lens function and lens development. Nucleic Acids Res. 1991 Mar 25;19(6):1279–1284. doi: 10.1093/nar/19.6.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitman M. L., Clapoff S., Rossant J., Tsui L. C., Glode L. M., Maxwell I. H., Bernstein A. Genetic ablation: targeted expression of a toxin gene causes microphthalmia in transgenic mice. Science. 1987 Dec 11;238(4833):1563–1565. doi: 10.1126/science.3685993. [DOI] [PubMed] [Google Scholar]
- Chien Y. H., Dawid I. B. Isolation and characterization of calmodulin genes from Xenopus laevis. Mol Cell Biol. 1984 Mar;4(3):507–513. doi: 10.1128/mcb.4.3.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasgupta S., Hohman T. C., Carper D. Hypertonic stress induces alpha B-crystallin expression. Exp Eye Res. 1992 Mar;54(3):461–470. doi: 10.1016/0014-4835(92)90058-z. [DOI] [PubMed] [Google Scholar]
- Delaye M., Tardieu A. Short-range order of crystallin proteins accounts for eye lens transparency. 1983 Mar 31-Apr 6Nature. 302(5907):415–417. doi: 10.1038/302415a0. [DOI] [PubMed] [Google Scholar]
- Dubin R. A., Gopal-Srivastava R., Wawrousek E. F., Piatigorsky J. Expression of the murine alpha B-crystallin gene in lens and skeletal muscle: identification of a muscle-preferred enhancer. Mol Cell Biol. 1991 Sep;11(9):4340–4349. doi: 10.1128/mcb.11.9.4340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubin R. A., Wawrousek E. F., Piatigorsky J. Expression of the murine alpha B-crystallin gene is not restricted to the lens. Mol Cell Biol. 1989 Mar;9(3):1083–1091. doi: 10.1128/mcb.9.3.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duguid J. R., Rohwer R. G., Seed B. Isolation of cDNAs of scrapie-modulated RNAs by subtractive hybridization of a cDNA library. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5738–5742. doi: 10.1073/pnas.85.15.5738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Goto K., Okada T. S., Kondoh H. Functional cooperation of lens-specific and nonspecific elements in the delta 1-crystallin enhancer. Mol Cell Biol. 1990 Mar;10(3):958–964. doi: 10.1128/mcb.10.3.958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grainger R. M. Embryonic lens induction: shedding light on vertebrate tissue determination. Trends Genet. 1992 Oct;8(10):349–355. doi: 10.1016/0168-9525(92)90280-h. [DOI] [PubMed] [Google Scholar]
- Harland R. M. In situ hybridization: an improved whole-mount method for Xenopus embryos. Methods Cell Biol. 1991;36:685–695. doi: 10.1016/s0091-679x(08)60307-6. [DOI] [PubMed] [Google Scholar]
- Hayashi S., Goto K., Okada T. S., Kondoh H. Lens-specific enhancer in the third intron regulates expression of the chicken delta 1-crystallin gene. Genes Dev. 1987 Oct;1(8):818–828. doi: 10.1101/gad.1.8.818. [DOI] [PubMed] [Google Scholar]
- Head M. W., Peter A., Clayton R. M. Evidence for the extralenticular expression of members of the beta-crystallin gene family in the chick and a comparison with delta-crystallin during differentiation and transdifferentiation. Differentiation. 1991 Dec;48(3):147–156. doi: 10.1111/j.1432-0436.1991.tb00253.x. [DOI] [PubMed] [Google Scholar]
- Henry J. J., Grainger R. M. Early tissue interactions leading to embryonic lens formation in Xenopus laevis. Dev Biol. 1990 Sep;141(1):149–163. doi: 10.1016/0012-1606(90)90110-5. [DOI] [PubMed] [Google Scholar]
- Hodin J., Wistow G. 5'-RACE PCR of mRNA for three taxon-specific crystallins: for each gene one promoter controls both lens and non-lens expression. Biochem Biophys Res Commun. 1993 Jan 29;190(2):391–396. doi: 10.1006/bbrc.1993.1060. [DOI] [PubMed] [Google Scholar]
- Ingolia T. D., Craig E. A. Four small Drosophila heat shock proteins are related to each other and to mammalian alpha-crystallin. Proc Natl Acad Sci U S A. 1982 Apr;79(7):2360–2364. doi: 10.1073/pnas.79.7.2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwaki T., Kume-Iwaki A., Liem R. K., Goldman J. E. Alpha B-crystallin is expressed in non-lenticular tissues and accumulates in Alexander's disease brain. Cell. 1989 Apr 7;57(1):71–78. doi: 10.1016/0092-8674(89)90173-6. [DOI] [PubMed] [Google Scholar]
- Jonas E. A., Snape A. M., Sargent T. D. Transcriptional regulation of a Xenopus embryonic epidermal keratin gene. Development. 1989 Jun;106(2):399–405. doi: 10.1242/dev.106.2.399. [DOI] [PubMed] [Google Scholar]
- Kato K., Shinohara H., Kurobe N., Goto S., Inaguma Y., Ohshima K. Immunoreactive alpha A crystallin in rat non-lenticular tissues detected with a sensitive immunoassay method. Biochim Biophys Acta. 1991 Oct 25;1080(2):173–180. doi: 10.1016/0167-4838(91)90146-q. [DOI] [PubMed] [Google Scholar]
- Klemenz R., Fröhli E., Steiger R. H., Schäfer R., Aoyama A. Alpha B-crystallin is a small heat shock protein. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3652–3656. doi: 10.1073/pnas.88.9.3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieg P. A., Varnum S. M., Wormington W. M., Melton D. A. The mRNA encoding elongation factor 1-alpha (EF-1 alpha) is a major transcript at the midblastula transition in Xenopus. Dev Biol. 1989 May;133(1):93–100. doi: 10.1016/0012-1606(89)90300-x. [DOI] [PubMed] [Google Scholar]
- McDevitt D. S., Brahma S. K. Ontogeny and localization of the crystallins during embryonic lens development in Xenopus laevis. J Exp Zool. 1973 Nov;186(2):127–140. doi: 10.1002/jez.1401860204. [DOI] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murer-Orlando M., Paterson R. C., Lok S., Tsui L. C., Breitman M. L. Differential regulation of gamma-crystallin genes during mouse lens development. Dev Biol. 1987 Jan;119(1):260–267. doi: 10.1016/0012-1606(87)90227-2. [DOI] [PubMed] [Google Scholar]
- Piatigorsky J. Lens crystallins. Innovation associated with changes in gene regulation. J Biol Chem. 1992 Mar 5;267(7):4277–4280. [PubMed] [Google Scholar]
- Rupp R. A., Weintraub H. Ubiquitous MyoD transcription at the midblastula transition precedes induction-dependent MyoD expression in presumptive mesoderm of X. laevis. Cell. 1991 Jun 14;65(6):927–937. doi: 10.1016/0092-8674(91)90545-a. [DOI] [PubMed] [Google Scholar]
- Saha M. S., Grainger R. M. Early opsin expression in Xenopus embryos precedes photoreceptor differentiation. Brain Res Mol Brain Res. 1993 Mar;17(3-4):307–318. doi: 10.1016/0169-328x(93)90016-i. [DOI] [PubMed] [Google Scholar]
- Shastry B. S. Immunological studies on gamma crystallins from Xenopus: localization, tissue specificity and developmental expression of proteins. Exp Eye Res. 1989 Sep;49(3):361–369. doi: 10.1016/0014-4835(89)90046-8. [DOI] [PubMed] [Google Scholar]
- Smolich B. D., Tarkington S. K., Saha M. S., Stathakis D. G., Grainger R. M. Characterization of Xenopus laevis gamma-crystallin-encoding genes. Gene. 1993 Jun 30;128(2):189–195. doi: 10.1016/0378-1119(93)90562-h. [DOI] [PubMed] [Google Scholar]
- Sullivan C. H., O'Farrell S., Grainger R. M. Delta-crystallin gene expression and patterns of hypomethylation demonstrate two levels of regulation for the delta-crystallin genes in embryonic chick tissues. Dev Biol. 1991 May;145(1):40–50. doi: 10.1016/0012-1606(91)90211-k. [DOI] [PubMed] [Google Scholar]
- Thomas G., Zelenka P. S., Cuthbertson R. A., Norman B. L., Piatigorsky J. Differential expression of the two delta-crystallin/argininosuccinate lyase genes in lens, heart, and brain of chicken embryos. New Biol. 1990 Oct;2(10):903–914. [PubMed] [Google Scholar]
- Van Leen R. W., Breuer M. L., Lubsen N. H., Schoenmakers J. G. Developmental expression of crystallin genes: in situ hybridization reveals a differential localization of specific mRNAs. Dev Biol. 1987 Oct;123(2):338–345. doi: 10.1016/0012-1606(87)90392-7. [DOI] [PubMed] [Google Scholar]
- Wistow G. J., Piatigorsky J. Lens crystallins: the evolution and expression of proteins for a highly specialized tissue. Annu Rev Biochem. 1988;57:479–504. doi: 10.1146/annurev.bi.57.070188.002403. [DOI] [PubMed] [Google Scholar]
- Wistow G. Evolution of a protein superfamily: relationships between vertebrate lens crystallins and microorganism dormancy proteins. J Mol Evol. 1990 Feb;30(2):140–145. doi: 10.1007/BF02099940. [DOI] [PubMed] [Google Scholar]
- Wistow G., Summers L., Blundell T. Myxococcus xanthus spore coat protein S may have a similar structure to vertebrate lens beta gamma-crystallins. 1985 Jun 27-Jul 3Nature. 315(6022):771–773. doi: 10.1038/315771a0. [DOI] [PubMed] [Google Scholar]
- Zwaan J., Ikeda A. Macromolecular events during differentiation of the chicken lens. Exp Eye Res. 1968 Apr;7(2):301–311. doi: 10.1016/s0014-4835(68)80081-8. [DOI] [PubMed] [Google Scholar]
- van Leen R. W., van Roozendaal K. E., Lubsen N. H., Schoenmakers J. G. Differential expression of crystallin genes during development of the rat eye lens. Dev Biol. 1987 Apr;120(2):457–464. doi: 10.1016/0012-1606(87)90249-1. [DOI] [PubMed] [Google Scholar]