Abstract
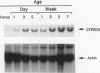
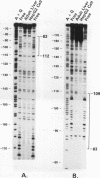
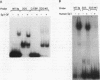
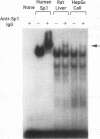
The rat CYP2D5 gene encodes a cytochrome P450 and is expressed in liver cells. Its expression commences a few days after birth, and maximal mRNA levels are achieved when animals reach puberty. Transfection and DNA binding studies were performed to investigate the mechanism controlling developmentally programmed, liver-specific expression of CYP2D5. Transfection studies using a series of CYP2D5 upstream DNA chloramphenicol acetyltransferase gene fusion constructs identified a segment of DNA between nucleotides -55 and -156 that conferred transcriptional activity in HepG2 cells. Activity was markedly increased by cotransfection with a vector expressing C/EBP beta but was unaffected by vectors producing other liver-enriched transcription factors (C/EBP alpha, HNF-1 alpha, and DBP). DNase I footprinting revealed a region protected by both HepG2 and liver cell nuclear extracts between nucleotides -83 and -112. This region displayed some sequence similarity to the Sp1 consensus sequence and was able to bind the Sp1 protein, as assessed by a gel mobility shift assay. The role of Sp1 in CYP2D5 transcription was confirmed by trans activation of the 2D5-CAT construct in Drosophila melanogaster cells by using an Sp1 expression vector. C/EBP beta alone was unable to directly bind the -83 to -112 region of the promoter but was able to produce a ternary complex when combined with HepG2 nuclear extracts or recombinant human Sp1. C/EBP alpha was unable to substitute for C/EBP beta in forming this ternary complex. A poor C/EBP binding site is present adjacent to the Sp1 site, and mutagenesis of this site abolished formation of the ternary complex with the CYP2D5 regulatory region. These result establish that two transcription factors can work in conjunction, possibly by protein-protein interaction, to activate the CYP2D5 gene.
Full text
PDF





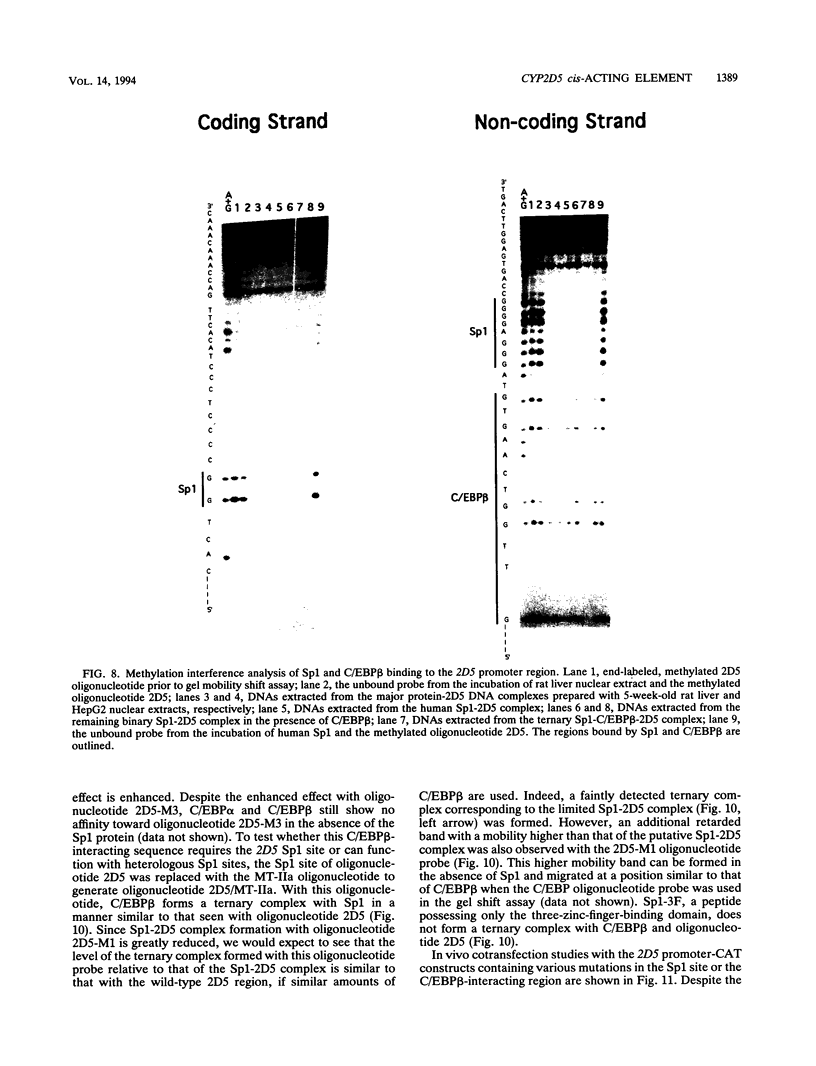
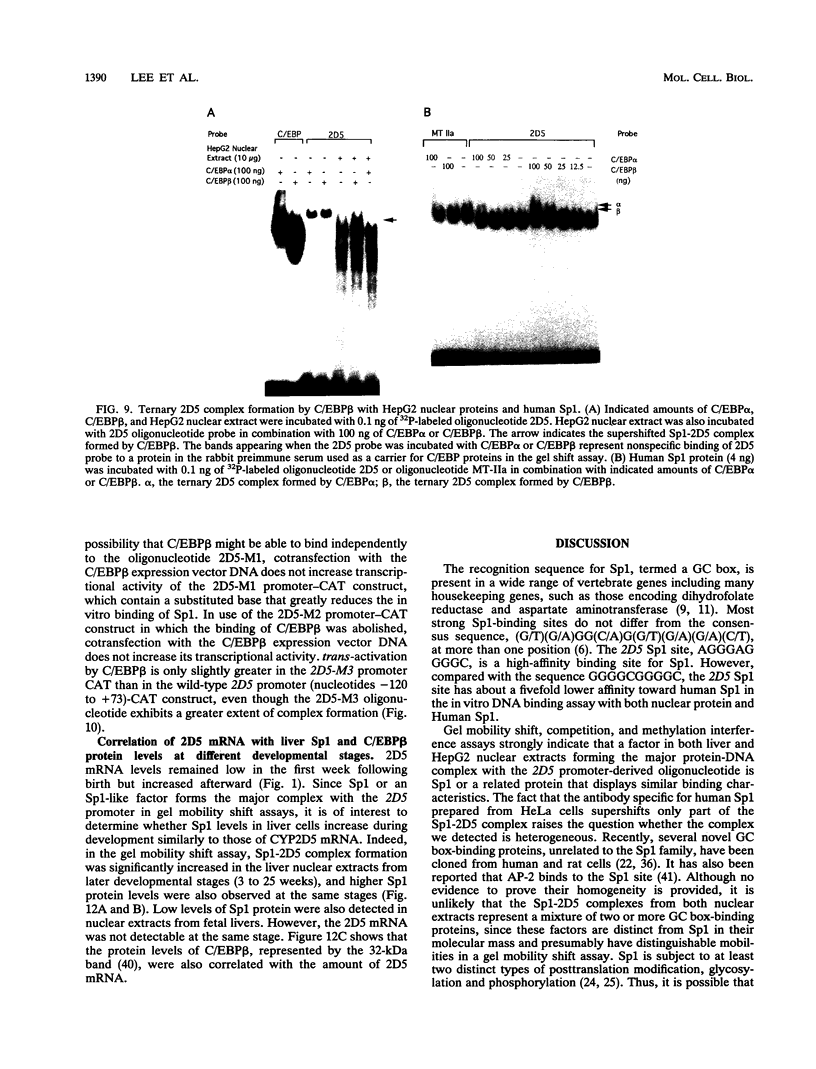

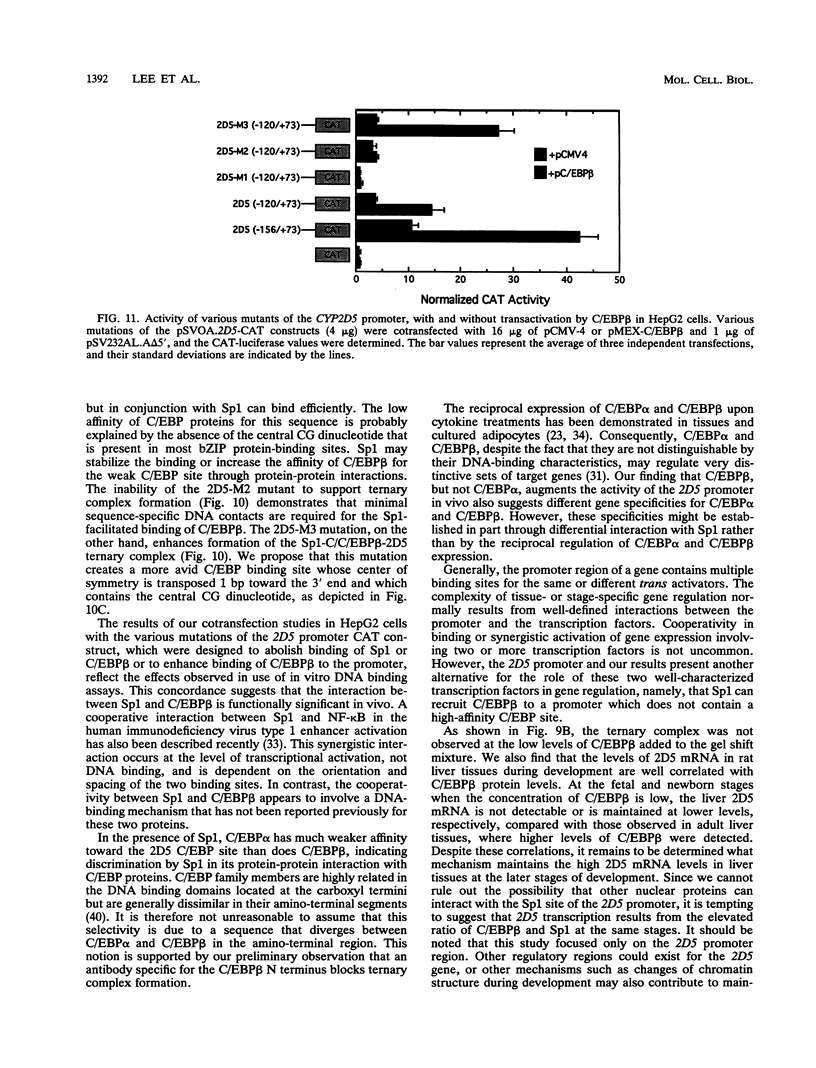


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burger H. J., Schuetz J. D., Schuetz E. G., Guzelian P. S. Paradoxical transcriptional activation of rat liver cytochrome P-450 3A1 by dexamethasone and the antiglucocorticoid pregnenolone 16 alpha-carbonitrile: analysis by transient transfection into primary monolayer cultures of adult rat hepatocytes. Proc Natl Acad Sci U S A. 1992 Mar 15;89(6):2145–2149. doi: 10.1073/pnas.89.6.2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cereghini S., Blumenfeld M., Yaniv M. A liver-specific factor essential for albumin transcription differs between differentiated and dedifferentiated rat hepatoma cells. Genes Dev. 1988 Aug;2(8):957–974. doi: 10.1101/gad.2.8.957. [DOI] [PubMed] [Google Scholar]
- Cereghini S., Raymondjean M., Carranca A. G., Herbomel P., Yaniv M. Factors involved in control of tissue-specific expression of albumin gene. Cell. 1987 Aug 14;50(4):627–638. doi: 10.1016/0092-8674(87)90036-5. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Costa R. H., Grayson D. R., Darnell J. E., Jr Multiple hepatocyte-enriched nuclear factors function in the regulation of transthyretin and alpha 1-antitrypsin genes. Mol Cell Biol. 1989 Apr;9(4):1415–1425. doi: 10.1128/mcb.9.4.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desjarlais J. R., Berg J. M. Redesigning the DNA-binding specificity of a zinc finger protein: a data base-guided approach. Proteins. 1992 Feb;12(2):101–104. doi: 10.1002/prot.340120202. [DOI] [PubMed] [Google Scholar]
- Felgner P. L., Gadek T. R., Holm M., Roman R., Chan H. W., Wenz M., Northrop J. P., Ringold G. M., Danielsen M. Lipofection: a highly efficient, lipid-mediated DNA-transfection procedure. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7413–7417. doi: 10.1073/pnas.84.21.7413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garlatti M., Tchesnokov V., Daheshia M., Feilleux-Duché S., Hanoune J., Aggerbeck M., Barouki R. CCAAT/enhancer-binding protein-related proteins bind to the unusual promoter of the aspartate aminotransferase housekeeping gene. J Biol Chem. 1993 Mar 25;268(9):6567–6574. [PubMed] [Google Scholar]
- Gonzalez F. J. Control of constitutively-expressed developmentally-activated rat hepatic cytochrome P450 genes. Keio J Med. 1992 Jun;41(2):68–75. doi: 10.2302/kjm.41.68. [DOI] [PubMed] [Google Scholar]
- Gonzalez F. J., Liu S. Y., Yano M. Regulation of cytochrome P450 genes: molecular mechanisms. Pharmacogenetics. 1993 Feb;3(1):51–57. doi: 10.1097/00008571-199302000-00006. [DOI] [PubMed] [Google Scholar]
- Gonzalez F. J., Meyer U. A. Molecular genetics of the debrisoquin-sparteine polymorphism. Clin Pharmacol Ther. 1991 Sep;50(3):233–238. doi: 10.1038/clpt.1991.131. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorski K., Carneiro M., Schibler U. Tissue-specific in vitro transcription from the mouse albumin promoter. Cell. 1986 Dec 5;47(5):767–776. doi: 10.1016/0092-8674(86)90519-2. [DOI] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Green S. Receptor-mediated mechanisms of peroxisome proliferators. Biochem Pharmacol. 1992 Feb 4;43(3):393–401. doi: 10.1016/0006-2952(92)90554-v. [DOI] [PubMed] [Google Scholar]
- Guengerich F. P. Enzymatic oxidation of xenobiotic chemicals. Crit Rev Biochem Mol Biol. 1990;25(2):97–153. doi: 10.3109/10409239009090607. [DOI] [PubMed] [Google Scholar]
- Hunt C. M., Guzelian P. S., Molowa D. T., Wright S. A. Regulation of rat hepatic cytochrome P450IIE1 in primary monolayer hepatocyte culture. Xenobiotica. 1991 Dec;21(12):1621–1631. doi: 10.3109/00498259109044410. [DOI] [PubMed] [Google Scholar]
- Imataka H., Sogawa K., Yasumoto K., Kikuchi Y., Sasano K., Kobayashi A., Hayami M., Fujii-Kuriyama Y. Two regulatory proteins that bind to the basic transcription element (BTE), a GC box sequence in the promoter region of the rat P-4501A1 gene. EMBO J. 1992 Oct;11(10):3663–3671. doi: 10.1002/j.1460-2075.1992.tb05451.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isshiki H., Akira S., Tanabe O., Nakajima T., Shimamoto T., Hirano T., Kishimoto T. Constitutive and interleukin-1 (IL-1)-inducible factors interact with the IL-1-responsive element in the IL-6 gene. Mol Cell Biol. 1990 Jun;10(6):2757–2764. doi: 10.1128/mcb.10.6.2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson S. P., MacDonald J. J., Lees-Miller S., Tjian R. GC box binding induces phosphorylation of Sp1 by a DNA-dependent protein kinase. Cell. 1990 Oct 5;63(1):155–165. doi: 10.1016/0092-8674(90)90296-q. [DOI] [PubMed] [Google Scholar]
- Jackson S. P., Tjian R. O-glycosylation of eukaryotic transcription factors: implications for mechanisms of transcriptional regulation. Cell. 1988 Oct 7;55(1):125–133. doi: 10.1016/0092-8674(88)90015-3. [DOI] [PubMed] [Google Scholar]
- Johnson P. F., Landschulz W. H., Graves B. J., McKnight S. L. Identification of a rat liver nuclear protein that binds to the enhancer core element of three animal viruses. Genes Dev. 1987 Apr;1(2):133–146. doi: 10.1101/gad.1.2.133. [DOI] [PubMed] [Google Scholar]
- Jones K. A., Kadonaga J. T., Rosenfeld P. J., Kelly T. J., Tjian R. A cellular DNA-binding protein that activates eukaryotic transcription and DNA replication. Cell. 1987 Jan 16;48(1):79–89. doi: 10.1016/0092-8674(87)90358-8. [DOI] [PubMed] [Google Scholar]
- Kriwacki R. W., Schultz S. C., Steitz T. A., Caradonna J. P. Sequence-specific recognition of DNA by zinc-finger peptides derived from the transcription factor Sp1. Proc Natl Acad Sci U S A. 1992 Oct 15;89(20):9759–9763. doi: 10.1073/pnas.89.20.9759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsunaga E., Gonzalez F. J. Specific cytosine demethylations within the first exons of the rat CYP2D3 and CYP2D5 genes are associated with activation of hepatic gene expression during development. DNA Cell Biol. 1990 Jul-Aug;9(6):443–452. doi: 10.1089/dna.1990.9.443. [DOI] [PubMed] [Google Scholar]
- Matsunaga E., Umeno M., Gonzalez F. J. The rat P450 IID subfamily: complete sequences of four closely linked genes and evidence that gene conversions maintained sequence homogeneity at the heme-binding region of the cytochrome P450 active site. J Mol Evol. 1990 Feb;30(2):155–169. doi: 10.1007/BF02099942. [DOI] [PubMed] [Google Scholar]
- Nebert D. W., Petersen D. D., Fornace A. J., Jr Cellular responses to oxidative stress: the [Ah] gene battery as a paradigm. Environ Health Perspect. 1990 Aug;88:13–25. doi: 10.1289/ehp.908813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins N. D., Edwards N. L., Duckett C. S., Agranoff A. B., Schmid R. M., Nabel G. J. A cooperative interaction between NF-kappa B and Sp1 is required for HIV-1 enhancer activation. EMBO J. 1993 Sep;12(9):3551–3558. doi: 10.1002/j.1460-2075.1993.tb06029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ron D., Brasier A. R., McGehee R. E., Jr, Habener J. F. Tumor necrosis factor-induced reversal of adipocytic phenotype of 3T3-L1 cells is preceded by a loss of nuclear CCAAT/enhancer binding protein (C/EBP). J Clin Invest. 1992 Jan;89(1):223–233. doi: 10.1172/JCI115566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sogawa K., Imataka H., Yamasaki Y., Kusume H., Abe H., Fujii-Kuriyama Y. cDNA cloning and transcriptional properties of a novel GC box-binding protein, BTEB2. Nucleic Acids Res. 1993 Apr 11;21(7):1527–1532. doi: 10.1093/nar/21.7.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno T., Gonzalez F. J. Transcriptional control of the rat hepatic CYP2E1 gene. Mol Cell Biol. 1990 Sep;10(9):4495–4505. doi: 10.1128/mcb.10.9.4495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umek R. M., Friedman A. D., McKnight S. L. CCAAT-enhancer binding protein: a component of a differentiation switch. Science. 1991 Jan 18;251(4991):288–292. doi: 10.1126/science.1987644. [DOI] [PubMed] [Google Scholar]
- Waxman D. J., Azaroff L. Phenobarbital induction of cytochrome P-450 gene expression. Biochem J. 1992 Feb 1;281(Pt 3):577–592. doi: 10.1042/bj2810577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams S. C., Cantwell C. A., Johnson P. F. A family of C/EBP-related proteins capable of forming covalently linked leucine zipper dimers in vitro. Genes Dev. 1991 Sep;5(9):1553–1567. doi: 10.1101/gad.5.9.1553. [DOI] [PubMed] [Google Scholar]
- Williams T., Tjian R. Analysis of the DNA-binding and activation properties of the human transcription factor AP-2. Genes Dev. 1991 Apr;5(4):670–682. doi: 10.1101/gad.5.4.670. [DOI] [PubMed] [Google Scholar]
- Yano M., Falvey E., Gonzalez F. J. Role of the liver-enriched transcription factor DBP in expression of the cytochrome P450 CYP2C6 gene. Mol Cell Biol. 1992 Jun;12(6):2847–2854. doi: 10.1128/mcb.12.6.2847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wet J. R., Wood K. V., DeLuca M., Helinski D. R., Subramani S. Firefly luciferase gene: structure and expression in mammalian cells. Mol Cell Biol. 1987 Feb;7(2):725–737. doi: 10.1128/mcb.7.2.725. [DOI] [PMC free article] [PubMed] [Google Scholar]