Abstract
Aims
Smooth muscle cells (SMC) play an important role in vascular homeostasis and disease. Although adult mesenchymal stem cells (MSC) have been used as a source of contractile SMC, they suffer from limited proliferation potential and culture senescence, particularly when originating from older donors. By comparison, human induced pluripotent stem cells (hiPSC) can provide an unlimited source of functional SMC for autologous cell-based therapies and for creating models of vascular disease. Our goal was to develop an efficient strategy to derive functional, contractile SMC from hiPSC.
Methods and results
We developed a robust, stage-wise, feeder-free strategy for hiPSC differentiation into functional SMC through an intermediate stage of multipotent MSC, which could be coaxed to differentiate into fat, bone, cartilage, and muscle. At this stage, the cells were highly proliferative and displayed higher clonogenic potential and reduced senescence when compared with parental hair follicle mesenchymal stem cells. In addition, when exposed to differentiation medium, the myogenic proteins such as α-smooth muscle actin, calponin, and myosin heavy chain were significantly upregulated and displayed robust fibrillar organization, suggesting the development of a contractile phenotype. Indeed, tissue constructs prepared from these cells exhibited high levels of contractility in response to receptor- and non-receptor-mediated agonists.
Conclusion
We developed an efficient stage-wise strategy that enabled hiPSC differentiation into contractile SMC through an intermediate population of clonogenic and multipotent MSC. The high yield of MSC and SMC derivation suggests that our strategy may facilitate an acquisition of the large numbers of cells required for regenerative medicine or for studying vascular disease pathophysiology.
Keywords: Induced pluripotent stem cells, Mesenchymal stem cells, Smooth muscle cells, 2D differentiation, Vascular contractility, Cellular senescence
1. Introduction
Smooth muscle cells (SMC) comprise the wall of multiple tissues including blood vessels, lymphatic vessels, urinary bladder, uterus, gastrointestinal tract, and respiratory tract. SMC surround the endothelial cell layer on the abluminal side of blood vessels and regulate blood flow by controlling the vessel diameter through contraction or dilation.1 SMC exist in two phenotypic states: synthetic and contractile.1,2 Synthetic SMC are proliferative and non-contractile, while contractile SMC are quiescent cells but contractile.2 The switch from the contractile to the synthetic phenotype has been implicated in the development of several vascular diseases such as intimal hyperplasia, hypertension, and atherosclerosis.1–3
Functional SMC may be useful for cell-based therapies to restore tissue function and/or promote vascular regeneration.4–6 These reasons led researchers to investigate adult stem cells as sources of functional SMC including bone marrow-derived mesenchymal stem cells (BM-MSC),6–8 adipose-derived stem cells,9 umbilical cord-derived MSC,10 muscle-derived stem cells,11 and hair follicle (HF)-derived MSC.12–14 However, the proliferation and differentiation capacity of MSC decrease significantly with donor aging,15 limiting the potential of cells originating from elderly patients, who are mostly in need for cellular therapies. MSC also suffer from culture senescence limiting their culture time to about 8–10 passages and preventing their expandability to the large cell numbers required for cell therapies.
The advent of induced pluripotent stem cells (iPSC) that can be generated from any adult cell in the body has spawned an excitement in the field of regenerative medicine,16,17 because iPSC can provide an inexhaustible source of autologous cells with broad differentiation potential. There is a limited number of studies that investigated differentiation strategies to generate SMC from human (h)iPSC.17–19 In addition, most studies were designed to provide a proof of concept that hiPSC can differentiate into SMC and not necessarily to obtain high efficiency as might be required for regenerative medicine. In this study, we developed a novel monolayer protocol of hiPSC differentiation towards contractile SMC through a multistep process that begins with epithelial-to-mesenchymal transition (EMT), giving rise to an intermediate population of highly proliferative and multipotent MSC that could be monitored and quantified using an novel lentiviral dual-promoter vector (LVDP). In turn, the hiPSC-derived MSC could be coaxed to differentiate into a homogeneous population of highly contractile SMC that may be useful for regenerative medicine or for the development of cellular models of vascular disease.
2. Methods
2.1. hiPSC culture
Human foreskin fibroblast derived iPSC (F-iPSC), generated from Thomson factors (OCT4, SOX2, NANOG, and LIN28), were purchased from WiCell Institute, Madison WI, USA. Human hair follicle MSC derived iPSC (HF-iPSC) were generated from HF-MSC with the four Yamanaka factors (OCT4, SOX2, KLF4, and cMYC). Both hiPSC lines were regularly maintained on mouse embryonic fibroblasts (MEF; Chemicon) in human embryonic stem cell medium (hESM) comprising of DMEM-F12 (Invitrogen), 20% knockout serum replacer (Invitrogen), 1% non-essential amino acid supplements (Invitrogen), 100 μM β-mercaptoethanol (Sigma), and 10 ng per mL basic fibroblast growth factor (bFGF; Invitrogen). hESM was daily changed and hiPSC were passaged every 5–6 days to new MEF feeders.
2.2. Lentivirus production
An lentiviral dual-promoter vector (LVDP) as described elsewhere20 was used to monitor cell fate in this study. The construct is described in more detail in Supplementary material online, Methods.
To generate lentivirus, 293T cells were simultaneously transfected with an LVDP plasmid (30 µg), psPAX2 (25 µg), and pMD2G (6 µg) using the standard calcium phosphate precipitation method. Virus-containing supernatant was harvested for two consecutive days. Subsequently, the supernatant was filtered through 0.45 µm filter, centrifuged at 4°C for 2 h, re-suspended in hESM, and stored in −80°C for further use. The virus titre was determined by transducing 293T cells with serially diluted viral particles using flow cytometry. A multiplicity of infection of 0.1 was used for all experiments.
2.3. Lentiviral transduction of hiPSC
For hiPSC LV transduction optimization on matrigel (growth factor-reduced matrigel, BD), F-iPSC line was used and two conditions were tested: (i) traditional transduction (TT), where hiPSC were plated on matrigel and LV was added next day and (ii) LV/matrigel (LVM) transduction; where LVDP was mixed with matrigel solution and hiPSC were plated afterwards. hiPSC were cultured in the presence of MEF- conditioned medium (CM; hESM conditioned by MEF) plus 10 ng per mL bFGF. The transduction efficiency and fluorescence intensity of transduced cells (see Supplementary material online, Figure S2B and C) were measured by flow cytometry on day 4.
2.4. Fluorescence microscopy and flow cytometry
ZsGreen fluorescence reporter activity was monitored and images were acquired using a Zeiss AxioObserver (Zeiss, Thornwood, NY, USA) inverted fluorescence microscope equipped with an ORCA-ER CCD camera (Hamamatsu, Japan). For flow cytometry, cells were trypsinized, centrifuged, and filtered through 40 μm nylon cell strainer (BD Biosciences, CA, USA) and subsequently resuspended in phosphate buffered saline (PBS), and fluorescence intensity was measured using FACS Calibur (BD Biosciences, CA, USA). Non-transduced hiPSC and hiPSC transduced with LV encoding for ZsGreen or DsRed only were used for gating.
2.5. Immunocytochemistry
Immunocytochemistry and histology were performed as previously described.6,21,22 Briefly, cells were fixed in 4% paraformaldehyde, permeabilized (PBS with 0.1% triton X-100), blocked (1% bovine serum albumin (BSA)/0.01% triton X-100), and incubated with one of the following antibodies: rabbit anti-human Nanog (1:100; 4°C overnight; BD), mouse anti-human SSEA4 (1:100; 4°C overnight; Invitrogen), mouse anti-human αSMA (1:50; 4°C overnight; Serotec, Raleigh, NC, USA), mouse anti-human calponin (1:50; 4°C overnight; Dako, Carpinteria, CA, USA), rabbit anti-human p21 (1:100; 4°C overnight; Cell Signaling, Danvers, MA, USA), rabbit anti-human Ki67 (1:100; 4°C overnight; Abcam, Cambridge, MA, USA), or rabbit anti-human myosin heavy chain (1:100; 4°C overnight; Biomedical Technologies, Stoughton, MA, USA) diluted in blocking buffer. Subsequently, the cells were incubated with Alexa Fluor 488 or 594-conjugated goat anti-mouse or goat anti-rabbit antibodies (1:100; 1 h at RT; Invitrogen) and counter-stained with Hoechst nuclear dye (1:400 in PBS; 10 min; Sigma). Cells that were incubated with secondary antibodies alone served as negative controls.
2.6. Adipogenic, chondrogenic, and osteogenic differentiation
hiPSC-derived MSC were induced to differentiate into osteogenic, chondrogenic, or adipogenic lineages as previously described.21 Briefly, 5 × 103 cells/cm2 were plated in each well of a six-well plate and cultured in medium M231 supplemented with bFGF, epidermal growth factor (EGF), Insulin-like growth factor (IGF), and insulin. Upon reaching confluence, medium was replaced with appropriate induction medium that was replaced every 3 days. Differentiation was assessed using functional assays, i.e. Von kossa, Alcian Blue, and Oil Red O staining for osteogenic, chondrogenic, and adipogenic lineages, respectively. See the Supplementary material online for details.
2.7. RNA isolation, cDNA synthesis, and Reverse transcription polymerase chain reaction (RT-PCR)
Total RNA was isolated using the Promega's RNA isolation kit (Promega Corporation, Madison, WI, USA) as per manufacturer's instructions. One microgram of total RNA was reverse transcribed using superscript III reverse transcriptase (GIBCO) and oligo dT primer (GIBCO). Forward and reverse primer sets used for RT-PCR are summarized in Supplementary material online, Table S1.
2.8. Engineered vascular constructs
Tissue-engineered vascular constructs containing hiPSC-MSC and hiPSC-SMC were fabricated and vascular contractility measurement was done as previously described.6,22–24 Briefly, hiPSC-MSC or hiPSC-SMC were polymerized in thrombin and fibrinogen around a mandrel and cultured in vessel medium for 2 weeks before mechanical properties were examined. For more information see Supplementary material online.
2.9. Contractility and mechanical properties of tissue equivalents
Tissue constructs were mounted on stainless steel hooks connected to a force transducer in an isolated tissue bath. Tissues were equilibrated at a basal tension, contractions were recorded in response to vasoagonists, and mechanical properties were determined. See Supplementary material online for details.
2.10. Clonogenic assay
Clonogenic assays were performed as previously described.12 Briefly, HF-MSC and HF-iPSC-MSC were seeded (10 cells/cm2) in a 100 mm culture dish and cultured for 10 days in DMEM containing 10% MSC-qualified foetal bovine serum (FBS) plus 2 ng per mL bFGF. Subsequently, the cells were fixed with a solution of methanol and acetic acid (3:1 v/v), stained with trypan blue, and photographed using a gel documentation imaging system (UVP, Upland, CA, USA). Images were analysed using ImageJ (NIH, USA) to determine the area and effective diameter of each clone.
2.11. Immunoblotting
Immunoblotting was performed as previously described.6 HF-MSC and HF-iPSC-MSC were lysed, run in a denaturing gel, transferred to membrane, and probed using anti-human p21 antibody. Chemiluminescence was detected using a commercial kit. See Supplementary material online for details.
2.12. Statistical analysis
Data were expressed as mean ± standard deviation and statistical significance (defined as P < 0.05) was determined using Student's t-test.
3. Results
3.1. Stage 1: inducing and monitoring EMT
Two hiPSC lines were used in this study. Foreskin fibroblast-derived hiPSC (F-iPSC; WiCell Institute) that was originally created from foreskin fibroblasts using the four Thomson pluripotency factors (OCT4, NANOG, SOX2, LIN28)25; and hiPSC that were derived from human HF-MSC12,13 using the four Yamanaka factors (OCT4, SOX2, KLF4, and cMYC).26,27 Both hiPSC lines were routinely cultured on MEF feeders and expressed pluripotency markers OCT4A and SSEA4 (see Supplementary material online, Figure S1).
In previous work, we used the promoter of the smooth muscle α-actin gene (ACTA2) to follow the myogenic differentiation of BM- and HF-derived MSC that were then purified from the total MSC population using flow cytometry sorting.6,12,13 We reasoned that the ACTA2 promoter could also be used to monitor hiPSC as they undergo mesenchymal fate transition. To this end, we employed an LVDP construct that we designed in our laboratory20 and encodes for ZsGreen under the ACTA2 promoter and DsRed2 under the constitutive human Phosphoglycerate kinase (hPGK) promoter (ACTA2-LVDP; see Supplementary material online, Figure S2A). Transduced cells are expected to express DsRed constitutively but express ZsGreen only upon ACTA2 promoter activation and therefore, they can be followed in real time using fluorescence microscopy.
In the first step of the differentiation protocol, hiPSC were plated on growth factor reduced matrigel-coated dishes in the presence of MEF-CM, and the next day, they were transduced with LVDP (see Supplementary material online, Figure S2A). Two approaches of lentiviral transduction were tested using a LV encoding for ZsGreen under the human phosphoglycerate kinase (hPGK) promoter to quantify the transduction efficiency and choose the optimum protocol: (i) LVM transduction, where LV was mixed with matrigel and coated on the surface of each dish for 2 h before plating hiPSC to initiate gene transfer; and (ii) TT, where hiPSC were first plated on matrigel-coated dishes, and 24 h later LV was introduced overnight. As measured by flow cytometry, the gene transfer efficiency (% ZsGreen+ cells) was 68.5 ± 6.5 and 80.8 ± 4.0% by the LVM and TT method, respectively (n = 3, P < 0.05; see Supplementary material online, Figure S2B). Similarly, the transgene expression level as determined by the mean fluorescent intensity was significantly higher in TT (571.6 ± 172.5 a.u.) when compared with the LVM method (276.1 ± 95.0 a.u.; n = 3, P < 0.05; see (Supplementary material online, Figure S2C).
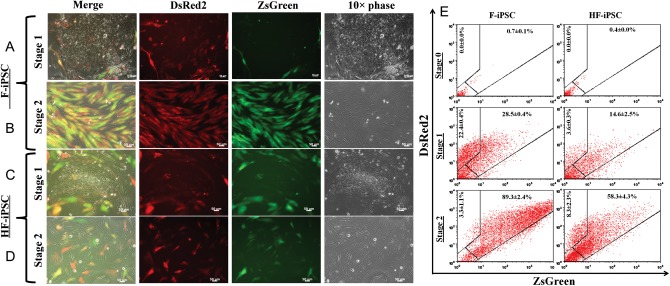
Based on these results, the TT method was employed in all further experiments for hiPSC transduction with the ACTA2-LVDP virus. By day 2 post-transduction, the peripheral cells of F-iPSC and HF-iPSC colonies started to assume fibroblastic morphology and to express ZsGreen (Figure 1A and C). By day 7, 56.1 ± 0.7% of transduced (DsRed2+) F-iPSC and 80.3 ± 2.5% of transduced HF-iPSC were ACTA2-ZsGreen+ (Figure 1E; Stage 1). RT-PCR showed that in Stage 1, cells continued to express pluripotency factors Sox2, Oct4, and Nanog, suggesting the presence of undifferentiated cells in the colonies (Figure 2A and B). At the same time, mesendodermal genes (T, EOMES, MIXL1, and GSC) were upregulated, while genes signifying definitive endoderm (FOXA2 and SOX17) or ectoderm (PAX6, OTX2v1, and SOX1) were absent. In addition, transcription factors regulating EMT (TWIST, SLUG, SNAIL, FOXC2) and EMT-related structural proteins (FSP1, FN1, N-cadherin) were upregulated in Stage 1 F-iPSC and HF-iPSC (Figure 2C and D). In agreement with the presence of pluripotency factors, the epithelial marker E-Cadherin was present in Stage 1 cells, possibly due to the presence of a mixture of differentiated and undifferentiated iPSC.
Figure 1.
The α-actin promoter activity captures iPSC as they undergo mesenchymal transition. (A and C) By day 3 of differentiation (Stage 1), cells in the periphery of F-iPSC (A) and HF-iPSC (C) colonies assumed fibroblastic morphology and started to express ZsGreen, indicating ACTA2 promoter activation. (B and D) In Stage 2, the fraction of ZsGreen expression of F-iPSC (B) and HF-iPSC increased dramatically. Bars, 50 μm. (E) The percentage of double-positive ACTA2-ZsGreen+/DsRed+ cells at the indicated stages of differentiation was measured by flow cytometry. Results are expressed as mean ± SD (n = 3).
Figure 2.
A gene expression profile of hiPSC as they undergo differentiation. (A and B) RT-PCR for pluripotency-associated genes and genes associated with mesendodermal, endodermal, or ectodermal lineages in (A) F-iPSC; (B) HF-iPSC. (C and D) RT-PCR for EMT-associated genes at the indicated stages of hiPSC differentiation.
To ensure that the ACTA2 promoter is not active in pluripotent stem cells, F-iPSC were transduced with ACTA2-LVDP, and colonies-containing DsRed+ cells were picked, dissociated, and re-plated in a new MEF monolayer to generate DsRed-expressing (transduced) F-iPSC clones. Under conditions that maintained pluripotency, none of the DsRed-expressing colonies appeared to express ZsGreen (see Supplementary material online, Figure S3A), indicating the absence of ACTA2 promoter activity in undifferentiated cells. Immunostaining confirmed that OCT4A was expressed in all cells within each colony but ACTA2 was absent, except in the MEFs surrounding each colony (see Supplementary material online, Figure S3B). Taken together, these data suggest that in Stage 1 of differentiation hiPSC undergo EMT that can be monitored by the ACTA2 promoter-driven gene expression.
3.2. Stage 2: enrichment with cells exhibiting MSC phenotype
After 7 days on matrigel, cells were plated on gelatin-coated (0.1%) dishes and cultured in M231 medium containing smooth muscle growth supplement with 5% FBS, 2 ng/mL bFGF, 0.5 ng/mL EGF, 5 ng/mL heparin, and 5 µg/mL insulin (Figure 1B and D). Under these conditions, the fraction of ACTA2-ZsGreen+ cells among the DsRed2+ cells increased from 56.1 ± 0.7% in Stage 1 to 96.4 ± 1.2% in Stage 2 for F-iPSC (n = 3, P < 0.001) and from 80.3 ± 2.5% in Stage 1 to 87.5 ± 3.1% Stage 2 for HF-iPSC (n = 3, P < 0.05; Figure 1E; Stage 2).
At this stage, pluripotency factors Oct4 and Sox2 were significantly decreased and only small levels of Nanog were detectable (Figure 2A and B). Expression of mesendodermal genes (T, EOMS, MIXL1) was also diminished (Figure 2A and B). On the other hand, expression of EMT-related genes continued to be high, while E-cadherin was diminished (Figure 2C and D). Taken together, these data suggest that during Stage 2 of differentiation, our culture was enriched in mesenchymal cells.
We also tested whether type IV collagen, which was previously shown to promote mesodermal28 and vascular lineage differentiation,29,30 could also promote Stage 2 differentiation more efficiently than gelatin. On type IV collagen, the percentage of ACTA2-ZsGreen+/DsRed2 + F-iPSC-derived cells increased to a similar extent as on gelatin, from 56.1 ± 0.7% in Stage 1 to 96.8 ± 1.6% in Stage 2 (n = 3, P < 0.001; see Supplementary material online, Figure S4).
3.3. Stage 2: cells exhibit MSC differentiation potential
Next, we tested the hypothesis that Stage 2 cells might have acquired the characteristics of multipotent MSC. Indeed, flow cytometry showed that Stage 2 cells derived from either F-hiPSC or HF-hiPSC exhibited molecular immunophenotype similar to BM-MSC, expressing high levels of CD73, CD90, CD49b, CD44, CD105 but lacking haematopoietic markers CD34 and CD45 (Figure 3A).
Figure 3.
Stage 2 hiPSC exhibit similar immunophenotype and differentiation potential as BM-MSC. (A) Immunophenotype of Stage 2 differentiated hiPSC and BM-MSC. Flow cytometry with MSC-specific antibodies as indicated. Insets depict control samples stained with IgG alone (n = 3). (B and C) Stage 2 hiPSC were induced to differentiate into mesenchymal lineages. (B) RT-PCR for the indicated genes before and after induction of differentiation. (C) Differentiation was assessed by functional assays as indicated: Von Kossa (osteogenesis), Alcian Blue (chondrogenesis), and Oil Red O (adipogenesis) staining. Non-induced controls are shown in the corresponding insets. Bars, 50 μm.
In addition, Stage 2 hiPSC could be coaxed to differentiate into osteogenic, adipogenic, and chondrogenic lineages as evidenced by RT-PCR and functional assays (Figure 3B and C). Upon osteogenic induction, the cells expressed osteogenic genes RUNX2 and alkaline phosphatase and exhibited high levels of calcium phosphate deposits, as shown by Von Kossa staining. Chondrogenic medium upregulated the SOX9 and Aggrecan (ACAN) genes and induced expression of glycosaminoglycans, as shown by Alcian Blue staining. Finally, adipogenic differentiation medium increased transcription of PPAR-γ (PPARG) and Leptin (LEP) genes and induced the formation of oil droplets, as demonstrated by Oil Red O staining.
3.4. Stage 3: differentiation of hiPSC-MSC into contractile SMC
Next, we hypothesized that hiPSC-derived MSC can be coaxed to differentiate into functional SMC. To this end, we treated hiPSC-MSC with the following combinations: (i) heparin (30 μg/mL); (ii) TGFβ1 (10 ng/mL); (iii) TGFβ1 (10 ng/mL) plus heparin (30 μg/mL); or (iv) TGFβ1 (10 ng/mL) plus insulin (2 μg/mL) for 5 days. All combinations increased expression of myogenic markers αSMA, calponin (CNN1), and caldesmon (CALD), but the combination of heparin and TGFβ1 induced the highest expression of the late SMC differentiation marker, myosin heavy chain (MYH11; see Supplementary material online, Figure S5). Therefore, we chose TGFβ1/heparin to induce hiPSC-MSC differentiation into mature, functional SMC.
RT-PCR analysis showed that expression of SMC-specific genes (ACTA2, MYH11, CNN1, CALD1, and SM22) increased in Stage 2; and the intermediate and late SMC markers, CNN1 and MYH11 increased further in Stage 3 of differentiation (Figure 4A and B). In agreement, immunostaining of undifferentiated hiPSC-MSC displayed diffuse, weak staining for ACTA2, CNN1, and MYH11 (Figure 4C). However, treatment with TGF-β1/heparin for 5 days increased expression and induced filamentous organization of all three proteins (Figure 4D), suggesting that cells in Stage 3 of differentiation assumed mature SMC phenotype.
Figure 4.
Stage 2 cells express SMC-specific genes but only Stage 3 cells express SMC proteins. (A and B) RT-PCR for SMC-specific genes (ACTA2, CNN1, MYH11, SM22, and CALD1) in F-iPSC (A) or HF-iPSC (B) at different stages of differentiation as indicated. (C and D) Immunostaining for ACTA2, CNN1, and MYH11 of Stage 2 (C) or Stage 3 (D) cells.
3.5. Stage 3: hiPSC exhibited strong contractile function
The defining property of mature SMC is their ability to generate force in response to vasoactive agonists. To measure contractile function, we fabricated small-diameter cylindrical tissue constructs by embedding Stage 2 or Stage 3 hiPSC in fibrin hydrogels that were allowed to polymerize around cylindrical mandrels. After 2 weeks of culture in vessel medium [TGFβ1 (2 ng/mL), insulin (2 µg/mL), and ascorbic acid (300 μM)], the tissue constructs were examined histologically and found to contain cells that were distributed uniformly and assumed circumferential alignment (Figure 5A), as previously shown with vascular SMC or SMC derived from HF or BM-MSC.6,12,13,22,24,31
Figure 5.
Stage 3 but not Stage 2 hiPSC are highly contractile SMC. Vascular constructs were generated with Stage 3 hiPSC and vascular contractility was measured in response to receptor-mediated (U46619 and Endothelin-1) and non-receptor-mediated (118 mM KCl) vasoconstrictors. (A) Hematoxylin and eosin stain (H&E) of paraffin sections of vascular constructs with Stage 3 cells showed uniform distribution and circumferential alignment. (B) Contraction force (Pa) of Stage 2- or Stage 3-based vascular constructs in response to vasoagonists as indicated. (C) Representative graphs of isometric contraction in response to U46619. (D) Ultimate tensile stress (UTS) of vascular constructs. Results are expressed as mean ± SD (n = 3). *P < 0.05. Bar, 20 μm.
After 2 weeks, we measured the isometric tension generated by tissue constructs in response to receptor- and non-receptor-mediated agonists using a force transducer as we reported previously.6,12,22 In agreement with immunostaining, Stage 2 cells from F-iPSC or HF-iPSC displayed very weak contractility, but Stage 3 F-hiPSC or HF-hiPSC exhibited robust force generation in response to all three agonists (Figure 5B and C). Specifically, Stage 3 cells generated 8- to 12-fold higher force in response to U46619 (F-iPSC Stage 3: 1807.5 ± 825.5 Pa vs. Stage 2: 222.1 ± 101.9 Pa; n = 3, P < 0.05; HF-iPSC Stage 3: 1465.9 ± 148.5 Pa vs. Stage 2: 118.9 ± 63.4; n = 3, P < 0.05); 5- to 7-fold increase in response to ET1 (F-hiPSC Stage 3: 1001.7 ± 259.7 Pa vs. Stage 2: 179.4 ± 51.1; n = 3, P < 0.05; and HF-iPSC Stage 3: 1076.2 ± 198.6 Pa vs. Stage 2: 147.8 ± 39.1; n = 3, P < 0.05); and 6- to 14-fold increased response to KCl (F-hiPSC Stage 3: 1080.6 ± 578.5 Pa vs. 172.3 ± 69.5, n = 3, P < 0.05; HF-hiPSC Stage 3: 1921.8 ± 808.25 Pa vs. Stage 2: 132.3 ± 48.0; n = 3, P < 0.05). Vascular tissue constructs from parental HF-MSC served as positive control exhibiting similar reactivity as iPSC-SMC (U46619: 983.2 ± 128.5 Pa; ET1: 1155.8 ± 285.3 Pa; and KCl: 823 ± 397.4 Pa).
In addition to vasoactivity, Stage 3 cells generated tissue constructs of significantly higher strength (Figure 5D). Specifically, the ultimate tensile stress (UTS) of tissues prepared from Stage 3 cells was 2- to 2.5 times higher than those from Stage 2 cells (F-hiPSC Stage 3: 259.5 ± 72.6 kPa vs. Stage 2: 108.6 ± 37.8 kPa, n = 3, P < 0.05; HF-iPSC Stage 3: 223.4 ± 58.4 kPa vs. Stage 2: 89.3 ± 9.2 kPa, n = 3, P < 0.05). Taken together, the higher contractility and superior mechanical properties of vascular constructs suggested that Stage 3 but not Stage 2 cells assumed functional characteristics of mature SMC.
3.5.1. HF-iPSC-derived MSC exhibited higher clonogenicity and reduced senescence than parental HF-MSC
Next, we compared the HF-iPSC-derived MSC with their parental HF-MSC in terms of clonogenic potential and senescence. To this end, P14 HF-iPSC-MSC (Stage 2) and P6 parental HF-MSC were seeded at a density of ∼10 cells/cm2 and cultured for 10 days in medium containing 10% MSC-qualified FBS plus 2 ng per mL bFGF. Single-cell-derived clones were imaged and quantified using Image J.
HF-iPSC-derived MSC gave rise to 2.5–4 times the higher number of clones than parental HF-MSC (Figure 6A(i) and (iii)) (>2 mm diameter clones: 11.33 ± 3.05 for HF-iPSC-MSC vs. 4.33 ± 1.52 for parent HF-MSC, P < 0.05; < 2mm diameter clones: 23.67 ± 9.29 for HF-IPSC-MSC vs. 6 ± 2 for HF-MSC, P < 0.05; Figure 6B). In addition, the cell density was significantly higher in HF-iPSC-MSC-derived clones (Figure 6A(ii) and (iv)). Immunostaining showed that significantly higher fraction of HF-iPSC-MSC expressed the proliferation marker Ki67 (HF-iPSC-MSC: 55.67 ± 12.82% vs. HF-MSC: 17.49 ± 4.11%, P < 0.005, n = 10) and significantly smaller fraction expressed the senescence marker p2132 when compared with parental HF-MSC (HF-iPSC-MSC: 15.87 ± 3.87% vs. HF-MSC: 86.53 ± 5.13%, P < 0.005, n = 10; Figure 6C and D).
Figure 6.
HF-iPSC-derived MSC were more proliferative and resistant to senescence than the parental HF-MSC. HF-iPSC-MSC (Stage 2) cells and HF-MSC (parental population) were seeded at clonal density (10 cells/cm2) and cultured for 10 days in medium containing 10% MSC-qualified FBS plus 2 ng per mL bFGF. (A) Images of representative cell culture dishes and colonies. Bar = 1000 μm. (B) The number of colonies larger or smaller than 2 mm in diameter was quantified using Image J. *P < 0.05 between the indicated samples. (C) Immunostaining of HF-iPSC-MSC and HF-MSC for Ki67 or p21 (green); nuclei were counterstained with Hoechst. Bars: 50 μm. (D) The percentage of Ki67+ and p21+ cells was determined from 10 images using Image J. **P < 0.005 between the indicated samples. (E) RT-PCR for the indicated genes. (F) Immunoblotting for p21.
RT-PCR analysis showed that inhibitors of G1/S cell cycle transition such as p2132 and p16INK4a33 were downregulated, while BMI1,33 a repressor of p16INK4a, and G1/S-specific cyclin-D134 were upregulated in HF-iPSC-MSC (Figure 6E). Finally, immunoblotting showed that HF-MSC expressed higher level of p21 protein compared with HF-iPSC-MSC (normalized band intensity for HF-iPSC-MSC: 0.33 ± 0.22 vs. HF-MSC: 0.92 ± 0.21, P < 0.03, n = 3; Figure 6F). Collectively, these results suggest that despite their more advanced passage number (P14) HF-iPSC-derived MSC were more proliferative and less senescent than the parental (P6) HF-MSC.
4. Discussion
Mature contractile SMC make up the vascular media and play an important role in maintaining homeostasis by regulating vessel tone in response to various vasoactive signals.1–3 Their phenotypic switching from contractile to synthetic is implicated in the development of several vascular diseases such as intimal hyperplasia, atherosclerosis, and restenosis.1–3 Although SMC have been derived from multipotent adult stem cells,7–9,13,35–39 iPSC have been investigated less frequently.17–19 These studies provided a proof of concept that hiPSC can differentiate into SMC, but the differentiation strategies employed so far obtained a mixture of many cell types, thereby requiring development of purification procedures to isolate the cell type of interest in this case, SMC.
In the present study, we developed a simple and robust 2D differentiation protocol of SMC differentiation using soluble signals and extracellular matrix molecules but without the use of feeder cells lines (see Supplementary material online, Figure S6). We demonstrated that hiPSC differentiated into contractile phenotype via an intermediate stage with broader MSC differentiation potential. At this intermediate stage, the cells were highly proliferative, exhibited the surface marker profile of MSC, and could differentiate into bone, fat, and cartilage lineages. In addition, they expressed SMC-related genes such as αSMA, CNN1, CALD1, SM22, but did not express the corresponding proteins, except when they were induced to differentiate (Stage 3). Under myogenic differentiation conditions, all myogenic proteins were expressed at high level and most important, they exhibited filamentous organization indicative of contractile SMC phenotype. This multistage strategy of differentiation allowed for expansion of an intermediate cell population, before induction of terminal differentiation into the contractile cells that may be ultimately used to recover the lost tissue function. Finally, this differentiation strategy was validated with two hiPSC lines that were generated using different sets of transcription factors, suggesting that the differentiation pathway may be independent of the pathways that were followed to attain pluripotency.
Under feeder-free conditions, the cells in the periphery of embryonic stem cells colonies were reported to undergo EMT.40–42 In agreement, when plated on matrigel-coated dishes, the peripheral cells of hiPSC colonies assumed fibroblastic morphology and exhibited activated ACTA2 promoter, similar to other epithelial cells undergoing EMT.43–45 Interestingly, the ACTA2 promoter was not active in undifferentiated hiPSC on MEF monolayers, suggesting that activation was likely the result of EMT due to the extracellular matrix (matrigel) and soluble factors (MEF-CM) present in the first step of differentiation. Indeed, as hiPSC cells expressed ZsGreen, they also upregulated EMT-related transcription factors SNAIL,46 SLUG,46 and TWIST47,48 and FOXC2,49 repressed ECAD,46,50 and upregulated mesenchymal markers46 such as NCAD, FSP-1,51,52 vimentin,50 and fibronectin.53 These data suggested that hiPSC underwent EMT that could be monitored and quantified by ACTA2-driven ZsGreen expression.
Stage 1 HF-iPSC did not express the mesodermal marker FSP1 and transiently upregulated OTX2v1 and its downstream mediator Pax6, genes that specify neuroectodermal commitment during development. Subsequent differentiation steps towards the myogenic fate reduced expression of OTX2v1 and Pax6 and increased expression of FSP1. Although the reasons for the differences between HF-iPSC and F-iPSC remain unknown, transient upregulation of OTX2v1 and Pax6 might reflect residual epigenetic memory of the parental HF cells. HF-MSC originate from the mesodermal portion of the hair follicle but they also abundantly express neural markers such as nestin, suggesting that these cells may possess mixed mesenchymal and neuroectodermal potential. Nevertheless, transient expression of these transcription factors did not prevent efficient differentiation of HF-iPSC into the SMC lineage.
Interestingly, while hiPSC-MSC had highly active ACTA2 promoter and expressed SMC-related genes such as αSMA, CNN1, CALD1, MYH11, and SM22 at the mRNA level, immunostaining showed that protein expression was very low, as was myogenic function. Treatment with TGF-β1 and heparin for 5–7 days increased protein expression and filamentous organization of αSMA, CNN1, and MYH11, indicating the development of contractile phenotype. Indeed, cylindrical vascular constructs prepared from Stage 3 cells displayed high levels of contractile function in response to receptor- and non-receptor-mediated agonists, demonstrating the ability to generate force under physiologically relevant conditions. These results suggested that under appropriate differentiation conditions, Stage 3 hiPSC assumed a mature and contractile SMC phenotype that could be used to engineer vascular grafts exhibiting high vascular contractility and improved mechanical properties.
It is interesting to note that HF-iPSC were derived with reprogramming of HF-MSC from a 69-year-old donor, while F-iPSC line were derived from foreskin (neonatal) fibroblasts. Nevertheless, SMC derived from either cell line demonstrated similar level of contractility, suggesting that induced pluripotency might have promoted rejuvenation of aged cells. Indeed, Stage 2 HF-iPSC-MSC could be propagated in culture for at least 40 population doublings without reaching senescence and still maintained the capacity to differentiate into contractile SMC. In addition, HF-iPSC-MSC were more clonogenic and expressed higher levels of cell cycle-promoting genes and lower levels of senescence-inducing genes when compared with their parental counterparts HF-MSC of lower passage. This result suggested that the reprogramming-endowed ‘rejuvenation’ was conveyed to the resulting MSC without compromising the high differentiation potential. Although this result reflects only one donor, it is in agreement with recent reports in the reprogramming literature54–56 and suggests that this strategy could be used to overcome the adverse effects of donor aging on proliferation and myogenic differentiation of adult MSC.15
While the field of reprogramming is still advancing many researchers started to examine iPSC as a model system to understand complex diseases including neurological, haematological, cardiac, and metabolic disorders.57,58 Others started to evaluate iPSC as a source of cells for regenerative medicine applications, primarily as an alternative source to adult stem cells,59 which suffer from limited lifespan due to organismal or culture senescence,15,60 posing significant challenges in engineering functional tissues, especially from the cells of elderly patients. In this regard, use of the differentiated progeny of iPSC in cell therapies requires robust differentiation protocols that yield large numbers of functional cells to replace the lost tissue function. In the current study, we developed a stage-wise strategy to obtain highly functional SMC through an intermediate population of highly proliferative and multipotent MSC that may be useful for studying vascular disease or for regenerative medicine applications.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Conflict of interest: none declared.
Funding
This work was supported by grants from the National Heart and Lung Institute (R01 HL086582) and the New York Stem Cell Science Fund (NYSTEM, Contract# C024316) to S.T.A.
Supplementary Material
References
- 1.Yoshida T, Owens GK. Molecular determinants of vascular smooth muscle cell diversity. Circ Res. 2005;96:280–291. doi: 10.1161/01.RES.0000155951.62152.2e. doi:10.2967/jnumed.108.055954. [DOI] [PubMed] [Google Scholar]
- 2.Beamish JA, He P, Kottke-Marchant K, Marchant RE. Molecular regulation of contractile smooth muscle cell phenotype: implications for vascular tissue engineering. Tissue Eng Part B Rev. 2010;16:467–491. doi: 10.1089/ten.teb.2009.0630. doi:10.1016/S0735-1097(84)80254-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Armulik A, Genove G, Betsholtz C. Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev Cell. 2011;21:193–215. doi: 10.1016/j.devcel.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 4.Yamahara K, Sone M, Itoh H, Yamashita JK, Yurugi-Kobayashi T, Homma K, et al. Augmentation of neovascularization [corrected] in hindlimb ischemia by combined transplantation of human embryonic stem cells-derived endothelial and mural cells. PLoS One. 2008;3:e1666. doi: 10.1371/journal.pone.0001666. doi:10.1016/S0735-1097(00)01191-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferreira LS, Gerecht S, Shieh HF, Watson N, Rupnick MA, Dallabrida SM, et al. Vascular progenitor cells isolated from human embryonic stem cells give rise to endothelial and smooth muscle like cells and form vascular networks in vivo. Circ Res. 2007;101:286–294. doi: 10.1161/CIRCRESAHA.107.150201. doi:10.1016/S0735-1097(02)02863-2. [DOI] [PubMed] [Google Scholar]
- 6.Liu JY, Swartz DD, Peng HF, Gugino SF, Russell JA, Andreadis ST. Functional tissue-engineered blood vessels from bone marrow progenitor cells. Cardiovasc Res. 2007;75:618–628. doi: 10.1016/j.cardiores.2007.04.018. doi:10.1161/01.CIR.85.1.237. [DOI] [PubMed] [Google Scholar]
- 7.Ross JJ, Hong Z, Willenbring B, Zeng L, Isenberg B, Lee EH, et al. Cytokine-induced differentiation of multipotent adult progenitor cells into functional smooth muscle cells. J Clin Invest. 2006;116:3139–3149. doi: 10.1172/JCI28184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gong ZD, Niklason LE. Small-diameter human vessel wall engineered from bone marrow-derived mesenchymal stem cells (hMSCs) FASEB J. 2008;22:1635–1648. doi: 10.1096/fj.07-087924. doi:10.1161/CIRCULATIONAHA.107.743963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rodriguez LV, Alfonso Z, Zhang R, Leung J, Wu B, Ignarro LJ. Clonogenic multipotent stem cells in human adipose tissue differentiate into functional smooth muscle cells. Proc Natl Acad Sci USA. 2006;103:12167–12172. doi: 10.1073/pnas.0604850103. doi:10.2967/jnumed.108.054395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu KH, Zhou B, Lu SH, Feng B, Yang SG, Du WT, et al. In vitro and in vivo differentiation of human umbilical cord derived stem cells into endothelial cells. J Cell Biochem. 2007;100:608–616. doi: 10.1002/jcb.21078. doi:10.2967/jnumed.111.091009. [DOI] [PubMed] [Google Scholar]
- 11.Hwang JH, Yuk SH, Lee JH, Lyoo WS, Ghil SH, Lee SS, et al. Isolation of muscle derived stem cells from rat and its smooth muscle differentiation [corrected] Mol Cells. 2004;17:57–61. doi:10.1016/j.jacc.2011.06.051. [PubMed] [Google Scholar]
- 12.Liu JY, Peng HF, Andreadis ST. Contractile smooth muscle cells derived from hair-follicle stem cells. Cardiovasc Res. 2008;79:24–33. doi: 10.1093/cvr/cvn059. doi:10.1056/NEJMra061889. [DOI] [PubMed] [Google Scholar]
- 13.Liu JY, Peng HF, Gopinath S, Tian J, Andreadis ST. Derivation of functional smooth muscle cells from multipotent human hair follicle mesenchymal stem cells. Tissue Eng Part A. 2010;16:2553–2564. doi: 10.1089/ten.tea.2009.0833. doi:10.1161/hc0202.102119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peng HF, Liu JY, Andreadis ST, Swartz DD. Hair follicle-derived smooth muscle cells and small intestinal submucosa for engineering mechanically robust and vasoreactive vascular media. Tissue Eng Part A. 2011;17:981–990. doi: 10.1089/ten.tea.2010.0109. doi:10.1016/j.amjcard.2007.07.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Han J, Liu JY, Swartz DD, Andreadis ST. Molecular and functional effects of organismal ageing on smooth muscle cells derived from bone marrow mesenchymal stem cells. Cardiovasc Res. 2010;87:147–155. doi: 10.1093/cvr/cvq024. doi:10.1016/j.jtcvs.2006.05.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. doi:10.1093/eurheartj/ehi171. [DOI] [PubMed] [Google Scholar]
- 17.Lee TH, Song SH, Kim KL, Yi JY, Shin GH, Kim JY, et al. Functional recapitulation of smooth muscle cells via induced pluripotent stem cells from human aortic smooth muscle cells. Circ Res. 2010;106:120–128. doi: 10.1161/CIRCRESAHA.109.207902. [DOI] [PubMed] [Google Scholar]
- 18.Cheung C, Bernardo AS, Trotter MW, Pedersen RA, Sinha S. Generation of human vascular smooth muscle subtypes provides insight into embryological origin-dependent disease susceptibility. Nat Biotechnol. 2012;30:165–173. doi: 10.1038/nbt.2107. doi:10.1016/j.jcmg.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taura D, Sone M, Homma K, Oyamada N, Takahashi K, Tamura N, et al. Induction and isolation of vascular cells from human induced pluripotent stem cells–brief report. Arterioscler Thromb Vasc Biol. 2009;29:1100–1103. doi: 10.1161/ATVBAHA.108.182162. doi:10.2967/jnumed.108.055954. [DOI] [PubMed] [Google Scholar]
- 20.Tian J, Andreadis ST. Independent and high-level dual-gene expression in adult stem-progenitor cells from a single lentiviral vector. Gene Ther. 2009;16:874–884. doi: 10.1038/gt.2009.46. doi:10.1016/S0735-1097(84)80254-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bajpai VK, Mistriotis P, Andreadis ST. Clonal multipotency and effect of long-term in vitro expansion on differentiation potential of human hair follicle derived mesenchymal stem cells. Stem Cell Res. 2012;8:74–84. doi: 10.1016/j.scr.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Swartz DD, Russell JA, Andreadis ST. Engineering of fibrin-based functional and implantable small-diameter blood vessels. Am J Physiol Heart Circ Physiol. 2005;288:H1451–H1460. doi: 10.1152/ajpheart.00479.2004. doi:10.1016/S0735-1097(00)01191-8. [DOI] [PubMed] [Google Scholar]
- 23.Liang MS, Andreadis ST. Engineering fibrin-binding TGF-beta1 for sustained signaling and contractile function of MSC based vascular constructs. Biomaterials. 2011;32:8684–8693. doi: 10.1016/j.biomaterials.2011.07.079. doi:10.1016/S0735-1097(02)02863-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yao L, Swartz DD, Gugino SF, Russell JA, Andreadis ST. Fibrin-based tissue-engineered blood vessels: differential effects of biomaterial and culture parameters on mechanical strength and vascular reactivity. Tissue Eng. 2005;11:991–1003. doi: 10.1089/ten.2005.11.991. doi:10.1161/01.CIR.85.1.237. [DOI] [PubMed] [Google Scholar]
- 25.Yu J, Hu K, Smuga-Otto K, Tian S, Stewart R, Slukvin II, et al. Human induced pluripotent stem cells free of vector and transgene sequences. Science. 2009;324:797–801. doi: 10.1126/science.1172482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lu Y, Loh YH, Li H, Ficarro SB, Parikh J, Salomonis N, et al. A self-sustaining feedback loop that regulates proteome diversity and supports self-renewal in pluripotent stem cells. 2012 In review doi:10.1161/CIRCULATIONAHA.107.743963. [Google Scholar]
- 27.Park IH, Zhao R, West JA, Yabuuchi A, Huo H, Ince TA, et al. Reprogramming of human somatic cells to pluripotency with defined factors. Nature. 2008;451:141–146. doi: 10.1038/nature06534. doi:10.2967/jnumed.108.054395. [DOI] [PubMed] [Google Scholar]
- 28.Nishikawa SI, Nishikawa S, Hirashima M, Matsuyoshi N, Kodama H. Progressive lineage analysis by cell sorting and culture identifies FLK1+VE-cadherin+ cells at a diverging point of endothelial and hemopoietic lineages. Development. 1998;125:1747–1757. doi: 10.1242/dev.125.9.1747. doi:10.2967/jnumed.111.091009. [DOI] [PubMed] [Google Scholar]
- 29.Yamashita J, Itoh H, Hirashima M, Ogawa M, Nishikawa S, Yurugi T, et al. Flk1-positive cells derived from embryonic stem cells serve as vascular progenitors. Nature. 2000;408:92–96. doi: 10.1038/35040568. doi:10.1016/j.jacc.2011.06.051. [DOI] [PubMed] [Google Scholar]
- 30.Gerecht-Nir S, Ziskind A, Cohen S, Itskovitz-Eldor J. Human embryonic stem cells as an in vitro model for human vascular development and the induction of vascular differentiation. Lab Invest. 2003;83:1811–1820. doi: 10.1097/01.lab.0000106502.41391.f0. doi:10.1056/NEJMra061889. [DOI] [PubMed] [Google Scholar]
- 31.Barocas VH, Girton TS, Tranquillo RT. Engineered alignment in media equivalents: magnetic prealignment and mandrel compaction. J Biomech Eng. 1998;120:660–666. doi: 10.1115/1.2834759. doi:10.1161/hc0202.102119. [DOI] [PubMed] [Google Scholar]
- 32.Gartel AL, Serfas MS, Tyner AL. p21–negative regulator of the cell cycle. Proc Soc Exp Biol Med. 1996;213:138–149. doi: 10.3181/00379727-213-44046. doi:10.1016/j.amjcard.2007.07.076. [DOI] [PubMed] [Google Scholar]
- 33.Ressler S, Bartkova J, Niederegger H, Bartek J, Scharffetter-Kochanek K, Jansen-Durr P, et al. p16INK4A is a robust in vivo biomarker of cellular aging in human skin. Aging Cell. 2006;5:379–389. doi: 10.1111/j.1474-9726.2006.00231.x. doi:10.1016/j.jtcvs.2006.05.051. [DOI] [PubMed] [Google Scholar]
- 34.Fu M, Wang C, Li Z, Sakamaki T, Pestell RG. Minireview: cyclin D1: normal and abnormal functions. Endocrinology. 2004;145:5439–5447. doi: 10.1210/en.2004-0959. doi:10.1093/eurheartj/ehi171. [DOI] [PubMed] [Google Scholar]
- 35.Yu H, Fang D, Kumar SM, Li L, Nguyen TK, Acs G, et al. Isolation of a novel population of multipotent adult stem cells from human hair follicles. Am J Pathol. 2006;168:1879–1888. doi: 10.2353/ajpath.2006.051170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Harris LJ, Abdollahi H, Zhang P, McIlhenny S, Tulenko TN, Dimuzio PJ. Differentiation of adult stem cells into smooth muscle for vascular tissue engineering. J Surg Res. 2011;168:306–314. doi: 10.1016/j.jss.2009.08.001. doi:10.1016/j.jcmg.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cho SW, Lim SH, Kim IK, Hong YS, Kim SS, Yoo KJ, et al. Small-diameter blood vessels engineered with bone marrow-derived cells. Ann Surg. 2005;241:506–515. doi: 10.1097/01.sla.0000154268.12239.ed. doi:10.2967/jnumed.108.055954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Beltrami AP, Cesselli D, Bergamin N, Marcon P, Rigo S, Puppato E, et al. Multipotent cells can be generated in vitro from several adult human organs (heart, liver, and bone marrow) Blood. 2007;110:3438–3446. doi: 10.1182/blood-2006-11-055566. doi:10.1016/S0735-1097(84)80254-5. [DOI] [PubMed] [Google Scholar]
- 39.Heydarkhan-Hagvall S, Schenke-Layland K, Yang JQ, Heydarkhan S, Xu Y, Zuk PA, et al. Human adipose stem cells: a potential cell source for cardiovascular tissue engineering. Cells Tissues Organs. 2008;187:263–274. doi: 10.1159/000113407. [DOI] [PubMed] [Google Scholar]
- 40.Xu C, Inokuma MS, Denham J, Golds K, Kundu P, Gold JD, et al. Feeder-free growth of undifferentiated human embryonic stem cells. Nat Biotechnol. 2001;19:971–974. doi: 10.1038/nbt1001-971. doi:10.1016/S0735-1097(00)01191-8. [DOI] [PubMed] [Google Scholar]
- 41.Eastham AM, Spencer H, Soncin F, Ritson S, Merry CL, Stern PL, et al. Epithelial-mesenchymal transition events during human embryonic stem cell differentiation. Cancer Res. 2007;67:11254–11262. doi: 10.1158/0008-5472.CAN-07-2253. doi:10.1016/S0735-1097(02)02863-2. [DOI] [PubMed] [Google Scholar]
- 42.Ullmann U, In't Veld P, Gilles C, Sermon K, De Rycke M, Van de Velde H, et al. Epithelial-mesenchymal transition process in human embryonic stem cells cultured in feeder-free conditions. Mol Hum Reprod. 2007;13:21–32. doi: 10.1093/molehr/gal091. doi:10.1161/01.CIR.85.1.237. [DOI] [PubMed] [Google Scholar]
- 43.Bedi S, Vidyasagar A, Djamali A. Epithelial-to-mesenchymal transition and chronic allograft tubulointerstitial fibrosis. Transplant Rev (Orlando) 2008;22:1–5. doi: 10.1016/j.trre.2007.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nakajima Y, Mironov V, Yamagishi T, Nakamura H, Markwald RR. Expression of smooth muscle alpha-actin in mesenchymal cells during formation of avian endocardial cushion tissue: a role for transforming growth factor beta3. Dev Dyn. 1997;209:296–309. doi: 10.1002/(SICI)1097-0177(199707)209:3<296::AID-AJA5>3.0.CO;2-D. doi:10.1161/CIRCULATIONAHA.107.743963. [DOI] [PubMed] [Google Scholar]
- 45.Venkov C, Plieth D, Ni T, Karmaker A, Bian A, George AL, Jr, et al. Transcriptional networks in epithelial-mesenchymal transition. PLoS One. 2011;6:e25354. doi: 10.1371/journal.pone.0025354. doi:10.2967/jnumed.108.054395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Barrallo-Gimeno A, Nieto MA. The Snail genes as inducers of cell movement and survival: implications in development and cancer. Development. 2005;132:3151–3161. doi: 10.1242/dev.01907. doi:10.2967/jnumed.111.091009. [DOI] [PubMed] [Google Scholar]
- 47.Venkov CD, Link AJ, Jennings JL, Plieth D, Inoue T, Nagai K, et al. A proximal activator of transcription in epithelial-mesenchymal transition. J Clin Invest. 2007;117:482–491. doi: 10.1172/JCI29544. doi:10.1016/j.jacc.2011.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kida Y, Asahina K, Teraoka H, Gitelman I, Sato T. Twist relates to tubular epithelial-mesenchymal transition and interstitial fibrogenesis in the obstructed kidney. J Histochem Cytochem. 2007;55:661–673. doi: 10.1369/jhc.6A7157.2007. doi:10.1056/NEJMra061889. [DOI] [PubMed] [Google Scholar]
- 49.Mani SA, Yang J, Brooks M, Schwaninger G, Zhou A, Miura N, et al. Mesenchyme Forkhead 1 (FOXC2) plays a key role in metastasis and is associated with aggressive basal-like breast cancers. Proc Natl Acad Sci USA. 2007;104:10069–10074. doi: 10.1073/pnas.0703900104. doi:10.1161/hc0202.102119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang J, Mani SA, Donaher JL, Ramaswamy S, Itzykson RA, Come C, et al. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell. 2004;117:927–939. doi: 10.1016/j.cell.2004.06.006. doi:10.1016/j.amjcard.2007.07.076. [DOI] [PubMed] [Google Scholar]
- 51.Strutz F, Okada H, Lo CW, Danoff T, Carone RL, Tomaszewski JE, et al. Identification and characterization of a fibroblast marker: FSP1. J Cell Biol. 1995;130:393–405. doi: 10.1083/jcb.130.2.393. doi:10.1016/j.jtcvs.2006.05.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Iwano M, Plieth D, Danoff TM, Xue C, Okada H, Neilson EG. Evidence that fibroblasts derive from epithelium during tissue fibrosis. J Clin Invest. 2002;110:341–350. doi: 10.1172/JCI15518. doi:10.1093/eurheartj/ehi171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Duband JL, Thiery JP. Appearance and distribution of fibronectin during chick embryo gastrulation and neurulation. Dev Biol. 1982;94:337–350. doi: 10.1016/0012-1606(82)90352-9. [DOI] [PubMed] [Google Scholar]
- 54.Lapasset L, Milhavet O, Prieur A, Besnard E, Babled A, Ait-Hamou N, et al. Rejuvenating senescent and centenarian human cells by reprogramming through the pluripotent state. Genes Dev. 2011;25:2248–2253. doi: 10.1101/gad.173922.111. doi:10.1016/j.jcmg.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Suhr ST, Chang EA, Rodriguez RM, Wang K, Ross PJ, Beyhan Z, et al. Telomere dynamics in human cells reprogrammed to pluripotency. PLoS One. 2009;4:e8124. doi: 10.1371/journal.pone.0008124. doi:10.2967/jnumed.108.055954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Suhr ST, Chang EA, Tjong J, Alcasid N, Perkins GA, Goissis MD, et al. Mitochondrial rejuvenation after induced pluripotency. PLoS One. 2010;5:e14095. doi: 10.1371/journal.pone.0014095. doi:10.1016/S0735-1097(84)80254-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Park IH, Arora N, Huo H, Maherali N, Ahfeldt T, Shimamura A, et al. Disease-specific induced pluripotent stem cells. Cell. 2008;134:877–886. doi: 10.1016/j.cell.2008.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Robinton DA, Daley GQ. The promise of induced pluripotent stem cells in research and therapy. Nature. 2012;481:295–305. doi: 10.1038/nature10761. doi:10.1016/S0735-1097(00)01191-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bajpai VK, Andreadis ST. Stem cell sources for vascular tissue engineering and regeneration. Tissue Eng Part B Rev. 2012 doi: 10.1089/ten.teb.2011.0264. ahead of print. doi:10.1089/ten.teb.2011.0264 doi:10.1016/S0735-1097(02)02863-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Poh M, Boyer M, Solan A, Dahl SL, Pedrotty D, Banik SS, et al. Blood vessels engineered from human cells. Lancet. 2005;365:2122–2124. doi: 10.1016/S0140-6736(05)66735-9. doi:10.1161/01.CIR.85.1.237. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.