Background: Polysialic acid (PSA) plays important roles in the developing and adult nervous system.
Results: The interaction of PSA with myristoylated alanine-rich C kinase substrate (MARCKS) at the plasma membrane regulates neurite outgrowth.
Conclusion: The MARCKS/PSA interaction regulates PSA-triggered signal transduction.
Significance: Study of the molecular mechanisms underlying PSA-induced cellular responses helps to understand the functions of PSA in the nervous system.
Keywords: Cell Adhesion, Membrane Bilayer, Neurite Outgrowth, Plasma Membrane, Polysaccharide, MARCKS, NCAM, Polysialic Acid
Abstract
Polysialic acid (PSA) is a homopolymeric glycan that plays crucial roles in the developing and adult nervous system. So far only a few PSA-binding proteins have been identified. Here, we identify myristoylated alanine-rich C kinase substrate (MARCKS) as novel PSA binding partner. Binding assays showed a direct interaction between PSA and a peptide comprising the effector domain of MARCKS (MARCKS-ED). Co-immunoprecipitation of PSA-carrying neural cell adhesion molecule (PSA-NCAM) with MARCKS and co-immunostaining of MARCKS and PSA at the cell membrane of hippocampal neurons confirm the interaction between PSA and MARCKS. Co-localization and an intimate interaction of PSA and MARCKS at the cell surface was seen by confocal microscopy and fluorescence resonance energy transfer (FRET) analysis after the addition of fluorescently labeled PSA or PSA-NCAM to live CHO cells or hippocampal neurons expressing MARCKS as a fusion protein with green fluorescent protein (GFP). Cross-linking experiments showed that extracellularly applied PSA or PSA-NCAM and intracellularly expressed MARCKS-GFP are in close contact, suggesting that PSA and MARCKS interact with each other at the plasma membrane from opposite sides. Insertion of PSA and MARCKS-ED peptide into lipid bilayers from opposite sides alters the electric properties of the bilayer confirming the notion that PSA and the effector domain of MARCKS interact at and/or within the plane of the membrane. The MARCKS-ED peptide abolished PSA-induced enhancement of neurite outgrowth from cultured hippocampal neurons indicating an important functional role for the interaction between MARCKS and PSA in the developing and adult nervous system.
Introduction
PSA3 is a linear homopolymer composed of at least 8 to >100 negatively charged sialic acid (5′-N-acetylneuraminic acid) residues in an unusual α2,8-linkage (1). PSA chains form large negatively charged and highly hydrated helical structures (2) that have been suggested to attenuate cellular interactions and increase cell motility (3). Thus, PSA belongs to a class of functionally important anionic glycans (4). In the mammalian brain, PSA is predominantly attached to the protein backbone of the neural cell adhesion molecule NCAM (4), but it has also been found on neuropilin 2 (5) and SynCam1 (6).
PSA is associated with morphogenetic changes during developmental processes, such as cell migration, axonal growth, neurite branching, and synaptogenesis and persists in the adult brain mainly in structures that display a high degree of functional plasticity (4, 7).
So far only brain-derived neurotrophic factor (8), histone H1 (9), and fibroblast growth factor 2 (10) have been identified as PSA-binding proteins and shown to directly interact with PSA. Here, we identified MARCKS as a novel interaction partner of PSA using an anti-idiotype approach.
MARCKS shows tissue-specific expression with high levels in certain brain regions, such as the hippocampus. It is expressed in the brain and spinal cord from early stages of development (11, 12), and MARCKS-deficient embryos die around birth showing severe abnormalities of the central nervous system (13). Heterozygous mice appear normal but exhibit impaired spatial learning (14). MARCKS is a protein kinase C (PKC)-specific substrate and binds calmodulin and actin (15, 16). MARCKS is a rod-shaped, acidic protein that possesses three highly conserved regions: 1) the N terminus, which contains a consensus sequence for myristoylation that is involved in membrane binding of MARCKS (17), 2) the MH2 domain, which resembles the cytoplasmic tail of the cation-independent mannose-6-phosphate receptor, and 3) the phosphorylation site domain, which contains the PKC phosphorylation sites and the effector domain (18, 19). The effector domain is highly basic, in contrast to the rest of the highly acidic protein, and has been shown to be crucial for the function of MARCKS, whereas myristoylation of MARCKS is not required for many of the in vivo functions of MARCKS as indicated by studies using expression of non-myristoylatable MARCKS in MARCKS-null mice (19). The effector domain mediates the membrane insertion of MARCKS as well as the cross-linking and membrane association of actin filaments (20). The phosphorylation of the effector domain by PKC and/or binding of calmodulin to the effector domain regulates the membrane insertion of MARCKS and the interaction of MARCKS with actin.
In the present study we show that PSA binds directly to the effector domain of MARCKS. We provide evidence that MARCKS and PSA interact at the cell membrane of hippocampal neurons and that the interaction between MARCKS and PSA modulates the neuritogenesis of hippocampal neurons.
EXPERIMENTAL PROCEDURES
Mice
C57BL/6J mice or NCAM-deficient (NCAM−/−) mice (21) that had been back-crossed onto the C57BL/6J background for more than nine generations and their wild-type (NCAM+/+) littermates were bred and maintained at the animal facility of the Universitätsklinikum Hamburg-Eppendorf.
Antibodies and Reagents
Rabbit polyclonal antibody against MARCKS has been described (22). Phospho-MARCKS antibody (sc-12971) recognizing Ser(P)-159/163 was from Santa Cruz Biotechnology (Santa Cruz, CA), and the GFP antibody was from Rockland Immunochemicals (Gilbertsville, PA). Polyclonal NCAM antibody 1β2 against the extracellular domain of NCAM has been described (23). Polyclonal antibody against tubulin was from Covance (Princeton, NJ). Mouse monoclonal IgG2a antibody 735 against PSA (24) and endoglycosidase N (EndoN) were kind gifts from Dr. Rita Gerardy-Schahn (Zentrum Biochemie, Zelluläre Chemie, Medizinische Hochschule, Hannover, Germany). Secondary antibodies were purchased from Dianova (New York). Colominic acid, chondroitin sulfate, and heparin were purchased from Sigma. PSA-mimicking peptide with the sequence NTHTDPYIYPID and a scrambled version of this peptide with the sequence TNYDITPPHDIYC have been described (25). MARCKS-ED peptide (KKKKKRFSFKKSFKLSGFSFKKNKK) and the corresponding control peptide (KKKKKRASAKKSAKLSGASAKKNKK; sequence differences are underlined) as well as two peptides deriving from brain acid soluble protein 1 (BASP1) and comprising putative effector domains similar to that present in MARCKS (BASP1-ED peptide 1, EEKPKDAADGEAKAEEKEADKAAAAKEAPKA; BASP1-ED peptide 2, GGKLSKKKKGYNVNDEKAKDKDKKA) were purchased from Schafer-N (Copenhagen, Denmark).
Polysialylated NCAM (PSA-NCAM) was produced as recombinant PSA-NCAM-Fc containing the extracellular portion of murine NCAM fused with the Fc fragment of human IgG (26, 27).
Fusion constructs of wild-type, non-myristoylatable or non-phosphorylatable MARCKS and the green fluorescent protein (GFP) have been described (17, 28). Non-myristoylatable or non-phosphorylatable MARCKS mutants were obtained by alanine replacement of the N-terminal glycine preventing myristoylation (A2G2 mutant) and by replacement of the four phosphorylatable serines within the phosphorylation site domain by alanine, glycine, asparagine, or aspartic acid (AS, GS, NS, or DS mutants), respectively. The F/A MARCKS-GFP mutant in which the phenylalanine residues in the effector domain are exchanged to alanine residues was generated using the GENEART® site-directed mutagenesis system (Invitrogen, Darmstadt).
Affinity Chromatography with a PSA Mimicking Anti-idiotypic Antibody
Propagation and screening of the phage library as well as selection, purification, and characterization of PSA-mimicking single chain variable fragment (scFv) antibody was performed as described (9). For the preparation of soluble brain proteins, 15 brains from postnatal (0–7 days old) or adult (12–16 weeks old) C57BL/6J mice were homogenized in 4 ml of TNE buffer (25 mm Tris-HCl, pH 7.5, 150 mm NaCl, 5 mm EDTA). This and all following steps were carried out at 4 °C. The homogenates were centrifuged at 1,000 × g for 10 min, and the resulting supernatants were centrifuged at 100,000 × g for 30 min. The supernatants containing soluble brain proteins were first run over a Sepharose 4B column (GE Healthcare) and then over a column with 3.2 mg of purified scFv antibodies immobilized on 2 ml of CNBr-activated Sepharose 4B (GE Healthcare) overnight at a flow rate of 0.2 ml/min. After washing the immunoaffinity column with 15 ml of PBS (0.3 ml/min), bound proteins were eluted with 200 mm glycine, 500 mm NaCl, pH 2.7 (0.3 ml/min). The eluted fractions were collected, neutralized immediately with 1 m Tris/HCl, pH 8.8, and subjected to protein precipitation (29).
Western Blot Analysis, Two-dimensional Gel Electrophoresis, Silver Staining, and Mass Spectrometry
Western blot analysis, silver staining, and mass spectrometry were performed as described (30). For two-dimensional gel electrophoresis, the protein precipitate of the eluate was resuspended in 5 m urea, 2 m thiourea, 65 mm dithiothreitol, 4% CHAPS, and 0.8% AmpholineTM pH3–10 (GE Healthcare) and incubated with ImmobilineTM DryStripe pH3–10 (GE Healthcare) overnight at room temperature. A Multiphor II apparatus (GE Healthcare) was used for isoelectric focusing at 17 °C and 2 mA applying the following protocol: 1 min at 200 V, continuous increase of voltage to 3500 V within 90 min, 3500 V for 60 min. After isoelectric focusing, the stripes were incubated two times for 10 min in equilibration buffer composed of 15 ml of 1 m Tris/HCl, pH 6.8, 36 g of urea, 30 ml of 87% glycerol, 100 mg of dithiothreitol, 15 ml of 10% SDS, 14.5 ml of H2O, and a spatula tip of bromphenol blue and then subjected to SDS-PAGE.
ELISA and Label-free Binding Assay
For ELISA, 384-well flat bottom microtiter plates with high binding surface (Corning, Lowell, MA) were coated in triplicate with 25 μl of MARCKS-ED or control peptide (100 μg/ml in PBS) or PBS alone at 4 °C overnight. All of the following steps were carried out at room temperature. After blocking with 1% BSA for 1 h, the wells were washed once with PBS containing 0.05% Tween 20 (PBST). Wells were incubated with increasing concentrations of soluble colominic acid for 1 h. After washing 3 times with PBST and once with PBS, 25 μl of PSA antibody 735 (5 μg/ml) were added to each well. After incubation for 1 h, the plates were washed 3 times with PBST. The wells were incubated with horseradish peroxidase (HRP)-conjugated secondary mouse antibody for 1 h and washed 3 times with PBST. Binding was visualized by incubating the wells with 50 μl of 0.5 mg/ml o-phenylenediamine dihydrochloride (Thermo Scientific, Waltham, MA) for 2–5 min. The reaction was stopped by the addition of 50 μl of 2.5 m H2SO4 and quantified using an ELISA reader at 490 nm and the software KCjunior (Bio-TEK, Winooski, VT).
As an alternative to ELISA, a label-free binding assay with photonic crystal optical biosensors (SRU Biosystems, Woburn, MA), which allows detection of interactions without labeling, was performed as described (31). Briefly, 384-well plates with a TiO2 surface (SRU Biosystems) were washed 3 times with PBS containing Ca2+ and Mg2+ (PAA, Cölbe, Germany) (PBS+) and coated overnight at 4 °C with 125 ng peptide/well. Wells were then washed 3 times with PBS+, and the peak wavelength shift was measured (BIND® PROFILER, SRU Biosystems) to control or to measure the coating efficiency. Wells were then blocked with 2% BSA in PBS+ for 3 h at room temperature. After three washes with PBS+, different amounts of ligands in PBS+ were added to the wells. Binding of ligands to substrate-coated peptide was measured by determination of peak wavelength shift every 30 s for 60 min. Values obtained from wells without ligands were set as background and subtracted from values obtained with ligand solution. All interactions were performed in triplicates.
Co-immunoprecipitation
Brains from 2-day-old C57BL/6J mice were homogenized in 50 mm Tris-HCl, pH 7.5, 1 mm CaCl2, 1 mm MgCl2, and 1 mm NaHCO3 plus complete EDTA-free protease inhibitor mixture (Roche Diagnostics). Brain protein extracts were generated by lysing brain homogenates with RIPA buffer containing 50 mm Tris-HCl, pH 7.5, 150 mm NaCl, 1% Nonidet P-40, 1 mm Na4P2O4, 1 mm NaF, 1 mm EDTA, 2 mm Na3VO4, and 1 mm PMSF for 1 h at 4 °C. After centrifugation at 700 × g for 10 min at 4 °C, the resulting supernatant was taken as detergent extract for immunoprecipitation. In parallel, supernatant containing 1 mg of total protein was incubated without or with 5 μg of EndoN at 37 °C for 3 h. EndoN-treated and untreated supernatants were then precleared with protein A/G-agarose beads (Santa Cruz Biotechnology) for 3 h at 4 °C and then incubated with polyclonal MARCKS antibody or control IgG overnight at 4 °C with gentle rotation. Protein A/G-agarose was added and incubated 6 h at 4 °C under constant agitation. After extensive washes with RIPA buffer, immunoprecipitated proteins were eluted from agarose beads by 2× SDS sample buffer (125 mm Tris-HCl, 4% SDS, 30% glycerol, 10% β-mercaptoethanol, and 0.00625% bromphenol blue, pH 6.8).
Immunostaining of Hippocampal Neurons
The hippocampi of 0–2-day-old C57BL/6J mice were isolated and dissociated using the method published previously (32). Dissociated hippocampal neurons were seeded at a density of 2 × 105 on 12-mm glass coverslips pretreated with 0.01% poly-l-lysine (PLL) and maintained for 20 h at 37 °C. For live staining of PSA and MARCKS, cells were washed 3 times with serum-free culture medium and incubated with antibodies against PSA and MARCKS for 20 min on ice. Cells were then gently washed with serum-free culture medium, fixed with 4% paraformaldehyde for 30 min, washed 3 times with PBS, and blocked with 1% BSA in PBS for 30 min at room temperature. Fixed cells were then incubated with secondary antibodies coupled to the fluorescent dyes Cy3 or Cy2 for 20 min, stained with DAPI for 2 min, washed 3 times with PBS, and mounted with Fluoromount-G (Southern Biotech, Birmingham, AL). For staining of fixed cells, hippocampal neurons were treated with 4% paraformaldehyde for 30 min, washed with PBS, blocked, and permeabilized with PBS containing 0.2% Tween 20 and 1% BSA for 30 min and incubated with primary antibodies for 30 min at room temperature. After washing with PBS, cells were incubated with secondary antibodies coupled to the fluorescent dye Cy2 or Cy3 for 30 min at room temperature, washed 3 times with PBS, stained with DAPI for 2 min, washed 3 times with PBS, and then mounted with Fluoromount-G. For co-staining of PSA, MARCKS, and actin, live neurons were incubated with the PSA antibody for 20 min on ice, fixed, incubated with MARCKS antibody for 30 min at room temperature followed by incubation with secondary antibodies coupled to the fluorescent dyes Cy2 or Cy5 and with Alexa Fluor® 594 phalloidin (Invitrogen) for 20 min at room temperature in the dark. Images were taken with an Olympus FluoView 1000 microscope (Olympus, Hamburg, Germany).
Stimulation and Transfection of Hippocampal Neurons and Neurite Outgrowth Measurements
For transient transfection of hippocampal neurons, Basic Neuron small cell number (SCN) Nucleofector® kit was used according to the manufacturer's instructions (Amaxa Biosystems, Cologne, Germany). In brief, 100 nm peptides (titration tests indicated 100 nm as optimal concentration for transfection) were dissolved in 10 μl of nucleofector solution equilibrated to room temperature. Four to six million cells were suspended in 90 μl of nucleofector solution and mixed with 10 μl of peptide-containing solution. After electroporation in the Nucleofector® device (Amaxa Biosystems) applying Basic Neuron SCN Program 7, the neurons were transferred from the cuvette into tubes with 0.5–1 ml of prewarmed neurobasal medium (Invitrogen) using the plastic pipettes supplied in the kit. The neurobasal medium was supplemented with 10% horse serum (Sigma) and 5 mg/ml gentamycin (Invitrogen). Cells were counted using trypan blue (Sigma) and plated on glass coverslips coated with 100 μg/ml PLL at a density of 300 or 1500 cells/mm2. Neurons were maintained for 24 h at 37 °C in the absence or presence of 10 μg/ml colominic acid, chondroitin sulfate, or heparin. For EndoN treatment, neurons were treated with 5 μg/ml EndoN for 3 h. For determination of the levels of phosphorylated MARCKS, neurons were incubated without or with 20 μg/ml colominic acid or chondroitin sulfate for 3 h, lysed in RIPA buffer supplemented with phosphatase inhibitor PhosStopTM (Roche Diagnostics), and kinase inhibitor K262a (Tocris Bioscience, Ellisville, MO), and subjected to Western blot analysis. For quantification of the level of phosphorylated MARCKS by confocal microscopy, neurons were treated without or with 20 μg/ml colominic acid or chondroitin sulfate for 3 h, fixed, and subjected to immunostaining using the phospho-MARCKS antibody and confocal microscopy followed by determination of the fluorescence intensities of 10 neurons per group using ImageJ software. Statistical comparisons between the groups were performed using Student's t test. For neurite outgrowth measurements, neurons were incubated with 20 μg/ml colominic acid, chondroitin sulfate or heparin, and/or with 10 μg/ml MARCKS-ED or control peptide, fixed with 2.5% glutaraldehyde, and stained with 1% methylene blue/toluidine blue in 1% borax. Morphological quantification of neurite lengths was performed as described (30). Neurites of hippocampal neurons with a length of at least one cell body diameter were counted, and total neurite length per cell was determined by counting 100 cells in each of two wells per experiment. Differences between the groups were statistically evaluated using Student's t test followed by one-way analysis of variance.
Conjugation of Fluorescent Dyes to Colominic Acid and Chondroitin Sulfate
For coupling of colominic acid or chondroitin sulfate to aminomethylcoumarin hydrazide (Sigma) or HiLyte FluorTM 405 hydrazide (MoBiTec, Göttingen, Germany), 15 mg of colominic acid or chondroitin sulfate were incubated with 1.5 ml of 100 mm sodium metaperiodate for 15 min and then with 1 ml of ethylene glycol for 1 h. The oxidized carbohydrates were precipitated by 3.75 ml of ethanol and collected by centrifugation for 15 min at 4000 × g and 4 °C. After resuspension of the carbohydrates in water (3 mg/ml), 100 μl of 2 mm aminomethylcoumarin hydrazide or HiLyte FluorTM 405 hydrazide in dimethyl formamide were added to 1 ml of oxidized carbohydrates. After incubation for 2 h, the carbohydrates conjugated to the dyes were precipitated by 1.5 ml of ethanol and centrifugation for 15 min at 4000 × g and 4 °C. The conjugates were resuspended in PBS (final concentration, 30 mg/ml). For preparation of fluorescently labeled PSA-NCAM, 100 μg of recombinant PSA-NCAM-Fc were subjected to mild periodate oxidation, incubation with HiLyte FluorTM 405 hydrazide, and dialysis against PBS. Mild periodate oxidation leaves the glycans intact because oxidation affects only the non-reducing end of the glycans.
Transfection of Cells with MARCKS-GFP Constructs and FRET Analysis
CHO cells (2 × 105 cells/ml) or primary hippocampal neurons (5 × 105 cells/ml) were seeded onto PLL coated glass coverslips. CHO cells were transfected using FuGENE® HD Transfection Reagent (Roche Diagnostics) or TurboFect (Thermo Scientific) 1 day after seeding. Hippocampal neurons were transfected using the CalPhosTM mammalian transfection kit (Clontech, Mountain View, CA) 2 days after seeding. The dye/carbohydrate conjugates were added to the cells 24 h after transfection. After incubation for 15 min, the coverslips were transferred to a plastic dish containing 3 ml of prewarmed medium. During the live imaging the cells were maintained in an incubation chamber at 37 °C, 5% CO2, and 70% humidity. FRET analysis was performed using the sensitized emission method at the confocal laser scanning microscope Olympus FluoView1000 (Olympus). The donor was the HiLyte FluorTM 405, and the acceptor was the GFP of the MARCKS fusion proteins.
Cross-linking Experiments Using l-Photo-Leucine
CHO cells (5 × 105 cells per 6-well) transfected with MARCKS constructs were maintained in DMEM-LM (Thermo Scientific) supplemented with 1% penicillin, 1% streptomycin, 4 mm l-Photo-Leucine (Thermo Scientific) and 2 mm methionine (Sigma). After 24 h, 10 μg/ml colominic acid/PSA or PSA-NCAM-Fc was added to the cells and incubated for 5 min at 37 °C. Cells were then exposed to UV light (365 nm, 3 × 15 watts, 5-cm distance) and lysed in 500 μl of RIPA buffer with protease inhibitor mixture (complete, EDTA-free; Roche Diagnostics). Cell lysates were subjected to immunoprecipitation using GFP or PSA antibodies and/or Protein A beads.
Lipid Bilayer Technology
For the formation and analysis of artificial lipid bilayers, the Ionovation Compact V02 system (Ionovation GmbH, Osnabrück, Germany) was used. In this system two chambers are separated by a Teflon septum with a 120-μm pinhole where bilayers are formed upon repetitive lowering and raising the level of a lipid mixture in one chamber. In all experiments, a 1:1 mixture of 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC) and 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoethanolamine (POPE) from Ionovation was used. Both chambers were filled with 1.4 ml of electrode buffer (250 mm KCl, 2 mm CaCl2, 10 mm Tris/MOPS, pH 7) from Ionovation. The bilayer formation was monitored optically and by measuring the capacitance (a stable bilayer displays a capacitance of 60–80 picofarads). After a stable bilayer had established, 3 μm colominic acid/PSA or chondroitin sulfate was added into the anode chamber (representing the extracellular compartment), and then 3 μm MARCKS-ED peptide or control peptide was applied to the cathode chamber (representing the intracellular compartment). During the experiments the capacitance of the bilayer was recorded.
RESULTS
MARCKS and BASP1 Are Potential PSA Binding Partners
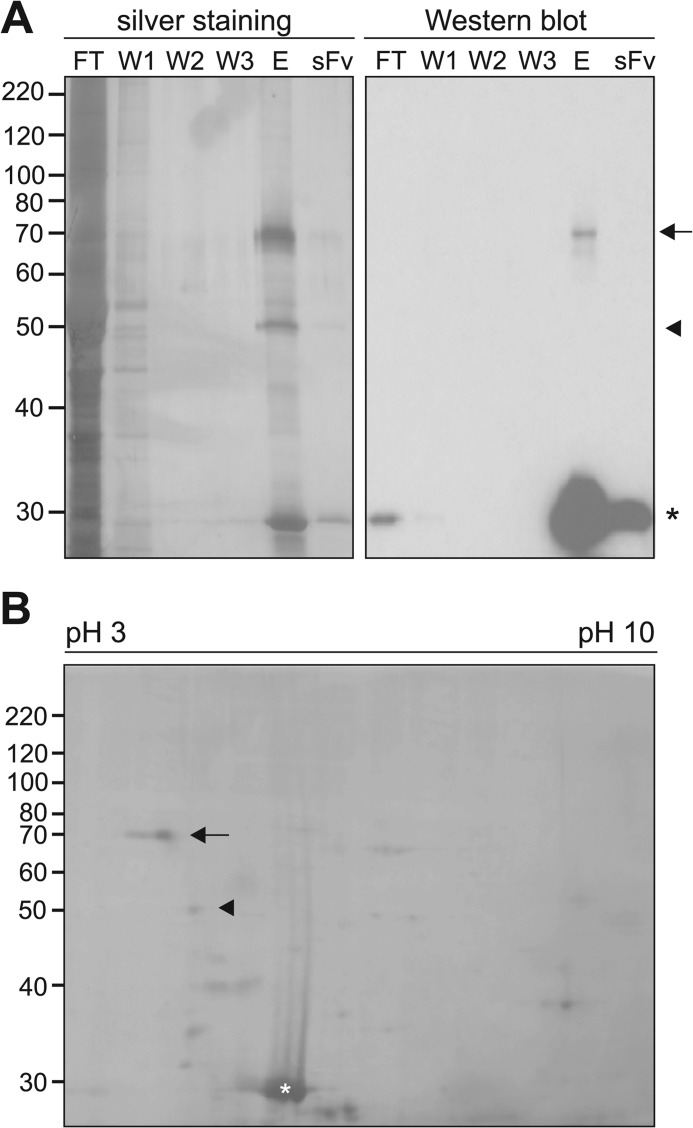
In a recent study we used a PSA-mimicking anti-idiotypic scFv antibody for immunoaffinity chromatography with a mouse brain fraction enriched in membrane-associated proteins and identified histone H1 as a novel PSA binding partner (9). Here, we applied soluble brain proteins isolated from postnatal or adult mice to the immobilized PSA-mimicking scFv antibody. By silver staining upon SDS-PAGE, mainly two proteins of ∼50 and ∼70 kDa were detected in the eluate obtained after immunoaffinity chromatography of soluble brain proteins from postnatal mice (Fig. 1A) and adult mice (data not shown). These proteins were not found in the wash fractions or after separation of the scFv antibody, which was seen as an ∼30-kDa band, and comparison with the flow-through showed that these proteins were highly enriched by immunoaffinity chromatography (Fig. 1A). By Western blot analysis with the scFv antibody, only the 70-kDa protein was detectable in the eluate, whereas no immunopositive protein band was observed in the flow-through, the wash fractions, or scFv preparation (Fig. 1A). The ∼50- and ∼70-kDa bands were analyzed by ESI-MS/MS mass spectrometry. After tryptic digestion of the ∼50- and ∼70-kDa bands, 2 of the detected peptides from the ∼70-kDa band and 3 of the detected peptides from the ∼50-kDa band could be assigned to MARCKS and BASP1, respectively. The MS/MS spectrum of a 1813.958- and 784.411-Da precursor mass (detected as triple- and double-charged ion at m/z = 605.660 and 393.213) matched the tryptic peptide GEATAERPGEAAVASSPSK (1814.887 Da) and LSGFSFK (785.419 Da) of mouse MARCKS, whereas the MS/MS spectrum of a 3354.370-, 3296.370-, and 2057.018-Da precursor mass (detected as 4-fold, 4-fold, or triple-charged ion at m/z = 839.600, 825.100, or 686.680, respectively) matched the tryptic peptide AGEASAESTGAADGAAPEEGEAKKTEAPAAAGPEAK (3355.577 Da), SDAAPAASDSKPSSAEPAPSSKETPAASEAPSSAAK (3297.535 Da), and SDAAPAASDSKPSSAEPAPSSK (2057.962 Da) of mouse BASP1. Separation of the eluate by two-dimensional gel electrophoresis and silver staining of the two-dimensional gel showed an intense staining of a ∼70-kDa spot with a pI of ∼4.2 and a faint staining of a ∼50-kDa spot with a pI of ∼4.5 (Fig. 1B). Because the pI values of the ∼70- and ∼50-kDa spots matched the theoretical pI of MARCKS and BASP1, which are 4.34 and 4.5, the result of the two-dimensional gel electrophoresis confirmed that the ∼50- and ∼70-kDa bands represented BASP1 and MARCKS.
FIGURE 1.
Identification of MARCKS and BASP1 as PSA binding partners using a PSA-mimicking scFv antibody for affinity chromatography. A, purified scFv antibody was immobilized and used for affinity chromatography with soluble brain proteins isolated from postnatal (0–7 days old) mice. After collecting the flow-through (FT) and three consecutive wash fractions (W1, W2, W3), proteins bound to the scFv antibody were eluted. The eluate (E), the flow-through, the wash fractions, and the purified scFv antibody (sFv) were separated by SDS-PAGE and subjected to silver staining or Western blot analysis using the scFv antibody. B, the eluate was separated by two-dimensional gel electrophoresis, pH 3–10, and the two-dimensional gel was subjected to silver staining. A and B, the position of the ∼70- and ∼50-kDa protein bands, which were identified by mass spectrometry as MARCKS and BASP1, are indicated by an arrow and arrowhead, respectively. The asterisk indicates the position of the scFv antibody.
Because the PSA-mimicking scFv antibody binds to the immunoaffinity-purified ∼70-kDa protein, which was identified as MARCKS, but not to the ∼50-kDa protein, which was also present in the eluate after immunoaffinity chromatography and was identified as BASP1, one can conclude that MARCKS binds to PSA in a native and denatured conformation, whereas BASP1 either does not bind to PSA but was co-purified with MARCKS or only binds PSA in a native but not denatured conformation.
PSA Directly Binds to the Effector Domain of MARCKS
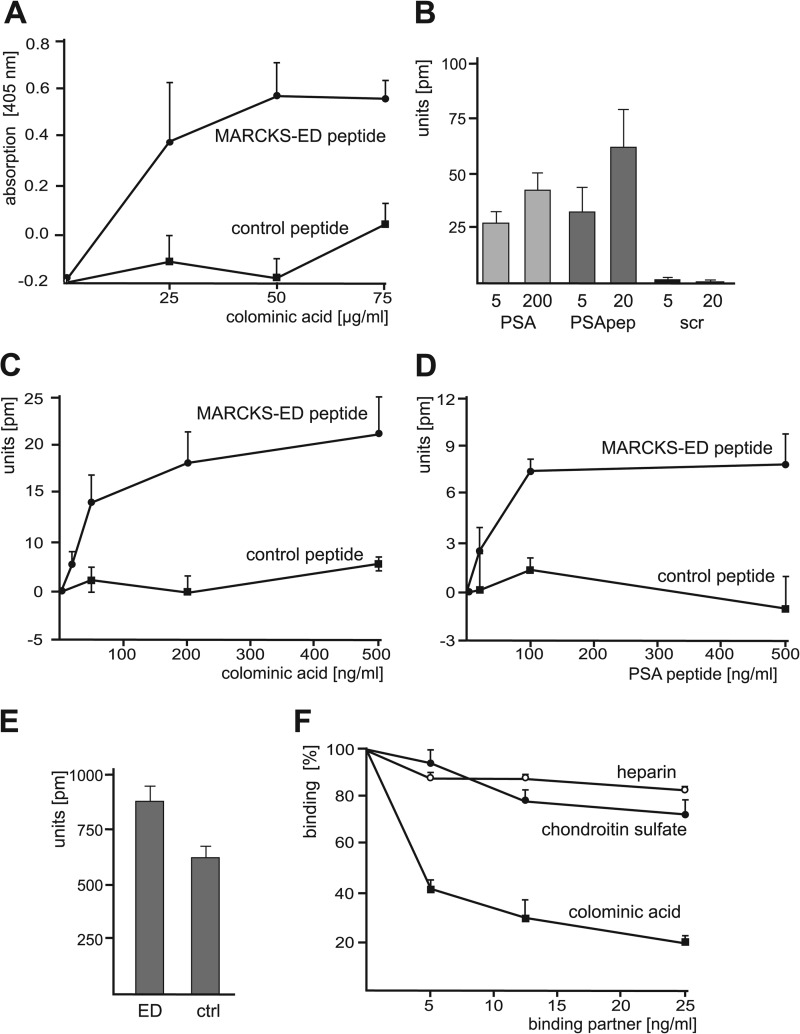
To analyze the direct binding of MARCKS or BASP1 to PSA, ELISA experiments were performed. Using recombinant MARCKS and BASP1 as the substrate coat, no binding of colominic acid, which is the bacterial homolog of PSA, to MARCKS and BASP1 was detectable (data not shown). Because the effector domain of MARCKS is crucial for the function of MARCKS, we tested a synthetic peptide that comprises the effector domain of MARCKS (MARCKS-ED peptide) and a corresponding control peptide as substrate coats and observed a concentration-dependent and saturable binding of colominic acid/PSA to the MARCKS-ED peptide but not to the control peptide (Fig. 2A).
FIGURE 2.
Identification of a direct binding of PSA to the MARCKS effector domain. A, MARCKS-ED peptide and the corresponding control peptide as negative control were substrate-coated and incubated with increasing concentrations of colominic acid/PSA. The binding of colominic acid/PSA was detected by ELISA using PSA antibody and HRP-conjugated secondary antibodies. Mean values ± S.D. from three independent experiments carried out in triplicate are shown. B, MARCKS-ED and control peptide were substrate-coated and incubated with 5 or 200 ng/ml colominic acid/PSA (PSA), PSA-mimicking peptide (PSApep), or a scrambled version of the PSA-mimicking peptide (scr). C and D, substrate-coated MARCKS-ED peptide or control peptide was incubated with increasing amounts of colominic acid/PSA (C) or PSA-mimicking peptide (D). B—D, binding was determined by measuring the peak wavelength shifts in a label-free binding assay. E, coating efficiency of MARCKS-ED peptide (ED) or control peptide (ctrl) was determined by measuring the peak wavelength shifts. F, MARCKS-ED and control peptide were substrate-coated and incubated with PSA-mimicking peptide in the absence or presence of increasing concentrations of colominic acid/PSA, chondroitin sulfate, or heparin. Binding was determined by measuring the peak wavelength shifts. B–F, mean values ± S.D. from of two independent experiments carried out in triplicates are shown.
We also used two synthetic BASP1 peptides as substrate coats. These peptides have similar structural features like the effector domain of MARCKS, e.g. amphipathic helical structure with positively charged amino acids on one side and hydrophobic on the other side, and thus might function as effector domains. However, in contrast to the MARCKS-ED peptide, no binding of colominic acid/PSA to the putative BASP1-ED peptides was detectable (data not shown). The results from the ELISA experiments indicated that PSA directly interacts with the effector domain of MARCKS, whereas no direct binding of BASP1 or putative BASP1-ED peptides was detectable.
In a label-free binding assay, binding of colominic acid/PSA to substrate-coated MARCKS-ED peptide was confirmed (Fig. 2B). In addition, we could also show that a PSA-mimicking peptide (25) binds to MARCKS-ED peptide, whereas a scrambled version of the PSA-mimicking peptide did not bind the MARCKS-ED peptide (Fig. 2B). A concentration-dependent binding of colominic acid/PSA and PSA-mimicking peptide to MARCKS-ED peptide, but not to control peptide, was observed in label-free binding assay (Fig. 2, C and D) when using substrate-coated MARCKS-ED and control peptides, which showed similar coating efficiencies (Fig. 2E). The binding of the PSA-mimicking peptide to substrate-coated MARCKS-ED peptide was inhibited in a concentration-dependent manner by colominic acid/PSA, but not by other negatively charged polymers, namely chondroitin sulfate and heparin (Fig. 2F).
MARCKS Interacts with PSA-NCAM via Its PSA Moiety
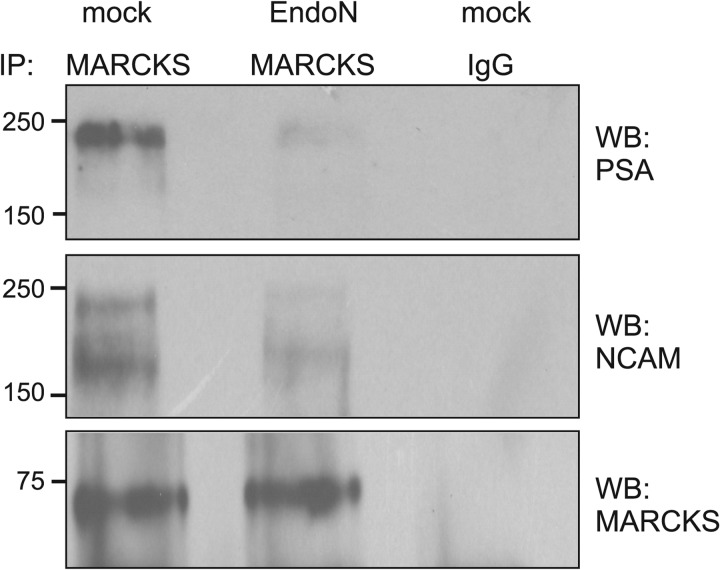
Next, we further investigated the binding of MARCKS to PSA by immunoprecipitation using a detergent extract of brain homogenate treated in the absence or presence of EndoN, which degrades PSA. Western blot analysis after immunoprecipitation using a MARCKS antibody revealed that a protein band of ∼250 kDa was recognized by the PSA antibody in the immunoprecipitates from untreated brain homogenate, whereas only very small amounts of this protein were detectable in immunoprecipitates from EndoN-treated homogenate (Fig. 3). Probing the Western blots with a NCAM antibody indicated that the MARCKS antibody co-immunoprecipitated large amounts of NCAM with an apparent molecular mass of ∼250 and ∼180 kDa from untreated brain homogenate, whereas only small amounts of these NCAM forms were detectable in the immunoprecipitates from EndoN-treated homogenate (Fig. 3). Western blot analysis with a MARCKS antibody showed that equal amounts of MARCKS were immunoprecipitated from the untreated and EndoN-treated homogenates (Fig. 3). Neither MARCKS nor PSA-carrying NCAM was detectable when a non-immune control antibody was used for immunoprecipitation (Fig. 3). These results indicated that PSA-carrying NCAM co-immunoprecipitates with MARCKS and that predominantly the PSA glycan moiety mediates the interaction between MARCKS and PSA-carrying NCAM, whereas the protein backbone of NCAM plays a minor or no role in the interaction between NCAM and MARCKS.
FIGURE 3.
MARCKS co-immunoprecipitates with PSA-carrying NCAM. Brain detergent extracts were treated without (mock) or with EndoN and subjected to immunoprecipitation with polyclonal rabbit MARCKS antibody or non-immune control rabbit antibody (IgG) as the negative control. The immunoprecipitates were subjected to Western blot analysis (WB) using PSA, NCAM, or MARCKS antibodies.
PSA Interacts with MARCKS at the Cell Membrane
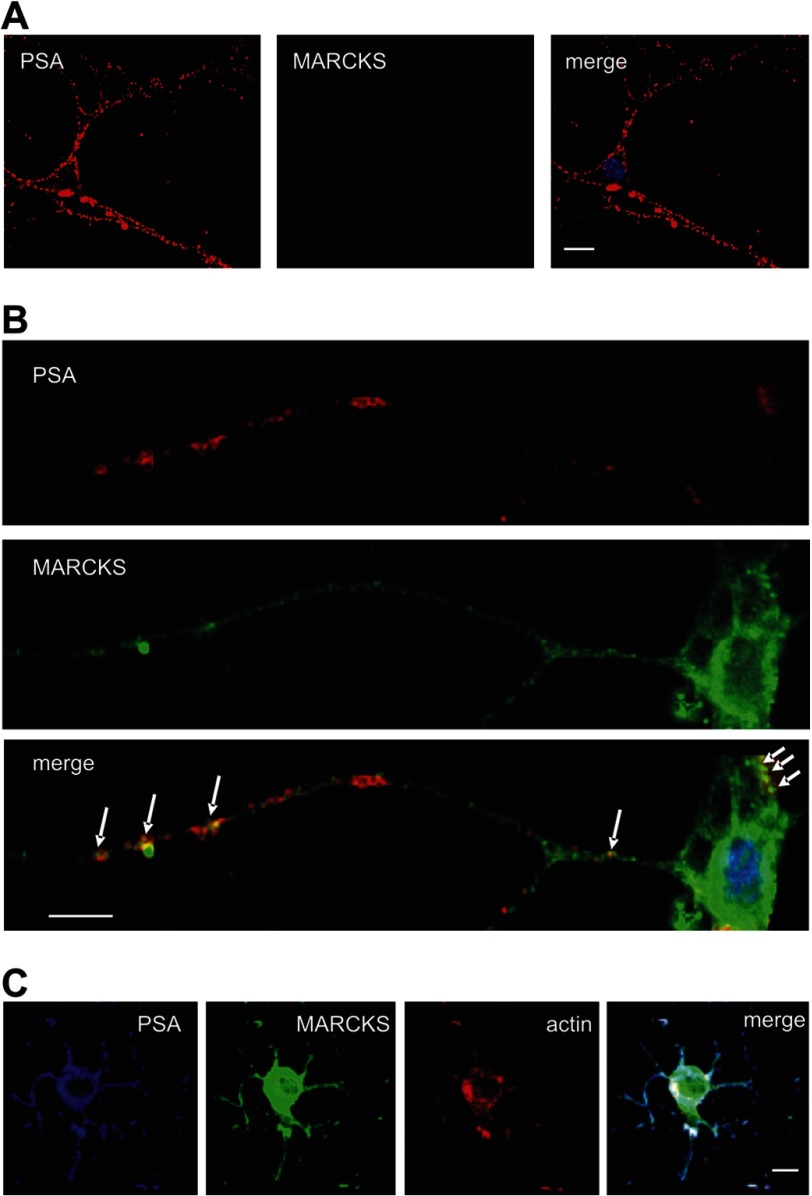
Because PSA is exposed at the cell surface, we tested the possibility of whether MARCKS, which is a cytoplasmic protein, is present extracellularly. To test this, cell surface biotinylation was carried out and revealed no evidence for extracellular MARCKS (data not shown). As an alternative method to test an extracellular localization of MARCKS, live hippocampal neurons in culture were immunocytochemically labeled with MARCKS and PSA antibodies. Confocal microscopic analysis only showed PSA immunoreactivity at the cell surface of hippocampal neurons, whereas no immunostaining of MARCKS was detectable (Fig. 4A). However, when immunocytochemical analysis of fixed and permeabilized hippocampal neurons was performed, a partial co-localization of PSA and MARCKS at the cell body and along neurites was observed (Fig. 4B), suggesting that PSA and MARCKS interact with each other at the cell membrane. A more pronounced co-localization of MARCKS and PSA was observed when live neurons were first incubated with PSA antibody and then subjected to fixation, permeabilization, and staining with MARCKS antibody (Fig. 4C), indicating that extracellular clustering of PSA by the PSA antibody induces a co-distribution of intracellular MARCKS and extracellular PSA. This notion suggests that PSA and MARCKS interact with each other from opposite sides of the plasma membrane. In addition, staining of actin using fluorescent-labeled phalloidin revealed a pronounced co-localization of actin with MARCKS and PSA (Fig. 4C), suggesting that the MARCKS/PSA interaction is involved in the organization of the actin cytoskeleton.
FIGURE 4.
Co-localization of MARCKS and PSA in cultured hippocampal neurons. A and B, cultured live hippocampal neurons (A) or fixed hippocampal neurons (B) were incubated with rabbit polyclonal MARCKS antibody and mouse monoclonal PSA antibody followed by incubation with corresponding fluorescent dye-labeled secondary antibodies. After fixation and permeabilization of cells, nuclei were stained with DAPI. Superimpositions of MARCKS and PSA staining show partial co-localization (seen in yellow) at the surface of cell bodies and processes. C, cultured live hippocampal neurons were incubated with mouse monoclonal PSA antibody followed by fixation and permeabilization of the cells and by incubation of the fixed cells with rabbit polyclonal MARCKS antibody, fluorescent dye-labeled secondary antibodies, and phalloidin. Superimpositions of MARCKS, PSA, and actin staining show partial co-localization (seen in white) at the surface of cell bodies and processes. The scale bar represents 5 μm.
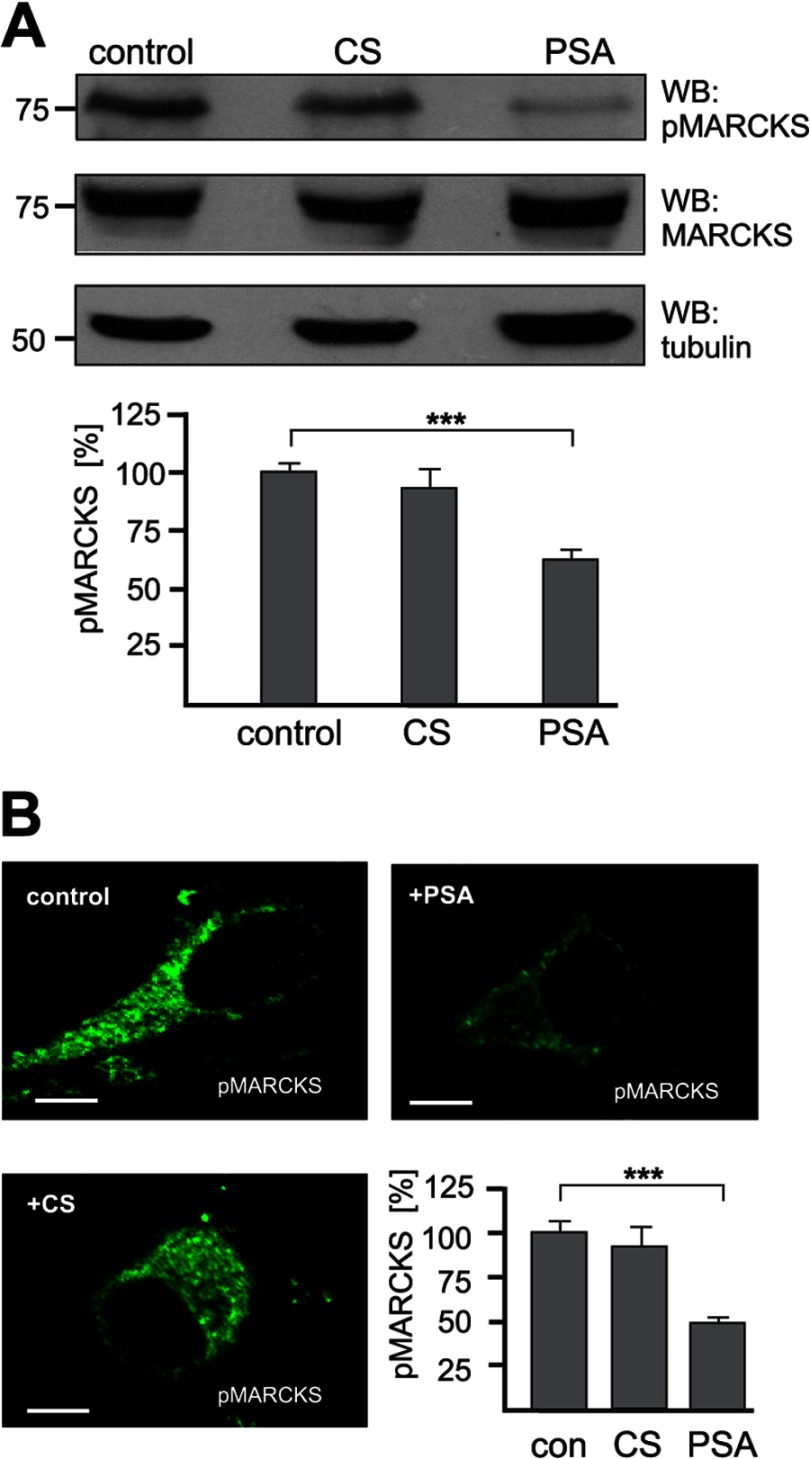
If PSA interacts with MARCKS at the plasma membrane, increasing amounts of MARCKS should be recruited to the plasma membrane in the presence of PSA. Because MARCKS can only associate with the plasma membrane when the effector domain is not phosphorylated by PKC (17), the level of PKC-phosphorylated MARCKS should decrease. To test this assumption, hippocampal neurons were incubated in the absence or presence of colominic acid/PSA or, for control, chondroitin sulfate, and then subjected to Western blot analysis using an antibody that recognizes only PKC-phosphorylated MARCKS. Indeed, in the presence of colominic acid/PSA, the amount of PKC-phosphorylated MARCKS was reduced by ∼40% when compared with the amount observed in its absence, whereas the level of total MARCKS was similar under both conditions (Fig. 5A). In the presence of chondroitin sulfate, the level of phosphorylated MARCKS was only slightly reduced in comparison to the level observed in its absence (Fig. 5A). Similarly, when using the antibody against phosphorylated MARCKS for immunocytochemical staining, a reduction of the level of phosphorylated MARCKS by ∼50% was observed in the presence of colominic acid/PSA relative to the presence of chondroitin sulfate or the absence of these polymeric glycans (Fig. 5B). This result suggests that PSA at least partially triggers signal transduction via an interaction with MARCKS at the plasma membrane and that this interaction interferes with PKC-dependent signal transduction.
FIGURE 5.
Reduction of the level of phosphorylated MARCKS by PSA. A and B, primary hippocampal neurons were incubated without (control) or with chondroitin sulfate (CS) or colominic acid (PSA). A, neurons were lysed with RIPA buffer and probed by Western blot (WB) analysis with an antibody against phosphorylated MARCKS (pMARCKS), total MARCKS, and tubulin as loading control (upper panel). Band intensities were quantified by densitometry. The levels of pMARCKS in the presence of colominic acid or chondroitin sulfate relative to the levels obtained in their absence, which was set to 100%, are shown (lower panel). Mean values ± S.E. (n = 3) are shown (***, p < 0.001, paired Student's t test). B, neurons were immunostained with an antibody against phosphorylated MARCKS. Representative images of neurons and the quantification of the fluorescence intensity are shown. The levels of pMARCKS in the presence of colominic acid or chondroitin sulfate relative to the levels obtained in their absence (set to 100%) are shown (lower panel). Mean values ± S.E. of 10 cells per group are shown (***, p < 0.001, paired Student's t test). The scale bar represents 5 μm.
PSA Interacts with MARCKS within the Plane of the Plasma Membrane but from Opposite Sides
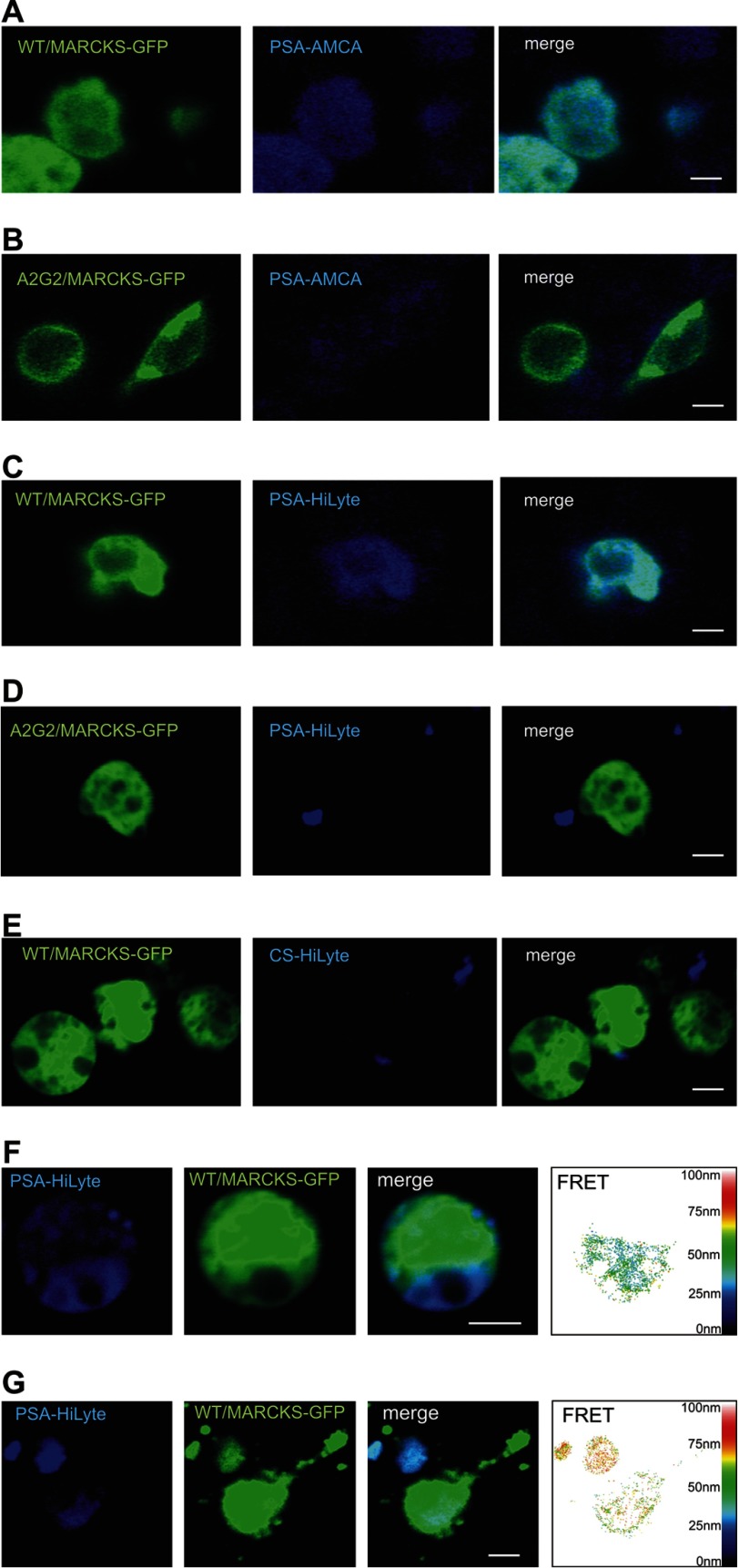
It has been shown that insertion of myristoylated MARCKS into membranes is mediated by its effector domain (33, 34). Exchanging the serine residues in the effector domain to alanine, asparagine, or aspartic acid or mutation of the myristoylation site prevents the membrane insertion of MARCKS (28). PSA has also been reported to insert into membranes (35–37). Because both MARCKS and PSA insert into membranes, it seemed likely to us that cytoplasmic MARCKS penetrates the inner leaflet of the plasma membranes and interacts with extracellular PSA, which penetrates the outer leaflet of the plasma membrane. To test this possible interaction within the plane of the plasma membrane, we performed confocal microscopic analysis of CHO cells after transfection with MARCKS-GFP constructs and incubation with colominic acid/PSA that was labeled with the fluorescent dye aminomethylcoumarin. Cells expressing non-mutated MARCKS fused to GFP showed pronounced binding of labeled colominic acid/PSA (Fig. 6A), whereas labeled colominic acid/PSA did not bind to cells expressing GFP fusions with the non-myristoylatable MARCKS mutant (Fig. 6B) or the non-phosphorylatable MARCKS mutants (data not shown), neither of which insert into membranes (19, 28). Similarly, colominic acid/PSA labeled with the dye HiLyte Fluor 405 also bound to cells expressing non-mutated MARCKS-GFP (Fig. 6C) but did not bind to cells expressing the non-myristoylatable MARCKS mutant fused to GFP (Fig. 6D) or the non-phosphorylatable MARCKS mutants fused to GFP (data not shown). Moreover, chondroitin sulfate labeled with HiLyte Fluor 405 did not bind to cells expressing non-mutated MARCKS-GFP (Fig. 6E). FRET analysis of cells shows a pronounced co-localization of labeled colominic acid/PSA and non-mutated MARCKS-GFP and revealed an approximate distance of 20–50 nm between the labeled colominic acid/PSA and MARCKS-GFP (Fig. 6F). A similar co-localization and approximate distance between the HiLyte Fluor 405-labeled colominic acid/PSA and non-mutated MARCKS-GFP was observed when transfected hippocampal neurons were used for confocal microscopy and FRET analysis (Fig. 6G).
FIGURE 6.
PSA and MARCKS interact at the cell membrane from opposite sides. CHO cells (A–F) or hippocampal neurons (G) were transfected with non-mutated MARCKS-GFP (A, C, and E–G) or with a non-myristoylatable MARCKS-GFP mutant (B and D). Colominic acid/PSA conjugated to the fluorescent dye aminomethylcoumarin (AMCA; A and B) or HiLyte Fluor 405 (C, D, F, and G) or chondroitin sulfate conjugated to HiLyte Fluor 405 (E) was applied to the transfected cells. A–G, images of representative cells from confocal microscopy and FRET analysis are shown. The scale bar represents 5 μm.
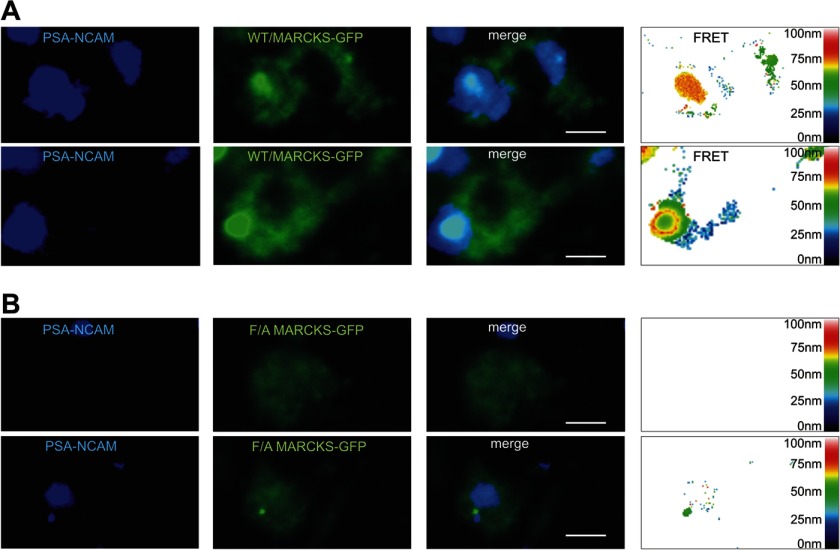
In a next step recombinant soluble PSA-NCAM, which contains PSA attached to the extracellular domain of NCAM, was labeled with the fluorescent dye HiLyte Fluor 405 and applied to cultures of NCAM-deficient hippocampal neurons transfected with non-mutated MARCKS- or with MARCKS-GFP containing exchanges of the phenylalanine residues to alanine residues in the effector domain. FRET analysis of neurons expressing non-mutated MARCKS-GFP showed a pronounced co-localization of labeled PSA-NCAM and non-mutated MARCKS-GFP and revealed a large number of FRET signals indicating a distance of 10–30 nm between PSA-NCAM and MARCKS-GFP (Fig. 7A). Binding of fluorescent-labeled PSA-NCAM to cells expressing mutated MARCKS-GFP was observed, but FRET analysis showed either no FRET signals or a low number of FRET signals for distances of less than 30 nm between the labeled PSA-NCAM and the mutated MARCKS-GFP (Fig. 7B). These results indicate that soluble PSA-NCAM interacts with the effector domain of MARCKS and that mutation of the effector domain leads to disruption or disturbance of this interaction.
FIGURE 7.
PSA-NCAM interacts with MARCKS at the cell membrane from opposite sides. NCAM-deficient hippocampal neurons were transfected with non-mutated MARCKS-GFP (A) or MARCKS-GFP mutant with phenylalanine to alanine exchanges (F/A) in the effector domain of MARCKS (B). Recombinant PSA-NCAM conjugated to the fluorescent dye HiLyte Fluor 405 (A and B) was applied to the transfected cells. Images of representative cells from confocal microscopy and FRET analysis are shown. Scale bar represents 5 μm.
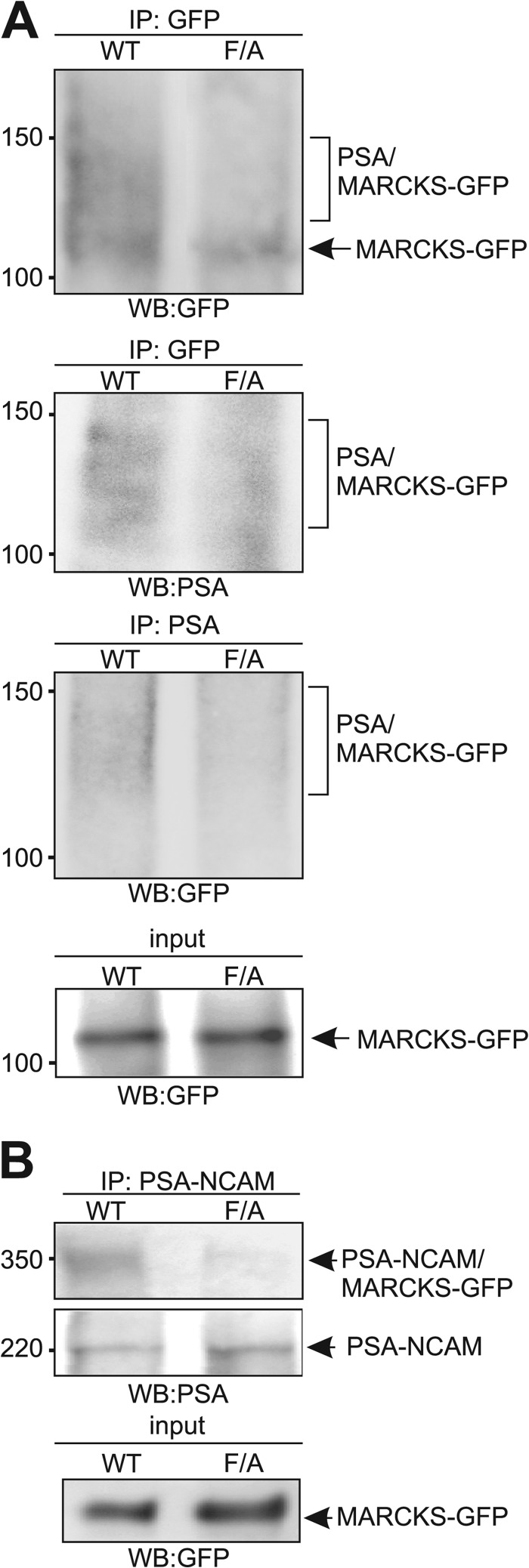
The results of the FRET analyses indicate an intimate interaction of PSA and MARCKS within the plane of the membrane. To further substantiate this idea, we performed cross-linking experiments using l-Photo-Leucine. CHO cells were transfected with MARCKS-GFP constructs and incubated with l-Photo-Leucine, allowing incorporation of this photo-active amino acid into newly synthesized proteins. After application of colominic acid/PSA to live cells, they were exposed to UV light. We expected that the photo-active leucine residue in the effector domain of intracellularly expressed MARCKS-GFP should form covalent bonds with extracellularly added colominic acid/PSA after photoactivation if colominic acid/PSA interacts with the effector domain at or in the plasma membrane and contacts the photo-activated leucine residues within a few angstrom. Colominic acid/PSA irreversibly cross-linked to MARCKS-GFP could then be isolated and detected by polysialy residues on MARCKS-GFP using immunoprecipitation and Western blot analysis with GFP and PSA antibodies. Because the colominic acid/PSA preparation contains molecules of different length, the molecular mass of colominic acid/PSA ranges from 10 to 50 kDa, and its attachment to the 110-kDa MARCKS-GFP protein should cause a molecular mass shift of MARCKS-GFP to 120–150 kDa. By Western blot analysis using a GFP antibody, a broad diffuse band ranging from ∼120 to 150 kDa and one distinct band of 110 kDa were observed in the GFP immunoprecipitates when cells were transfected with non-mutated MARCKS, whereas only the GFP-positive 110-kDa band was seen in the GFP immunoprecipitates from cells transfected with the mutated MARCKS (Fig. 8A). Western blot analysis with the PSA antibody revealed staining only of the broad diffuse band ranging from 120 to 150 kDa in the GFP immunoprecipitates from cells expressing the non-mutated MARCKS-GFP, whereas only a diffuse background staining was seen in immunoprecipitates from cells expressing the MARCKS mutant (Fig. 8A). Similarly, when probing the PSA immunoprecipitates in Western blot analysis with the GFP antibody, only a diffuse immunoreactivity ranging from 120 to 150 kDa was observed in immunoprecipitates from cells expressing non-mutated MARCKS, whereas only a faint diffuse background staining was seen in immunoprecipitates from cells expressing the MARCKS mutant (Fig. 8A). Importantly, for both MARCKS-GFP constructs, similar expression levels of the 110-kDa MARCKS-GFP proteins were observed by Western blot analysis of the cell lysates with GFP antibody (Fig. 8A). The shift in apparent molecular mass to 120–150 kDa, observed only for non-mutated MARCKS-GFP, indicates that colominic acid/PSA was cross-linked to non-mutated MARCKS-GFP but not to mutated MARCKS-GFP.
FIGURE 8.
PSA and MARCKS interact within the plane of the plasma membrane. CHO cells were transfected with non-mutated MARCKS-GFP or MARCKS-GFP mutant with phenylalanine to alanine exchanges (F/A). After incubation with L-Photo-Leucine and colominic acid/PSA (A) or PSA-NCAM (B), cells were exposed to UV light and subjected to immunoprecipitation (IP) with PSA or GFP antibody (A) or to precipitation with Protein A (B). The immunoprecipitates were probed by Western blot (WB) analysis using GFP and PSA antibodies. Expression levels were determined by Western blot analysis of the cell lysates (input) using the GFP antibody.
We then used recombinant PSA-NCAM in the cross-linking experiment with CHO cells transfected with non-mutated MARCKS or mutated MARCKS. After exposure to UV light and isolation of Fc-carrying PSA-NCAM using protein A beads, the precipitates were subjected to Western blot analysis with PSA antibody. A band of ∼220 kDa was observed in both precipitates, whereas an additional band of ∼330 kDa was detectable only in the precipitate isolated from cells expressing non-mutated MARCKS (Fig. 8B). Both MARCKS-GFP constructs showed similar expression levels of the 110-kDa MARCKS-GFP proteins in cell lysates (Fig. 8B). The shift in apparent molecular mass to 330 kDa observed for non-mutated MARCKS-GFP indicates cross-linking of the 220-kDa PSA-NCAM and the non-mutated 110-kDa MARCKS-GFP. In summary, the results of the cross-linking approach provide further evidence that the effector domain of MARCKS tightly interacts with PSA at or within the plasma membrane from opposite sides.
PSA and the MARCKS Effector Domain Peptide Interact within Lipid Bilayers from Opposite Sides
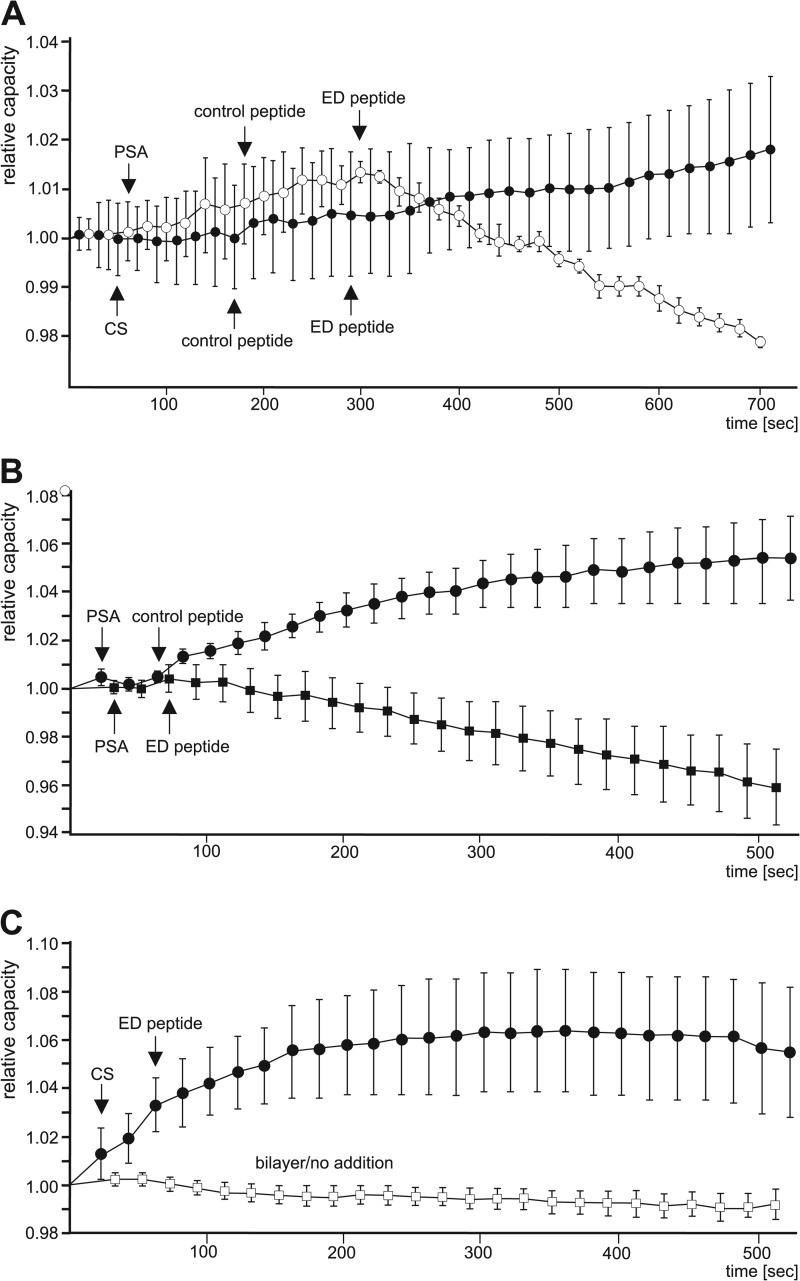
To provide further evidence for the interaction between PSA and the effector domain of MARCKS at or within membranes, the capacitance of lipid bilayers was monitored after the addition of colominic acid/PSA or chondroitin sulfate and MARCKS-ED peptide or the corresponding control peptide to opposite sides of the bilayer.
Application of colominic acid/PSA to the stable bilayer did not lead to significant alterations in relative capacitance of the membrane (Fig. 9A). Subsequent addition of the control peptide to the opposite side of colominic acid/PSA or chondroitin sulfate application led to a slight increase in relative membrane capacitance (Fig. 9A). After application of MARCKS-ED peptide, the relative capacitance reached a plateau when chondroitin sulfate was present on the opposite side, whereas the capacitance constantly decreased in the presence of colominic acid/PSA on the opposite side (Fig. 9A). Similarly, the capacitance was also reduced when the MARCKS-ED peptide was directly applied to colominic acid/PSA on the opposite site but was not lowered upon direct application of the control peptide (Fig. 9B). Moreover, when MARCKS-ED peptide was directly added to the opposite side of chondroitin sulfate application, a decrease in membrane capacitance was also not observed (Fig. 9C). In addition, the relative membrane capacitance of the lipid bilayer without the addition of peptides, chondroitin sulfate, and colominic acid/PSA did not change significantly with time (Fig. 9C), demonstrating the stability of the bilayer. These results indicate that colominic acid/PSA and MARCKS-ED peptide interact with each other after their membrane insertion and that this interaction within the membrane affects the membrane properties, allowing the flow of ions through the bilayer.
FIGURE 9.
PSA and MARCKS-ED peptide insert and interact with each other in lipid bilayers. After formation of stable lipid bilayers, colominic acid/PSA (A and B) or chondroitin sulfate (CS, A and B) was applied to the anode chamber and MARCKS-ED peptide or control peptide was added to the cathode chamber (A–C). For control, bilayers without any addition were analyzed (C). The capacitance was monitored, and mean values ± S.E. of relative capacitance (Ct/Ct = 0) from four independent experiments are shown.
MARCKS Mediates PSA-induced Neurite Outgrowth of Hippocampal Neurons
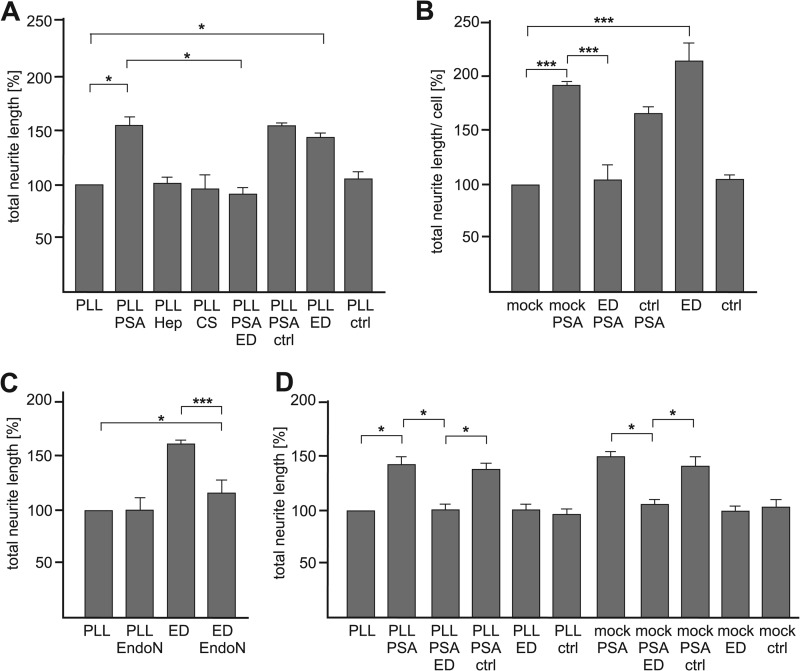
Next, we investigated whether the interaction between extracellular PSA and intracellular MARCKS has functional consequences. To test whether PSA influences neurite outgrowth and if the effects of PSA on neurite outgrowth were mediated via MARCKS, hippocampal neurons were grown on substrate-coated PLL in the absence or presence of soluble colominic acid/PSA and without or with soluble MARCKS-ED or control peptide. In comparison to neurite outgrowth on PLL without additives, neurite outgrowth was increased in the presence of soluble colominic acid/PSA (Fig. 10A) but was not altered in the presence of chondroitin sulfate or heparin (Fig. 10A). In the concomitant presence of colominic acid/PSA and soluble MARCKS-ED peptide, which binds to PSA in vitro (Fig. 2), neurite outgrowth was not increased and was similar to that observed in the absence of MARCKS-ED peptide and colominic acid/PSA (Fig. 10A), indicating that soluble MARCKS-ED peptide had interacted with PSA and thus PSA was no longer able to trigger neurite outgrowth. In contrast to the MARCKS-ED peptide, the control peptide, which did not bind to colominic acid/PSA (Fig. 2), had no effect on the enhancement of neurite outgrowth by colominic acid/PSA. These results indicate that the interaction between extracellular PSA and intracellular MARCKS via its effector domain is involved in neurite outgrowth.
FIGURE 10.
PSA-induced neurite outgrowth is mediated by the MARCKS effector domain. A, wild-type hippocampal neurons were seeded onto PLL and incubated in the absence or presence of colominic acid (PSA), heparin (Hep), chondroitin sulfate (CS), MARCKS-ED peptide (ED), control peptide (ctrl), or colominic acid and MARCKS-ED peptide or control peptide. B, hippocampal neurons were mock-transfected (mock) or transfected with MARCKS-ED peptide (ED) or the control peptide (ctrl) and maintained on PLL in the absence or presence of colominic acid (PSA). C, hippocampal neurons were incubated without and with EndoN in the absence or presence of soluble MARCKS-ED peptide (ED). D, NCAM-deficient hippocampal neurons were either maintained on PLL in the absence or presence of colominic acid (PSA) and/or MARCKS-ED peptide (ED) or control peptide (ctrl) or were mock-transfected (mock) or transfected with MARCKS-ED peptide (ED) or control peptide (ctrl) and incubated in the absence or presence of colominic acid (PSA). A–D, The total length of neurites per hippocampal neuron was determined. Mean values ± S.E. for total neurite lengths are shown and related to the values obtained on PLL only, which was set to 100% (*, p < 0.05; ***, p < 0.001 obtained by two-tailed Student's t test from n = ∼100 neurons in three (A and C) or two (B and D) independent experiments).
To further investigate whether MARCKS is involved in PSA-mediated neurite outgrowth, neurite outgrowth was determined after perturbing the interaction of endogenous cytoplasmic MARCKS with colominic acid/PSA by transfecting hippocampal neurons with the MARCKS-ED peptide. The experiment was performed with the expectation that the MARCKS-ED peptide competes with the interaction of endogenous MARCKS effector domain with colominic acid/PSA at the membrane and thus should block neurite outgrowth. Transfection of MARCKS-ED peptide indeed blocked neurite outgrowth promotion by soluble colominic acid/PSA, whereas transfection of the control peptide had no effect on the promotion of neurite outgrowth by colominic acid/PSA (Fig. 10B). The combined results show that MARCKS regulates PSA-induced neurite outgrowth and that perturbation of the interaction between PSA and the MARCKS effector domain impairs neurite outgrowth.
Unexpectedly, neurite outgrowth was enhanced in the presence of soluble MARCKS-ED peptide or upon transfection of the MARCKS-ED peptide when compared with neurite outgrowth obtained in the presence or upon transfection of the control peptide and in the absence of peptides or upon mock transfection (Fig. 10, A and B). To test whether this enhancement of neurite outgrowth by the MARCKS-ED peptide is PSA-dependent, neurite outgrowth of hippocampal neurons was determined after incubation without or with EndoN in the absence or presence of MARCKS-ED peptide. In parallel, neurite outgrowth of NCAM-deficient hippocampal neurons, which are devoid of PSA, was measured in the presence or upon transfection of MARCKS-ED peptide. In the absence of peptide, neurite outgrowth was not altered by EndoN treatment, whereas EndoN treatment abolished the neurite outgrowth enhanced by the MARCKS-ED peptide (Fig. 10C), indicating that the MARCKS-ED peptide-triggered neurite outgrowth is PSA-dependent. Moreover, application or transfection of the MARCKS-ED peptide had no effect on neurite outgrowth of NCAM-deficient neurons (Fig. 10D), confirming that neurite outgrowth induced by MARCKS-ED depends on PSA and indicating that the interaction between PSA on NCAM and MARCKS via its effector domain is involved in regulation of neurite outgrowth.
Interestingly, as seen with wild-type neurons, neurite outgrowth from NCAM-deficient neurons was also increased in the presence of colominic acid/PSA, although to a lesser extent (20% reduction of the neurite lengths of NCAM-deficient neurons in the presence of colominic acid/PSA when compared with the length of WT neurons). In addition, the concomitant application of MARCKS-ED peptide and colominic acid/PSA did not promote neurite outgrowth from NCAM-deficient neurons, whereas neurite outgrowth was enhanced in the concomitant presence of the control peptide and colominic acid/PSA. These results indicate that the effect of soluble PSA on neurite outgrowth is mostly independent of cellular NCAM and PSA-carrying NCAM but is mainly mediated via the effector domain of MARCKS.
DISCUSSION
In the present study we identified MARCKS as a PSA binding partner using a PSA-mimicking anti-idiotypic antibody for affinity chromatography of soluble mouse brain proteins. Although we could not show a direct interaction between PSA and full-length MARCKS, we showed direct binding of colominic acid/PSA to the MARCKS-ED peptide comprising the effector domain of MARCKS, indicating that this effector domain interacts with PSA. We also showed an association between PSA and MARCKS by co-immunoprecipitation of PSA-carrying NCAM and by co-localization of MARCKS and PSA at the plasma membrane of hippocampal neurons.
It has been reported that MARCKS inserts into cell membranes via electrostatic interactions between positively charged residues within the effector domain and negatively charged phospholipids and via a penetration of the phenylalanine side chains within the effector domain into the lipid bilayer (33, 34). Similar to MARCKS, it has been shown that PSA associates with liposomal membranes and penetrates lipid bilayers (35–37). This intimate interaction of PSA with membranes is mediated or facilitated by the helical and random-coil conformations of PSA (38). Based on the similar properties of PSA and MARCKS to insert into lipid bilayers, we propose that MARCKS and PSA interact at or within the plasma membrane from opposite sides. Cross-linking of extracellularly applied colominic acid/PSA or PSA-NCAM and intracellularly expressed MARCKS-GFP via a photo-active leucine residue in the effector domain of MARCKS as well as co-localization studies and FRET analysis using fluorescently labeled colominic acid/PSA or PSA-NCAM and MARCKS-GFP-expressing cells supports this idea. Moreover, colominic acid/PSA and the MARCKS-ED peptide insert into lipid bilayers from opposite sides and the insertion and interaction of colominic acid/PSA and the MARCKS-ED peptide leads to an ion flow through the bilayer and a concomitant reduction in membrane capacitance. These findings suggest that the interaction of PSA and MARCKS at and/or within the plasma membrane modulate the electric properties of the plasma membrane.
The interaction between PSA and MARCKS at or within the plasma membrane from opposite sides appears necessary to interpret our observation that transfection of the MARCKS-ED peptide or individual application of colominic acid/PSA or MARCKS-ED peptide in the culture medium promotes neurite outgrowth of hippocampal neurons, whereas the promoting effect of colominic acid/PSA and MARCKS-ED peptide is perturbed when the MARCKS-ED peptide and colominic acid/PSA are concomitantly applied to hippocampal neurons.
MARCKS shows high affinity binding to phosphatidylinositol 4,5-bisphosphate (PIP2) and plays an important role in regulating the availability of PIP2 by sequestration of PIP2 (39), which has been implicated in signal transduction (40). The MARCKS-ED peptide binds PIP2 with high affinity like the full-length MARCKS and inhibits PIP2 hydrolysis in vitro (41), indicating that the effector domain mediates the sequestration of PIP2. This notion is supported by the finding that a MARCKS mutant lacking the effector domain is not able to sequester PIP2 (41). Furthermore, overexpression of MARCKS or inhibition of the phosphoinositide metabolism results in reduction of PIP2 levels and in enhanced neurite outgrowth, whereas overexpression of a MARCKS mutant lacking the effector domain does not enhance neurite outgrowth (41). These findings indicate that sequestration of PIP2 by MARCKS regulates neurite outgrowth. In our view it is unlikely that the MARCKS-ED peptide enhances neurite outgrowth by binding to PIP2, leading to enhanced sequestrations of PIP2, as the MARCKS-ED peptide has no effect on neurite outgrowth of NCAM-deficient neurons, which lack PSA. This view is supported by our observation that MARCKS-ED peptide also promotes neurite outgrowth when applied to the neurons extracellularly. Because PIP2 is present predominantly in the inner leaflet of the plasma membrane, it is unlikely that the peptide sequesters PIP2 and regulates signal transduction from outside of the cell.
The MARCKS-ED peptide promotes neurite outgrowth from untreated wild-type neurons upon transfection or application but has no effect on neurite outgrowth of EndoN-treated, PSA-ablated wild-type neurons or on neurite outgrowth of NCAM-deficient neurons, which lack the predominant neuronal PSA carrier. Thus, we propose that the MARCKS-ED peptide interferes with the interaction between MARCKS and membrane-bound PSA-carrying NCAM at the cell surface, leading to the dissociation of PSA chains from intracellular MARCKS. The dissociated PSA chains may interact with PSA-binding proteins present in the near vicinity of PSA-NCAM. This interaction then could trigger, most likely MARCKS-independently, signal transduction pathways leading to promotion of neurite outgrowth. Alternatively, it is also possible that the dissociation of PSA chains from MARCKS leads to conformational changes of the protein backbone of PSA-NCAM. Upon conformational change, PSA-NCAM may trigger signal transduction events and neurite outgrowth directly or indirectly through an interaction with an associated or nearby binding partner. Because only NCAM and/or the fibroblast growth factor receptor have been suggested to be involved in PSA signaling (25, 42), we believe that the promotion of neurite outgrowth by MARCKS-ED peptide is mediated by NCAM and may involve the fibroblast growth factor receptor.
Because application of colominic acid/PSA to NCAM-deficient neurons lacking PSA-carrying NCAM promotes neurite outgrowth, although to a lesser extent, we propose that promotion of neurite outgrowth by soluble PSA depends mainly on the direct binding of soluble PSA to MARCKS. In this context it is important to stress that we have detected significant levels or elevated levels of soluble PSA-NCAM in cerebrospinal fluids of healthy patients and of patients with Alzheimer disease, respectively (43). This finding and the observation of high PSA-NCAM levels in cerebrospinal fluids of patients with medulloblastoma metastasis (44) indicate that soluble PSA occurs in vivo as soluble PSA-NCAM, which can be generated by proteolytic cleavage (45), and suggests that soluble PSA attached to NCAM not only plays an important role under normal conditions but also under pathological conditions such as neurodegeneration.
The interaction of soluble PSA-NCAM and plasma membrane-bound MARCKS may not only modulate membrane properties but also may play an important role in regulation of MARCKS-triggered signal transduction pathways and/or MARCKS-dependent functions, e.g. PIP2 sequestration and the cross-linking of actin to the membrane. Disturbance of the direct interaction between colominic acid/PSA and MARCKS by the MARCKS-ED peptide abolishes the PSA-triggered and MARCKS-dependent intracellular functions and blocks MARCKS-mediated promotion of neurite outgrowth. In addition, binding of the MARCKS-ED peptide to colominic acid/PSA also inhibits the promotion of neurite outgrowth induced by the peptide.
The important role of PSA in brain development and in synaptic plasticity of the adult brain has been shown in a number of studies using EndoN for removal of PSA or using mice deficient in the PSA-synthesizing enzymes and thus deficient in PSA (46–53). The ablation of PSA results in reduction of neurite outgrowth and in deficits in cell migration, axonal targeting, and development of hippocampal mossy fibers as well as long term potentiation and learning and memory (26, 46–50, 53–55). Most of these deficits have also been observed in NCAM-deficient mice (52, 56–58), indicating that NCAM is the major and most important PSA carrier in the nervous system and that the PSA moiety on NCAM plays a crucial role in nervous system development and function. Furthermore, the importance of PSA, in particular of PSA on NCAM, in synaptic plasticity associated with memory and learning has been shown by in vitro and in vivo application of PSA. The addition of colominic acid/PSA or of soluble PSA-carrying NCAM to acute hippocampal slices of NCAM-deficient mice restores long term potentiation that is impaired in NCAM-deficient mice, whereas application to the hippocampus of wild-type mice leads to impairment in formation of hippocampus-dependent contextual memory (26). The addition of colominic acid/PSA increased the open time of the AMPA receptors (57, 59), whereas colominic acid/PSA addition prevents activation of GluN2B-containing NMDA receptors at low glutamate concentrations (60).
Similarly to PSA, a number of different in vitro and in vivo studies on the functional role of MARCKS in the nervous system indicated that MARCKS is expressed in regions undergoing neuronal proliferation and migration as well as neurite outgrowth (12). In addition, MARCKS is involved in cell adhesion, lamellipodia formation, initiation of neuritogenesis, neurite outgrowth, growth cone adhesion, pathfinding, dendrite branching, dendritic spine morphogenesis, and synaptic plasticity (13, 28, 39, 41, 61–71). Knockdown of MARCKS by RNA interference and overexpression of MARCKS mutants revealed that non-phosphorylated MARCKS stabilizes growth cone adhesion, enhances branching and growth of dendrites, elongates dendritic spines, and enhances initiation of neuritogenesis and neurite outgrowth (65, 66, 68, 69). Expression of different mutants such as the effector domain-deficient mutant indicated that binding of MARCKS to the surface membrane via its effector domain is essential for MARCKS-induced dendritic morphogenesis and neurite outgrowth (66, 68). The observations that the level of membrane-bound, non-phosphorylated MARCKS increases in a learning paradigm (67) and that heterozygous MARCKS mice and transgenic mice overexpressing MARCKS show deficits in spatial learning (13, 69) and an impairment of long term potentiation in the hippocampal mossy fiber-CA3 pathway (70) implicate MARCKS in hippocampus-dependent learning processes. In addition, because infusion of MARCKS-ED peptide into the hippocampus impairs learning and memory, it is highly likely that the effector domain of MARCKS plays a significant role in regulation of learning and memory (68).
The findings on the functional role of PSA and MARCKS in nervous system development and function and our data on neurite outgrowth mediated by the PSA/MARCKS interaction at the plasma membrane suggest that the interaction between PSA and MARCKS not only regulates important events in the developing, but also in the adult nervous system, such as regeneration and synaptic plasticity. Because colominic acid/PSA-induced promotion of neurite outgrowth is blocked by the MARCKS-ED peptide and because the addition of colominic acid/PSA enhances the level of non-phosphorylated MARCKS, it is very likely that PSA triggers signaling and cellular responses at least partially via its interaction with non-phosphorylated MARCKS at the plasma membrane. It will thus be important to study the role of MARCKS in PSA-dependent signal transduction during nervous system development as well as in regeneration after injury and synaptic plasticity in the adult nervous system. In addition, the study of the MARCKS-PSA connection may allow insights in the functions of PSA in the pathogenesis of neuropsychiatric and neurodegenerative disorders, such as schizophrenia, bipolar disorder, depression, anxiety, and Alzheimer disease (72).
Acknowledgments
We are grateful to Drs. Alexandre Podtelejnikov and Matthias Mann (Protein Bioinformatics, Odense, Denmark) for protein sequencing and Dr. Rita Gerardy-Schahn (Medizinische Hochschule Hannover, Germany) for the PSA antibody and endoglycosidase N.
Footnotes
- PSA
- polysialic acid
- NCAM
- neural cell adhesion molecule
- MARCKS
- myristoylated alanine-rich C kinase substrate
- MARCKS-ED
- MARCKS effector domain
- EndoN
- endoglycosidase N
- BASP1
- brain acid soluble protein 1
- PLL
- poly-l-lysine
- PIP2
- phosphatidylinositol 4,5-bisphosphate
- scFv
- single chain variable fragment
- RIPA
- radioimmune precipitation assay buffer.
REFERENCES
- 1. Finne J., Finne U., Deagostini-Bazin H., Goridis C. (1983) Occurrence of α-2–8-linked polysialosyl units in a neural cell adhesion molecule. Biochem. Biophys. Res. Commun. 112, 482–487 [DOI] [PubMed] [Google Scholar]
- 2. Michon F., Brisson J. R., Jennings H. J. (1987) Conformational differences between linear α(2–8)-linked homosialooligosaccharides and the epitope of the group B meningococcal polysaccharide. Biochemistry 26, 8399–8405 [DOI] [PubMed] [Google Scholar]
- 3. Mühlenhoff M., Eckhardt M., Gerardy-Schahn R. (1998) Polysialic acid. Three dimensional structure, biosynthesis, and function. Curr. Opin. Struct. Biol. 8, 558–564 [DOI] [PubMed] [Google Scholar]
- 4. Rutishauser U. (2008) Polysialic acid in the plasticity of the developing and adult vertebrate nervous system. Nat. Rev. Neurosci. 9, 26–35 [DOI] [PubMed] [Google Scholar]
- 5. Curreli S., Arany Z., Gerardy-Schahn R., Mann D., Stamatos N. M. (2007) Polysialylated neuropilin-2 is expressed on the surface of human dendritic cells and modulates dendritic cell-T lymphocyte interactions. J. Biol. Chem. 282, 30346–30356 [DOI] [PubMed] [Google Scholar]
- 6. Galuska S. P., Rollenhagen M., Kaup M., Eggers K., Oltmann-Norden I., Schiff M., Hartmann M., Weinhold B., Hildebrandt H., Geyer R., Mühlenhoff M., Geyer H. (2010) Synaptic cell adhesion molecule SynCAM 1 is a target for polysialylation in postnatal mouse brain. Proc. Natl. Acad. Sci. U.S.A. 107, 10250–10255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kleene R., Schachner M. (2004) Glycans and neural cell interactions. Nat. Rev. Neurosci. 5, 195–208 [DOI] [PubMed] [Google Scholar]
- 8. Kanato Y., Kitajima K., Sato C. (2008) Direct binding of polysialic acid to a brain-derived neurotrophic factor depends on the degree of polymerization. Glycobiology 18, 1044–1053 [DOI] [PubMed] [Google Scholar]
- 9. Mishra B., von der Ohe M., Schulze C., Bian S., Makhina T., Loers G., Kleene R., Schachner M. (2010) Functional role of the interaction between polysialic acid and extracellular histone H1. J. Neurosci. 30, 12400–12413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ono S., Hane M., Kitajima K., Sato C. (2012) Novel regulation of fibroblast growth factor 2 (FGF2)-mediated cell growth by polysialic acid. J. Biol. Chem. 287, 3710–3722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Albert K. A., Nairn A. C., Greengard P. (1987) The 87-kDa protein, a major specific substrate for protein kinase C. Purification from bovine brain and characterization. Proc. Natl. Acad. Sci. U.S.A. 84, 7046–7050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. McNamara R. K., Lenox R. H. (1998) Distribution of the protein kinase C substrates MARCKS and MRP in the postnatal developing rat brain. J. Comp. Neurol. 397, 337–356 [PubMed] [Google Scholar]
- 13. Stumpo D. J., Bock C. B., Tuttle J. S., Blackshear P. J. (1995) MARCKS deficiency in mice leads to abnormal brain development and perinatal death. Proc. Natl. Acad. Sci. U.S.A. 92, 944–948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. McNamara R. K., Stumpo D. J., Morel L. M., Lewis M. H., Wakeland E. K., Blackshear P. J., Lenox R. H. (1998) Effect of reduced myristoylated alanine-rich C kinase substrate expression on hippocampal mossy fiber development and spatial learning in mutant mice. Transgenic rescue and interactions with gene background. Proc. Natl. Acad. Sci. U.S.A. 95, 14517–14522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Graff J. M., Young T. N., Johnson J. D., Blackshear P. J. (1989) Phosphorylation-regulated calmodulin binding to a prominent cellular substrate for protein kinase C. J. Biol. Chem. 264, 21818–21823 [PubMed] [Google Scholar]
- 16. Hartwig J. H., Thelen M., Rosen A., Janmey P. A., Nairn A. C., Aderem A. (1992) MARCKS is an actin filament cross linking protein regulated by protein kinase C and calcium calmodulin. Nature 356, 618–622 [DOI] [PubMed] [Google Scholar]
- 17. Swierczynski S. L., Blackshear P. J. (1995) Membrane association of the myristoylated alanine-rich C kinase substrate (MARCKS) protein. Mutational analysis provides evidence for complex interactions. J. Biol. Chem. 270, 13436–13445 [DOI] [PubMed] [Google Scholar]
- 18. Arbuzova A., Schmitz A. A., Vergères G. (2002) Cross-talk unfolded. MARCKS proteins. Biochem. J. 362, 1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Swierczynski S. L., Siddhanti S. R., Tuttle J. S., Blackshear P. J. (1996) Nonmyristoylated MARCKS complements some but not all of the developmental defects associated with MARCKS deficiency in mice. Dev. Biol. 179, 135–147 [DOI] [PubMed] [Google Scholar]
- 20. Vergères G., Ramsden J. J. (1998) Binding of MARCKS (myristoylated alanine-rich C kinase substrate)-related protein (MRP) to vesicular phospholipid membranes. Biochem. J. 330, 5–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cremer H., Lange R., Christoph A., Plomann M., Vopper G., Roes J., Brown R., Baldwin S., Kraemer P., Scheff S. (1994) Inactivation of the N-CAM gene in mice results in size reduction of the olfactory bulb and deficits in spatial learning. Nature 367, 455–459 [DOI] [PubMed] [Google Scholar]
- 22. Lobaugh L. A., Blackshear P. J. (1990) Neuropeptide Y stimulation of myosin light chain phosphorylation in cultured aortic smooth muscle cells. J. Biol. Chem. 265, 18393–18399 [PubMed] [Google Scholar]
- 23. Niethammer P., Delling M., Sytnyk V., Dityatev A., Fukami K., Schachner M. (2002) Cosignaling of NCAM via lipid rafts and the FGF receptor is required for neuritogenesis. J. Cell Biol. 157, 521–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Frosch M., Görgen I., Boulnois G. J., Timmis K. N., Bitter-Suermann D. (1985) NZB mouse system for production of monoclonal antibodies to weak bacterial antigens. Isolation of an IgG antibody to the polysaccharide capsules of Escherichia coli K1 and group B meningococci. Proc. Natl. Acad. Sci. U.S.A. 82, 1194–1198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mehanna A., Mishra B., Kurschat N., Schulze C., Bian S., Loers G., Irintchev A., Schachner M. (2009) Polysialic acid glycomimetics promote myelination and functional recovery after peripheral nerve injury in mice. Brain 132, 1449–1462 [DOI] [PubMed] [Google Scholar]
- 26. Senkov O., Sun M., Weinhold B., Gerardy-Schahn R., Schachner M., Dityatev A. (2006) Polysialylated neural cell adhesion molecule is involved in induction of long term potentiation and memory acquisition and consolidation in a fear-conditioning paradigm. J. Neurosci. 26, 10888–109898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Vutskits L., Djebbara-Hannas Z., Zhang H., Paccaud J. P., Durbec P., Rougon G., Muller D., Kiss J. Z. (2001) PSA-NCAM modulates BDNF-dependent survival and differentiation of cortical neurons. Eur. J. Neurosci. 13, 1391–1402 [DOI] [PubMed] [Google Scholar]
- 28. Spizz G., Blackshear P. J. (2001) Overexpression of the myristoylated alanine-rich C-kinase substrate inhibits cell adhesion to extracellular matrix components. J. Biol. Chem. 276, 32264–32273 [DOI] [PubMed] [Google Scholar]
- 29. Wessel D., Flügge U. I. (1984) A method for the quantitative recovery of protein in dilute solution in the presence of detergents and lipids. Anal. Biochem. 138, 141–143 [DOI] [PubMed] [Google Scholar]
- 30. Makhina T., Loers G., Schulze C., Ueberle B., Schachner M., Kleene R. (2009) Extracellular GAPDH binds to L1 and enhances neurite outgrowth. Mol. Cell. Neurosci. 41, 206–218 [DOI] [PubMed] [Google Scholar]
- 31. Loers G., Makhina T., Bork U., Dörner A., Schachner M., Kleene R. (2012) The interaction between cell adhesion molecule L1, matrix metalloproteinase 14, and adenine nucleotide translocator at the plasma membrane regulates L1-mediated neurite outgrowth of murine cerebellar neurons. J. Neurosci. 32, 3917–3930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dityatev A., Dityateva G., Schachner M. (2000) Synaptic strength as a function of post- versus presynaptic expression of the neural cell adhesion molecule NCAM. Neuron 26, 207–217 [DOI] [PubMed] [Google Scholar]
- 33. Qin Z., Cafiso D. S. (1996) Membrane structure of protein kinase C and calmodulin binding domain of myristoylated alanine rich C kinase substrate determined by site-directed spin labeling. Biochemistry 35, 2917–2925 [DOI] [PubMed] [Google Scholar]
- 34. Victor K., Jacob J., Cafiso D. S. (1999) Interactions controlling the membrane binding of basic protein domains. Phenylalanine and the attachment of the myristoylated alanine-rich C-kinase substrate protein to interfaces. Biochemistry 38, 12527–12536 [DOI] [PubMed] [Google Scholar]
- 35. Janas T., Janas T., Krajiński H. (2000) Membrane transport of polysialic acid chains. Modulation of transmembrane potential. Eur. Biophys. J. 29, 507–514 [DOI] [PubMed] [Google Scholar]
- 36. Janas T., Krajiński H., Timoszyk A., Janas T. (2001) Translocation of polysialic acid across model membranes. Kinetic analysis and dynamic studies. Acta Biochim. Pol. 48, 163–173 [PubMed] [Google Scholar]
- 37. Janas T., Nowotarski K., Janas T. (2010) Polysialic acid can mediate membrane interactions by interacting with phospholipids. Chem. Phys. Lipids 163, 286–291 [DOI] [PubMed] [Google Scholar]
- 38. Brisson J. R., Baumann H., Imberty A., Pérez S., Jennings H. J. (1992) Helical epitope of the group B meningococcal α-(2–8)-linked sialic acid polysaccharide. Biochemistry 31, 4996–5004 [DOI] [PubMed] [Google Scholar]
- 39. Gambhir A., Hangyás-Mihályné G., Zaitseva I., Cafiso D. S., Wang J., Murray D., Pentyala S. N., Smith S. O., McLaughlin S. (2004) Electrostatic sequestration of PIP2 on phospholipid membranes by basic/aromatic regions of proteins. Biophys. J. 86, 2188–2207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kwiatkowska K. (2010) One lipid, multiple functions: how various pools of PI(4,5)P(2) are created in the plasma membrane. Cell. Mol. Life Sci. 67, 3927–3946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Laux T., Fukami K., Thelen M., Golub T., Frey D., Caroni P. (2000) GAP43, MARCKS, and CAP23 modulate PI(4,5)P(2) at plasmalemmal rafts and regulate cell cortex actin dynamics through a common mechanism. J. Cell Biol. 149, 1455–1472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Li J., Dai G., Cheng Y. B., Qi X., Geng M. Y. (2011) Polysialylation promotes neural cell adhesion molecule-mediated cell migration in a fibroblast growth factor receptor-dependent manner but independent of adhesion capability. Glycobiology 21, 1010–1018 [DOI] [PubMed] [Google Scholar]
- 43. Strekalova H., Buhmann C., Kleene R., Eggers C., Saffell J., Hemperly J., Weiller C., Müller-Thomsen T., Schachner M. (2006) Elevated levels of neural recognition molecule L1 in the cerebrospinal fluid of patients with Alzheimer disease and other dementia syndromes. Neurobiol. Aging 27, 1–9 [DOI] [PubMed] [Google Scholar]
- 44. Figarella-Branger D., Dubois C., Chauvin P., De Victor B., Gentet J. C., Rougon G. (1996) Correlation between polysialic-neural cell adhesion molecule levels in CSF and medulloblastoma outcomes. J. Clin. Oncol. 14, 2066–2072 [DOI] [PubMed] [Google Scholar]
- 45. Kalus I., Bormann U., Mzoughi M., Schachner M., Kleene R. (2006) Proteolytic cleavage of the neural cell adhesion molecule by ADAM17/TACE is involved in neurite outgrowth. J. Neurochem. 98, 78–88 [DOI] [PubMed] [Google Scholar]
- 46. Rønn LC., Berezin V., Bock E. (2000) The neural cell adhesion molecule in synaptic plasticity and ageing. Int. J. Dev. Neurosci. 18, 193–199 [DOI] [PubMed] [Google Scholar]
- 47. Venero C., Herrero A. I., Touyarot K., Cambon K., López-Fernández M. A., Berezin V., Bock E., Sandi C. (2006) Hippocampal up-regulation of NCAM expression and polysialylation plays a key role on spatial memory. Eur. J. Neurosci. 23, 1585–1595 [DOI] [PubMed] [Google Scholar]
- 48. Becker C. G., Artola A., Gerardy-Schahn R., Becker T., Welzl H., Schachner M. (1996) The polysialic acid modification of the neural cell adhesion molecule is involved in spatial learning and hippocampal long-term potentiation. J. Neurosci. Res. 45, 143–152 [DOI] [PubMed] [Google Scholar]
- 49. Seki T., Rutishauser U. (1998) Removal of polysialic acid-neural cell adhesion molecule induces aberrant mossy fiber innervation and ectopic synaptogenesis in the hippocampus. J. Neurosci. 18, 3757–3766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Markram K., Gerardy-Schahn R., Sandi C. (2007) Selective learning and memory impairments in mice deficient for polysialylated NCAM in adulthood. Neuroscience 144, 788–796 [DOI] [PubMed] [Google Scholar]
- 51. Angata K., Long J. M., Bukalo O., Lee W., Dityatev A., Wynshaw-Boris A., Schachner M., Fukuda M., Marth J. D. (2004) Sialyltransferase ST8Sia-II assembles a subset of polysialic acid that directs hippocampal axonal targeting and promotes fear behavior. J. Biol. Chem. 279, 32603–32613 [DOI] [PubMed] [Google Scholar]
- 52. Stoenica L., Senkov O., Gerardy-Schahn R., Weinhold B., Schachner M., Dityatev A. (2006) In vivo synaptic plasticity in the dentate gyrus of mice deficient in the neural cell adhesion molecule NCAM or its polysialic acid. Eur. J. Neurosci. 23, 2255–2264 [DOI] [PubMed] [Google Scholar]
- 53. Dityatev A., Dityateva G., Sytnyk V., Delling M., Toni N., Nikonenko I., Muller D., Schachner M. (2004) Polysialylated neural cell adhesion molecule promotes remodeling and formation of hippocampal synapses. J. Neurosci. 24, 9372–9382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Brocco M. A., Frasch A. C., (2006) Interfering polysialyltransferase ST8SiaII/STX mRNA inhibits neurite growth during early hippocampal development. FEBS Lett. 580, 4723–4726 [DOI] [PubMed] [Google Scholar]
- 55. Muller D., Wang C., Skibo G., Toni N., Cremer H., Calaora V., Rougon G., Kiss J. Z. (1996) PSA-NCAM is required for activity-induced synaptic plasticity. Neuron 17, 413–422 [DOI] [PubMed] [Google Scholar]
- 56. Eckhardt M., Bukalo O., Chazal G., Wang L., Goridis C., Schachner M., Gerardy-Schahn R., Cremer H., Dityatev A. (2000) Mice deficient in the polysialyltransferase ST8SiaIV/PST-1 allow discrimination of the roles of neural cell adhesion molecule protein and polysialic acid in neural development and synaptic plasticity. J. Neurosci. 20, 5234–5244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hammond M. S., Sims C., Parameshwaran K., Suppiramaniam V., Schachner M., Dityatev A. (2006) Neural cell adhesion molecule-associated polysialic acid inhibits NR2B-containing N-methyl-d-aspartate receptors and prevents glutamate-induced cell death. J. Biol. Chem. 281, 34859–34869 [DOI] [PubMed] [Google Scholar]
- 58. Cremer H., Chazal G., Carleton A., Goridis C., Vincent J. D., Lledo P. M. (1998) Long-term but not short-term plasticity at mossy fiber synapses is impaired in neural cell adhesion molecule-deficient mice. Proc. Natl. Acad. Sci. U.S.A. 95, 13242–13247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Vaithianathan T., Matthias K., Bahr B., Schachner M., Suppiramaniam V., Dityatev A., Steinhaüser C. (2004) Neural cell adhesion molecule-associated polysialic acid potentiates α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptor currents. J. Biol. Chem. 279, 47975–47984 [DOI] [PubMed] [Google Scholar]
- 60. Kochlamazashvili G., Senkov O., Grebenyuk S., Robinson C., Xiao M. F., Stummeyer K., Gerardy-Schahn R., Engel A. K., Feig L., Semyanov A., Suppiramaniam V., Schachner M., Dityatev A. (2010) Restoration of synaptic plasticity and learning in young and aged NCAM-deficient mice by enhancing neurotransmission mediated by GluN2A-containing NMDA receptors. J. Neurosci. 30, 4171–4183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Yamaguchi H., Shiraishi M., Fukami K., Tanabe A., Ikeda-Matsuo Y., Naito Y., Sasaki Y. (2009) MARCKS regulates lamellipodia formation induced by IGF-I via association with PIP2 and β-actin at membrane microdomains. J. Cell. Physiol. 220, 748–755 [DOI] [PubMed] [Google Scholar]
- 62. Watterson J. M., Watson D. G., Meyer E. M., Lenox R. H. (2002) A role for protein kinase C and its substrates in the action of valproic acid in the brain. Implications for neural plasticity. Brain Res. 934, 69–80 [DOI] [PubMed] [Google Scholar]
- 63. Shiraishi M., Tanabe A., Saito N., Sasaki Y., (2006) Unphosphorylated MARCKS is involved in neurite initiation induced by insulin-like growth factor-I in SH-SY5Y cells. J. Cell. Physiol. 209, 1029–1038 [DOI] [PubMed] [Google Scholar]
- 64. Gatlin J. C., Estrada-Bernal A., Sanford S. D., Pfenninger K. H. (2006) Myristoylated, alanine-rich C-kinase substrate phosphorylation regulates growth cone adhesion and pathfinding. Mol. Biol. Cell 17, 5115–5130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Li H., Chen G., Zhou B., Duan S. (2008) Actin filament assembly by myristoylated alanine-rich C kinase substrate-phosphatidylinositol-4,5-diphosphate signaling is critical for dendrite branching. Mol. Biol. Cell 19, 4804–4813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Calabrese B., Halpain S. (2005) Essential role for the PKC target MARCKS in maintaining dendritic spine morphology. Neuron 48, 77–90 [DOI] [PubMed] [Google Scholar]
- 67. Solomonia R. O., Apkhazava D., Nozadze M., Jackson A. P., McCabe B. J., Horn G. (2008) Different forms of MARCKS protein are involved in memory formation in the learning process of imprinting. Exp. Brain Res. 188, 323–330 [DOI] [PubMed] [Google Scholar]
- 68. Timofeeva O. A., Eddins D., Yakel J. L., Blackshear P. J., Levin E. D. (2010) Hippocampal infusions of MARCKS peptides impair memory of rats on the radial-arm maze. Brain Res. 1308, 147–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. McNamara R. K., Hussain R. J., Simon E. J., Stumpo D. J., Blackshear P. J., Abel T., Lenox R. H. (2005) Effect of myristoylated alanine-rich C kinase substrate (MARCKS) overexpression on hippocampus-dependent learning and hippocampal synaptic plasticity in MARCKS transgenic mice. Hippocampus 15, 675–683 [DOI] [PubMed] [Google Scholar]
- 70. Hussain R. J., Stumpo D. J., Blackshear P. J., Lenox R. H., Abel T., McNamara R. K. (2006) Myristoylated alanine-rich C kinase substrate (MARCKS) heterozygous mutant mice exhibit deficits in hippocampal mossy fiber-CA3 long term potentiation. Hippocampus 16, 495–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Gascard P., Tran D., Sauvage M., Sulpice J. C., Fukami K., Takenawa T., Claret M., Giraud F. (1991) Asymmetric distribution of phosphoinositides and phosphatidic acid in the human erythrocyte membrane. Biochim. Biophys. Acta 1069, 27–36 [DOI] [PubMed] [Google Scholar]
- 72. Senkov O., Tikhobrazova O., Dityatev A. (2012) PSA-NCAM. Synaptic functions mediated by its interactions with proteoglycans and glutamate receptors. Int. J. Biochem. Cell Biol. 44, 591–595 [DOI] [PubMed] [Google Scholar]