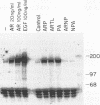
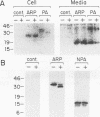
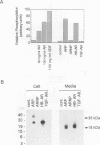
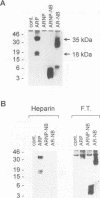
Abstract
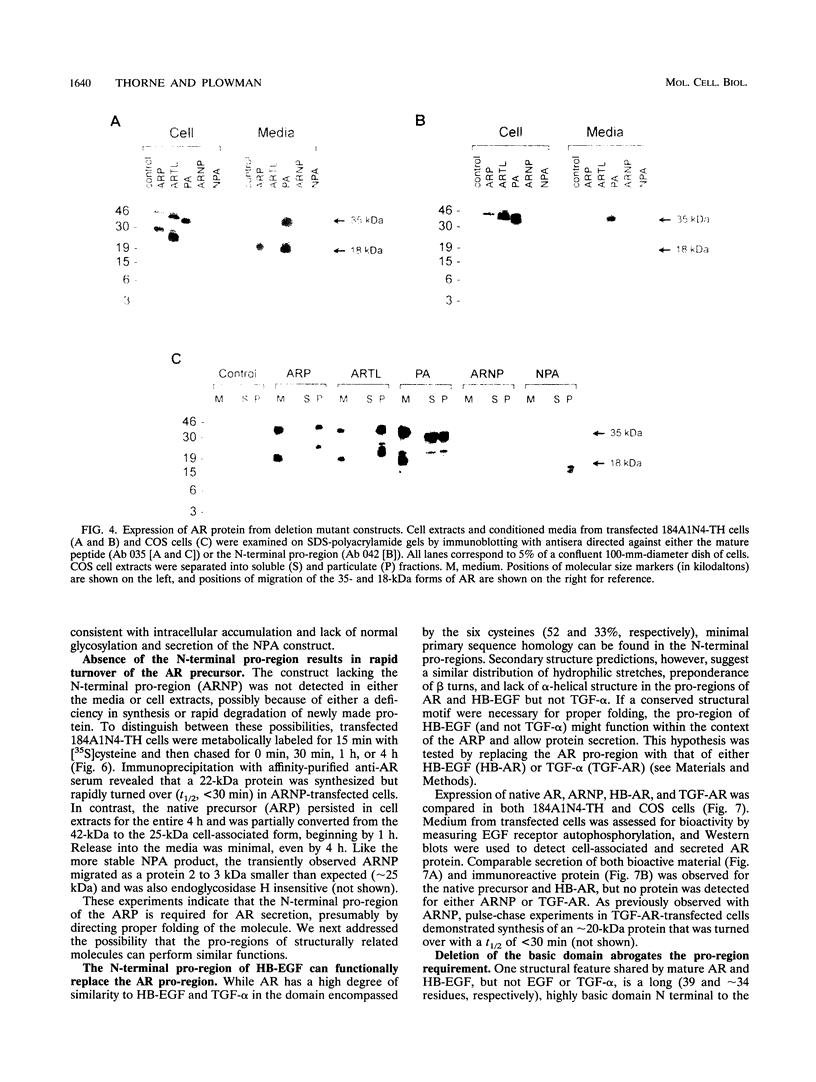
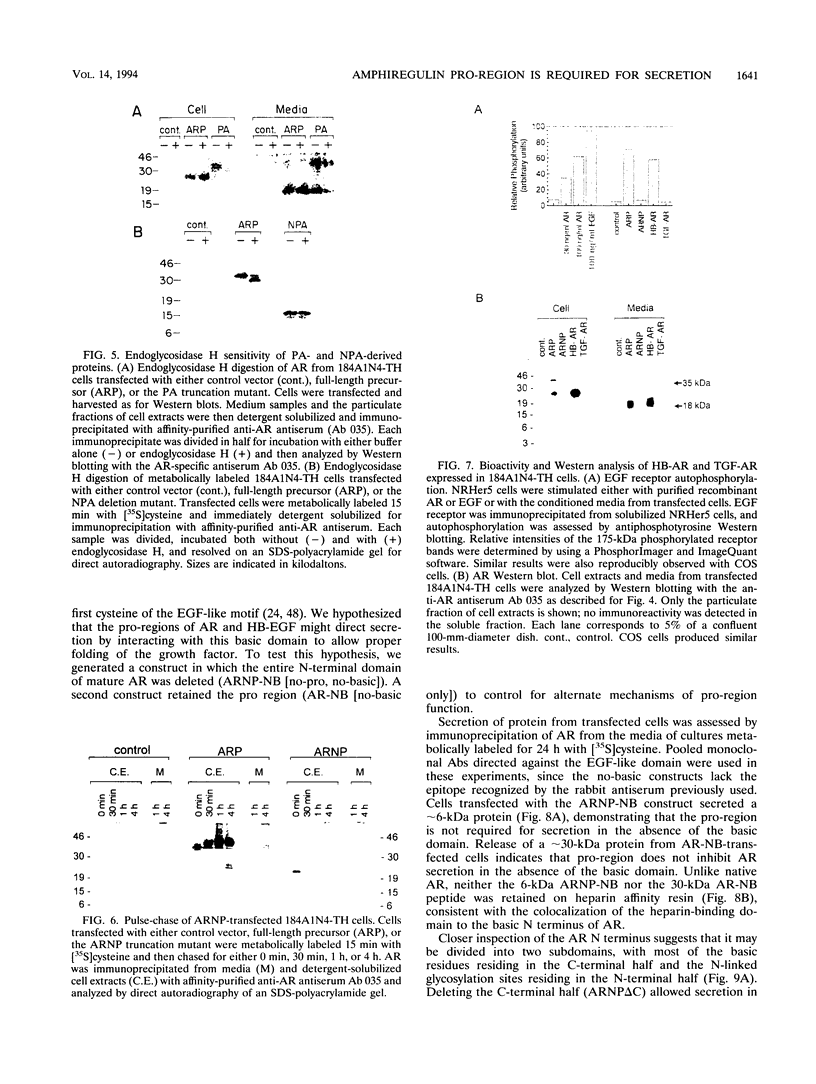
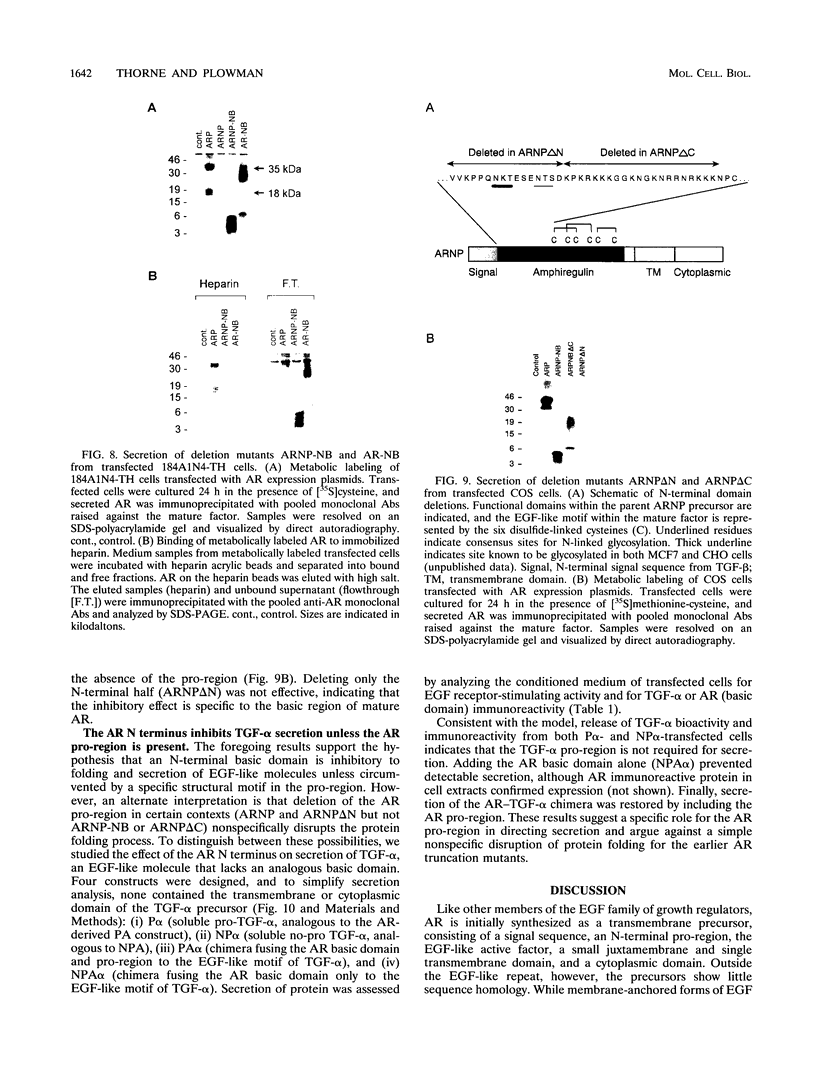
The five members of the human epidermal growth factor (EGF) family (EGF, transforming growth factor alpha [TGF-alpha], heparin-binding EGF-like growth factor [HB-EGF], betacellulin, and amphiregulin [AR]) are synthesized as transmembrane proteins whose extracellular domains are proteolytically processed to release the biologically active mature growth factors. These factors all activate the EGF receptor, but in contrast to EGF and TGF-alpha, the mature forms of HB-EGF and AR are also glycosylated, heparin-binding proteins. We have constructed a series of mutants to examine the influence of the distinct precursor domains in the biosynthesis of AR. The transmembrane and cytoplasmic domains of the precursor are not required for secretion of bioactive AR from either COS or mammary epithelium-derived cells, although proteolytic removal of the N-terminal pro-region is less efficient in the absence of the membrane anchor. Deletion of the N-terminal pro-region, however, results in rapid intracellular degradation of the molecule with no detectable secretion of active growth factor. AR secretion is preserved by replacing the native pro-region with the corresponding domain of the HB-EGF precursor but not with that of the TGF-alpha precursor. In the absence of any N-terminal pro-region, secretion of the molecule is restored by deleting the N-terminal heparin-binding domain of mature AR. Both EGF and TGF-alpha, in contrast, can be secreted without their pro-regions. However, if the protein is fused with the AR heparin-binding domain, TGF-alpha secretion is inhibited unless the AR pro-region is also present. We propose that the heparin-binding domain of mature AR necessitates the presence of a specific structural motif in an N-terminal pro-region to permit proper folding, and thus secretion, of a bioactive molecule.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anklesaria P., Teixidó J., Laiho M., Pierce J. H., Greenberger J. S., Massagué J. Cell-cell adhesion mediated by binding of membrane-anchored transforming growth factor alpha to epidermal growth factor receptors promotes cell proliferation. Proc Natl Acad Sci U S A. 1990 May;87(9):3289–3293. doi: 10.1073/pnas.87.9.3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker D., Sohl J. L., Agard D. A. A protein-folding reaction under kinetic control. Nature. 1992 Mar 19;356(6366):263–265. doi: 10.1038/356263a0. [DOI] [PubMed] [Google Scholar]
- Bell G. I., Fong N. M., Stempien M. M., Wormsted M. A., Caput D., Ku L. L., Urdea M. S., Rall L. B., Sanchez-Pescador R. Human epidermal growth factor precursor: cDNA sequence, expression in vitro and gene organization. Nucleic Acids Res. 1986 Nov 11;14(21):8427–8446. doi: 10.1093/nar/14.21.8427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betsholtz C., Johnsson A., Heldin C. H., Westermark B., Lind P., Urdea M. S., Eddy R., Shows T. B., Philpott K., Mellor A. L. cDNA sequence and chromosomal localization of human platelet-derived growth factor A-chain and its expression in tumour cell lines. Nature. 1986 Apr 24;320(6064):695–699. doi: 10.1038/320695a0. [DOI] [PubMed] [Google Scholar]
- Bjorge J. D., Chan T. O., Antczak M., Kung H. J., Fujita D. J. Activated type I phosphatidylinositol kinase is associated with the epidermal growth factor (EGF) receptor following EGF stimulation. Proc Natl Acad Sci U S A. 1990 May;87(10):3816–3820. doi: 10.1073/pnas.87.10.3816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasband A. J., Rogers K. T., Chen X. R., Azizkhan J. C., Lee D. C. Characterization of the rat transforming growth factor alpha gene and identification of promoter sequences. Mol Cell Biol. 1990 May;10(5):2111–2121. doi: 10.1128/mcb.10.5.2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosenberg M. W., Pandiella A., Massagué J. The cytoplasmic carboxy-terminal amino acid specifies cleavage of membrane TGF alpha into soluble growth factor. Cell. 1992 Dec 24;71(7):1157–1165. doi: 10.1016/s0092-8674(05)80064-9. [DOI] [PubMed] [Google Scholar]
- Brachmann R., Lindquist P. B., Nagashima M., Kohr W., Lipari T., Napier M., Derynck R. Transmembrane TGF-alpha precursors activate EGF/TGF-alpha receptors. Cell. 1989 Feb 24;56(4):691–700. doi: 10.1016/0092-8674(89)90591-6. [DOI] [PubMed] [Google Scholar]
- Brannan C. I., Lyman S. D., Williams D. E., Eisenman J., Anderson D. M., Cosman D., Bedell M. A., Jenkins N. A., Copeland N. G. Steel-Dickie mutation encodes a c-kit ligand lacking transmembrane and cytoplasmic domains. Proc Natl Acad Sci U S A. 1991 Jun 1;88(11):4671–4674. doi: 10.1073/pnas.88.11.4671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunner A. M., Marquardt H., Malacko A. R., Lioubin M. N., Purchio A. F. Site-directed mutagenesis of cysteine residues in the pro region of the transforming growth factor beta 1 precursor. Expression and characterization of mutant proteins. J Biol Chem. 1989 Aug 15;264(23):13660–13664. [PubMed] [Google Scholar]
- Cook P. W., Mattox P. A., Keeble W. W., Pittelkow M. R., Plowman G. D., Shoyab M., Adelman J. P., Shipley G. D. A heparin sulfate-regulated human keratinocyte autocrine factor is similar or identical to amphiregulin. Mol Cell Biol. 1991 May;11(5):2547–2557. doi: 10.1128/mcb.11.5.2547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derynck R., Jarrett J. A., Chen E. Y., Eaton D. H., Bell J. R., Assoian R. K., Roberts A. B., Sporn M. B., Goeddel D. V. Human transforming growth factor-beta complementary DNA sequence and expression in normal and transformed cells. Nature. 1985 Aug 22;316(6030):701–705. doi: 10.1038/316701a0. [DOI] [PubMed] [Google Scholar]
- Derynck R., Roberts A. B., Winkler M. E., Chen E. Y., Goeddel D. V. Human transforming growth factor-alpha: precursor structure and expression in E. coli. Cell. 1984 Aug;38(1):287–297. doi: 10.1016/0092-8674(84)90550-6. [DOI] [PubMed] [Google Scholar]
- Dobashi Y., Stern D. F. Membrane-anchored forms of EGF stimulate focus formation and intercellular communication. Oncogene. 1991 Jul;6(7):1151–1159. [PubMed] [Google Scholar]
- Ehlers M. R., Riordan J. F. Membrane proteins with soluble counterparts: role of proteolysis in the release of transmembrane proteins. Biochemistry. 1991 Oct 22;30(42):10065–10074. doi: 10.1021/bi00106a001. [DOI] [PubMed] [Google Scholar]
- Engler D. A., Matsunami R. K., Campion S. R., Stringer C. D., Stevens A., Niyogi S. K. Cloning of authentic human epidermal growth factor as a bacterial secretory protein and its initial structure-function analysis by site-directed mutagenesis. J Biol Chem. 1988 Sep 5;263(25):12384–12390. [PubMed] [Google Scholar]
- Flanagan J. G., Chan D. C., Leder P. Transmembrane form of the kit ligand growth factor is determined by alternative splicing and is missing in the Sld mutant. Cell. 1991 Mar 8;64(5):1025–1035. doi: 10.1016/0092-8674(91)90326-t. [DOI] [PubMed] [Google Scholar]
- Gentry L. E., Lioubin M. N., Purchio A. F., Marquardt H. Molecular events in the processing of recombinant type 1 pre-pro-transforming growth factor beta to the mature polypeptide. Mol Cell Biol. 1988 Oct;8(10):4162–4168. doi: 10.1128/mcb.8.10.4162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez S., Boileau G., Zollinger L., Nault C., Rholam M., Cohen P. Site-specific mutagenesis identifies amino acid residues critical in prohormone processing. EMBO J. 1989 Oct;8(10):2911–2916. doi: 10.1002/j.1460-2075.1989.tb08440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray A. M., Mason A. J. Requirement for activin A and transforming growth factor--beta 1 pro-regions in homodimer assembly. Science. 1990 Mar 16;247(4948):1328–1330. doi: 10.1126/science.2315700. [DOI] [PubMed] [Google Scholar]
- Higashiyama S., Abraham J. A., Miller J., Fiddes J. C., Klagsbrun M. A heparin-binding growth factor secreted by macrophage-like cells that is related to EGF. Science. 1991 Feb 22;251(4996):936–939. doi: 10.1126/science.1840698. [DOI] [PubMed] [Google Scholar]
- Higashiyama S., Lau K., Besner G. E., Abraham J. A., Klagsbrun M. Structure of heparin-binding EGF-like growth factor. Multiple forms, primary structure, and glycosylation of the mature protein. J Biol Chem. 1992 Mar 25;267(9):6205–6212. [PubMed] [Google Scholar]
- Hurtley S. M., Helenius A. Protein oligomerization in the endoplasmic reticulum. Annu Rev Cell Biol. 1989;5:277–307. doi: 10.1146/annurev.cb.05.110189.001425. [DOI] [PubMed] [Google Scholar]
- Ignotz R. A., Kelly B., Davis R. J., Massagué J. Biologically active precursor for transforming growth factor type alpha, released by retrovirally transformed cells. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6307–6311. doi: 10.1073/pnas.83.17.6307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikemura H., Takagi H., Inouye M. Requirement of pro-sequence for the production of active subtilisin E in Escherichia coli. J Biol Chem. 1987 Jun 5;262(16):7859–7864. [PubMed] [Google Scholar]
- Jameson B. A., Wolf H. The antigenic index: a novel algorithm for predicting antigenic determinants. Comput Appl Biosci. 1988 Mar;4(1):181–186. doi: 10.1093/bioinformatics/4.1.181. [DOI] [PubMed] [Google Scholar]
- Johnson G. R., Saeki T., Gordon A. W., Shoyab M., Salomon D. S., Stromberg K. Autocrine action of amphiregulin in a colon carcinoma cell line and immunocytochemical localization of amphiregulin in human colon. J Cell Biol. 1992 Aug;118(3):741–751. doi: 10.1083/jcb.118.3.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen M. J., Cantor A. B., Furie B. C., Brown C. L., Shoemaker C. B., Furie B. Recognition site directing vitamin K-dependent gamma-carboxylation resides on the propeptide of factor IX. Cell. 1987 Jan 30;48(2):185–191. doi: 10.1016/0092-8674(87)90422-3. [DOI] [PubMed] [Google Scholar]
- Kriegler M., Perez C., DeFay K., Albert I., Lu S. D. A novel form of TNF/cachectin is a cell surface cytotoxic transmembrane protein: ramifications for the complex physiology of TNF. Cell. 1988 Apr 8;53(1):45–53. doi: 10.1016/0092-8674(88)90486-2. [DOI] [PubMed] [Google Scholar]
- Lazar E., Watanabe S., Dalton S., Sporn M. B. Transforming growth factor alpha: mutation of aspartic acid 47 and leucine 48 results in different biological activities. Mol Cell Biol. 1988 Mar;8(3):1247–1252. doi: 10.1128/mcb.8.3.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S., Plowman G. D., Buckley S. D., Shipley G. D. Heparin inhibition of autonomous growth implicates amphiregulin as an autocrine growth factor for normal human mammary epithelial cells. J Cell Physiol. 1992 Oct;153(1):103–111. doi: 10.1002/jcp.1041530114. [DOI] [PubMed] [Google Scholar]
- Luetteke N. C., Michalopoulos G. K., Teixidó J., Gilmore R., Massagué J., Lee D. C. Characterization of high molecular weight transforming growth factor alpha produced by rat hepatocellular carcinoma cells. Biochemistry. 1988 Aug 23;27(17):6487–6494. doi: 10.1021/bi00417a043. [DOI] [PubMed] [Google Scholar]
- Massagué J., Pandiella A. Membrane-anchored growth factors. Annu Rev Biochem. 1993;62:515–541. doi: 10.1146/annurev.bi.62.070193.002503. [DOI] [PubMed] [Google Scholar]
- Mercola M., Deininger P. L., Shamah S. M., Porter J., Wang C. Y., Stiles C. D. Dominant-negative mutants of a platelet-derived growth factor gene. Genes Dev. 1990 Dec;4(12B):2333–2341. doi: 10.1101/gad.4.12b.2333. [DOI] [PubMed] [Google Scholar]
- Miyazono K., Hellman U., Wernstedt C., Heldin C. H. Latent high molecular weight complex of transforming growth factor beta 1. Purification from human platelets and structural characterization. J Biol Chem. 1988 May 5;263(13):6407–6415. [PubMed] [Google Scholar]
- Miyazono K., Olofsson A., Colosetti P., Heldin C. H. A role of the latent TGF-beta 1-binding protein in the assembly and secretion of TGF-beta 1. EMBO J. 1991 May;10(5):1091–1101. doi: 10.1002/j.1460-2075.1991.tb08049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mroczkowski B., Reich M., Chen K., Bell G. I., Cohen S. Recombinant human epidermal growth factor precursor is a glycosylated membrane protein with biological activity. Mol Cell Biol. 1989 Jul;9(7):2771–2778. doi: 10.1128/mcb.9.7.2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naglich J. G., Metherall J. E., Russell D. W., Eidels L. Expression cloning of a diphtheria toxin receptor: identity with a heparin-binding EGF-like growth factor precursor. Cell. 1992 Jun 12;69(6):1051–1061. doi: 10.1016/0092-8674(92)90623-k. [DOI] [PubMed] [Google Scholar]
- Pandiella A., Massagué J. Cleavage of the membrane precursor for transforming growth factor alpha is a regulated process. Proc Natl Acad Sci U S A. 1991 Mar 1;88(5):1726–1730. doi: 10.1073/pnas.88.5.1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandiella A., Massagué J. Multiple signals activate cleavage of the membrane transforming growth factor-alpha precursor. J Biol Chem. 1991 Mar 25;266(9):5769–5773. [PubMed] [Google Scholar]
- Pelham H. R. Control of protein exit from the endoplasmic reticulum. Annu Rev Cell Biol. 1989;5:1–23. doi: 10.1146/annurev.cb.05.110189.000245. [DOI] [PubMed] [Google Scholar]
- Perez C., Albert I., DeFay K., Zachariades N., Gooding L., Kriegler M. A nonsecretable cell surface mutant of tumor necrosis factor (TNF) kills by cell-to-cell contact. Cell. 1990 Oct 19;63(2):251–258. doi: 10.1016/0092-8674(90)90158-b. [DOI] [PubMed] [Google Scholar]
- Plowman G. D., Green J. M., McDonald V. L., Neubauer M. G., Disteche C. M., Todaro G. J., Shoyab M. The amphiregulin gene encodes a novel epidermal growth factor-related protein with tumor-inhibitory activity. Mol Cell Biol. 1990 May;10(5):1969–1981. doi: 10.1128/mcb.10.5.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rall L. B., Scott J., Bell G. I., Crawford R. J., Penschow J. D., Niall H. D., Coghlan J. P. Mouse prepro-epidermal growth factor synthesis by the kidney and other tissues. Nature. 1985 Jan 17;313(5999):228–231. doi: 10.1038/313228a0. [DOI] [PubMed] [Google Scholar]
- Ray P., Moy F. J., Montelione G. T., Liu J. F., Narang S. A., Scheraga H. A., Wu R. Structure-function studies of murine epidermal growth factor: expression and site-directed mutagenesis of epidermal growth factor gene. Biochemistry. 1988 Sep 20;27(19):7289–7295. doi: 10.1021/bi00419a017. [DOI] [PubMed] [Google Scholar]
- Rose J. K., Doms R. W. Regulation of protein export from the endoplasmic reticulum. Annu Rev Cell Biol. 1988;4:257–288. doi: 10.1146/annurev.cb.04.110188.001353. [DOI] [PubMed] [Google Scholar]
- Sasada R., Ono Y., Taniyama Y., Shing Y., Folkman J., Igarashi K. Cloning and expression of cDNA encoding human betacellulin, a new member of the EGF family. Biochem Biophys Res Commun. 1993 Feb 15;190(3):1173–1179. doi: 10.1006/bbrc.1993.1173. [DOI] [PubMed] [Google Scholar]
- Scott J., Selby M., Urdea M., Quiroga M., Bell G. I., Rutter W. J. Isolation and nucleotide sequence of a cDNA encoding the precursor of mouse nerve growth factor. Nature. 1983 Apr 7;302(5908):538–540. doi: 10.1038/302538a0. [DOI] [PubMed] [Google Scholar]
- Seed B., Aruffo A. Molecular cloning of the CD2 antigen, the T-cell erythrocyte receptor, by a rapid immunoselection procedure. Proc Natl Acad Sci U S A. 1987 May;84(10):3365–3369. doi: 10.1073/pnas.84.10.3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevarino K. A., Stork P., Ventimiglia R., Mandel G., Goodman R. H. Amino-terminal sequences of prosomatostatin direct intracellular targeting but not processing specificity. Cell. 1989 Apr 7;57(1):11–19. doi: 10.1016/0092-8674(89)90167-0. [DOI] [PubMed] [Google Scholar]
- Shing Y., Christofori G., Hanahan D., Ono Y., Sasada R., Igarashi K., Folkman J. Betacellulin: a mitogen from pancreatic beta cell tumors. Science. 1993 Mar 12;259(5101):1604–1607. doi: 10.1126/science.8456283. [DOI] [PubMed] [Google Scholar]
- Shoyab M., Plowman G. D., McDonald V. L., Bradley J. G., Todaro G. J. Structure and function of human amphiregulin: a member of the epidermal growth factor family. Science. 1989 Feb 24;243(4894 Pt 1):1074–1076. doi: 10.1126/science.2466334. [DOI] [PubMed] [Google Scholar]
- Stein J., Borzillo G. V., Rettenmier C. W. Direct stimulation of cells expressing receptors for macrophage colony-stimulating factor (CSF-1) by a plasma membrane-bound precursor of human CSF-1. Blood. 1990 Oct 1;76(7):1308–1314. [PubMed] [Google Scholar]
- Stern D. F., Hare D. L., Cecchini M. A., Weinberg R. A. Construction of a novel oncogene based on synthetic sequences encoding epidermal growth factor. Science. 1987 Jan 16;235(4786):321–324. doi: 10.1126/science.3492043. [DOI] [PubMed] [Google Scholar]
- Suter U., Heymach J. V., Jr, Shooter E. M. Two conserved domains in the NGF propeptide are necessary and sufficient for the biosynthesis of correctly processed and biologically active NGF. EMBO J. 1991 Sep;10(9):2395–2400. doi: 10.1002/j.1460-2075.1991.tb07778.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixido J., Wong S. T., Lee D. C., Massagué J. Generation of transforming growth factor-alpha from the cell surface by an O-glycosylation-independent multistep process. J Biol Chem. 1990 Apr 15;265(11):6410–6415. [PubMed] [Google Scholar]
- Teixidó J., Massagué J. Structural properties of a soluble bioactive precursor for transforming growth factor-alpha. J Biol Chem. 1988 Mar 15;263(8):3924–3929. [PubMed] [Google Scholar]
- Toksoz D., Zsebo K. M., Smith K. A., Hu S., Brankow D., Suggs S. V., Martin F. H., Williams D. A. Support of human hematopoiesis in long-term bone marrow cultures by murine stromal cells selectively expressing the membrane-bound and secreted forms of the human homolog of the steel gene product, stem cell factor. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7350–7354. doi: 10.1073/pnas.89.16.7350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakefield L. M., Smith D. M., Flanders K. C., Sporn M. B. Latent transforming growth factor-beta from human platelets. A high molecular weight complex containing precursor sequences. J Biol Chem. 1988 Jun 5;263(16):7646–7654. [PubMed] [Google Scholar]
- Weissman J. S., Kim P. S. The pro region of BPTI facilitates folding. Cell. 1992 Nov 27;71(5):841–851. doi: 10.1016/0092-8674(92)90559-u. [DOI] [PubMed] [Google Scholar]
- Winther J. R., Sørensen P. Propeptide of carboxypeptidase Y provides a chaperone-like function as well as inhibition of the enzymatic activity. Proc Natl Acad Sci U S A. 1991 Oct 15;88(20):9330–9334. doi: 10.1073/pnas.88.20.9330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiren K. M., Potts J. T., Jr, Kronenberg H. M. Importance of the propeptide sequence of human preproparathyroid hormone for signal sequence function. J Biol Chem. 1988 Dec 25;263(36):19771–19777. [PubMed] [Google Scholar]
- Wise R. J., Pittman D. D., Handin R. I., Kaufman R. J., Orkin S. H. The propeptide of von Willebrand factor independently mediates the assembly of von Willebrand multimers. Cell. 1988 Jan 29;52(2):229–236. doi: 10.1016/0092-8674(88)90511-9. [DOI] [PubMed] [Google Scholar]
- Wong S. T., Winchell L. F., McCune B. K., Earp H. S., Teixidó J., Massagué J., Herman B., Lee D. C. The TGF-alpha precursor expressed on the cell surface binds to the EGF receptor on adjacent cells, leading to signal transduction. Cell. 1989 Feb 10;56(3):495–506. doi: 10.1016/0092-8674(89)90252-3. [DOI] [PubMed] [Google Scholar]
- Young R. M., Mendoza A. E., Collins T., Orkin S. H. Alternatively spliced platelet-derived growth factor A-chain transcripts are not tumor specific but encode normal cellular proteins. Mol Cell Biol. 1990 Nov;10(11):6051–6054. doi: 10.1128/mcb.10.11.6051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X. L., Ohta Y., Jordan F., Inouye M. Pro-sequence of subtilisin can guide the refolding of denatured subtilisin in an intermolecular process. Nature. 1989 Jun 8;339(6224):483–484. doi: 10.1038/339483a0. [DOI] [PubMed] [Google Scholar]