Abstract
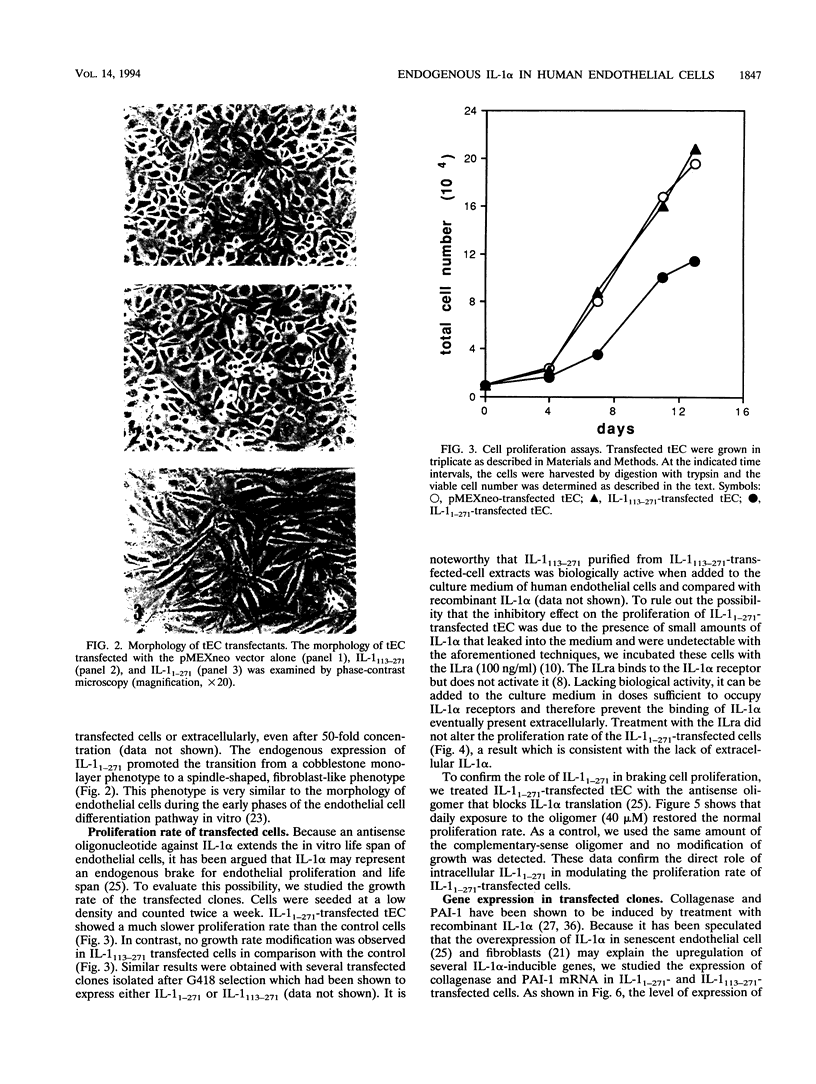
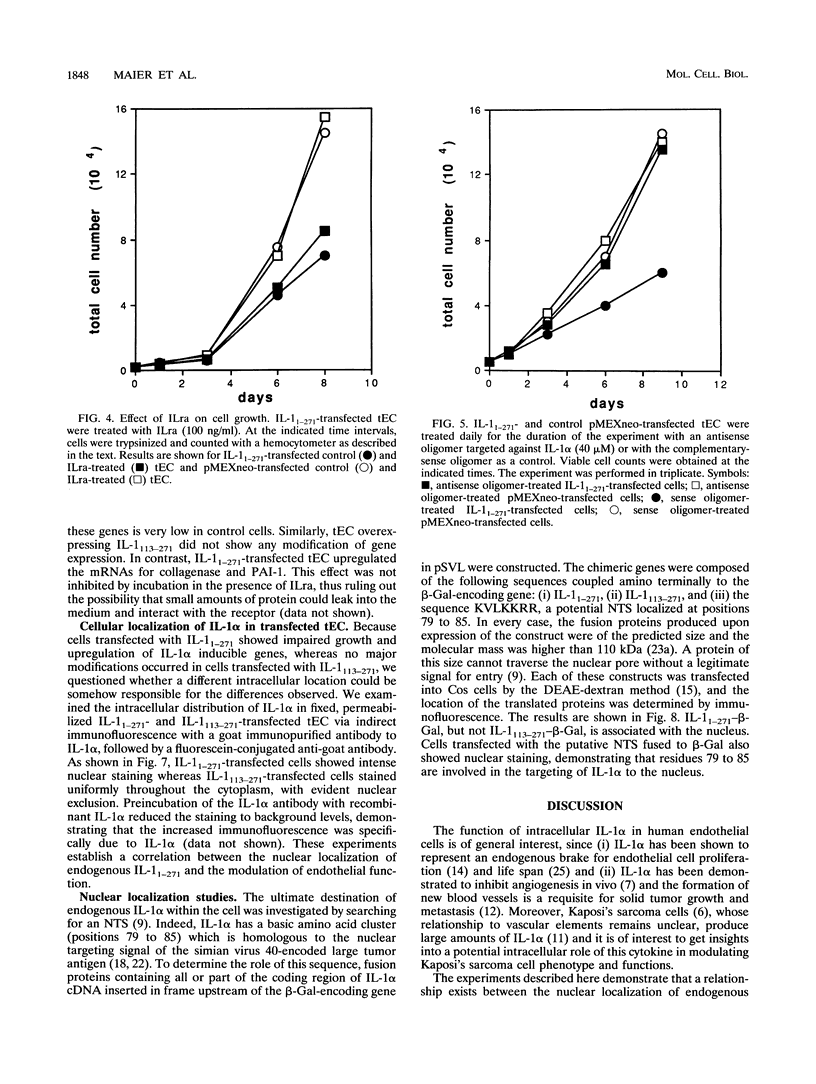
We have previously shown that the signal peptideless cytokine interleukin 1 alpha (IL-1 alpha) may play a role as an intracellular regulator of human endothelial cell senescence (J. A. M. Maier, P. Voulalas, D. Roeder, and T. Maciag, Science 249:1570-1574, 1990). To investigate the potential intracellular function of IL-1 alpha, transformed endothelial cells were transfected with the human cDNAs that code for the two forms of IL-1 alpha, the precursor molecule IL-1(1-271) and the mature protein IL-1(113-271). The subcellular localization of the two different polypeptides was investigated directly or by using chimeric genes constructed by fusion of different fragments of the IL-1 alpha gene and the beta-galactosidase open reading frames. The IL-1(113-271) protein was cytoplasmic, while IL-1(1-271) was nuclear. The basic cluster at the NH2 terminus of IL-1, KVLKKRR, has been shown to mediate IL-1 alpha nuclear targeting. Moreover, nuclear localization of IL-1 alpha correlates with impaired cell growth and expression of some IL-1 alpha-inducible genes. These results suggest that transport of endogenous IL-1(1-271) into the nucleus is required for it to modulate endothelial cell function.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acland P., Dixon M., Peters G., Dickson C. Subcellular fate of the int-2 oncoprotein is determined by choice of initiation codon. Nature. 1990 Feb 15;343(6259):662–665. doi: 10.1038/343662a0. [DOI] [PubMed] [Google Scholar]
- Baldin V., Roman A. M., Bosc-Bierne I., Amalric F., Bouche G. Translocation of bFGF to the nucleus is G1 phase cell cycle specific in bovine aortic endothelial cells. EMBO J. 1990 May;9(5):1511–1517. doi: 10.1002/j.1460-2075.1990.tb08269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boraschi D., Rambaldi A., Sica A., Ghiara P., Colotta F., Wang J. M., de Rossi M., Zoia C., Remuzzi G., Bussolino F. Endothelial cells express the interleukin-1 receptor type I. Blood. 1991 Sep 1;78(5):1262–1267. [PubMed] [Google Scholar]
- Carruth L. M., Demczuk S., Mizel S. B. Involvement of a calpain-like protease in the processing of the murine interleukin 1 alpha precursor. J Biol Chem. 1991 Jul 5;266(19):12162–12167. [PubMed] [Google Scholar]
- Colotta F., Bussolino F., Polentarutti N., Guglielmetti A., Sironi M., Bocchietto E., De Rossi M., Mantovani A. Differential expression of the common beta and specific alpha chains of the receptors for GM-CSF, IL-3, and IL-5 in endothelial cells. Exp Cell Res. 1993 Jun;206(2):311–317. doi: 10.1006/excr.1993.1151. [DOI] [PubMed] [Google Scholar]
- Corbeil J., Evans L. A., Vasak E., Cooper D. A., Penny R. Culture and properties of cells derived from Kaposi sarcoma. J Immunol. 1991 May 1;146(9):2972–2976. [PubMed] [Google Scholar]
- Cozzolino F., Torcia M., Aldinucci D., Ziche M., Almerigogna F., Bani D., Stern D. M. Interleukin 1 is an autocrine regulator of human endothelial cell growth. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6487–6491. doi: 10.1073/pnas.87.17.6487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello C. A. Interleukin-1 and interleukin-1 antagonism. Blood. 1991 Apr 15;77(8):1627–1652. [PubMed] [Google Scholar]
- Dingwall C., Laskey R. A. Protein import into the cell nucleus. Annu Rev Cell Biol. 1986;2:367–390. doi: 10.1146/annurev.cb.02.110186.002055. [DOI] [PubMed] [Google Scholar]
- Eisenberg S. P., Evans R. J., Arend W. P., Verderber E., Brewer M. T., Hannum C. H., Thompson R. C. Primary structure and functional expression from complementary DNA of a human interleukin-1 receptor antagonist. Nature. 1990 Jan 25;343(6256):341–346. doi: 10.1038/343341a0. [DOI] [PubMed] [Google Scholar]
- Ensoli B., Nakamura S., Salahuddin S. Z., Biberfeld P., Larsson L., Beaver B., Wong-Staal F., Gallo R. C. AIDS-Kaposi's sarcoma-derived cells express cytokines with autocrine and paracrine growth effects. Science. 1989 Jan 13;243(4888):223–226. doi: 10.1126/science.2643161. [DOI] [PubMed] [Google Scholar]
- Folkman J., Klagsbrun M. Angiogenic factors. Science. 1987 Jan 23;235(4787):442–447. doi: 10.1126/science.2432664. [DOI] [PubMed] [Google Scholar]
- Gao M., Knipe D. M. Distal protein sequences can affect the function of a nuclear localization signal. Mol Cell Biol. 1992 Mar;12(3):1330–1339. doi: 10.1128/mcb.12.3.1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garfinkel S., Haines D. S., Brown S., Wessendorf J., Gillespie D. H., Maciag T. Interleukin-1 alpha mediates an alternative pathway for the antiproliferative action of poly(I.C) on human endothelial cells. J Biol Chem. 1992 Dec 5;267(34):24375–24378. [PubMed] [Google Scholar]
- Grenfell S., Smithers N., Miller K., Solari R. Receptor-mediated endocytosis and nuclear transport of human interleukin 1 alpha. Biochem J. 1989 Dec 15;264(3):813–822. doi: 10.1042/bj2640813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haskill S., Martin G., Van Le L., Morris J., Peace A., Bigler C. F., Jaffe G. J., Hammerberg C., Sporn S. A., Fong S. cDNA cloning of an intracellular form of the human interleukin 1 receptor antagonist associated with epithelium. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3681–3685. doi: 10.1073/pnas.88.9.3681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalderon D., Roberts B. L., Richardson W. D., Smith A. E. A short amino acid sequence able to specify nuclear location. Cell. 1984 Dec;39(3 Pt 2):499–509. doi: 10.1016/0092-8674(84)90457-4. [DOI] [PubMed] [Google Scholar]
- Kimura H. Schwannoma-derived growth factor must be transported into the nucleus to exert its mitogenic activity. Proc Natl Acad Sci U S A. 1993 Mar 15;90(6):2165–2169. doi: 10.1073/pnas.90.6.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi Y., Yamamoto K., Saido T., Kawasaki H., Oppenheim J. J., Matsushima K. Identification of calcium-activated neutral protease as a processing enzyme of human interleukin 1 alpha. Proc Natl Acad Sci U S A. 1990 Jul;87(14):5548–5552. doi: 10.1073/pnas.87.14.5548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S., Millis A. J., Baglioni C. Expression of interleukin 1-inducible genes and production of interleukin 1 by aging human fibroblasts. Proc Natl Acad Sci U S A. 1992 May 15;89(10):4683–4687. doi: 10.1073/pnas.89.10.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanford R. E., Butel J. S. Construction and characterization of an SV40 mutant defective in nuclear transport of T antigen. Cell. 1984 Jul;37(3):801–813. doi: 10.1016/0092-8674(84)90415-x. [DOI] [PubMed] [Google Scholar]
- Maciag T., Kadish J., Wilkins L., Stemerman M. B., Weinstein R. Organizational behavior of human umbilical vein endothelial cells. J Cell Biol. 1982 Sep;94(3):511–520. doi: 10.1083/jcb.94.3.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier J. A., Hla T., Maciag T. Cyclooxygenase is an immediate-early gene induced by interleukin-1 in human endothelial cells. J Biol Chem. 1990 Jul 5;265(19):10805–10808. [PubMed] [Google Scholar]
- Maier J. A., Voulalas P., Roeder D., Maciag T. Extension of the life-span of human endothelial cells by an interleukin-1 alpha antisense oligomer. Science. 1990 Sep 28;249(4976):1570–1574. doi: 10.1126/science.2218499. [DOI] [PubMed] [Google Scholar]
- Mantovani A., Bussolino F., Dejana E. Cytokine regulation of endothelial cell function. FASEB J. 1992 May;6(8):2591–2599. doi: 10.1096/fasebj.6.8.1592209. [DOI] [PubMed] [Google Scholar]
- March C. J., Mosley B., Larsen A., Cerretti D. P., Braedt G., Price V., Gillis S., Henney C. S., Kronheim S. R., Grabstein K. Cloning, sequence and expression of two distinct human interleukin-1 complementary DNAs. Nature. 1985 Jun 20;315(6021):641–647. doi: 10.1038/315641a0. [DOI] [PubMed] [Google Scholar]
- Martin-Zanca D., Oskam R., Mitra G., Copeland T., Barbacid M. Molecular and biochemical characterization of the human trk proto-oncogene. Mol Cell Biol. 1989 Jan;9(1):24–33. doi: 10.1128/mcb.9.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushima K., Taguchi M., Kovacs E. J., Young H. A., Oppenheim J. J. Intracellular localization of human monocyte associated interleukin 1 (IL 1) activity and release of biologically active IL 1 from monocytes by trypsin and plasmin. J Immunol. 1986 Apr 15;136(8):2883–2891. [PubMed] [Google Scholar]
- Miossec P., Cavender D., Ziff M. Production of interleukin 1 by human endothelial cells. J Immunol. 1986 Apr 1;136(7):2486–2491. [PubMed] [Google Scholar]
- Mizel S. B., Kilian P. L., Lewis J. C., Paganelli K. A., Chizzonite R. A. The interleukin 1 receptor. Dynamics of interleukin 1 binding and internalization in T cells and fibroblasts. J Immunol. 1987 May 1;138(9):2906–2912. [PubMed] [Google Scholar]
- Mosley B., Urdal D. L., Prickett K. S., Larsen A., Cosman D., Conlon P. J., Gillis S., Dower S. K. The interleukin-1 receptor binds the human interleukin-1 alpha precursor but not the interleukin-1 beta precursor. J Biol Chem. 1987 Mar 5;262(7):2941–2944. [PubMed] [Google Scholar]
- Nigg E. A., Baeuerle P. A., Lührmann R. Nuclear import-export: in search of signals and mechanisms. Cell. 1991 Jul 12;66(1):15–22. doi: 10.1016/0092-8674(91)90135-l. [DOI] [PubMed] [Google Scholar]
- Oberyszyn T. M., Sabourin C. L., Bijur G. N., Oberyszyn A. S., Boros L. G., Robertson F. M. Interleukin-1 alpha gene expression and localization of interleukin-1 alpha protein during tumor promotion. Mol Carcinog. 1993;7(4):238–248. doi: 10.1002/mc.2940070406. [DOI] [PubMed] [Google Scholar]
- Pober J. S., Cotran R. S. Cytokines and endothelial cell biology. Physiol Rev. 1990 Apr;70(2):427–451. doi: 10.1152/physrev.1990.70.2.427. [DOI] [PubMed] [Google Scholar]
- Quarto N., Talarico D., Florkiewicz R., Rifkin D. B. Selective expression of high molecular weight basic fibroblast growth factor confers a unique phenotype to NIH 3T3 cells. Cell Regul. 1991 Sep;2(9):699–708. doi: 10.1091/mbc.2.9.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakowicz-Szulczynska E. M., Rodeck U., Herlyn M., Koprowski H. Chromatin binding of epidermal growth factor, nerve growth factor, and platelet-derived growth factor in cells bearing the appropriate surface receptors. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3728–3732. doi: 10.1073/pnas.83.11.3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubartelli A., Cozzolino F., Talio M., Sitia R. A novel secretory pathway for interleukin-1 beta, a protein lacking a signal sequence. EMBO J. 1990 May;9(5):1503–1510. doi: 10.1002/j.1460-2075.1990.tb08268.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigler P. B. Transcriptional activation. Acid blobs and negative noodles. Nature. 1988 May 19;333(6170):210–212. doi: 10.1038/333210a0. [DOI] [PubMed] [Google Scholar]
- Silver P. A. How proteins enter the nucleus. Cell. 1991 Feb 8;64(3):489–497. doi: 10.1016/0092-8674(91)90233-o. [DOI] [PubMed] [Google Scholar]
- Singer I. I., Scott S., Hall G. L., Limjuco G., Chin J., Schmidt J. A. Interleukin 1 beta is localized in the cytoplasmic ground substance but is largely absent from the Golgi apparatus and plasma membranes of stimulated human monocytes. J Exp Med. 1988 Feb 1;167(2):389–407. doi: 10.1084/jem.167.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M. R., Muegge K., Keller J. R., Kung H. F., Young H. A., Durum S. K. Direct evidence for an intracellular role for IFN-gamma. Microinjection of human IFN-gamma induces Ia expression on murine macrophages. J Immunol. 1990 Mar 1;144(5):1777–1782. [PubMed] [Google Scholar]
- Smith M. R., Munger W. E., Kung H. F., Takacs L., Durum S. K. Direct evidence for an intracellular role for tumor necrosis factor-alpha 1. Microinjection of tumor necrosis factor kills target cells. J Immunol. 1990 Jan 1;144(1):162–169. [PubMed] [Google Scholar]
- Stevenson F. T., Torrano F., Locksley R. M., Lovett D. H. Interleukin 1: the patterns of translation and intracellular distribution support alternative secretory mechanisms. J Cell Physiol. 1992 Aug;152(2):223–231. doi: 10.1002/jcp.1041520202. [DOI] [PubMed] [Google Scholar]
- Takahashi K., Sawasaki Y., Hata J., Mukai K., Goto T. Spontaneous transformation and immortalization of human endothelial cells. In Vitro Cell Dev Biol. 1990 Mar;26(3 Pt 1):265–274. doi: 10.1007/BF02624456. [DOI] [PubMed] [Google Scholar]
- Wessendorf J. H., Garfinkel S., Zhan X., Brown S., Maciag T. Identification of a nuclear localization sequence within the structure of the human interleukin-1 alpha precursor. J Biol Chem. 1993 Oct 15;268(29):22100–22104. [PubMed] [Google Scholar]
- Wigler M., Sweet R., Sim G. K., Wold B., Pellicer A., Lacy E., Maniatis T., Silverstein S., Axel R. Transformation of mammalian cells with genes from procaryotes and eucaryotes. Cell. 1979 Apr;16(4):777–785. doi: 10.1016/0092-8674(79)90093-x. [DOI] [PubMed] [Google Scholar]
- Young P. R., Hazuda D. J., Simon P. L. Human interleukin 1 beta is not secreted from hamster fibroblasts when expressed constitutively from a transfected cDNA. J Cell Biol. 1988 Aug;107(2):447–456. doi: 10.1083/jcb.107.2.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan X., Hu X., Friedman S., Maciag T. Analysis of endogenous and exogenous nuclear translocation of fibroblast growth factor-1 in NIH 3T3 cells. Biochem Biophys Res Commun. 1992 Nov 16;188(3):982–991. doi: 10.1016/0006-291x(92)91328-n. [DOI] [PubMed] [Google Scholar]