ABSTRACT
The acp gene encoding the 13-kDa adhesin complex protein (ACP) from Neisseria meningitidis serogroup B strain MC58 was cloned and expressed in Escherichia coli, and the purified recombinant ACP (rACP) was used for immunization studies. Analysis of the ACP amino acid sequences from 13 meningococcal strains, isolated from patients and colonized individuals, and 178 strains in the Bacterial Isolate Genome Sequence (BIGS) database showed the presence of only three distinct sequence types (I, II, and III) with high similarity (>98%). Immunization of mice with type I rACP in detergent micelles and liposomes and in saline solution alone induced high levels of serum bactericidal activity (SBA; titers of 1/512) against the homologous strain MC58 and killed strains of heterologous sequence types II and III with similar SBA titers (1/128 to 1/512). Levels of expression of type I, II, or III ACP by different meningococcal strains were similar. ACP functioned as an adhesin, as demonstrated by reduced adherence of acp knockout (MC58 ΔACP) meningococci to human cells in vitro and the direct surface binding of rACP and by the ability of anti-rACP sera to inhibit adherence of wild-type bacteria. ACP also mediated the invasion of noncapsular meningococci into human epithelial cells, but it was not a particularly impressive invasin, as the internalized bacterial numbers were low. In summary, the newly identified ACP protein is an adhesin that induces cross-strain bactericidal activity and is therefore an attractive target antigen for incorporation into the next generation of serogroup B meningococcal vaccines.
IMPORTANCE
Infections caused by Neisseria meningitidis serogroup B are still significant causes of mortality and morbidity worldwide, and broadly protective vaccines of defined antigen composition are not yet licensed. Here, we describe the properties of the adhesin complex protein (ACP), which we demonstrate is a newly recognized molecule that is highly conserved and expressed to similar levels in meningococci and facilitates meningococcal interactions with human cells. We also report that a recombinant ACP protein vaccine induces murine antibodies that significantly kill meningococci expressing different ACP. Taken together, these properties demonstrate that ACP merits serious consideration as a component of a broadly protective vaccine against serogroup B meningococci.
Introduction
Diseases caused by Neisseria meningitidis (meningococcus) remain significant causes of mortality and morbidity worldwide. Despite the successful introduction of capsular polysaccharide-protein conjugate vaccines against serogroups A, C, Y, and W-135 (1), this approach is not recommended for serogroup B meningococci (MenB) due to molecular mimicry between the B polysaccharide capsule and human fetal brain neural cell adhesion molecules (2). Current licensed MenB vaccines have been prepared by detergent treatment of outer membranes (OM) to form lipopolysaccharide (LPS)-depleted OM vesicle (OMV) vaccines, which provide only strain-specific protection (3). Many individual OM and secreted proteins have been tested for their ability to induce serum bactericidal antibodies, the generally accepted correlate of protection against meningococcal infection (4). The “reverse vaccinology” approach used by Novartis has led to the development of a MenB vaccine (Bexsero) that contains defined OM antigens—the factor H binding protein (fHbp, fused to GNA2091 carrier protein), neisserial heparin binding protein (NHBA, fused to GNA1030 carrier protein), and an adhesin, NadA (5)—mixed with the OMV from the New Zealand MenB outbreak strain (PorA 1.4), which appears to be essential for immunogenicity of the antigens (6, 7). Bexsero has received “positive opinions” from the European Medicines Agency, and is expected to be licensed in 2013. Trials of another vaccine (Pfizer, LP2086) containing two subfamilies of fHbp have reached phase II (8). Despite this progress, due to considerable variation in amino acid sequences and antigen expression levels among meningococci in the population, strain coverage remains a potential issue with this first generation of MenB vaccines; e.g., in the United Kingdom, it is predicted to be 73% for Bexsero (9). Hence, there is a continuing need to investigate the vaccine potential of other MenB antigens, and a goal for effective vaccine development is to identify those antigens that are more conserved and capable of inducing cross-protective antibody responses.
In the current study, we have investigated the properties of a novel protein, the product of gene NMB2095 in the MC58 genome (10), which is annotated as encoding adhesin complex protein (ACP; 124 amino acids; Mr, 13.3 kDa) and which has been identified in several proteomics studies of meningococcal OM and OMV (11, 12). The acp gene was also shown to be upregulated under iron-depleted conditions (13). Here, we demonstrate the ability of (i) a recombinant ACP (rACP) to induce antibodies that promote complement-mediated serum bactericidal activity (SBA) against a diverse range of meningococcal strains and (ii) ACP to mediate meningococcal interactions with human cells in vitro, as there is no direct information regarding the role of ACP in meningococcal pathogenesis.
RESULTS
Cloning, expression, and purification of rACP.
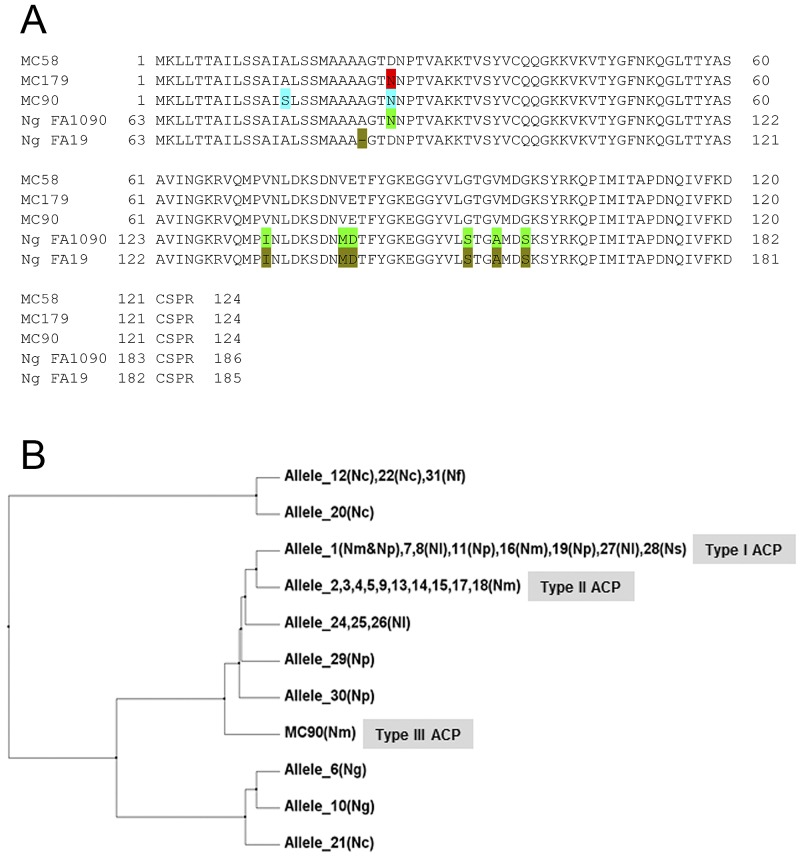
The acp gene was cloned into the pRSETA system, and the recombinant plasmids, pRSETA-acp, were transformed into Escherichia coli BL21(DE3) pLysS for isopropyl-β-d-thiogalactopyranoside (IPTG)-induced protein expression. Analysis of the IPTG-induced E. coli lysate revealed that the recombinant ACP (rACP) was insoluble. Protein purification required denaturing conditions to yield pure protein as judged by SDS-PAGE (Fig. 1). The Mr of rACP, which has an N-terminal leader sequence of 39 amino acids that contains the 6×His tag, was 17.8 kDa, and the Limulus Amebocyte Lysate (LAL) assay showed no detectable LPS (<0.125 pg/mg rACP).
FIG 1 .
Purification of recombinant ACP. The protein was purified to homogeneity using nickel affinity chromatography under denaturing conditions. Lane 1 contains molecular mass markers, and lane 2 shows rACP (10 µg) as a single band of ~17-kDa molecular mass.
Antigenicity of rACP.
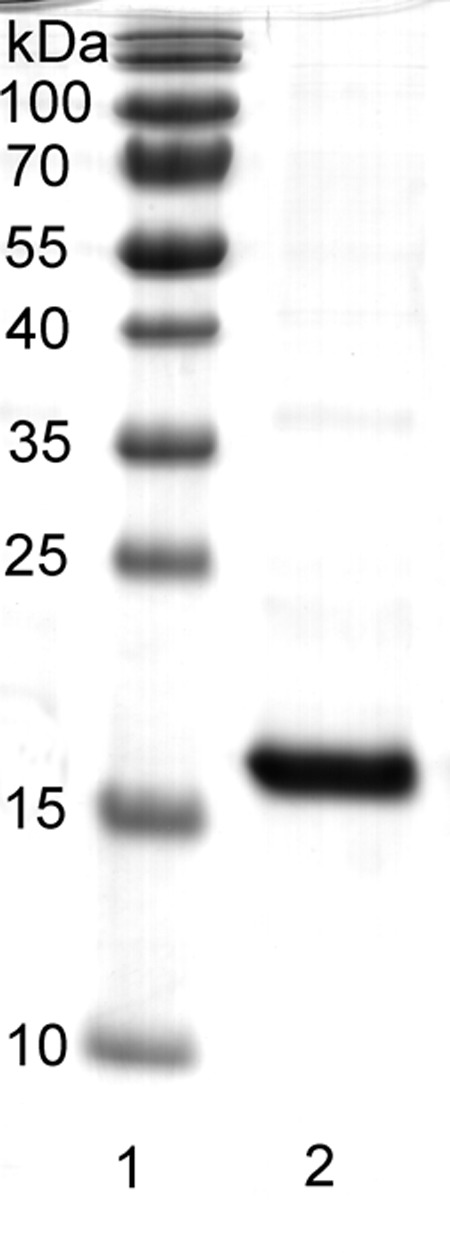
Purified rACP was used for immunization studies, and murine humoral immune responses were investigated initially by an enzyme-linked immunosorbent assay (ELISA) of rACP (Fig. 2A). High titers of antibodies that reacted with rACP protein were raised using all the different adjuvant and delivery systems. The rACP adsorbed to Al(OH)3 induced statistically higher mean titers (133,000) than rACP in saline solution (22,000), in ZW 3-14 micelles (10,000), or in liposomes (8,600) (P < 0.05). The addition of MonoPhosphoryl Lipid A (MPLA) into ZW 3-14 micelles and liposomes increased mean antibody titers to 49,000 and 41,000, respectively, but these values did not reach statistical significance compared to mixtures without MPLA (P > 0.05). In addition, immunization with MC58 OM on Al(OH)3 also induced antibodies that reacted with rACP (Fig. 2A). All of the antisera raised to formulations containing rACP reacted with ACP present in MC58 OM, but there were no significant differences between the different adjuvant groups (P > 0.05) (Fig. 2B). As expected, significantly higher (P < 0.05) anti-OM antibody titers were induced by immunization with OM than with rACP preparations.
FIG 2 .
ELISA reactivity of antisera raised against different rACP formulations. Anti-rACP sera were reacted against (A) rACP and (B) MC58 OM. The columns represent the geometric means of reciprocal ELISA titers (n = 5 animals per group), and the error bars show the 95% confidence limits. No reactivity against either rACP or homologous OM was observed with sera from sham immunized animals. NMS, Normal Mouse Serum.
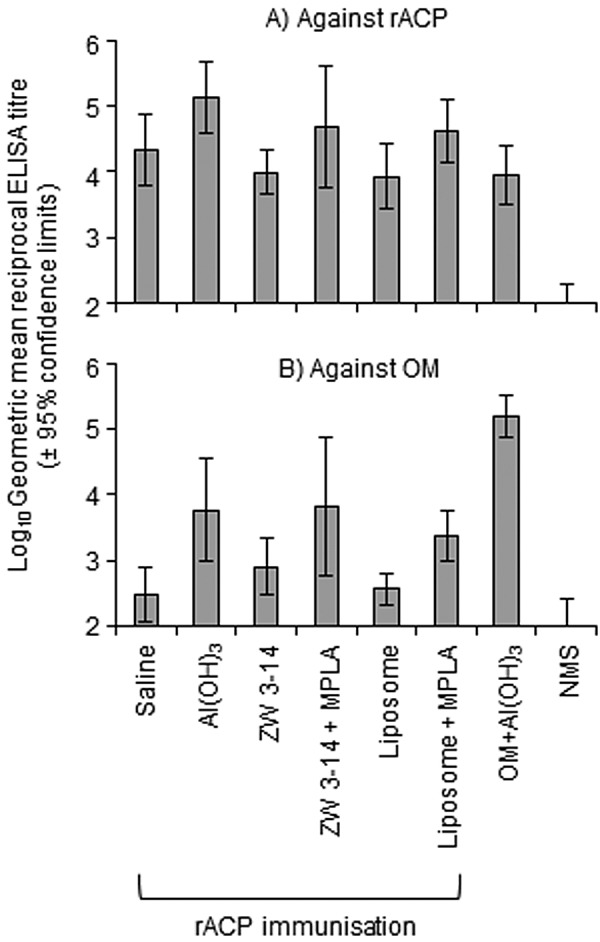
The specificity of the immune response against rACP was also investigated by Western blotting. Pooled murine antisera raised against rACP in the different formulations reacted with both a whole-cell lysate and an OM preparation of the homologous strain MC58, recognizing ACP with Mr ~13 kDa (Fig. 3).
FIG 3 .
Reactivity of murine anti-rACP sera with MC58 OM in Western blot analysis. Pooled antisera (1/200 dilution) recognized ACP as a single 13-kDa band in OM. All sham antisera were nonreactive, as shown by a representative strip blot.
Expression of ACP on the surface of meningococci was examined by fluorescence-activated cell sorter (FACS) analysis using rabbit anti-rACP sera. Postimmune rabbit antisera (with ELISA titers of ~140,000 to 160,000 against rACP and ~3,000 against MC58 OM) were tested on wild-type (ACP+) MC58 bacteria and showed a significant increase in fluorescein isothiocyanate (FITC) fluorescence-recorded events with a right shift (Fig. 4A). In contrast, postimmune sera showed no significant reactivity with the MC58 ∆ACP (ACP−) strain (Fig. 4B). In addition, all murine anti-rACP sera raised using the different adjuvants and delivery vehicles reacted with wild-type MC58 as shown by FACS analysis (Fig. 4C).
FIG 4 .
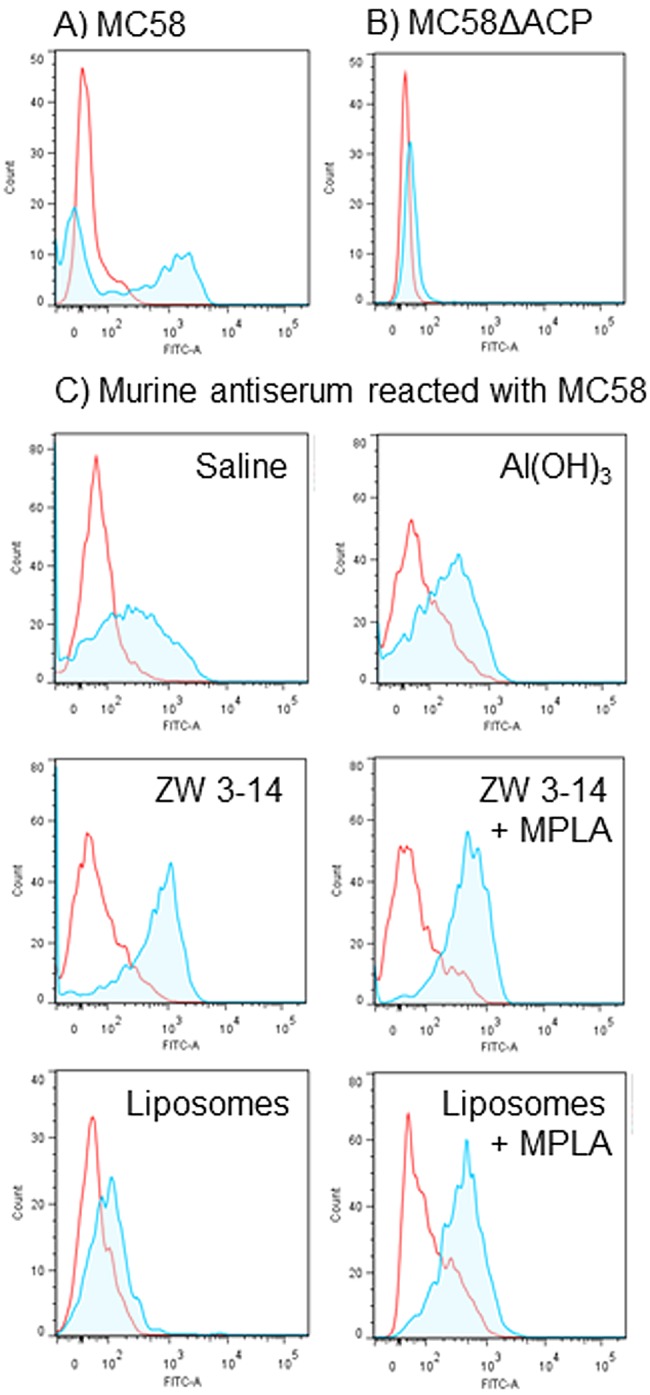
FACS analysis demonstrates expression of ACP on the surface of meningococci. (A and B) Reactivity of rabbit anti-rACP sera with MC58 and MC58 ∆ACP. The area within the red line shows the reactivity with preimmune rabbit sera (neat). The shaded area within the blue line shows the reactivity of rabbit anti-rACP sera (neat) with (A) MC58 (ACP+) and (B) MC58 ∆ACP (ACP−). (C) Reactivity of murine anti-rACP sera with MC58. Pooled murine antisera to rACP raised with the different adjuvants were reacted (1/10) with MC58. The areas within the red lines show the reactivity of sera from sham-immunized mice, and the shaded areas within the blue lines show the reactivity of sera from rACP-immunized mice.
ACP is highly conserved among meningococci.
The DNA sequencing results of the acp gene in our collection of isolates from colonized individuals and from patients were translated to amino acid sequences and aligned (Fig. 5A). There were 3 types of ACP identified: type I (strains MC58 and MC168), type II (strains MC54, L2470, MC161, MC162, MC172, MC173, MC174, MC179, MC180, and MENC11), and type III (strain MC90 only). The different ACP proteins shared a high degree of similarity (98% to 99%): type II ACP protein had only 1 amino acid difference from type I ACP (Asp25 → Asn25), whereas type III ACP had 2 substitutions (Asp25 → Asn25 and Ala12 → Ser12) compared to type I ACP.
FIG 5 .
(A) Alignment of ACP amino acid sequences from MC58, the surveyed strains in our collection, and gonococci. Colors denote the amino acid differences compared with MC58. Ng, N. gonorrhoeae; Nl, N. lactamica; Nm, N. meningitidis; Np, N. polysaccharea; Ns, N. sicca; Nc, N. cinerea; Nf, N flavscens. (B) A dendrogram showing the clustering of 11 ACP proteins and the relationships between type I, II, and III ACP in Neisseria spp.
Using the Basic Local Alignment Search Tool (NCBI website), we showed that there were only 2 distinct inferred ACP protein sequences in meningococcal strains and that the sequences were identical to those of the type I and type II proteins identified in our strain collection. We also accessed the BIGS database (14), which includes the complete genome sequence data for 205 Neisseria strains (31 DNA alleles in total), of which 173 were meningococci (N. meningitidis). In this collection, meningococci possessed genes corresponding only to type I ACP (encoded by 15 strains represented by allele 1) or to type II ACP (encoded by 158 strains represented by allele 2). Type II ACP protein was exclusively encoded by meningococci, while a variety of Neisseria strains, including N. sicca, N. polysaccharea, and N. lactamica, contained the acp gene encoding type I ACP. Notably, genes encoding type I, II, and III ACP proteins were not present in N. gonorrhoeae, but gonococci expressed 2 different ACP proteins that showed 94% similarity with meningococcal ACP (Fig. 5).
In order to investigate ACP expression, bacterial lysates from our strains that encoded type I, II, or III proteins were reacted with rabbit anti-rACP sera in Western blots. Antisera reacted with a ~13-kDa band present in all the strains and with similar intensities (Fig. 6). In addition, a lysate of MC58 ΔACP was used as a negative control and, as expected, showed no reactivity.
FIG 6 .
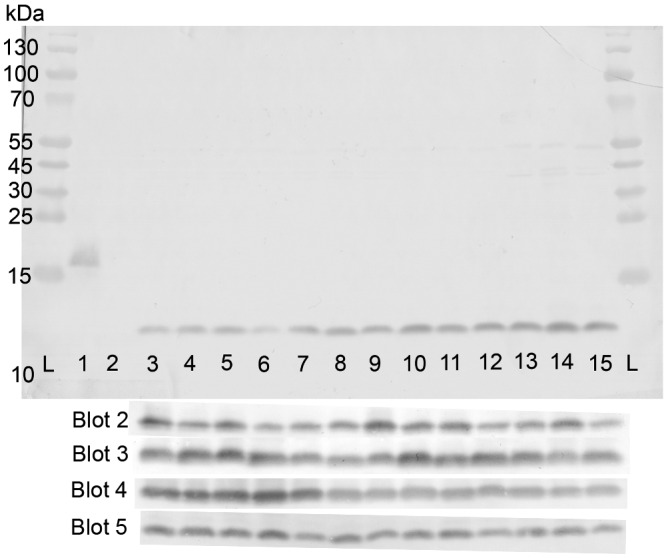
Western blot reactivity of rabbit antisera (1/400 dilution) with ACP in lysates of meningococcal strains. Lanes L, protein markers; lane 1, rACP (0.1 µg); lane 2, MC58 ΔACP (20 µg); lanes 3 and 4, MC58 and MC168 (type 1; 20 µg); lanes 5 to 14, L2470, MC54, MC161, MC162, MC172, MC173, MC174, MC179, MC180, and MENC11 (type II; 20 µg); lane 15, MC90 (type III; 20 µg). Similar reactivities for all strains were observed with four additional and independent blots of the same samples.
rACP elicits cross-strain bactericidal antibody.
Initially, murine antisera were tested for their ability to promote complement-mediated killing of the homologous meningococcal strain MC58 (Table 1). rACP in liposomes or ZW 3-14 micelles or in saline solution alone induced antisera with the highest bactericidal antibody titers (512). Notably, rACP adsorbed to Al(OH)3 also induced bactericidal antibodies (128). However, addition of MPLA to rACP in liposomes and micelles resulted in a complete loss of bactericidal activity. Importantly, pooled murine antisera with bactericidal antibodies to type I ACP (strain MC58) in liposomes, saline solution, or Al(OH)3 also showed identical bactericidal activities for heterologous strains MC179 (type II ACP) and MC90 (type III ACP) (Table 2). No bactericidal activity was observed for antisera from sham-immunized animals.
TABLE 1 .
Bactericidal activity of pooled antisera raised against rACP formulations, for the homologous strain MC58
| Formulation | Serum bactericidal titer (range)a against MC58 |
|
|---|---|---|
| + rACP | − rACP | |
| Saline solution | 512 | <4 |
| Al(OH)3 | 128 (128, 512) | <4 |
| ZW 3–14 micelle | 512 | <4 |
| ZW 3–14 micelle + MPLA | <4 | <4 |
| Liposome | 512 (128, 1,024) | <4 |
| Liposome + MPLA | <4 | <4 |
The titers are expressed as the reciprocal of the highest dilution at which 50% killing was observed. Titers for normal mouse serum and sera from mice immunized with MC58 OM were <4 and 20,000, respectively. Data are the median values, with the range of values in parentheses, for SBA from three or more independent measurements of bactericidal activity of all pooled serum samples. Single values denote that the SBA titers from the independent experiments were identical.
TABLE 2 .
Bactericidal activity of pooled murine anti-rACP sera for heterologous strains
| ACP type/representative strain | Serum bactericidal titer (range)a elicited by rACP in: |
||
|---|---|---|---|
| Saline solution | Liposomes | Al(OH)3 | |
| I/MC58 | 512 | 512 (128, 1,024) | 128 (128, 512) |
| II/MC179 | 512 (128, 4,096) | 512 (128, 2,048) | 64 (64, 128) |
| III/MC90 | 512 (128, 4,096) | 256 (128, 2,048) | 64 (64, 128) |
The titers are expressed as the reciprocal of the highest dilution at which 50% killing was observed. Data are the median values, with the range of values in parentheses, for SBA from three or more independent measurements of bactericidal activity of all pooled serum samples. Single values denote that the SBA titers from the independent experiments were identical.
The role of ACP in meningococcal adhesion and invasion of human cells.
The potential role of ACP in pathogenesis was investigated by infecting human cell cultures in vitro with wild-type MC58 and MC58 ∆ACP and comparing bacterial association and invasion. Compared with MC58, there was a significant reduction in association of MC58 ΔACP bacteria with all human cell types (Fig. 7A). Reduced association of MC58 ΔACP was greatest with both Chang and Hep2 epithelial cells (~75% reduction; P < 0.05), and approximately 30% to 50% with both human umbilical vein endothelial cells (HUVECs) and meningioma cells (P < 0.05). Furthermore, the MC58 ΔACP complemented strain restored the numbers of associated bacteria for all three cell types to levels similar to those of the wild-type strain (P > 0.05).
FIG 7 .

(A) ACP mediates adhesion of meningococci to human cells. Chang, Hep2, HUVEC, and meningioma monolayers were infected at an MOI of 100 to 200 with MC58, MC58 ∆ACP, and the MC58 ΔACP complemented (com) strains. Representative experiments for each cell line are shown, as reported by Virji et al. (15). Experiments were repeated 3 to 4 times for each cell line, and the patterns of association of the different bacterial strains were reproducible between experiments, regardless of any quantitative variations between experiments. (B) Anti-rACP serum inhibits bacterial association to epithelial cells. Chang epithelial cells were infected with different MOI of MC58 (ACP+) in the presence of decomplemented rabbit preimmune (1% vol/vol) and postimmune (1% vol/vol) anti-rACP serum, and bacterial association was measured after 3 h. The data are representative of n = 4 experiments, and the percentage of reduction in bacterial adherence was calculated from the following formula: 100 × (adherent bacteria with preimmune serum − adherent bacteria with postimmune serum)/adherent bacteria with preimmune serum. For both panels, the columns represent the mean associated bacterial numbers and the error bars show the standard deviations of the results determined with triplicate wells. *, P < 0.05.
To demonstrate further that ACP played a role in mediating meningococcal association with epithelial cells, decomplemented rabbit anti-rACP serum was added to cell cultures during infection with different multiplicities of infection (MOI) of MC58. Addition of anti-rACP serum showed a MOI-dependent reduction in total association of MC58 (Fig. 7B). At low MOI (0.02 to 0.2), association of MC58 was reduced by 50% with postimmune serum and this inhibitory effect decreased with increasing MOI (2 to 200).
A potential role for ACP in cellular invasion was investigated using the gentamicin assay (15). Initially, internalization of the wild-type capsulated (Cap+) MC58 and MC58 ∆ACP strains by Chang, Hep2, HUVECs, and meningioma cells was examined, but bacterial recovery after gentamicin treatment was very low and differences between the variants were not significant (P > 0.05). These data showed that ACP did not play a role in invasion of encapsulated strains, so we compared the interactions of noncapsulated (Cap−) ACP-expressing and MC58 ΔACP strains. Moreover, to avoid the potentially masking effects of the important meningococcal host binding factors of pili and Opa and Opc proteins (16), invasion of a noncapsular strain lacking their expression, i.e., MC58 ¢18 (Cap− Pil− Opa− Opc− ACP+), was compared with that of its ACP knockout mutant, MC58 ¢18 ∆ACP (Cap− Pil− Opa− Opc− ACP−). Compared to the MC58 ¢18 strain, absence of ACP reduced association of the mutant strain to all the cell lines by ~50% to 75% (P < 0.05) (Fig. 8A). Internalization of MC58 ¢18 by human cells was observed, but the numbers of bacteria recovered per monolayer after gentamicin treatment were low (Fig. 8B). Nevertheless, there was a significant reduction in the numbers of internalized MC58 ¢18 ∆ACP in Chang and Hep2 epithelial cells compared to the strain expressing ACP (P < 0.05) and also after taking into account the differences in association between the ACP+ and ACP− strains. In contrast, although there was an apparent reduction in the numbers of internalized MC58 ¢18 ∆ACP bacteria in HUVECs, this was not genuine, since the percentages of reduction in association and internalization of MC58 ¢18 ∆ACP were similar (P > 0.05) to those seen with MC58 ¢18. Very low numbers of MC58 ¢18 bacteria were recovered from meningioma cell monolayers, and no significant difference was observed with the MC58 ¢18 ∆ACP strain (P = 0.181); the inability of meningococci to invade these cells is consistent with our previous findings (17).
FIG 8 .
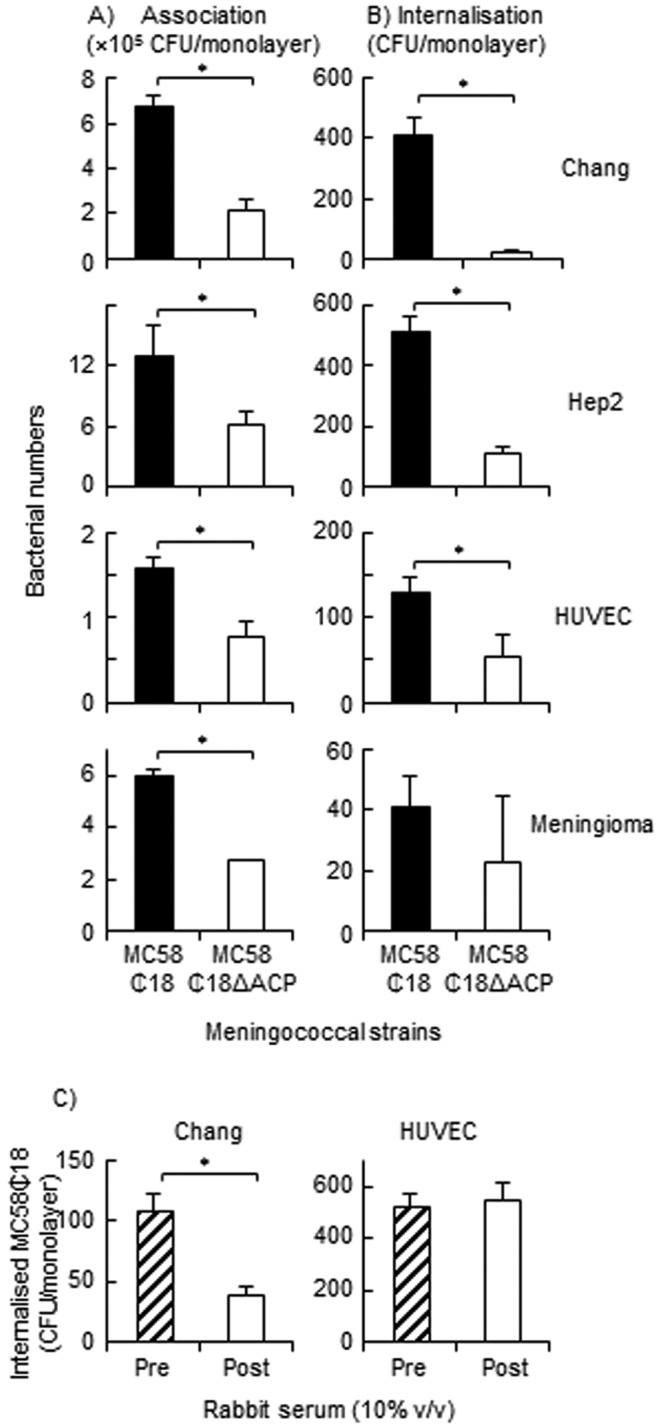
(A and B) Role of ACP in meningococcal invasion of human cells. Chang, Hep2, HUVEC, and meningioma cell monolayers were challenged with MC58 ¢18 and MC58 ¢18 ΔACP strains (MOI, 100 to 200), and the numbers of both associated and internalized bacteria were quantified. Results of representative experiments for each cell line are shown, as reported by Virji et al. (15). Experiments were repeated 3 times, and the differences in association and/or invasion between the strains were reproducible between experiments, regardless of any quantitative variations between experiments. (C) Effect of rabbit anti-rACP serum on internalization of MC58 ¢18 strain into Chang epithelial cells and HUVECs. Cell monolayers (n = 3 experiments for each cell line) were infected for 4 h with strain MC58 ¢18 in the presence of 10% (vol/vol) preimmune and postimmune rabbit anti-rACP serum and the numbers of internalized bacteria quantified. For all figures, the columns represent the numbers of associated and/or internalized bacteria and the error bars the standard deviations of the results determined with triplicate wells. *, P < 0.05.
To further demonstrate that ACP played a role in internalization, decomplemented rabbit anti-rACP serum was added to Chang cells and HUVECs during infection with MC58 ¢18. Addition of 10% (vol/vol) serum significantly (P < 0.05) inhibited the internalization by Chang cells of MC58 ¢18 (Fig. 8C): however, there was no difference in the numbers of internalized bacteria in HUVECs in the presence or absence of antiserum (P = 0.6), confirming that ACP is unlikely to play a role in invasion of this cell type.
Next, we treated human cell cultures with rACP to confirm binding of the protein. Initially, Chang epithelial cells were treated with various doses of rACP and binding was quantified using specific rabbit anti-rACP serum and FACS analysis (Fig. 9A). Dose-dependent binding of rACP to Chang cells was observed, and the binding was specific since there was no significant reactivity of anti-rACP serum with recombinant macrophage infectivity potentiator (rMIP) (18), used as a control protein. Finally, we treated the other human cells with rACP and observed dose-dependent, high-level binding of rACP to Chang and Hep2 epithelial cells and moderate levels of protein binding to HUVECs and meningioma cells (Fig. 9B).
FIG 9 .
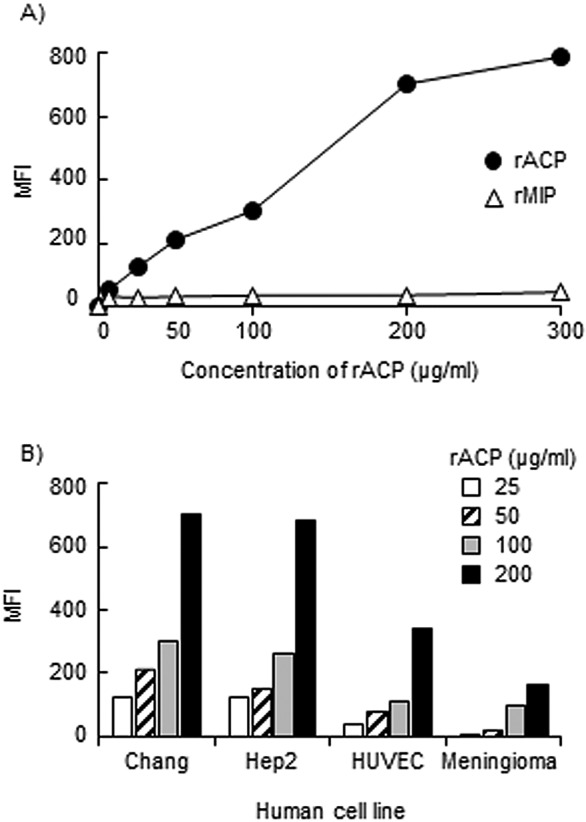
Binding of rACP to human cells. (A) Concentration-dependent binding of rACP and rMIP to Chang epithelial cells, expressed as net mean fluorescence intensity (MFI). Results of a representative experiment (n = 3) is shown. (B) Cellular binding of rACP to different human cell lines. The assay was done with different concentrations of rACP, and representative data are shown (n = 3 experiments with each cell line).
DISCUSSION
ACP fulfills several criteria for a meningococcal vaccine antigen: the protein is located on the surface on meningococci and expressed by all strains from a collection of patient and carriage strains, and it is highly conserved, with >98% similarity in protein amino acid sequences. Only two predicted proteins (type I and II) were found in the 205 meningococcal strains in the BIGS database, with one additional protein (type III) expressed by a single strain from our collection. Interestingly, type II ACP is present exclusively in N. meningitidis, whereas type I ACP is found not only in meningococci but also in other Neisseria strains colonizing the nasopharyngeal mucosal epithelium, including N. sicca, N. polysaccharea, and N. lactamica. In contrast, the gonococcal ACP was different from the meningococcal type I, II, and III proteins.
The rACP induced significant complement-mediated SBA against meningococci. Notably, the SBA toward the homologous strain induced by rACP in saline solution was similar to the SBA induced by rACP in liposomes, suggesting that purification of rACP under denaturing conditions did not adversely influence functional immunogenicity. Moreover, antisera to type I ACP showed cross-protection by killing heterologous strains expressing type II and III ACP at similar SBA levels. The nature of the antigen and careful matching of the adjuvant are both critical to the quality of the SBA response: thus, for rACP, adsorption to Al(OH)3 reduced bactericidal titers and introduction of exogenous MPLA abolished SBA. It is possible that MPLA can bind to ACP and alter protein folding and native protein-like conformation, thereby generating antibodies that probably do not recognize the functional epitope(s) on ACP. Similar observations were reported for rPorA porin and rMIP incorporated into liposomes, where the addition of MPLA resulted in significant reduction of bactericidal activity that was attributed to interference with the native conformation of the proteins in the liposome bilayer (18, 19).
By comparing the interactions of wild-type (ACP+), mutant (ACP−), and complemented strains, ACP was identified as a new adhesin showing the greatest levels of association with epithelial cells, followed by endothelial cells and meningeal cells. Binding assays confirmed that rACP protein attached to these different host cell types and that binding to epithelial cells was inhibited by anti-rACP sera. It is generally accepted that initial interactions between Cap+ meningococci and human cells are mediated by pili that subsequently retract or disassemble, thereby drawing the organism toward the host cell surface (16). The OM-associated Opa and Opc proteins do not mediate adherence of Cap+ meningococci (16), whereas ACP and other adhesins such as App (20), NadA (21), and NhhA (22) do contribute to Cap+ meningococcal adhesion. It is possible that a complex of ACP and these other adhesins is involved in the initial adhesion of Cap+ meningococci to the nasopharyngeal mucosa.
We investigated whether expression of ACP facilitated meningococcal internalization into human cells, and this was examined in a Cap− background, since Cap+ meningococci were not internalized in significant numbers. ACP was also observed to mediate the association of Cap− meningococci with the different host cell types. Our data also show that deletion of ACP led to a reduction in the numbers of internalized Cap− meningococci bacteria from epithelial cells. This suggested that ACP could also function as an invasin for Cap− meningococci in the absence of other important molecules, i.e., pili, Opa, and Opc. The addition of anti-rACP sera reduced ACP-mediated internalization, confirming a role for this protein for epithelial cell invasion. However, as the numbers of recovered meningococci per monolayer were particularly low, ACP appears not to be an impressive invasin. In addition, ACP does not facilitate significant internalization of meningococci into endothelial and meningeal cells.
In summary, ACP is a newly identified adhesin and the recombinant protein is capable of inducing cross-protective bactericidal antibodies. In many respects, ACP is remarkably similar to NadA in biological function and in its potential as a vaccine candidate antigen (21). However, the nadA gene is present in only 50% of disease-associated strains (23) whereas the acp gene is present in all meningococcal strains studied thus far and the amino acid sequences are more highly conserved. In addition, ACP is expressed to similar levels in meningococcal strains studied. Taken together, our data suggest that ACP merits serious consideration as a candidate antigen for inclusion in the next generation of serogroup B meningococcal vaccines.
MATERIALS AND METHODS
Bacteria and growth conditions.
Neisseria meningitidis strain MC58 (B: 15:P1.7,16b) and other meningococcal strains of different serogroups, serotypes, and serosubtypes, originally isolated from patients or colonized individuals, have been described previously (18). A noncapsular mutant strain, MC58 ¢18 (PilE− Opa− Opc−), was kindly provided by M. Virji (University of Bristol, United Kingdom) (24). Meningococci were grown on supplemented GC agar plates (25) incubated at 37°C in an atmosphere containing 5% (vol/vol) CO2, and OM were prepared by extraction of whole cells by the use of lithium acetate as previously described (18). E. coli strains DH5α and BL21(DE3) pLysS (Invitrogen, United Kingdom) were used for cloning and protein expression and were grown on Luria-Bertani (LB) agar and in LB broth. The growth curves of the wild-type strain and corresponding ΔACP mutants were similar (P > 0.05) in cell culture medium with or without human cell monolayers present (data not shown).
Cloning and expression of the acp gene in E. coli.
Genomic DNA of MC58 was extracted by alkaline lysis, as described previously (18), and used as the PCR template. The acp gene (375 bp) sequence was accessed from the NCBI website and amplified using a forward primer (NMB2095F; 5′ GGCTATCTCGAGATGAAACTTCTGACCACCGC 3′), a reverse primer (NMB2095R; 5′ GGCTATAAGCTTCTATTAACGTGGGGAACAGTCTT 3′), and 2× Phusion PCR master mix (Finnzymes, United Kingdom) under the following PCR conditions: initial denaturation (98°C, 30 s), 30 cycles of denaturation (98°C, 10 s), annealing (65°C, 30 s), and extension (72°C, 10 s), and a final extension at 72°C for 5 min. The CTCGAG and AAGCTT sequences (indicated in bold) represent the restriction sites for XhoI and HindIII enzymes, respectively. The method for gene cloning into the pRSETA system was described previously (18). Next, recombinant plasmids carrying the sequence-proved acp gene were transformed into E. coli BL21(DE3) pLysS for protein expression, which was induced by addition of IPTG to reach a final concentration of 1 mM, followed by bacterial growth for 4 h.
Purification of rACP.
The insoluble rACP was purified using nickel-nitrilotriacetic acid metal-affinity chromatography under denaturing conditions (QIAexpressionist system manual; Qiagen, United Kingdom). The bound protein was eluted using 100 mM NaH2PO4-10 mM Tris buffer containing 6 M GuHCl (pH 4.5) and then precipitated by adding trichloroacetic acid (TCA; BDH, United Kingdom) to reach a 10% (vol/vol) final concentration. Samples obtained during the purification procedure were analyzed using 10% to 25% (wt/vol) gradient SDS-PAGE (26) or Tricine–SDS-PAGE (27), and protein concentrations were determined using the bicinchoninic acid (BCA) assay (Pierce Thermo Scientific, United Kingdom). The presence of any contaminating LPS was detected with the Limulus Amebocyte Lysate (LAL) assay (Lonza, United Kingdom).
Immunization of animals.
BALB/c mice (H-2d) and New Zealand White rabbits were housed under standard conditions of temperature and humidity. Groups of five mice of approximate equal sizes and weights (6 to 7 weeks of age) were immunized intraperitoneally with rACP-saline solution, rACP-Al(OH)3, rACP-liposomes, rACP plus MPLA-liposomes, rACP–ZW 3-14 micelles, or rACP plus MPLA–ZW3-14 micelles. The methods for preparing liposomes and other adjuvant mixtures have been described previously (18). The immunization schedule was three doses of rACP (20 µg/mouse) on days 0, 14, and 28. Groups of five mice were also injected with the same preparations without rACP (sham controls); one group was injected with MC58 OM (20 µg/mouse) adsorbed to Al(OH)3, and another group was kept for normal serum. Mice were terminally bled by cardiac puncture under anesthesia on day 42.
Rabbits (n = 2) were immunized subcutaneously with rACP (20 µg/dose) emulsified in Freund’s complete adjuvant for the primary injection and Freund’s incomplete adjuvant for a subsequent three injections at 14-day intervals. Rabbits were terminally bled from the middle ear vein and by cardiac puncture under anesthesia, 14 days after the last dose.
All sera were stored at −20°C until required. This study complied with the animal experimentation guidelines of the Home Office and the authors’ institutions, and no animals suffered significant adverse effects.
Characterization of biological and functional properties of antibodies to rACP. (i) ELISA.
Individual murine antisera were reacted in ELISA with both rACP and MC58 OM, as described previously (19). Absorbance was measured at 450 nm after 10 min of incubation with enzyme substrate, and the ELISA titer, extrapolated from the linear portion of the serum titration curve, was taken as the reciprocal dilution which gave an increase in absorbance of 0.1 U after 10 min. A two-sample t test was used to compare differences between mean values for ELISA data, with P values <0.05 considered significant.
(ii) Western immunoblotting.
Samples containing rACP, OM, and whole-cell lysate preparations were separated on SDS-PAGE and then transferred to nitrocellulose by semidry blotting. After incubation with murine or rabbit sera, immunological reactivity was detected by using anti-mouse/rabbit immunoglobulin-alkaline phosphatase conjugate (Bio-Rad, United Kingdom) as described previously (19).
(iii) Fluorescence-activated cell sorter (FACS) analysis.
An overnight culture of bacteria was collected by centrifugation, and cold 70% (vol/vol) ethanol (2 ml) was added to the pellet, which was then stored at −20°C for 1 h to permeabilize the capsule. Bacteria were washed twice with sterile phosphate-buffered saline (PBS) containing 1% (wt/vol) bovine serum albumin (BSA) and suspended to 2 × 108 CFU/ml. Next, bacteria (l ml) were centrifuged (2,200 × g for 3 min), suspended in 200 µl rabbit sera (neat) or pooled murine sera (1/10), and incubated at 37°C for 30 min. After washes with PBS, bacteria were incubated with 100 µl of FITC-conjugated goat anti-rabbit or rabbit anti-mouse IgG (Dako, United Kingdom) (1/50 dilution) at room temperature for 30 min. Bacteria were fixed with a 0.4% (wt/vol) paraformaldehyde solution at room temperature for 30 min. Samples were analyzed on a FACSAria flow cytometer (BD Biosciences).
(iv) Complement-activated killing of meningococci.
The bactericidal activities of pooled antisera were determined with 5% (vol/vol) baby rabbit serum (AbD Serotec, United Kingdom) as a source of exogenous complement, as previously described (19). Murine antisera raised to OM were used as a positive control. Complement-dependent bactericidal activity was determined from the numbers of bacteria surviving in the presence of serum and complement compared to the numbers surviving with complement but without test serum. Sera that showed bactericidal activity (>50%) in two or more dilutions were considered positive.
Sequencing the acp gene of meningococcal strains.
The acp gene of selected meningococcal strains was sequenced commercially (Geneservice, Oxford, United Kingdom) using the primer Seq2095 (5′ CGGGATACGCCGACATTAGA 3′).
Constructing an acp knockout mutant.
Mutagenesis was achieved by heterologous allelic exchange. Primers KO2095F (5′ CGGGCTGAACCAGATAGACT 3′) and KO2095R (5′ GCTCCAGTTTGGTACGGAGA 3′) were used to amplify the 2.9-kb DNA segment from the genomic DNA of the ACP− mutant 35/11, while the amplified PCR product from the wild-type MC58 should be 1.3 kb. The ACP− strain 35/11, derived from strain 8013 (serogroup C), was one of the strains in the mutant library generated by random insertion of a minitransposon (1.6 kb) (28). The 2.9-kb PCR product was gel purified, cleaned, and used for transformation of MC58. Transformants were screened by PCR, and the selected MC58 ΔACP strain(s) was confirmed by Western blot analysis using rabbit antisera. Sequencing of the 2.9-kb DNA segment amplified from MC58 ΔACP showed that genes encoding the adjacent proteins NMB2094 and NMB2096 were unaffected by the mutagenesis procedure. Due to the low transformation rate of nonpiliated strains, the transformation protocol of van Dam and Bos (29) was used for strain MC58 ¢18.
Constructing acp-complemented strains.
Primers Com2095F (5′ GGCTATTTAATTAAATGAAACTTCTGACCACCGC 3′) and Com2095R (5′ TTAACGTGGGGAACAGTCTT 3′) were used to amplify acp gene from MC58. The sequence TTAATTAA (in bold) represents the restriction site for PacI. The other restriction enzyme used was PmeI. The acp gene was cloned into the pGCC4 vector and confirmed by sequencing using an upstream primer, LacP (5′ CGGTTCTGGCAAATATTCTG 3′). Next, pGCC4-acp was transformed into MC58 ΔACP using the method of Stohl and Seifert (30) and the complementary strains were identified by PCR screening.
Cell culture.
Human Chang conjunctival epithelial cells and Hep2 (epidermoid laryngeal carcinoma) cells (European Type Culture Collection, Porton Down, United Kingdom) were cultured in Dulbecco’s modified Eagle’s medium supplemented with Glutamax-1 and sodium pyruvate (DMEM) (Lonza, United Kingdom) and 5% (vol/vol) decomplemented fetal calf serum (dFCS) (Lonza). Human umbilical vein endothelial cells (HUVECs; PromoCell, Heidelberg, Germany) were grown in Medium 199 supplemented with 20% (vol/vol) dFCS and endothelial cell growth supplement (PromoCell). Human meningothelial meningioma cells were obtained from surgically removed tumors and characterized cytologically as described previously (17). Cell lines (passages 5 to 9) were grown in DMEM with 10% (vol/vol) dFCS on collagen-coated (type I collagen from rat tail [BD Biosciences] [50 µg/ml]-0.02 M acetic acid) tissue cultureware. All cells were cultured in a humidified atmosphere at 37°C with 5% (vol/vol) CO2.
Measurement of total bacterial association to human cells and internalization.
Cells in triplicate wells of a 24-well tissue culture plate were infected with bacteria in DMEM containing 1% (vol/vol) dFCS. After 3 h of incubation (37°C with 5% [vol/vol] CO2), the monolayers were washed gently 4 times with PBS and 250 µl of a lysis solution of PBS containing 1% (wt/vol) saponin (Sigma-Aldrich, United Kingdom) and 1% (vol/vol) dFCS, added to each well (15). After incubation for 15 min, viable counts of bacteria were made on GC agar plates.
A gentamicin assay was used to measure internalization of bacteria (15). Cell monolayers were infected with bacteria as described above, and after washing with PBS, 1 ml of gentamicin (200 µg/ml) in DMEM containing 1% (vol/vol) dFCS was added per well and incubated for 90 min to eliminate the extracellular and attached bacteria. The monolayers were washed 4 times and then lysed with saponin lysis solution to release the internalized bacteria. All association and internalization data were analyzed by independent t test, with P < 0.05 considered significant.
Measurement of rACP protein binding to human cells.
The ability of rACP to bind directly to human cells was determined using the method described by Serruto et al. (20), with modifications. Human cells were detached from cell culture flasks using Versene (Lonza, United Kingdom), harvested, and suspended in DMEM containing 1% (vol/vol) dFCS. Different concentrations of rACP, rMIP (18), or medium alone were mixed with cells (1 × 105) and incubated at 37°C for 1 h. Cells were then washed twice using PBS containing 5% (vol/vol) dFCS and centrifuged at 350 × g for 5 min. Next, cells were incubated with neat rabbit anti-rACP serum at room temperature for 30 min and then washed twice and reacted with FITC-conjugated goat anti-rabbit IgG (Dako, United Kingdom) (1/50 dilution) at 4°C for 30 min. Samples were analyzed on a FACSAria flow cytometer (BD Biosciences).
ACKNOWLEDGEMENTS
M.C. Hung was a Ph.D. student funded by the government of Taiwan. This work was supported by the Wessex Medical Trust. This publication made use of the Neisseria MultiLocus Sequence Typing website (http://pubmlst.org/neisseria/) developed by Keith Jolley and sited at the University of Oxford. The development of this site has been funded by the Wellcome Trust and European Union.
We are grateful to Vladimir Pelicic (Imperial College London, London, United Kingdom) for technical expertise in generating the MC58 ACP− mutant and to Hank Steven Seifert (Northwestern University, Chicago, IL) for generously providing the pGCC4 plasmid for complementation studies.
Footnotes
Citation Hung M, Heckels JE, Christodoulides M. 2013. The adhesin complex protein (ACP) of Neisseria meningitidis is a new adhesin with vaccine potential. mBio 4(2):e00041-13. doi:10.1128/mBio.00041-13.
REFERENCES
- 1. Snape MD, Perrett KP, Ford KJ, John TM, Pace D, Yu LM, Langley JM, McNeil S, Dull PM, Ceddia F, Anemona A, Halperin SA, Dobson S, Pollard AJ. 2008. Immunogenicity of a tetravalent meningococcal glycoconjugate vaccine in infants: a randomized controlled trial. JAMA 299:173–184 [DOI] [PubMed] [Google Scholar]
- 2. Finne J, Leinonen M, Mäkelä PH. 1983. Antigenic similarities between brain components and bacteria causing meningitis. Implications for vaccine development and pathogenesis. Lancet ii:355–357 [DOI] [PubMed] [Google Scholar]
- 3. Holst J, Martin D, Arnold R, Huergo CC, Oster P, O’Hallahan J, Rosenqvist E. 2009. Properties and clinical performance of vaccines containing outer membrane vesicles from Neisseria meningitidis. Vaccine 27(Suppl 2):B3–12 [DOI] [PubMed] [Google Scholar]
- 4. Granoff DM. 2010. Review of meningococcal group B vaccines. Clin. Infect. Dis. 50(Suppl 2):S54–S65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Palumbo E, Fiaschi L, Brunelli B, Marchi S, Savino S, Pizza M. 2012. Antigen identification starting from the genome: a “Reverse vaccinology” approach applied to MenB, p. 361–403 In Christodoulides M, Neisseria meningitidis: advanced methods and protocols. Humana Press, New York, NY. [DOI] [PubMed] [Google Scholar]
- 6. Serruto D, Bottomley MJ, Ram S, Giuliani MM, Rappuoli R. 2012. The new multicomponent vaccine against meningococcal serogroup B, 4CMenB: immunological, functional and structural characterization of the antigens. Vaccine 30(Suppl 2):B87–B97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bai X, Findlow J, Borrow R. 2011. Recombinant protein meningococcal serogroup B vaccine combined with outer membrane vesicles. Expert Opin. Biol. Ther. 11:969–985 [DOI] [PubMed] [Google Scholar]
- 8. Nissen MD, Marshall HS, Richmond PC, Jiang Q, Harris SL, Jones TR, Jansen KU, Perez JL. 30 October 2012. A randomized, controlled, phase 1/2 trial of a Neisseria meningitidis serogroup B bivalent rLP2086 vaccine in healthy children and adolescents. Pediatr. Infect. Dis. J. (Epub ahead of print.) [DOI] [PubMed] [Google Scholar]
- 9. Stephens DS. 2012. Prevention of serogroup B meningococcal disease. Lancet 379:592–594 [DOI] [PubMed] [Google Scholar]
- 10. Tettelin H, Saunders NJ, Heidelberg J, Jeffries AC, Nelson KE, Eisen JA, Ketchum KA, Hood DW, Peden JF, Dodson RJ, Nelson WC, Gwinn ML, DeBoy R, Peterson JD, Hickey EK, Haft DH, Salzberg SL, White O, Fleischmann RD, Dougherty BA, Mason T, Ciecko A, Parksey DS, Blair E, Cittone H, Clark EB, Cotton MD, Utterback TR, Khouri H, Qin H, Vamathevan J, Gill J, Scarlato V, Masignani V, Pizza M, Grandi G, Sun L, Smith HO, Fraser CM, Moxon ER, Rappuoli R, Venter JC. 2000. Complete genome sequence of Neisseria meningitidis serogroup B strain MC58. Science 287:1809–1815 [DOI] [PubMed] [Google Scholar]
- 11. Post DM, Zhang D, Eastvold JS, Teghanemt A, Gibson BW, Weiss JP. 2005. Biochemical and functional characterization of membrane blebs purified from Neisseria meningitidis serogroup B. J. Biol. Chem. 280:38383–38394 [DOI] [PubMed] [Google Scholar]
- 12. Ferrari G, Garaguso I, Adu-Bobie J, Doro F, Taddei AR, Biolchi A, Brunelli B, Giuliani MM, Pizza M, Norais N, Grandi G. 2006. Outer membrane vesicles from group B Neisseria meningitidis delta gna33 mutant: proteomic and immunological comparison with detergent-derived outer membrane vesicles. Proteomics 6:1856–1866 [DOI] [PubMed] [Google Scholar]
- 13. Grifantini R, Sebastian S, Frigimelica E, Draghi M, Bartolini E, Muzzi A, Rappuoli R, Grandi G, Genco CA. 2003. Identification of iron-activated and -repressed Fur-dependent genes by transcriptome analysis of Neisseria meningitidis group B. Proc. Natl. Acad. Sci. U. S. A. 100:9542–9547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jolley KA, Maiden MC. 2010. BIGSdb: scalable analysis of bacterial genome variation at the population level. BMC Bioinformatics 11:595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Virji M, Makepeace K, Peak IR, Ferguson DJ, Jennings MP, Moxon ER. 1995. Opc- and pilus-dependent interactions of meningococci with human endothelial cells: molecular mechanisms and modulation by surface polysaccharides. Mol. Microbiol. 18:741–754 [DOI] [PubMed] [Google Scholar]
- 16. Virji M. 2009. Pathogenic neisseriae: surface modulation, pathogenesis and infection control. Nat. Rev. Microbiol. 7:274–286 [DOI] [PubMed] [Google Scholar]
- 17. Hardy SJ, Christodoulides M, Weller RO, Heckels JE. 2000. Interactions of Neisseria meningitidis with cells of the human meninges. Mol. Microbiol. 36:817–829 [DOI] [PubMed] [Google Scholar]
- 18. Hung MC, Salim O, Williams JN, Heckels JE, Christodoulides M. 2011. The Neisseria meningitidis macrophage infectivity potentiator protein induces cross-strain serum bactericidal activity and is a potential serogroup B vaccine candidate. Infect. Immun. 79:3784–3791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Christodoulides M, Brooks JL, Rattue E, Heckels JE. 1998. Immunization with recombinant class 1 outer-membrane protein from Neisseria meningitidis: influence of liposomes and adjuvants on antibody avidity, recognition of native protein and the induction of a bactericidal immune response against meningococci. Microbiology 144(Pt 11):3027–3037 [DOI] [PubMed] [Google Scholar]
- 20. Serruto D, Adu-Bobie J, Scarselli M, Veggi D, Pizza M, Rappuoli R, Aricò B. 2003. Neisseria meningitidis App, a new adhesin with autocatalytic serine protease activity. Mol. Microbiol. 48:323–334 [DOI] [PubMed] [Google Scholar]
- 21. Capecchi B, Adu-Bobie J, Di Marcello F, Ciucchi L, Masignani V, Taddei A, Rappuoli R, Pizza M, Aricò B. 2005. Neisseria meningitidis NadA is a new invasin which promotes bacterial adhesion to and penetration into human epithelial cells. Mol. Microbiol. 55:687–698 [DOI] [PubMed] [Google Scholar]
- 22. Scarselli M, Serruto D, Montanari P, Capecchi B, Adu-Bobie J, Veggi D, Rappuoli R, Pizza M, Aricò B. 2006. Neisseria meningitidis NhhA is a multifunctional trimeric autotransporter adhesin. Mol. Microbiol. 61:631–644 [DOI] [PubMed] [Google Scholar]
- 23. Comanducci M, Bambini S, Brunelli B, Adu-Bobie J, Aricò B, Capecchi B, Giuliani MM, Masignani V, Santini L, Savino S, Granoff DM, Caugant DA, Pizza M, Rappuoli R, Mora M. 2002. NadA, a novel vaccine candidate of Neisseria meningitidis. J. Exp. Med. 195:1445–1454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. McNeil G, Virji M. 1997. Phenotypic variants of meningococci and their potential in phagocytic interactions: the influence of opacity proteins, pili, PilC and surface sialic acids. Microb. Pathog. 22:295–304 [DOI] [PubMed] [Google Scholar]
- 25. Zak K, Diaz JL, Jackson D, Heckels JE. 1984. Antigenic variation during infection with Neisseria gonorrhoeae: detection of antibodies to surface proteins in sera of patients with gonorrhea. J. Infect. Dis. 149:166–173 [DOI] [PubMed] [Google Scholar]
- 26. Heckels JE. 1981. Structural comparison of Neisseria gonorrhoeae outer membrane proteins. J. Bacteriol. 145:736–742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Schägger H. 2006. Tricine-SDS-PAGE. Nat. Protoc. 1:16–22 [DOI] [PubMed] [Google Scholar]
- 28. Rusniok C, Vallenet D, Floquet S, Ewles H, Mouzé-Soulama C, Brown D, Lajus A, Buchrieser C, Médigue C, Glaser P, Pelicic V. 2009. NeMeSys: a biological resource for narrowing the gap between sequence and function in the human pathogen Neisseria meningitidis. Genome Biol. 10:R110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. van Dam V, Bos MP. 2012. Generating knockout and complementation strains of Neisseria meningitidis, p. 55–72 In Christodoulides M, Neisseria meningitidis: advanced methods and protocols. Humana Press, New York, NY. [DOI] [PubMed] [Google Scholar]
- 30. Stohl EA, Seifert HS. 2001. The recX gene potentiates homologous recombination in Neisseria gonorrhoeae. Mol. Microbiol. 40:1301–1310 [DOI] [PubMed] [Google Scholar]