Abstract
Adipose tissue (AT) is composed of mature adipocytes and stromal vascular fraction (SVF) cells, including adipose stem/stromal cells (ASCs). We characterized hematopoietic cells residing in human nonobese AT by analyzing the SVF isolated from human lipoaspirates and peripheral blood (PB). Flow cytometry revealed that AT-resident hematopoietic cells consisted of AT-resident macrophages (ATMs) or lymphocytes with a negligible number of granulocytes. AT-resident lymphocytes were composed of helper T cells and natural killer cells. Almost no B cells and few cytotoxic T cells were observed in nonobese AT. More than 90% of ATMs were M2 state CD206+ macrophages (CD45+/CD14+) that were located in the periendothelium or interstitial spaces between adipocytes. We also discovered a novel subpopulation of CD34+/CD206+ ATMs (11.1% of CD206+ATMs) that localized in the perivascular region. Microarray of noncultured CD34+/CD206+ ATMs, CD34−/CD206+ ATMs, CD45−/CD31−/CD34+ ASCs, and PB-derived circulating monocytes revealed that CD34+/CD206+ ATMs shared characteristics with ASCs and circulating monocytes. Unlike CD34−/CD206+ ATMs, CD34+/CD206+ ATMs could grow in adherent culture and were capable of differentiating into multiple mesenchymal (adipogenic, osteogenic, and chondrogenic) lineages, similar to ASCs. CD34+/CD206+ ATMs grew rapidly and lost expression of CD45, CD14, and CD206 by passage 3, which resulted in a similar expression profile to ASCs. Thus, this novel ATM subpopulation (CD45+/CD14+/CD34+/CD206+) showed distinct biological properties from other ATMs and circulating monocytes/macrophages. The CD34+/CD206+ ATMs possessed characteristics similar to ASCs, including adherence, localization, morphology, and mesenchymal multipotency. This AT-resident subpopulation may have migrated from the bone marrow and may be important to tissue maintenance and remolding.
Introduction
Adipose tissue (AT) contains mature adipocytes and stromal vascular fraction (SVF) cells, including AT-resident hematopoietic cells. Our previous work shows that AT-resident hematopoietic cells constitute ∼10% of the total cells in intact human AT [1]. These AT-resident hematopoietic/immune cell populations have recently gained attention for their pivotal roles in the pathogenesis of obesity-related AT endocrine dysfunction, type 2 diabetes induced insulin resistance, and metabolic syndrome [2–6]. In the AT of obese individuals, inflammatory cells, such as macrophages and T lymphocytes increase in number with the degree of adiposity. This increase is followed by low-grade chronic inflammation, which may be responsible for metabolic syndrome-related traits and type 2 diabetes.
In addition to their involvement in inflammation, AT-resident hematopoietic cells exhibit a broad range of physiological functions during homeostasis, remodeling, and repair. For example, AT-associated c-Kit+/Sca-1+ lymphocytes were demonstrated to regulate self-renewal and antibody production of other lymphocytes [7]. Additionally, AT-resident macrophages (ATMs) that originate from blood-circulating monocytes exhibit a high degree of heterogeneity, which depends mostly on their specific microenvironment [8]. Two types of macrophages, inflammatory macrophages (M1) and anti-inflammatory macrophages (M2), are regarded as the 2 extremes in a continuum of functional macrophage stages [9,10] and are detected at different levels depending on the adiposity of the AT. Specifically, very few M1 stage macrophages are detected in lean AT where inflammatory signals are low or nonexistent [11,12]; however, M2 anti-inflammatory macrophages are highly prevalent in lean AT and appear to function in tissue repair/remodeling and prevention of inflammation [13,14].
AT has also been shown to act as a reservoir for hematopoietic stem/progenitor cells [15,16]. In fact, 1 report demonstrated that murine adipose-derived hematopoietic stem/progenitor cells contain precursors committed to the mast cell lineage [17]. Another report demonstrated the existence of hemangioblasts in human AT that exhibit the capacity for hematopoietic and endothelial differentiation in vitro [18].
Furthermore, an increasing number of reports suggest that cells belonging to adipogenic and hematopoietic lineages share common features [19–24], making it difficult to discern between the 2 populations. Features of AT-resident hematopoietic (stem/progenitor) cells that remain unknown include composition, localization, biological potential, physiological function, and their relationship with other adipose cells, such as adipose progenitor cells and vascular endothelial cells. Therefore, in the present study, we characterized resident hematopoietic cells in human nonobese AT. We show that an AT-resident macrophage population of adherent cells expressing CD34 and CD206 localizes around the vasculature, expresses anti-inflammatory macrophage markers, and displays mesenchymal multipotency.
Materials and Methods
Human tissue sampling and cell isolation
Liposuction aspirates were obtained from nonobese healthy donors (mean age, 42.4±5.4; mean body mass index, 21.9±0.9; n=8) undergoing liposuction of the abdomen and thigh. The SVF was isolated from the aspirated AT as described previously [25]. Briefly, the aspirated AT was washed with phosphate-buffered saline (PBS) and digested on a shaker at 37°C in PBS containing 0.075% collagenase (crude type, cat No. 032-10534; Wako Pure Chemical Industries) for 30 min. Mature adipocytes and connective tissue were separated from the cell pellet by centrifugation. The cell pellets were resuspended, filtered through 100-μm, 70-μm, and 40-μm meshes, and hemolyzed. Peripheral blood (PB) was obtained from healthy volunteers (mean age, 35.0±1.1; mean body mass index, 20.4±1.3; n=4). Hemolyzed blood was used for analyses. Small excised AT samples were harvested from the subcutaneous AT of patients undergoing plastic and reconstructive procedures.
Each patient provided informed consent to the institutional review board-approved protocol before the procedure (University of Tokyo Hospital, approved number 2489). All AT and PB samples used in this study were provided by nonobese patients or volunteers whose body mass index was under 25.
Immunohistochemistry
Harvested AT samples were zinc-fixed (Zinc Fixative; BD Biosciences), paraffin-embedded, and sliced into 6-μm-thick sections. Immunostaining was performed using a mouse anti-human CD45 monoclonal antibody (1:50, clone 2B11 + PD7/26; Dako) and a goat anti-human CD206 polyclonal antibody (1:40, R&D systems), and secondary antibodies conjugated with Alexa Fluor 488, 546, or 568 (1:200, Molecular Probes). Cell nuclei were visualized with TO-PRO-3 (1:2000, Molecular Probes). An isotypic primary antibody served as a negative control for each stain. Photographic images of the stained sections were acquired using a fluorescence microscope equipped with a digital camera (BioZero; Keyence).
For whole-mount staining, AT was cut into 3-mm pieces and immediately incubated with antibodies against human antigens CD14 (1:50, clone UCH-M1; Santa Cruz Biotechnology), CD34 (1:300, clone QBEnd10; Dako), CD45, and CD206. This was followed by incubation with secondary antibodies and Alexa Fluor 647-conjugated isolectin (1:100, Molecular Probes) to stain the endothelial cells. The samples were washed and directly observed with a confocal microscope.
Flow cytometric analyses and cell sorting
We used the following fluorochrome-conjugated monoclonal antibodies: anti-CD45-fluorescein isothiocyanate (FITC) (clone HI30), CD14-phycoerythrin (PE) (clone M5E2), CD206-Allophycocyanin (APC) (clone 19.2), CD3-PE (clone UCHT1), CD8-PE (clone HIT8a), CD19-PE (clone HIB19), CD56-PE (clone B159), CD34-PE (clone 8G12) (1:100, BD Biosciences), and CD45-PE-Cy7 (clone J.33; Beckman Coulter). Cells were incubated with each antibody for 30 min, and then analyzed using a multicolor flow cytometer (BD LSR II; BD Biosciences). Control gates were set based on staining with an irrelevant [matched labeled isotype control immunoglobulin G (IgG)] antibody; no more than 0.1% of the cells stained positive using the irrelevant antibody.
A multiparameter fluorescent-activated cell sorter (BD FACS Aria; BD Biosciences) was used for sorting of freshly isolated SVF cells. For multicolor analysis, we used antibodies with a combination of CD45-FITC (clone HI30), CD14-PE, CD206-APC, and CD34-PE-Cy7 (clone 8G12) or another combination of CD45-PE-Cy7 (clone HI30), CD14-PE, CD206-APC, and CD-68-FITC (clone Y1/82A). CD45+/CD14+/CD34+/CD206+ cells (CD34+/CD206+ ATMs), CD45+/CD14+/CD34−/CD206+ cells (CD34−/CD206+ ATMs), and CD45−/CD31−/CD34+ cells (adipose-derived stem/stromal cells; ASCs) were individually isolated from the SVF of aspirated AT from nonobese patients. PB-derived CD45+/CD14+ cells (circulating monocytes; PB-MCs) were also sorted.
Immunocytochemistry
AT-derived sorted cells were cultured in the M199 medium containing 10% fetal bovine serum (FBS). Each passage was performed when the cells were ∼80% confluent. At the first or third passage, the cells were seeded onto an 8-well glass bottom slide (Lab-Tek II Chamber Slide System; Nalge Nunc International KK). Immunocytochemistry was performed for CD34, CD45, CD14, and CD206 3 days after seeding. The same primary antibodies were used as those used in immunohistochemistry. Cells cultured on the slides were washed, fixed in 4% paraformaldehyde, and incubated with primary antibodies. Next, they were incubated with a secondary antibody, either Alexa Fluor 488- or 546-conjugated IgG, and subjected to nuclear staining with Hoechst 33342 (1:200, Dojindo). Stained slides were observed under a fluorescence microscope.
Induced mesenchymal differentiation of cultured cells
CD34+/CD206+ ATMs and ASCs were induced to differentiate into adipogenic, chondrogenic, and osteogenic lineages. The methods of differentiation and analyses have been confirmed to be reliable for induction of ASC differentiation [25]. Cells cultured in the control medium [the Dulbecco's modified Eagle's medium (DMEM) containing 10% FBS] were used as negative controls. For adipogenic differentiation, cells were incubated 21 days in the DMEM containing 10% FBS, 0.5 mM isobutyl-methylxanthine, 1 μM dexamethasone, 10 μM insulin, and 200 μM indomethacin. Adipogenic differentiation was confirmed by Oil Red O staining. We used Nile Red fluorescence to quantitatively measure lipid droplets by AdipoRed™ (Cambrex) at an excitation wavelength of 485 nm and an emission wavelength of 535 nm with a fluorescent plate reader (DTX 880 Multimode Detector; Beckman Coulter). For chondrogenic differentiation, cells were incubated 21 days in the DMEM containing 1% FBS supplemented with 6.25 mg/mL insulin, 10 ng/mL TGF-β1, and 50 nm ascorbate-2-phosphate. Chondrogenic differentiation was confirmed by Alcian Blue staining. We used a micromass culture system to quantitatively analyze differentiation, as reported previously [26]. Briefly, cells were plated in a 15-mL tube and cultured in the chondrogenic medium for 21 days. After 21 days, the diameter of the micromass was measured. For osteogenic differentiation, cells were incubated 21 days in the DMEM containing 10% FBS supplemented with 0.1 mM dexamethasone, 50 mM ascorbate-2-phosphate, and 10 mM β-glycerophosphate (Nacalai Tesque). Osteogenic differentiation was confirmed by von Kossa staining. Total calcium deposition was quantitatively evaluated based on the orthocresolphthalein complexone method using a Calcium C-Test Wako Kit (Wako Chemicals) according to the manufacturer's instructions.
Microarray analysis
For microarray analysis, 4 cell populations, CD34+/CD206+ ATMs, CD34−/CD206+ ATMs, ASCs, and PB-MCs, were sorted by FACS from the freshly isolated SVF or PB, and then immediately treated with an RNA extractant, ISOGEN-LS (nippongene). cDNA was synthesized from the extracted total RNA from each population. Labeled cRNA was then synthesized using a Low Input Quick Amp Labeling Kit (Agilent Technology). Microarray analysis was performed using SurePrint G3 Human GE 8x60K (Agilent). Feature Extraction ver10.7 (Agilent) and GeneSpringGX ver11.5 (Agilent) were used for data analysis. All array data sets were deposited into the National Center for Biotechnology Information Gene Expression Omnibus database (ID no. GSE37660).
Statistical analysis
Results are expressed as mean±SEM. A paired t-test was used to evaluate the differences in differentiation capacity between CD34-positive ATMs and ASCs. A value of P<0.05 was considered significant.
Results
Human AT-derived SVF, not PB, contains CD206-positive macrophages
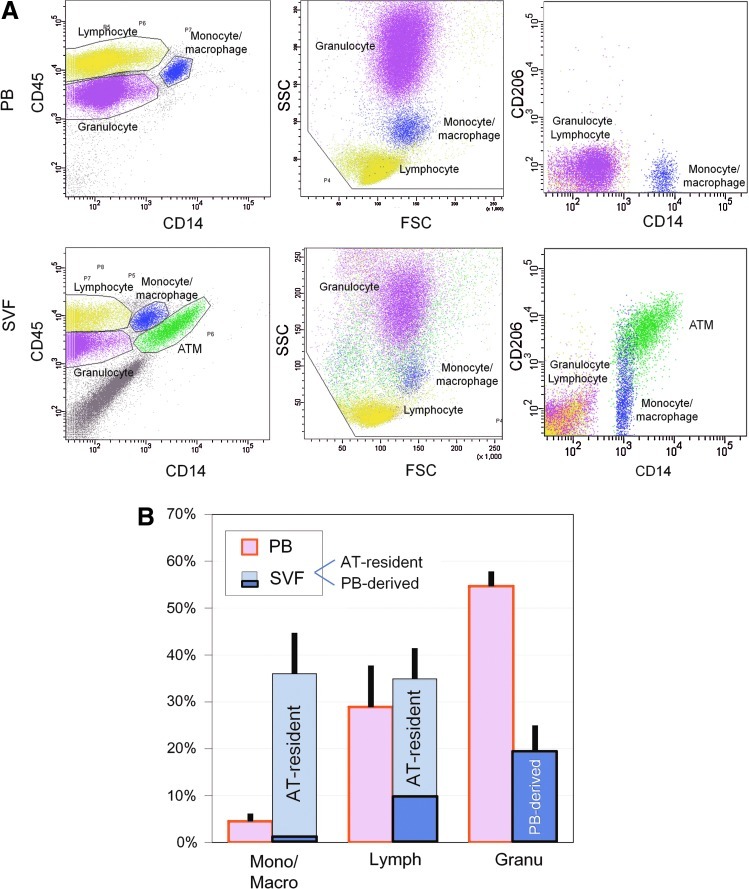
SVF cells obtained through collagenase digestion of human aspirated AT were substantially contaminated with PB cells resulting from hemorrhaging during the liposuction surgery. Therefore, PB and SVF were individually examined by flow cytometry to analyze AT-resident hematopoietic cells by excluding contaminated PB-derived circulating cells (Fig. 1A). PB-derived leukocytes were analyzed and separated into 3 populations by cell size [forward scatter (FSC)], complexity [side scatter (SSC)], and CD14 expression: (1) granulocytes (FSChigh/SSChigh/CD14−), (2) lymphocytes (FSClow/SSClow/CD14−), and (3) monocytes/macrophages (FSChigh/SSCmedium/CD14+). The averaged composition of each population in our sample was 54.7% (granulocytes), 28.9% (lymphocytes), and 4.6% (monocytes/macrophages) (n=4; Fig. 1B, Supplementary Table S1; Supplementary Data are available online at www.liebertpub.com/scd). The analyzed SVF cells indicated that the hematopoietic cells of the SVF were distinct from those of the PB. Specifically, granulocytes, lymphocytes, and monocytes/macrophages constituted 19.3(±5.6)%, 34.9(±6.5)%, and 36.1(±8.4)% of hematopoietic (CD45+) cells in the SVF, respectively (n=6; Fig. 1A, Supplementary Table S1). Unlike PB, 2 distinct monocyte/macrophage populations were observed in the SVF, a CD14+/CD206+ population and a CD14+/CD206− population (Fig. 1A). The former was not detected in PB, suggesting that all of CD14+/CD206+ cells were ATMs as reported previously [14]. In the samples from nonobese patients, CD14+/CD206+ ATMs were observed at a much higher rate (31.7%±7.8% of hematopoietic cells) than the CD14+/CD206− population (4.4%±0.9%), which was composed of PB-derived monocytes/macrophages and a population of M1 ATMs (Fig. 1B).
FIG. 1.
Flow cytometric analysis of hematopoietic (CD45+) cells in peripheral blood (PB) and stromal vascular fraction (SVF) freshly isolated from aspirated adipose tissue (AT). (A) By using FSC (forward scatter, representing cell size), SSC (side scatter, representing granularity), and expression of CD45 and CD14, nucleated PB cells (leukocytes) were classified into 3 predominant populations; granulocytes (purple; CD45+/FSChigh/SSChigh/CD14−), lymphocytes (yellow; CD45+/FSClow/SSClow/CD14−), and monocytes/macrophages (blue; CD45+/FSChigh/SSCmedium/CD14+), and all of them were negative for CD206. In contrast, adipose-derived SVF cells contain CD45− cells, which are AT-derived cells, such as adipose stromal cells (ASCs) and vascular endothelial cells. In comparison of CD45+ cells in SVF to those in PB, CD206+ cell population (green) was detected only in SVF. Thus, it was indicated that CD45+/FSChigh/SSCmiddle/CD14+/CD206+ were AT-resident macrophages (ATMs), though not all ATMs were CD206-positive. (B) Summarized data of hematopoietic (CD45+) cell composition in PB and SVF. SVF cells were composed of AT-resident cells and PB-derived cells. Almost all granulocytes in SVF were derived from PB, while most of monocytes/macrophages in SVF were found to be AT-resident in nonobese AT. Mono/Macro: monocytes/macrophages, Lymph: lymphocytes, Granu: granulocytes. Color images available online at www.liebertpub.com/scd
Composition of hematopoietic cells residing in human AT
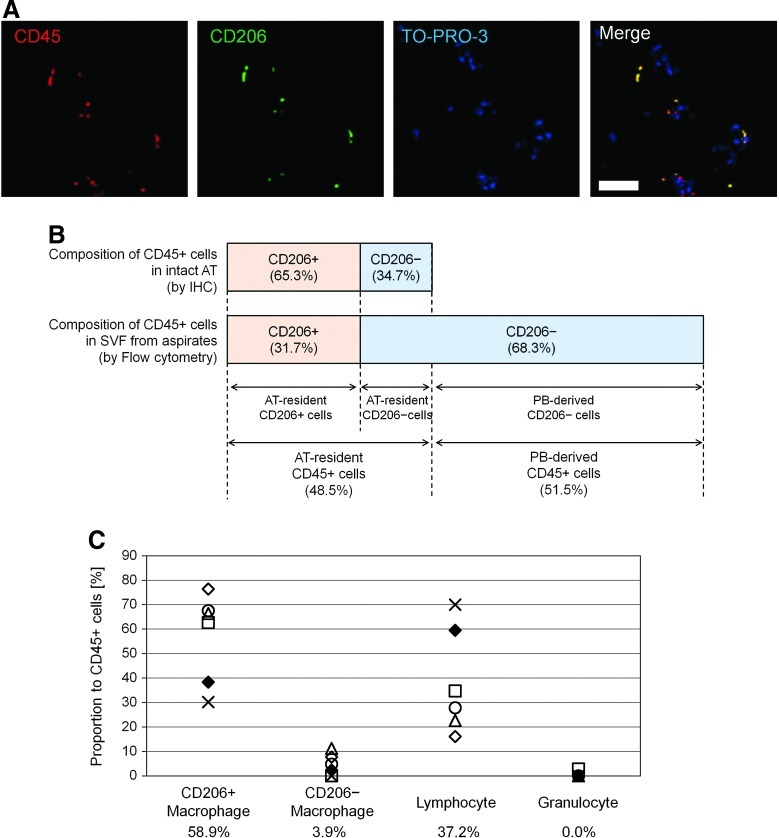
Immunohistochemistry using paraffin-embedded sections of human excised AT from nonobese patients was additionally performed to examine the number and localization of CD206+ ATMs (n=5). Histology revealed that all CD206+ cells also expressed CD45 (Fig. 2A) and CD206+ ATMs constituted 65.3%±3.5% of hematopoietic (CD45+) cells in the nonobese AT (Fig. 2B). However, FACS analysis of the SVF from aspirated AT (n=6) showed the CD206+ ATMs constituted 31.7%±7.8% of CD45+ cells (Fig. 2B), because aspirated AT (and also the freshly isolated SVF from aspirated AT) was substantially contaminated with PB through the operative procedure. The important finding that all CD206+ cells reside in AT, not in PB, enabled us to calculate the proportion of contaminated PB cells in SVF from aspirated AT theoretically; the rate of contaminating PB-derived CD45+ cells and AT-resident CD45+ cells is 51.5% and 48.5% respectively in the SVF isolated from aspirated AT (Fig. 2B).
FIG. 2.
Cellular composition of AT-resident hematopoietic (CD45+) cells. (A) Paraffin-embedded sections of intact (excised) AT were stained for CD45 (nucleated hematopoietic cells; red), CD206 (green), and TO-PRO3 (nuclei; blue). All of CD206+ cells were positive for CD45, suggesting that no other cells than ATMs express CD206 in intact AT. Scale Bar=50 μm. (B) CD206+ ATMs were measured, histologically using immunostained sections of intact AT, or by flow cytometry of SVF from aspirated AT. CD206+ ATMs accounted for 65.3% of AT-resident hematopoietic cells in intact AT, whereas CD206+ATMs account for 31.7% of hematopoietic cells from aspirated AT, which contains PB contamination. Considering together, we estimated the average ratio of PB-derived leukocyte contamination was 51.5% in our aspirated AT samples. (C) Composition of each hematopoietic cell population residing in intact AT was calculated for a rough estimation. The mean value of each cell type is listed at the bottom. CD206+ATMs appeared to constitute more than half of AT-resident hematopoietic cells in intact AT and most of the rest were composed of AT-resident lymphocytes, while CD206-negative monocyte/macrophage population constitutes less than 10% in nonobese AT. Color images available online at www.liebertpub.com/scd
Now, we could also calculate the theoretical proportion of AT-resident hematopoietic cells with the exception of contaminating PB cells from the FACS data on SVF cells isolated from aspirated AT. AT-resident hematopoietic cells were composed of 58.9% CD206+ ATMs (on average), 3.8% CD206− ATMs (on average), and 37.2% lymphocytes (on average). The number of granulocytes was negligible (Fig. 2C).
The composition of lymphocytes in SVF and PB was also compared by flow cytometry (Supplementary Fig. S1 and Supplementary Table S2). The most remarkable difference was observed in T cell subpopulations. The percentage of memory T cells (CD45+/CD3+/CD45RA−/CD45RO+) was much higher in the SVF than in the PB. Naïve T cells (CD45+/CD3+/CD45RA+/CD45RO−) and cytotoxic T cells (CD45+/CD3+/CD8+) were more abundant in the PB (Supplementary Fig. S1A, B). We virtually calculated the composition of adipose-resident lymphocytes based on the lymphocyte quantity in the SVF and PB and on contamination of PB in the SVF (Supplementary Fig. S1C). This revealed that adipose-resident lymphocytes are predominantly composed of helper T cells (CD45+/CD3+/CD8−) and natural killer (NK) cells (CD45+/CD3+/CD56+) and that almost no B cells (CD45+/CD3+/CD19+) and few cytotoxic T cells reside in nonobese AT.
Two distinct CD206+ ATM populations were found, CD34+ population and CD34− population
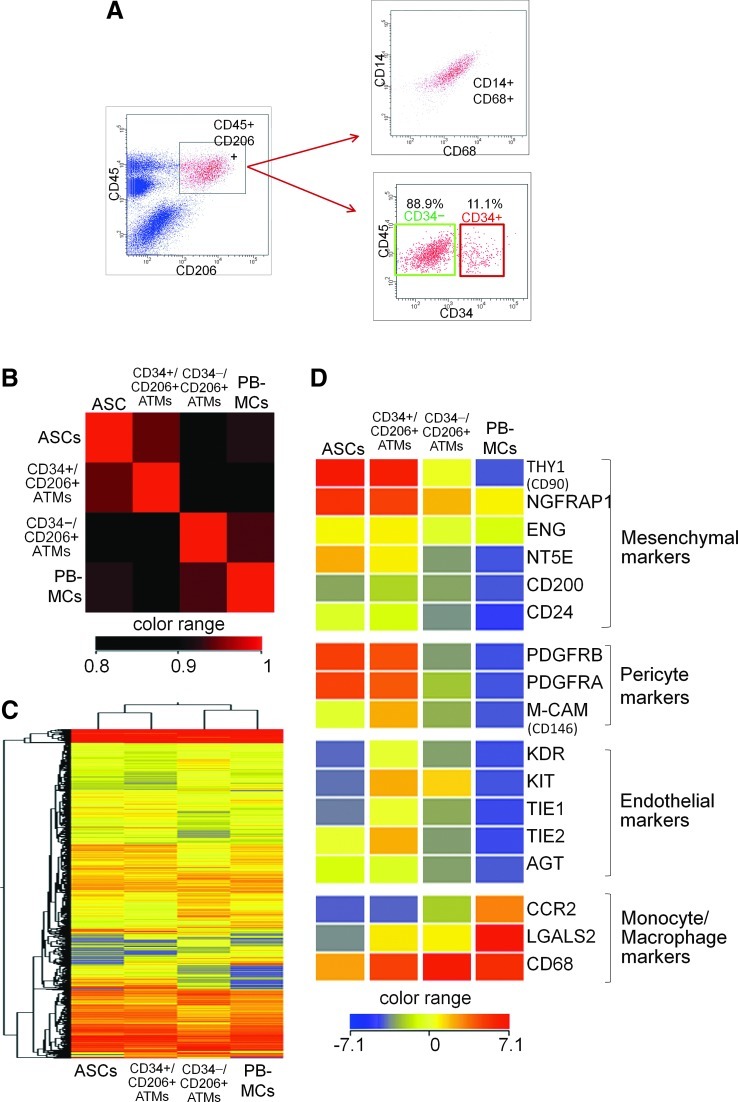
We analyzed CD206+ ATMs in the SVF by multicolor flow cytometry and found that all CD206+ cells in the SVF coexpressed CD45. Gated CD45+/CD206+ cells were shown to be positive for CD14 and CD68. The CD45+/CD14+/CD68+/CD206+ ATMs were clearly divided into 2 subpopulations according to CD34 expression, CD34+/CD206+ATMs (11.1%±1.7%, n=5) and CD34−/CD206+ ATMs (88.9%±1.7%, n=5) (Fig. 3A).
FIG. 3.
Flow cytometry and microarray analysis of distinct ATM populations. (A) Flow cytometric analysis of SVF cells obtained from aspirated AT showed that CD45+/CD206+ cell population expressed both CD14 and CD68, confirming that it is a population of monocytes/macrophages. The CD45+/CD206+/CD14+/CD68+ ATMs were further divided into 2 subpopulation, CD34+ population (11.1%) and CD34− population (88.9%). (B) Correlation plot of the gene expression of 4 samples, CD34+/CD206+ ATMs, CD34−/CD206+ ATMs, ASCs, and PB-derived monocytes/macrophages (PB-MCs). CD34+/CD206+ ATMs, CD34−/CD206+ ATMs, and ASCs were freshly sorted from SVF and PB-MCs were obtained from PB. The plot revealed that the gene expression profiles of CD34+/CD206+ ATMs were more similar to that of ASCs compared with CD34−/CD206+ ATMs or PB-MCs. All array data sets were deposited into the National Center for Biotechnology Information Gene Expression Omnibus database (ID no. GSE30742, www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE30742). (C) Cluster analysis of the 4 samples. This analysis confirmed that CD34+/CD206+ ATMs show similar gene expression pattern as ASCs rather than CD34−/CD206+ ATMs. (D) Gene expression of mesenchymal, pericyte, endothelial, and monocyte/macrophage markers in samples. Both CD34+/CD206+ ATMs and ASCs highly expressed mesenchymal markers and pericytes markers. Only CD34+/CD206+ ATMs showed endothelial markers. Color images available online at www.liebertpub.com/scd
Significant overlap of gene clusters was observed between CD34+ ATMs and ASCs
Gene expression patterns were compared by microarray analysis to elucidate the relationships between the 4 freshly isolated cell populations, SVF-derived CD34+/CD206+ ATMs, SVF-derived CD34−/CD206+ ATMs, SVF-derived ASCs (CD34+/CD31−/CD45−), and PB-MCs (CD14+/CD45+). A correlation plot of these samples revealed that CD34+/CD206+ ATM cells are similar to ASCs and that they are both quite different from CD34−/CD206+ ATMs (Fig. 3B). Consistent with this observation, unsupervised hierarchical clustering of all genes using a decision tree algorithm based on the average Euclidean distance placed CD34+/CD206+ ATMs and ASCs into the same group (Fig. 3C). Similar results were also obtained by clustering analysis using the Centroid or Ward algorithms.
ATMs and PB-monocytes showed high expression of monocyte/macrophage markers. Many genes were similarly expressed in CD34+/CD206+ ATMs and ASCs, including high expression of mesenchymal markers and pericyte markers (Fig. 3D). This suggests a potential link in physiological roles of the mesenchymal progenitors shared by these 2 populations. Interestingly, only CD34+/CD206+ ATMs displayed a relatively high expression of a series of vascular endothelial markers.
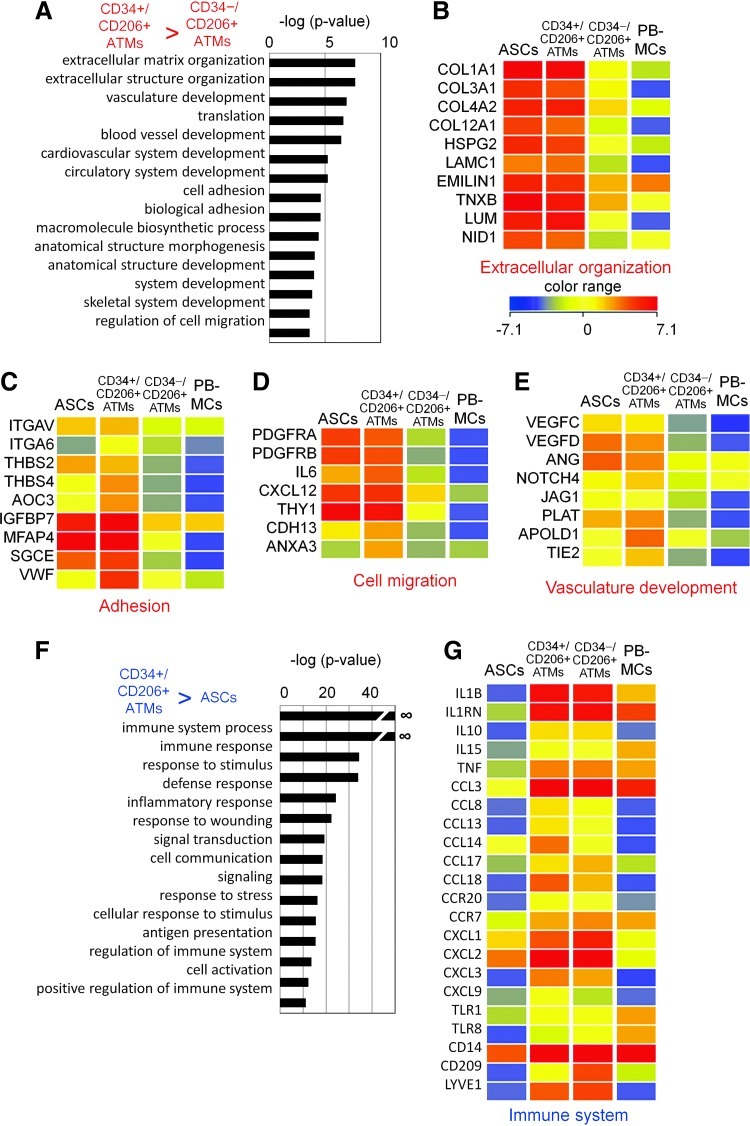
Gene ontology (GO) analysis of the genes highly expressed in CD34+/CD206+ ATMs compared to CD34−/CD206+ ATMs identified several characteristic ontologies (Fig. 4A, Supplementary Table S3). The expression of extracellular matrix components collagen, laminin, and heparin sulfate proteoglycans (Fig. 4B) in addition to the expression of a combination of molecules responsible for cell adhesion, such as integrins (Fig. 4C), raises the possibility that CD34+/CD206+ ATMs might have unique adhesive properties that are not evident in normal macrophages. More interestingly, CD34+/CD206+ ATMs expressed a series of genes involved in cell migration (Fig. 4D), vasculature, and blood vessel development, including the vascular endothelial growth factor-C and angiotensin (Fig. 4E). This suggests that CD34+/CD206+ ATMs may be involved in blood vessel formation.
FIG. 4.
Gene ontology (GO) analysis of the genes specifically expressed in CD34+/CD206+ ATMs. (A) GO analysis of the genes that show more than 5 times higher expression in CD34+/CD206+ ATMs comparing to CD34−/CD206+ ATMs. The top 15 biological process terms were shown. (B)–(E) Expression profile of the genes in the selected ontology; (B) Extracellular matrix organization, (C) Adhesion, (D) Cell migration, and (E) vasculature development. (F) GO analysis of the genes that show more than 5 times higher expression in CD34+/CD206+ ATMs comparing to ASCs. (G) Expression profile of the genes in the ontology, immune system. Color images available online at www.liebertpub.com/scd
In contrast, GO analysis of the genes highly expressed in CD34+/CD206+ ATMs compared to ASCs identified immune system-related genes (Fig. 4F, Supplementary Table S4). These included genes involved in response to stimulus and wounding, such as M2 macrophage-specific markers IL10, IL1RN, CCL3, CCL18, CXCL9, TNF, CD14, and LYVE1. The expression profile of immune-related genes is comparable between CD34+/CD206+ ATMs and CD34−/CD206+ ATMs. These results suggest that CD34+/CD206+ ATMs may be unique peripheral macrophages with characteristic expression of genes involved in cell adhesion, migration, and vasculature formation.
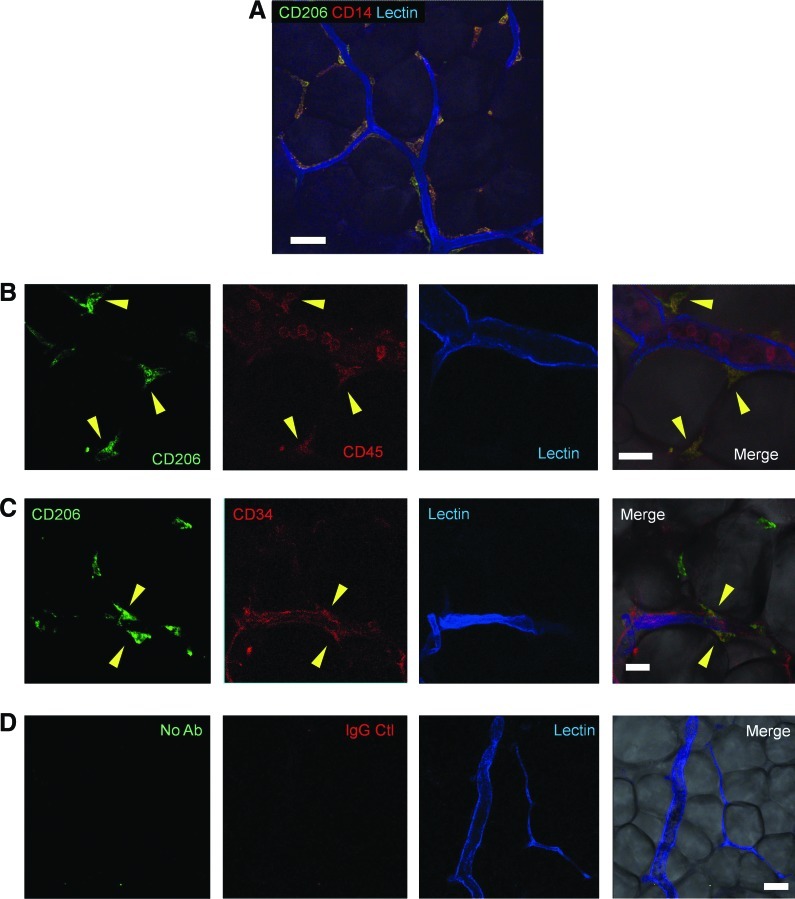
Whole-mount staining for localization and immunophenotyping of ATMs
Flow cytometry and immunohistology of human AT suggest that more than half of the AT-resident hematopoietic cells are CD206+ ATMs. Immunohistochemical whole-mount staining confirmed that all CD206+ cells in intact human AT expressed both CD14 and CD45 (Fig. 5A,B). This indicates that these cells are a subpopulation of monocytes/macrophages. Most of the CD206+ ATMs (CD45+/CD14+/CD206+) were at periendothelial locations, while a small number of them were observed in interstitial spaces between adipocytes. Some of the CD206+ ATMs coexpressed CD34 and localized in the perivascular area (Fig. 5C).
FIG. 5.
Whole-mount histological examination for localization of ATMs (A) Immunostaining of whole-mount AT indicated that CD206+ ATMs (CD14+/CD206+ cells) located mostly periendothelially in capillaries and a small number of CD206+ATMs were seen also in interstitial spaces between adipocytes. Lectin (blue) was used to visualize vascular endothelium. Scale bar=50 μm. (B) CD206+ ATMs (CD206+/CD45+ cells; arrowhead) localized outside of endothelial cells, while intravascular hematopoietic cells (CD45+ circulating leukocytes) did not express CD206. Scale bar=25 μm. (C) ATMs (CD206+ cells), coexpressing CD34, were found to be localized perivascularly, although not all ATMs expressed CD34. Scale bar=25 μm. (D) Staining images with isotype control. Scale bar=50 μm. Color images available online at www.liebertpub.com/scd
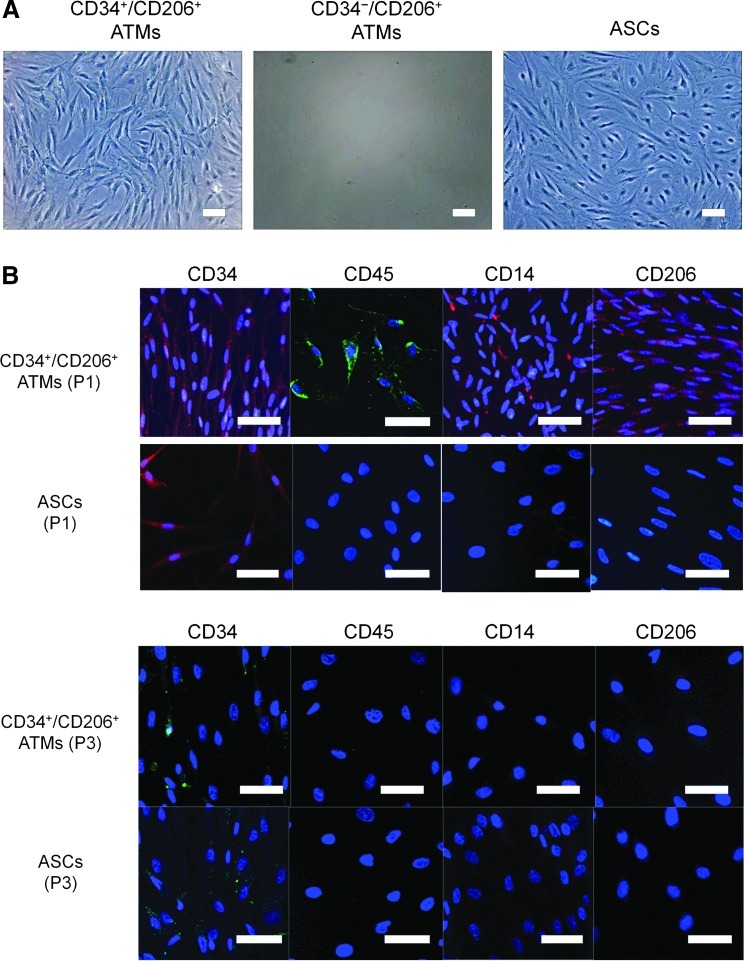
Subculture of CD34+/CD206+ ATMs and CD34−/CD206+ ATMs
CD34+/CD206+ and CD34−/CD206+ ATMs, and nonhematopoietic cell populations (CD45−) from the SVF were sorted and cultured separately on tissue culture plastic dishes. ASCs can be selectively expanded from CD45− populations by adherent culture in the M199 medium [26]. Interestingly, the CD34+/CD206+ ATMs expanded as rapidly as ASCs; however, CD34−/CD206+ ATMs did not grow in adherent culture (Fig. 6A). Although cultured CD34+/CD206+ ATMs partly preserved their expression of CD45, CD14, and CD206 at passage 1, they lost expression of CD45, CD14, and CD206 by passage 3 (Fig. 6B). Thus, CD34+/CD206+ ATMs displayed a similar expression profile to cultured ASCs after a few weeks of adherent culture and they could not be distinguished from each other by expression profiles or morphology.
FIG. 6.
Adherent culture for characterization of CD34+/CD206+ ATMs. (A) CD34+/CD206+ ATMs and CD34−/CD206+ ATMs were sorted and individually plated for adherent culture. ASCs were also cultured as control. CD34+/CD206+ ATMs proliferated with fibroblast-like morphology on a plate, while CD34− ATMs did not grow in the adherent culture. Scale bars=100μm. (B) Immunocytochemistry of cultured CD34+/CD206+ ATMs and ASCs. Cultured CD34+/CD206+ ATMs remained expressing CD34, CD45, CD14, and CD206 at passage 1 (P1), while cultured ASCs expressed only CD34. After culture for several weeks (at passage 3), CD34+/CD206+ ATMs completely lost their expression of CD45, CD14, and CD206, although CD34 was still partly expressed. Thus, the cultured ATMs were not discriminated from cultured ASCs with their surface marker expression and morphology. Scale bars=50 μm. Color images available online at www.liebertpub.com/scd
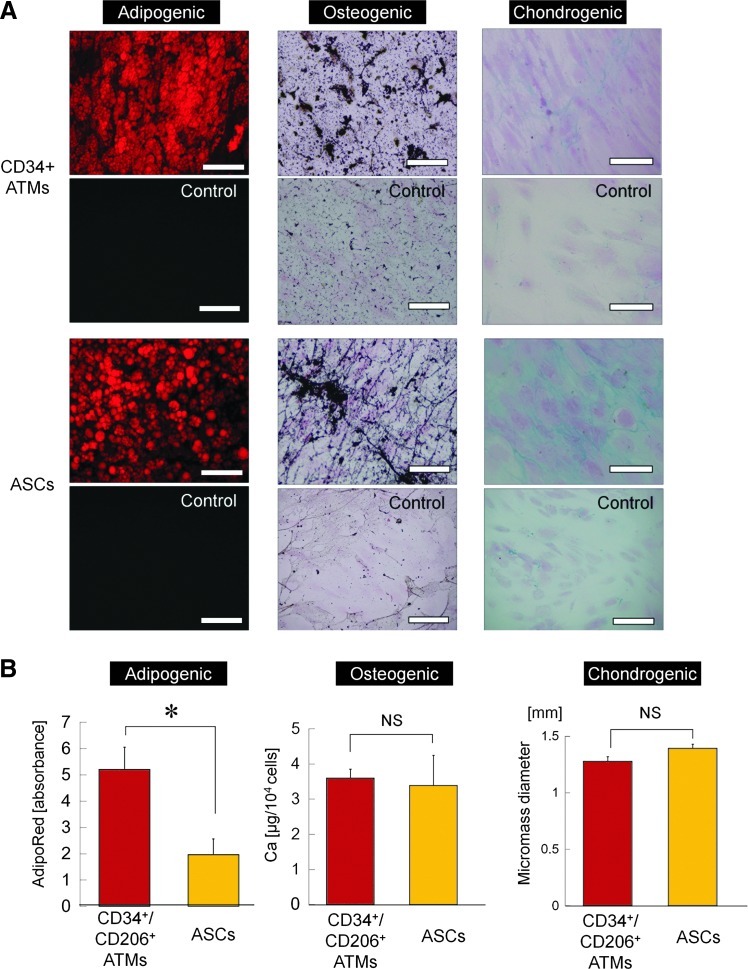
CD34+/CD206+ ATMs differentiate into multiple mesenchymal lineages
We next investigated whether adherent CD34+/CD206+ ATMs have mesenchymal multidifferentiation potentials, such as ASCs. Our results indicated substantial differentiating capacities of cultured CD34+/CD206+ ATMs into adipogenic, osteogenic, and chondrogenic lineages (Fig. 7A). Rather, CD34+/CD206+ ATMs displayed significantly enhanced adipogenic differentiation compared to ASCs (Fig. 7B). Thus, CD34+/CD206+ ATMs may be involved in AT remodeling as adipose-resident mesenchymal stem cells or adipocyte progenitor cells.
FIG. 7.
Mesenchymal differentiation of CD34+/CD206+ ATMs in comparison with ASCs. (A) Representative microscopic images of differentiation assays for adipogenic, osteogenic, and chondrogenic pathways. Lipid droplets of differentiated adipocytes were stained in red (Oil red O staining), while calcium deposits resulting from osteogenic differentiation were stained in black with von Kossa staining. Chondrogenesis was assessed by Alcian blue staining. Control cells cultured in control medium were also stained in each assay. Scale bars=100 μm. (B) Quantitative evaluations of mesenchymal differentiation into adipogenic, osteogenic, and chondrogenic lineages (n=3). Each differentiation capacity was evaluated by measuring lipid droplet content (adipogenic), total calcium content (osteogenic), or micromass diameter (chondrogenic). *P<0.05. Error bars represent SEM. Color images available online at www.liebertpub.com/scd
Discussion
The SVF isolated from aspirated AT is contaminated by numerous PB-derived erythrocytes and leukocytes. This is one factor that makes it difficult to analyze AT-resident hematopoietic cells. The composition of CD45+ cells contained in the SVF was analyzed previously by FACS [27]. Of these cells, 19% were CD14+ (macrophages), 6% were CD3+ (T cells), and 1.5% were CD56+ (NK cells); very few of the cells were CD19+ (B cells) [27]. In this previous report, however, contaminating PB cells were not excluded. Therefore, we aimed to accurately measure the composition of CD45+ cells residing in the AT. We confirmed that CD206-expressing cells were not detected in the PB, but were detected in the AT. We were able to estimate the contamination of aspirated AT by PB cells by using calculations based on the cell composition of the PB (examined by FACS), the SVF (from aspirated AT examined by FACS), and the AT (excised samples embedded in paraffin and examined by immunohistochemistry). Our estimation may not be exact because of the possible remaining of PB cells inside vessels in the paraffin-embedded AT. Regardless, this is the first report on the cellular composition of AT-resident hematopoietic cells that takes into account and subtracts out contaminating PB cells. It was concluded that AT-resident hematopoietic cells in nonobese subjects were composed predominantly of macrophages (constituting ∼60%) and lymphocytes (constituting ∼40%), the latter of which were predominantly composed of helper T cells (60%–70%), NK cells (30%–40%), and no B cells. T lymphocytes, especially cytotoxic T lymphocytes, have been shown to infiltrate obese AT, consistent to the degree of adiposity, and secrete inflammatory factors [5,6]. In this study, nonobese AT contained a negligible number of cytotoxic T cells. This was also observed for macrophages; CD206− macrophages that correspond to the M1 state inflammatory macrophages can constitute up to 40% of the cells in obese AT [28,29]. These cells were detected only in small numbers in our nonobese AT samples.
The molecular repertoire of M2 macrophages includes proteins, such as the mannose receptor (CD206), that are expressed at very low levels by M1 macrophages [10,13,30–32]. Our analyses demonstrated that cells expressing CD206 and CD14 accounted for the main population (about 60%) of hematopoietic cells and for more than 90% of CD45+/CD14+ ATMs in lean patients. Because almost no CD206+ cells were detected in the PB and all CD206+ cells were CD14-positive, we used the phenotypic marker CD206 to identify anti-inflammatory ATMs as described in previous studies [32]. Whole-mount staining demonstrated that there were quite a few CD206+ ATMs in intact AT. Some of them reside in the interstitial space between adipocytes, but most reside in periendothelial regions where ASCs are thought to be localized [33–35]. A previous study on ATMs in lean mice [11] reported that M2 ATMs reside between adipocytes and occasionally associated with blood vessels, which is supported by the present study. More recently, it was shown that some adipocytes originate from fetal vascular endothelium [36] and ASCs may be physiological progenitors for both adipocytes and vascular endothelial cells [37], which may also suggest a close relationship among CD206+ ATMs, adipocytes, and vascular cells.
Our results indicated that some of the perivascular ATMs coexpressed CD34. Flow cytometric analysis showed that 11.2% of ATMs (CD45+/CD14+/CD206+ cells) in the SVF expressed CD34. This provides new insights for translating previous studies. For example, CD45+/CD34+ cells that reside in the AT are phenotypically macrophages, at least in part, and some researchers, including us, have already pointed out that the proportion of CD45+/CD34+ cells in AT is higher than in PB. However, the identity of these cells remains unknown. Cousin B et al. [15] demonstrated that CD45+/CD34+ cells in mice AT are hematopoietic stem/progenitor cells due to their ability to form hematopoietic colonies in methylcellulose. Recent reports also suggest the presence of functional hematopoietic stem/progenitor cells in AT [16]. Hematopoietic stem/progenitor cells predisposed to forming monocytes/macrophages are located in the adventitia of murine aortas [38], which harbor tissue-specific progenitors with the potential to differentiate into vascular endothelial and smooth muscle cells [39]. Thus, the CD34+ ATMs might also function physiologically as hematopoietic progenitor cells in the AT.
Although CD34 is a hallmark of hematopoietic stem cells [40,41], it is also expressed in a wide variety of nonhematopoietic cells, such as vascular endothelial cells and ASCs. We previously attempted to compare CD34+ and CD34− fractions within shortly cultured ASCs and the results suggested that CD34 expression may correlate more with immaturity or the endothelial lineage than with mesenchymal or pericyte lineages [28]. In the present study, we observed many distinct features between CD34+/CD206+ ATMs and CD34−/CD206+ ATMs. Only CD34+/CD206+ ATMs acted like stromal cells and were capable of growing in adherent culture, suggesting that CD34 expression in ATMs might be greatly involved in cell adhesion. Comprehensive gene expression analyses also revealed that both cell populations had clear differences in gene expression patterns related to cell adhesion, vascular development, and extracellular matrix organization.
Our results revealed that CD34+/CD206+ATMs share many biological features with ASCs. First, both are localized in the perivascular region, like pericytes in the AT. Second, CD34+/CD206+ ATMs were adherent and lost expression of CD45 and CD14 over time in culture and they possessed similar expression profiles and morphologies as ASCs. Third, CD34+/CD206+ ATMs display mesenchymal multipotency by differentiating toward the adipogenic, osteogenic, and chondrogenic lineages. Finally, microarray analysis revealed that CD206+/CD34+ ATMs and ASCs share many gene clusters related to extracellular organization, cell adhesion, cell migration, and vascular development. Both populations displayed a higher expression of mesenchymal markers and pericyte markers than CD34−/CD206+ ATMs. Previous studies demonstrate that ASCs can be converted into macrophages depending on the microenvironment [42] and vice versa [43]. This suggests that both ASCs and macrophages have substantial plasticity and are closely related to each other. In addition, some studies suggest that certain adipocytes and adipose progenitor cells originate from bone marrow-derived cells [44–46], although there was a contradictory report that failed to demonstrate transdifferentiation of bone marrow-derived cells into adipocytes [47]. Our study demonstrated high adipogenic differentiation capacity of CD34+/CD206+ ATMs. Previous findings that some ASCs are derived from trafficked bone marrow-derived cells [46] may explain the origin and function of CD34+/CD206+ ATMs. Furthermore, there have also been reports that suggest a close relationship between endothelial progenitor cells and monocyte/macrophages, which mimic each other in many aspects [48,49]. Our microarray data indicates that CD34+/CD206+ ATMs display a higher expression of endothelial progenitor markers, such as KDR, Tie-1, and Tie-2 compared to ASCs, CD34−/CD206+ATMs, and PB-monocytes, suggesting CD34+/CD206+ ATMs may be associating in some way with endothelial progenitor cells. The relationship among CD34+/CD206+ ATMs, ASCs, and endothelial cells is quite an interesting question, so further study from various angles is needed.
In the present study, many experiments were based mainly on marker expressions of cells. In this regard, we should consider that some epitopes of antigens may have a cross reaction with doubtful antibodies. For example, it was reported that human mesenchymal stromal cells expressed CD14 cross-reactive epitopes depending on the clone of antibody [50].
In conclusion, we identified a novel population of ATMs (M2 macrophages) that express CD34 and CD206, share properties of monocytes/macrophages and ASCs, and are distinct from CD34−/CD206+ ATMs. These CD34+/CD206+ ATMs exist in nonobese AT in high numbers (constituting 5%–10% of AT-resident hematopoietic cells) and are located perivascularly like pericytes and ASCs. They highly express various gene clusters related to cell adhesion, cell migration, vascular development, extracellular matrix organization, and macrophage-specific and endothelial markers. They also demonstrated a high differentiation capacity toward adipogenic, osteogenic, and chondrogenic lineages. Altogether this report, along with previous reports, suggests that CD34+/CD206+ ATMs may migrate from bone marrow, home to AT, and functionally differentiate into various lineages, including adipocytes, endothelial cells, and macrophages for AT maintenance and remodeling.
Supplementary Material
Acknowledgments
We thank Ayako Kurata for technical assistance. This work was supported by a grant from the Japanese Ministry of Education, Culture, Sports, Science, and Technology (contact grant number: B2-21390477).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Eto H. Suga H. Matsumoto D. Inoue K. Aoi N. Kato H. Araki J. Yoshimura K. Characterization of structure and cellular components of aspirated and excised adipose tissue. Plast Reconstr Surg. 2009;124:1087–1097. doi: 10.1097/PRS.0b013e3181b5a3f1. [DOI] [PubMed] [Google Scholar]
- 2.Curat CA. Miranville A. Sengenès C. Diehl M. Tonus C. Busse R. Bouloumié A. From blood monocytes to adipose tissue-resident macrophages: induction of diapedesis by human mature adipocytes. Diabates. 2004;53:1285–1292. doi: 10.2337/diabetes.53.5.1285. [DOI] [PubMed] [Google Scholar]
- 3.Curat CA. Wegner V. Sengenès C. Miranville A. Tonus C. Busse R. Bouloumié A. Macrophages in human visceral adipose tissue: increased accumulation in obesity and a source of resistin and visfatin. Diabetologia. 2006;49:744–747. doi: 10.1007/s00125-006-0173-z. [DOI] [PubMed] [Google Scholar]
- 4.Cancello R. Tordjman J. Poitou C. Guilhem G. Bouillot JL. Hugol D. Coussieu C. Basdevant A. Bar Hen A, et al. Increased infiltration of macrophages in omental adipose tissue is associated with marked hepatic lesions in morbid human obesity. Diabetes. 2006;55:1554–1561. doi: 10.2337/db06-0133. [DOI] [PubMed] [Google Scholar]
- 5.Nishimura S. Manabe I. Nagasaki M. Eto K. Yamashita H. Ohsugi M. Otsu M. Hara K. Ueki K, et al. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med. 2009;15:914–920. doi: 10.1038/nm.1964. [DOI] [PubMed] [Google Scholar]
- 6.Kintscher U. Hartge M. Hess K. Foryst-Ludwig A. Clemenz M. Wabitsch M. Fischer-Posovszky P. Barth TF. Dragun D, et al. T-lymphocyte infiltration in visceral adipose tissue: a primary event in adipose tissue inflammation and the development of obesity-mediated insulin resistance. Arteriorscler Thromb Vasc Biol. 2008;28:1304–1310. doi: 10.1161/ATVBAHA.108.165100. [DOI] [PubMed] [Google Scholar]
- 7.Moro K. Yamada T. Tanabe M. Takeuchi T. Ikawa T. Kawamoto H. Furusawa J. Ohtani M. Fujii H. Koyasu S. Innate production of TH2 cytokines by adipose tissue-associated c-Kit+Sca-1+ lymphoid cells. Nature. 2010;463:540–544. doi: 10.1038/nature08636. [DOI] [PubMed] [Google Scholar]
- 8.Gordon S. Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5:953–964. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- 9.Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 10.Montvani A. Sozzani S. Locati M. Allavena P. Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23:549–555. doi: 10.1016/s1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- 11.Lumeng CN. DelProposto JB. Westcott DJ. Saltiel AR. Phenotypic switching of adipose tissue macrophages with obesity is generated by spatiotemporal differences in macrophage subtypes. Diabetes. 2008;57:3239–3246. doi: 10.2337/db08-0872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fujisaka S. Usui I. Bukhari A. Ikutani M. Oya T. Kanatani Y. Tsuneyama K. Nagai Y. Takatsu K, et al. Regulatory mechanisms for adipose tissue M1 and M2 macrophages in diet-induced obese mice. Diabetes. 2009;58:2574–2582. doi: 10.2337/db08-1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bourlier V. Zakaroff-Girard A. Miranville A. De Barros S. Maumus M. Sengenes C. Galitzky J. Lafontan M. Karpe F. Frayn KN. Bouloumié A. Remodeling phenotype of human subcutaneous adipose tissue macrophages. Circulation. 2008;117:806–815. doi: 10.1161/CIRCULATIONAHA.107.724096. [DOI] [PubMed] [Google Scholar]
- 14.Suganami T. Ogawa Y. Adipose tissue macrophages: their role in adipose tissue remodeling. J Leukocyte Biol. 2010;88:33–39. doi: 10.1189/jlb.0210072. [DOI] [PubMed] [Google Scholar]
- 15.Cousin B. Andre M. Arnaud E. Pénicaud L. Casteilla L. Reconstitution of lethally irradiated mice by cells isolated from adipose tissue. Biochem Biophys Res Commun. 2003;301:1016–1022. doi: 10.1016/s0006-291x(03)00061-5. [DOI] [PubMed] [Google Scholar]
- 16.Han J. Koh YJ. Moon HR. Ryoo HG. Cho CH. Kim I. Koh GY. Adipose tissue is an extramedullary reservoir for functional hematopoietic stem and progenitor cells. Blood. 2010;115:957–964. doi: 10.1182/blood-2009-05-219923. [DOI] [PubMed] [Google Scholar]
- 17.Poglio S. De Toni-Costes F. Arnaud E. Laharrague P. Espinosa E. Casteilla L. Cousin B. Adipose tissue as a dedicated reservoir of functional mast cell progenitors. Stem Cells. 2010;11:2065–2072. doi: 10.1002/stem.523. [DOI] [PubMed] [Google Scholar]
- 18.Miñana MD. Carbonell-Uberos F. Mirabet V. Marín S. Encabo A. IFATS collection: identification of hemangioblasts in the adult human adipose tissue. Stem Cells. 2008;10:2696–2704. doi: 10.1634/stemcells.2007-0988. [DOI] [PubMed] [Google Scholar]
- 19.Ho IC. Kim JH. Rooney JW. Spiegelman BM. Glimcher LH. A potential role for the nuclear factor of activated T cells family of transcriptional regulatory proteins in adipogenesis. Proc Natl Acad Sci U S A. 1998;95:15537–15541. doi: 10.1073/pnas.95.26.15537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ricote M. Li AC. Wilson TM. Kelly CJ. Glass CK. The peroxisome proliferator-activated receptor-gamma is a negative regulator of macrophage activation. Nature. 1998;391:79–82. doi: 10.1038/34178. [DOI] [PubMed] [Google Scholar]
- 21.Pelton PD. Zhou L. Demarest KT. Burris TP. PPARgamma activation induces the expression of the adipocyte fatty acid binding protein gene in human monocytes. Biochem Biophys Res Commun. 1999;261:456–458. doi: 10.1006/bbrc.1999.1071. [DOI] [PubMed] [Google Scholar]
- 22.Tong Q. Dalgin G. Xu H. Ting CN. Leiden JM. Hotamisligil GS. Function of GATA transcription factors in preadipocyte-adipocyte transition. Science. 2000;290:134–138. doi: 10.1126/science.290.5489.134. [DOI] [PubMed] [Google Scholar]
- 23.Moore KJ. Rosen ED. Fitzgerald ML. Randow F. Andersson LP. Altshuler D. Milstone DS. Mortensen RM. Spiegelman BM. Freeman MW. The role of PPAR-gamma in macrophage differentiation and cholesterol uptake. Nat Med. 2001;7:41–47. doi: 10.1038/83328. [DOI] [PubMed] [Google Scholar]
- 24.Cousin B. Munoz O. Andre M. Fontanilles AM. Dani C. Cousin JL. Laharrague P. Casteilla L. Pénicaud L. A role for preadipocytes as macrophage-like cells. FASEB J. 1999;13:305–312. doi: 10.1096/fasebj.13.2.305. [DOI] [PubMed] [Google Scholar]
- 25.Yoshimura K. Shigeura T. Matsumoto D. Sato T. Takaki Y. Aiba-Kojima E. Sato K. Inoue K. Nagase T. Koshima I. Gonda K. Characterization of freshly isolated and cultured cells derived from the fatty and fluid portions of liposuction aspirates. J Cell Physiol. 2006;208:64–76. doi: 10.1002/jcp.20636. [DOI] [PubMed] [Google Scholar]
- 26.Johnstone B. Hering TM. Caplan AI. In vitro chondrogenesis of bone marrow-derived mesenchymal progenitor cells. Exp Cell Res. 1998;238:265–272. doi: 10.1006/excr.1997.3858. [DOI] [PubMed] [Google Scholar]
- 27.Duffaut C. Zakaroff-Girard A. Bourlier V. Decaunes P. Maumus M. Chiotasso P. Sengenès C. Lafontan M. Galitzky J. Bouloumié A. Interplay between human adipocytes and T lymphocytes in obesity: CCL20 as an adipochemokine and T lymphocytes as lipogenic modulators. Arteriorscler Thromb Vasc Biol. 2009;10:1608–1614. doi: 10.1161/ATVBAHA.109.192583. [DOI] [PubMed] [Google Scholar]
- 28.Weisberg SP. McCann D. Desai M. Rosenbaum M. Leibel RL. Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu H. Barnes GT. Yang Q. Tan G. Yang D. Chou CJ. Sole J. Nichols A. Ross JS. Tartaglia LA. Chen H. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goerdt S. Orfanos CE. Other functions, other genes: alternative activation of antigen-presenting cells. Immunity. 1990;10:137–142. doi: 10.1016/s1074-7613(00)80014-x. [DOI] [PubMed] [Google Scholar]
- 31.McGreal EP. Miller JL. Gordon S. Ligand recognition by antigen-presenting cell C-type lectin receptors. Curr Opin Immunol. 2005;17:18–24. doi: 10.1016/j.coi.2004.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zeyda M. Farmer D. Todoric J. Aszmann O. Speiser M. Györi G. Zlabinger GJ. Stulnig TM. Human adipose tissue macrophages are of an anti-inflammatory phenotype but capable of excessive pro-inflammatory mediator production. Int J Obes (Lond) 2007;9:1420–1428. doi: 10.1038/sj.ijo.0803632. [DOI] [PubMed] [Google Scholar]
- 33.Tang W. Zeve D. Suh JM. Bosnakovski D. Kyba M. Hammer RE. Tallquist MD. Graff JM. White fat progenitor cells reside in the adipose vasculature. Science. 2008;322:583–586. doi: 10.1126/science.1156232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Traktuev DO. Merfeld-Clauss S. Li J. Khor F. Itescu S. Gimble JM. Gronthos S. Multipotential human adipose-derived stromal stem cells exhibit a perivascular phenotype in vitro and in vivo. J Cell Physiol. 2008;214:413–421. doi: 10.1002/jcp.21210. [DOI] [PubMed] [Google Scholar]
- 35.Crisan M. Yap S. Casteilla L. Chen CW. Corselli M. Park TS. Andriolo G. Sun B. Zheng B, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3:301–313. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 36.Tran KV. Gealekman O. Frontini A. Zingaretti MC. Morroni M. Giordano A. Smorlesi A. Perugini J. De Matteis R, et al. The vascular endothelium of the adipose tissue gives rise to both white and brown fat cells. Cell Metab. 2012;15:222–229. doi: 10.1016/j.cmet.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Planat-Benard V. Silvestre JS. Cousin B. André M. Nibbelink M. Tamarat R. Clergue M. Manneville C. Saillan-Barreau C, et al. Plasticity of human adipose lineage cells toward endothelial cells: physiological and therapeutic perspectives. Circulation. 2004;109:656–663. doi: 10.1161/01.CIR.0000114522.38265.61. [DOI] [PubMed] [Google Scholar]
- 38.Psaltis PJ. Harbuzariu A. Delacroix S. Witt TA. Holroyd EW. Spoon DB. Hoffman SJ. Pan S. Kleppe LS, et al. Identification of a monocyte-predisposed hierarchy of hematopoietic progenitor cells in the adventitia of postnatal murine aorta. Circulation. 2012;125:592–603. doi: 10.1161/CIRCULATIONAHA.111.059360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Psaltis PJ. Harbuzariu A. Delacroix S. Holroyd EW. Simari RD. Resident vascular progenitor cells: diverse origins, phenotype, and function. J Cardiovasc Transl Res. 2011;4:161–176. doi: 10.1007/s12265-010-9248-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Krause DS. Fackler MJ. Civin CI. May WS. CD34: structure, biology, and clinical utility. Blood. 1996;87:1–13. [PubMed] [Google Scholar]
- 41.Lange C. Li Z. Fang L. Baum C. Fehse B. CD34 modulates the trafficking behavior of hematopoietic cells in vivo. Stem Cells Dev. 2007;16:297–304. doi: 10.1089/scd.2006.0056. [DOI] [PubMed] [Google Scholar]
- 42.Charrière G. Cousin B. Arnaud E. André M. Bacou F. Penicaud L. Casteilla L. Preadipocyte conversion to macrophage. Evidence of plasticity. J Biol Chem. 2003;278:9850–9855. doi: 10.1074/jbc.M210811200. [DOI] [PubMed] [Google Scholar]
- 43.Chazenbalk G. Bertolotto C. Heneidi S. Jumabay M. Trivax B. Aronowitz J. Yoshimura K. Simmons CF. Dumesic DA. Azziz R. Novel pathway of adipogenesis through cross-talk between adipose tissue macrophages, adipose stem cells and adipocytes: evidence of cell plasticity. Plos One. 2011;6:e17834. doi: 10.1371/journal.pone.0017834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Crossno JT., Jr. Majka SM. Grazia T. Gill RG. Klemm DJ. Rosiglitazone promotes development of a novel adipocyte population from bone marrow-derived circulating progenitor cells. J Clin Invest. 2006;116:3220–3228. doi: 10.1172/JCI28510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sera Y. LaRue AC. Moussa O. Mehrotra M. Duncan JD. Williams CR. Nishimoto E. Schulte BA. Watson PM. Watson DK. Ogawa M. Hematopoietic stem cell origin of adipocytes. Exp Hematol. 2009;37:1108–1120. doi: 10.1016/j.exphem.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Majka SM. Fox KE. Psilas JC. Helm KM. Childs CR. Acosta AS. Janssen RC. Friedman JE. Woessner BT, et al. De novo generation of white adipocytes from the myeloid lineage via mesenchymal intermediates is age, adipose depot, and gender specific. Proc Natl Acad U S A. 2010;107:14781–14786. doi: 10.1073/pnas.1003512107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Koh YJ. Kang S. Lee HJ. Choi TS. Lee HS. Cho CH. Koh GY. Bone marrow-derived circulating progenitor cells fail to transdifferentiate into adipocytes in adult adipose tissues in mice. J Clin Invest. 2007;117:3684–3695. doi: 10.1172/JCI32504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rehman J. Li J. Orschell CM. March KL. Peripheral blood “endothelial progenitor cells” are derived from monocyte/macrophages and secrete angiogenic growth factors. Circulation. 2003;107:1164–1169. doi: 10.1161/01.cir.0000058702.69484.a0. [DOI] [PubMed] [Google Scholar]
- 49.Rohde E. Malischnik C. Thaler D. Maierhofer T. Linkesch W. Lanzer G. Guelly C. Strunk D. Blood monocytes mimic endothelial progenitor cells. Stem Cells. 2006;24:357–367. doi: 10.1634/stemcells.2005-0072. [DOI] [PubMed] [Google Scholar]
- 50.Pilz GA. Braun J. Ulrich C. Felka T. Warstat K. Ruh M. Schewe B. Abele H. Larbi A. Aicher WK. Human mesenchymal stromal cells express CD14 cross-reactive epitopes. Cytometry A. 2011;79A:635–645. doi: 10.1002/cyto.a.21073. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.