Abstract
Background
Bloodstream infections from central venous catheters (CVC-BSIs) increase morbidity and costs in intensive care units (ICUs). Substantial reductions in CVC-BSI rates have been reported using a combination of technical and non-technical interventions.
Methods
We conducted a 2-year, four-cluster, stepped non-randomised study of technical and non-technical (behavioural) interventions to prevent CVC-BSIs in adult and paediatric ICUs in England. Random-effects Poisson regression modelling was used to compare infection rates. A sample of ICUs participated in data verification.
Results
Of 223 ICUs in England, 215 (196 adult, 19 paediatric) submitted data on 2479 of 2787 possible months and 147 (66%) provided complete data. The exposure rate was 438 887 (404 252 adult and 34 635 paediatric) CVC-patient days. Over 20 months, 1092 CVC-BSIs were reported. Of these, 884 (81%) were ICU acquired. For adult ICUs, the mean CVC-BSI rate decreased over 20 months from 3.7 in the first cluster to 1.48 CVC-BSIs/1000 CVC-patient days (p<0.0001) for all clusters combined, and for paediatric ICUs from 5.65 to 2.89 (p=0.625). The trend for infection rate reduction did not accelerate following interventions training. CVC utilisation rates remained stable. Pre-ICU infections declined in parallel with ICU-acquired infections. Criterion-referenced case note review showed high agreement between adjudicators (κ 0.706) but wide variation in blood culture sampling rates and CVC utilisation. Generic infection control practices varied widely.
Conclusions
The marked reduction in CVC-BSI rates in English ICUs found in this study is likely part of a wider secular trend for a system-wide improvement in healthcare-associated infections. Opportunities exist for greater harmonisation of infection control practices. Future studies should investigate causal mechanisms and contextual factors influencing the impact of interventions directed at improving patient care.
Introduction
Blood stream infections (BSIs) from central venous catheters (CVCs) increase morbidity and are estimated to increase mortality risk by 25% and costs of care in the USA by US$16 550 on average per patient1 2 (box 1). A substantial body of evidence suggests that rates of CVC-BSIs are modifiable.3–13 The Michigan-Keystone project13 in 103 intensive care units (ICUs) in the USA reported a major reduction in CVC-BSIs from 7.7 to 1.4 CVC-BSIs per 1000 CVC-patient days using a complex intervention targeting specific technical practices (box 2), combined with support for cultural, behavioural and systemic change.14 A 3-year follow-up study reported sustained improvement15 and accelerated the trend for a reduction in case mix-adjusted mortality rates.16
Box 1. Background.
Central venous catheters (CVCs) are widely used in patients in intensive care units (ICUs) and other hospital locations for monitoring, drug delivery, and dialysis
CVCs increase the risk of blood stream infections (BSIs) which increase mortality and costs of care
CVC-BSIs can substantially be prevented when clinicians use best practice guidance during catheter insertion and subsequent maintenance
CVC-BSI rates in the NHS in England are unknown
This study examined the impact of benchmarking and best practice guidance on minimising CVC-BSIs in English ICUs
Box 2. Technical interventions to reduce central venous catheters (CVC)-blood stream infections.
Hand hygiene, gown, gloves, hat, mask. Eye protection when indicated
Skin antisepsis: 2% chlorhexidine gluconate in 70% isopropyl alcohol
Maximal sterile precautions including full barrier drapes
Site of insertion: avoid the femoral route
CVC maintenance: aseptic access technique, daily site review, and remove CVCs at earliest opportunity
The NHS Next Stage Review in 200817 announced that the National Patient Safety Agency (NPSA) would run a ‘national patient safety initiative to tackle central line catheter-related blood stream infections, drawing lessons from a remarkably successful Michigan initiative’. This 2-year programme, known as Matching Michigan, ran in England from April 2009 to the end of March 2011. It aimed to minimise CVC-BSI rates in adult and paediatric ICUs in England to at least the mean level (1.4 per 1000 CVC-patient days) seen in the Michigan-Keystone project. It involved three components: technical interventions, which sought to ensure consistent use of evidence-based measures for reducing risks of CVC-BSIs; non-technical interventions, which sought to intervene in culture and systems; and establishment of a standardised national reporting system for CVC-BSIs. All participating sites were invited to take part in two training sessions, the first focused on data collection and the second focused on the technical and non-technical interventions.
Matching Michigan followed, and took place during, heightened media interest and policy initiatives focused on healthcare-associated infections and BSIs (table 1) including the introduction by the Department of Health (DoH) in 2007 of best practice guidance on CVC insertion and management18 through its multicomponent ‘Saving Lives’ programme.19 Other improvement activities relevant to CVC-BSIs included the Health Foundation's Safer Patients Initiative, which ran in two phases from 2004 to 2008,20 and the Patient Safety First campaign, which began in 2008.21 However, in the absence of a national reporting system, it was not possible to assess the impact of any of these or any other efforts on CVC-BSI rates.
Table 1.
The context: national infection control initiatives in England before and during Matching Michigan
| 2001 | Mandatory reporting to the Health Protection Agency (HPA) of MRSA bacteraemia. |
| //www.hpa.org.uk/web/HPAweb&HPAwebStandard/HPAweb_C/1244763936373 | |
| 2003 | Report of the Chief Medical Officer: Winning ways: guidance to reduce healthcare associated infection in England. |
| //www.dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsPolicyAndGuidance/DH_4064682 | |
| 2004 | Mandatory reporting of Clostridium difficile infection (HPA website) |
| 2004 | Hospital in Europe Link for Infection Control through Surveillance of Nosocomial Infections in ICUs protocol. |
| http://helics.univ-lyon1.fr/helicshome.htm | |
| 2004 to 2008 | Health Foundation's Safer Patients Initiative (24 hospitals): includes CVC bundle. http://www.health.org.uk/areas-of-work/programmes/safer-patients-initiative/ |
| 2005 | DoH Saving Lives programme—NHS High Impact Interventions (NHS-HII), modelled on Institute for Healthcare Improvement bundles. |
| http://webarchive.nationalarchives.gov.uk/20120118164404/hcai.dh.gov.uk/whatdoido/high-impact-interventions/ | |
| 2006 | Health Act 2006: Department of Health Code of Practice gives new powers of inspection to the Healthcare Commission. Superseded by the Health & Social Care Act 2008 |
| 2008 | Health and Social Care Act 2008: required registration with the Care Quality Commission: duty to protect patients against HCAIs. New code of practice. |
| http://www.dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsPolicyAndGuidance/DH_081927 | |
| 2008 | Patient Safety First sponsored by National Patient Safety Agency (NPSA), NHS HII, and Health Foundation, includes interventions to reduce CVC-BSIs |
| http://www.patientsafetyfirst.nhs.uk/content.aspx?path=/ | |
| 2008 | High Quality Care For All: NHS Next Stage Review (Darzi report) states that the NPSA will run an ‘initiative to tackle central line catheter-related bloodstream infections’. |
| http://www.dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsPolicyAndGuidance/DH_085825 | |
| 04/2009 to 03/2011 | Matching Michigan project. http://www.patientsafetyfirst.nhs.uk/Content.aspx?path=/interventions/relatedprogrammes/matchingmichigan/ |
| 2011 | Mandatory reporting of MRSA and Escherichia coli bacteraemia (HPA website) |
BSI, blood stream infections; CVC, central venous catheter; HPA, Health Protection Agency; ICU, intensive care unit; MRSA, methicillin-resistant Staphylococcus aureus.
In this article, we report an analysis of the impact of Matching Michigan on rates of reported CVC-BSIs in adult and paediatric ICUs in England.
Methods
Design
This was a prospective, interventional, non-randomised, stepped, four-cluster, 2-year quality improvement project with continuous feedback of results to participating ICUs. The National Research Ethics Committee waived the requirement for informed patient consent on the basis that the intent was to improve uptake of established best practice care, and no patient-identifiable information would be collected centrally.
Delivery and recruitment
The NPSA established a national project team and an External Reference Group representing professional and governmental organisations. The scientific leads from the original Michigan-Keystone project acted as advisors and provided their improvement tools. Chief executive officers (CEOs) of all acute hospitals in England with ICUs were invited to participate in the programme. Participating hospitals agreed to appoint a local project team comprising an ICU physician, an ICU nurse, a microbiologist or infection control specialist and an executive or non-executive director.
Clusters
ICUs were grouped into four clusters with stepped implementation (table 2). Cluster 1 (North-Eastern Strategic Health Authority) allowed piloting of data collection, training and interventions. Clusters 2 and 3 comprised ICUs in southern and northern England respectively. Cluster 4 consisted of ICUs unable to join the project in the earlier phases.
Table 2.
ICU clusters, duration in project, training day attendance and reliability of submission of infection data
| Training day dates and no. (%) of total ICUs attending | Maximum opportunity to submit data and ICU-months submitted | No. ICUs submitting data | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Data collection | Interventions | |||||||||||||||
| Cluster | Adult ICUs | Paediatric ICUs | Duration in project (months) | Date | No. (%) attended | Date | No. (%) attended | Attended both | Max ICU-months | All | Adult | Paediatric | All | Adult | Paediatric | No. (%) ICUs with 100% submission |
| 1 | 15 | 4 | 20 | 30/3/09 | 17 (8) | 18/09/09 | 19 (9) | 17 (8) | 380 | 350 | 273 | 77 | 19 | 15 | 4 | 13 (68%) |
| 2 | 73 | 7 | 12 | 20/10/09 | 70 (31) | 16/03/10 | 57 (23) | 54 (24) | 2015 | 1776 | 1642 | 134 | 150 | 139 | 11 | 103 (66%) |
| 3 | 70 | 5 | 12 | 3/11/09 | 71 (32) | 18/03/10 | 72 (32) | 69 (31) | ||||||||
| 4 | 44 | 5 | 9 | 29/4/10 | 46 (21) | 15/07/10 | 41 (18) | 39 (17) | 392 | 353 | 319 | 34 | 46 | 42 | 4 | 31 (63%) |
| Total | 202 | 21 | 53 | 204 (91) | 183 (82) | 179 (80.3) | 2787 | 2479 | 2234 | 245 | 215 | 196 | 19 | 147 (66%) | ||
ICU, intensive care unit.
Definitions
Definitions of CVC, BSI, catheter-related (CRBSI) and catheter-associated BSI (CABSI) and measures of exposure are not straightforward. There is considerable evidence of variability in these definitions or a lack of clarity in their application in prior publications.22–25 The definitions we used, which were current in 2009, were from the Hospital In Europe Link for Infection Control through Surveillance programme,26 and the US National Nosocomial Infection Surveillance System from the Centre for Disease Control & Prevention,27 28 and were piloted and refined to ensure applicability and ease of understanding for an English context (see electronic supplementary material 1 (ESM 1)). The definitions distinguish between the surveillance definition of CRBSI and the clinical definition of CABSI. The key distinction between these definitions lies in the type of microbiological analysis undertaken to determine whether the source of any individual BSI can be attributed to a CVC.
ICUs were asked to submit data monthly to a specially created web-based system and to identify which definition they used for each infection at the time of reporting. Infections reported as either CRBSI or CABSI were summed to calculate infection rates. Measures of exposure were recorded through a daily census in each ICU involving a count of the number of CVCs in situ at a set time each day. ICUs were asked to complete a survey on generic infection control practices (table 3). Infection data could be submitted until 31 March 2011. However, to permit data cleaning before project closure, analysis was limited to the 20-month period from May 2009 to December 2010.
Table 3.
ICU infection control practices (127 respondents of 223 ICUs, response rate 57%)
| No. (%) of respondents | |
|---|---|
| Joint ward round with microbiology/infection control | |
| Daily weekday round | 56 (44%) |
| Less frequent | 54 (43%) |
| Never | 17 (13%) |
| Chlorhexidine bed baths | |
| Routine | 19 (15%) |
| If MRSA positive | 63 (50%) |
| Never | 27 (21%) |
| Information not given | 18 (14%) |
| Oral hygiene | |
| Chlorhexidine mouthwash | 25 (20%) |
| Corsodyl gel | 31 (24%) |
| Corsodyl mouthwash | 10 (8%) |
| Toothpaste | 41 (32%) |
| None of above | 2 (2%) |
| Information not given | 18 (14%) |
| Antimicrobial-coated CVCs | 35 (28%) |
| Antiseptic-coated CVCs | 37 (29%) |
| Bionnector valve use | |
| Yes | 86 (68%) |
| No | 26 (20%) |
| Information not given | 15 (12%) |
| Three-way tap use | |
| Routine | 55 (43%) |
| Sometimes or rare | 34 (27%) |
| Never | 23 (18%) |
| Information not given | 15 (12%) |
| Chlorhexidine-impregnated patch at CVC insertion site | |
| Yes | 21 (17%) |
| No | 90 (71%) |
| Information not given | 16 (13%) |
CVC, central venous catheter; ICU, intensive care unit; MRSA, methicillin-resistant Staphylococcus aureus.
Training and support
Each cluster was invited to attend two training days, the first on the data definitions developed for the programme (ESM 1) and the second some months later on the technical and non-technical interventions (table 4) adapted from the Michigan-Keystone project.14 Training was held in a centralised location and involved plenary and small group interactive sessions. ICUs started baseline data collection as soon as possible after the first training day.
Table 4.
Technical and non-technical interventions
| Resource or tool | Content, format |
|---|---|
| Technical | |
| Evidence based | |
| Effective hand hygiene |
|
| 2% chlorhexidine skin antiseptic | |
| Full-barrier precautions | |
| Avoidance of the femoral route | |
| Review and prompt removal | |
| Facilitators | |
| CVC insertion checklist |
|
| Colocated materials | |
| Non-technical | |
| Science of safety | |
| Guidance and teaching resources on safety |
|
| Clinical stories and safety incidents |
|
| Attendance at training sessions |
|
| Identifying and learning from incidents | |
| Identifying hazards, learning from safety incidents |
|
| LFD framework/root cause analysis |
|
| Staff safety assessment |
|
| Executive–clinician partnerships | |
| Senior executive/shadowing partnership |
|
| Safety issues worksheet for executive partnership |
|
| Teamwork and communication | |
| Establishing a unit safety team |
|
| Safety ‘climate’ and teamworking |
|
| Safety culture survey |
|
| Daily goals checklist |
|
Also available via: http://www.patientsafetyfirst.nhs.uk/content.aspx?path=/
AHRQ, Agency for Healthcare Research and Quality; BSI, blood stream infections; CVC, central venous catheter; DoH, Department of Health; LFD, Learning from Defects.
Teleconference calls and internet-based teaching sessions were offered over the course of the programme. Guidance was provided by telephone and email and, if appropriate, on-site visits by two quality improvement facilitators (ICU nurses). The Patient Safety First website was used to host information on the interventions and on the programme more generally.21 The project clinical leads provided additional ad hoc support and guidance when required.
Data verification
Data limits and rules programmed into the software allowed erroneously entered data to be detected and corrected through the web-based tool. Extreme values were examined by clinical members of the project team, and discussed with local project leads. We also undertook verification of consistency between ICUs in identifying and reporting CVC-BSIs in a purposive sample of ICUs. To conduct the verification, we used on-site criterion-referenced case note review and contemporaneous telephone discussion with a second remote and blinded reviewer. Following institutional approval, each ICU in the verification sample provided a list of all blood cultures (BCs) performed over 3 months, and the case records of 5–20 patients with positive BCs. The number of BCs performed and the number of CVC-patient days were compared with the number of patient days to determine the frequency of sampling for BCs, and the CVC-utilisation ratio. Local adjudication and reporting of each CVC-BSI was compared with external review. Inter-observer agreement was determined using the κ statistic. ICUs were not asked to provide self-reported data on compliance or implementation of the technical and non-technical interventions because there was no method of assuring data reliability or completeness.
Statistical analysis
Random-effects Poisson regression modelling was used for the primary outcome, based on mean monthly CVC-BSIs related to CVC-patient days, anchored by time since the second training day for each cluster (zero pre-intervention, number of months from month of intervention onwards), and using as covariates the time trend (months from May 2009), teaching status, size of unit, random effect of unit, and cluster. This tests the hypothesis that the intervention (the second training day) will change the slope of an underlying secular trend. To explore whether changes in ICU infection rates were independent of, or potentially part of, a whole-hospital trend, and in the absence of a measure of pre-ICU exposure rates, we compared quarterly pre-ICU with ICU-acquired infection rates expressed as the proportion of all CVC-BSIs which were ICU acquired (ICU-acquired CVC-BSIs divided by the sum of ICU-acquired and pre-ICU CVC-BSIs). A stable ratio over time would suggest ICU trends were part of a wider whole-hospital effect. A χ2 test for trend was performed to evaluate changes in this ratio. All p values are two sided, with p≤0.05 considered statistically significant. Stata (V.9) was used for all analyses.
Results
Participant characteristics
Chief executives of all (139) acute hospitals in England with ICUs agreed that their organisations would participate. Of these, 32 (23%) were university hospitals. The study sample represented 223 ICUs, of which 176 (79%) were general adult ICUs, 21 (9%) paediatric, and 26 (11.6%) subspeciality. The mean (range) number of ICU beds per unit was 12 (3–43); the mean (range) annual admissions was 685 (166–2423). More than 80% of ICUs attended both training days (table 2), though the size of the team attending training ranged from single individuals (doctor or nurse) to large groups including executive leads.
Most (96.4%, 215) ICUs submitted at least some infection data to Matching Michigan. Responses (57%) to the survey of generic infection control practices demonstrated wide variation between ICUs (table 3).
Infection rates
Infection data were submitted on 2479 ICU-months of a maximum 2787, giving a reliability rate of 0.89. Complete data were submitted for every possible month by 147 (66%) ICUs (range between clusters 63–68%) (table 2). The first cluster of 19 ICUs (15 adult, 4 paediatric) provided baseline comparator infection data for subsequent clusters. Clusters 2 and 3 received their training a few weeks apart and their infection data were merged into a single cluster for analysis.
Of 1092 CVC-BSIs reported over 20 months, 884 (81%) were ICU acquired. A majority (66.7%) were diagnosed using the catheter-associated definition (table 5). Paediatric CVC-BSIs accounted for 14.6% of total declared infections, but only 7.89% of CVC-patient days. A total of 438 887 (404 252 adult and 34 635 paediatric) CVC-patient days were reported, giving a mean ICU-acquired infection rate for the entire project of 2.01 CVC-BSIs per 1000 CVC-patient days (adult ICUs 1.88, paediatric ICUs 3.58). Detailed monthly infection and CVC utilisation rates are given in ESM 2.
Table 5.
1092 CVC-BSIs by infection classification and location
| Pre-ICU acquired | ICU acquired | |||||||
|---|---|---|---|---|---|---|---|---|
| Infection classification | CVC associated | CVC related | Total pre-ICU | CVC associated | CVC related | Total in ICU | CVC-patient days | ICU CVC-BSI rate/1000 CVC-patient days |
| Adult | 114 | 57 | 171 | 503 | 258 | 761 | 404252 | 1.88 |
| Paediatric | 28 | 9 | 37 | 84 | 39 | 123 | 34635 | 3.55 |
| Total | 142 | 66 | 208 | 587 | 297 | 884 | 438887 | 2.01 |
BSI, blood stream infection; CVC, central venous catheter; ICU, intensive care unit.
Changes in infection rates
Aggregated adult and paediatric ICU infection rates diminished with time from a first month rate of 4.4 CVC-BSIs/1000 CVC-patient days for cluster 1, to 1.7 CVC-BSIs in December 2010 (all clusters) (ESM 2 monthly, figure 1A quarterly). The ratio between ICU-acquired CVC-BSIs and all CVC-BSIs remained stable during the project (test of homogeneity χ2=16.11, p=0.6497; test for trend of odds χ2=0.12, p=0.7237), suggesting a possible common cause for the reduction in infection rates in ICU and non-ICU locations (figure 1B).
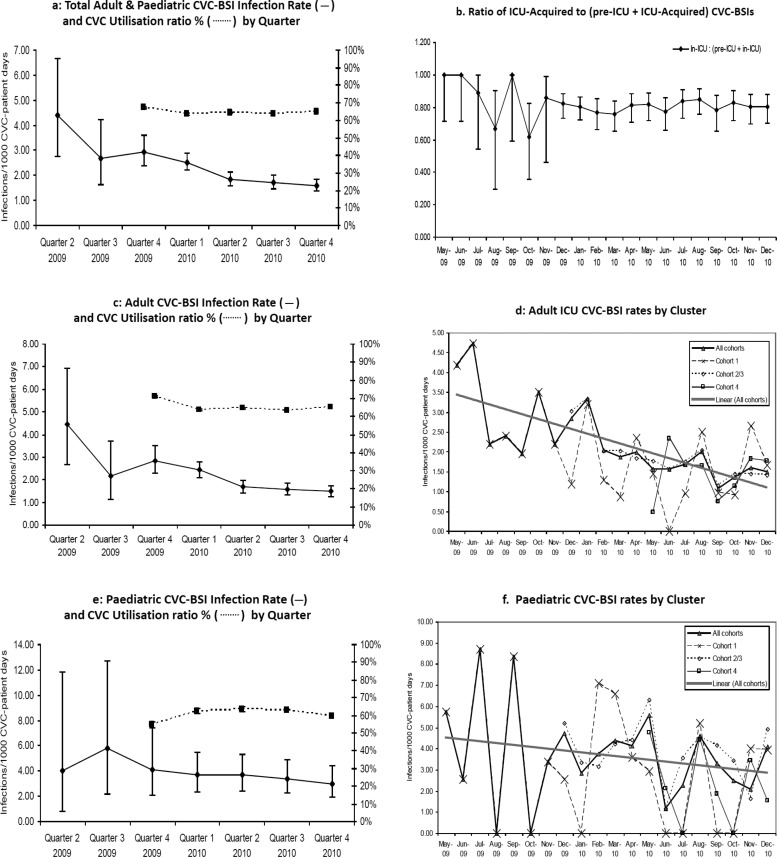
Figure 1.
Central venous catheter (CVC)-blood stream infection (BSI) rates. (A) Total adult and paediatric CVC-BSI infection rate (——) and CVC utilisation ratio % (……) by quarter. (B) Ratio of intensive care unit (ICU)-acquired to (pre-ICU+ICU-acquired) CVC-BSIs. (C) Adult CVC-BSI infection rate (——) and CVC utilisation ratio % (……) by quarter. (D) Adult ICU CVC-BSI rates by cluster. (E) Paediatric CVC-BSI infection rate (——) and CVC utilisation ratio % (……) by quarter. (F) Paediatric CVC-BSI rates by cluster.
Mean adult ICU CVC-BSIs diminished from 3.7 CVC-BSIs/1000 CVC-patient days in the first quarter (inception of cluster 1), to 1.48 in the last quarter (figure 1C), and for paediatric ICUs from 5.65 (four paediatric ICUs) to 2.89 (18 paediatric ICUs) (figure 1E). The progressive reduction in infection rates was statistically highly significant for adult ICUs (Z statistic −4.45, χ2 p<0.0001), but not paediatric ICUs (Z statistic −0.79, χ2 p=0.625).
The rate of change in reduction in infection rates did not accelerate following the second training day. For adult ICUs, each successive cluster to join the project had an entry-level infection rate close to the post-intervention level of the preceding cluster (figure 1D) (Z statistic 1.29 and 0.87, χ2 probability 0.19 and 0.38 for clusters 2 and 3 and cluster 4 respectively). Late engagement (cluster 4) was not associated with poorer performance in any metric. Numbers were too small, and the variation in infection rates too great, to draw secure conclusions from the paediatric data (figure 1F).
Associations
The trend for reduction in infection rates was not associated with hospital type or the number of CVC-patient days for either adult or paediatric ICUs. CVC utilisation ratios could only be determined from December 2009; utilisation rates remained stable (66.3/100 patient days for December 2009–February 2010, 64.6/100 for October–December 2010) (ESM 2 and figure 1A,C,E), despite the continuing fall in pre-ICU and ICU-acquired CVC-BSI rates for this period.
Attendance at both training days was achieved by 179 ICUs (80.3%), 127 of which also provided 100% complete infection data (of 147 ICUs achieving this). Training day attendance was strongly associated with more reliable data submission (χ2 10.2187, p<0.005), but not with infection rates (Z statistic −0.29, p=0.773).
Data verification
Twenty-eight of 45 ICUs responded to an invitation to participate in data verification and 17 actually participated (one paediatric ICU, two university, 14 adult general). Reasons for non-participation included no response to further contacts (10), clinical workload (3), inadequate administrative support (4), absence of timely authority to access medical records (7), and inadequate project team resources (4).
The 17 ICUs participating in the verification sub-study performed 2357 BCs during 17 020 patient-days and 10 601 CVC-patient days, of which 328 (13.9%) BCs were positive (ICU range 5.7–23%). Frequency of sampling and CVC use varied widely: the BC:patient-days ratio was 2357/17 020=13.8 BCs/100 patient-days (range 4.8–39.6) and the CVC utilisation ratio was 0.62 (range 0.42–0.78).
Criterion-referenced case note review was conducted in 177 patients with 187 positive BCs; in 54 patients (30.5%) no CVC was in situ within 48 hours of the positive BC, which excluded potential CVC-BSIs. Of the 177 patients with positive BCs, 17 had been declared as CVC-BSIs and 160 as non-attributable. External adjudication agreed with local adjudication in 167 instances (seven reclassified as attributable, three as non-attributable, overall correct classification 94.3%). The kappa for agreement between local and external adjudicators was 0.706 (SE of kappa=0.088; 95% CI 0.534 to 0.877). The method did not permit determination of CVC infection in the absence of a blood culture.
Discussion
On initial examination, and using the metrics employed by the majority of studies in this area, Matching Michigan was a success. The programme demonstrated a 60% reduction in reported CVC-BSIs in adult ICUs in England, despite starting with less headroom for improvement than the original Keystone-Michigan project13 (baseline 4.4 CVC-BSIs per 1000 patient catheter days in the first Matching Michigan cluster compared with 7.7 at baseline in Michigan). For paediatric ICUs the 48% reduction did not achieve statistical significance; the difficulty of reducing CVC-BSIs in paediatric intensive care is well recognised.29–32 A conventional narrative might run thus: training in technical and non-technical interventions to improve patient safety combined with measurement and performance feedback stimulated a change in behaviour which resulted in a reduction in BSIs from CVCs.
Closer examination of the data reveals a more complex picture requiring a nuanced interpretation. Attributing the impressive reduction in adult ICU CVC-BSIs rates solely to programme participation is complicated by two novel insights. First, each successive cluster joined the project on the trend line for the post-intervention level of the preceding cluster, thus indicating a strong secular trend. Second, pre-ICU infections (which were not targeted by Matching Michigan) diminished in line with ICU-acquired infections, indicating that the secular trend was not limited to the ICU. These findings suggest the possibility that the reduction in infection rates could be attributable as much to concurrent and preceding improvement efforts and to the consciousness-raising effect of a nationwide programme as to any specific component of the Matching Michigan programme itself.
This study is an example of the challenges of conducting field evaluations of complex interventions to improve care in real time in rapidly moving fields. It illustrates in particular the challenges of identifying causal mechanisms during ‘rising tides’ when multiple policy pressures and the emergence of professional and scientific consensus combine to produce improvements across the board.33–35 Falling rates of CVC-BSIs have been reported in a number of studies worldwide36 37 and our study was undertaken during a period of intense national activity in England directed towards reducing hospital-acquired infections, including methicillin-resistant Staphylococcus aureus BSI rates (which fell by 22% between April 2009 and March 2011, and by 50% since 2008).38 For example, many hospitals had already introduced 2% alcoholic chlorhexidine skin disinfectant, full-barrier drapes were becoming more widely available, and alcohol hand rub had become universally available.
Our stepped before and after design reduces the risk of bias,39 and the analysis therefore emphasises the need for caution in attributing the reduction in infection rates to specific elements in the programme. Lack of a specific causative link between complex behavioural interventions and improved outcomes has been reported for end-of-life care,40 stroke care,33 coronary balloon angioplasty34 and multifaceted safety programmes,35 while others have reported strong secular trends for improvement in CVC-BSI rates in conjunction with national reporting but in the absence of specific targeted interventions.36 Financial penalties as a further stimulus for improvement do not appear to have had an additional impact on the adoption of self-reported CVC-BSI prevention measures in the USA.41
Study designs involving randomisation, which could help to determine quality improvement programme effects more precisely, are challenged by ethical considerations when best practice is already well established, and practical considerations of isolating intervention from controls. Cluster-randomised designs are particularly important for interventions involving behavioural change,40 42 since the component elements may be rooted in specific cultures, locations and periods, and require testing in the same way as a pharmaceutical intervention in a new population.43 44
A design such as that used in our study—involving clusters joining in a pre-determined sequence, with each successive cluster acting as a de facto control for the preceding cluster—although not formally randomised is one of the more robust approaches that can feasibly be deployed. However, it is subject to a number of threats to internal validity. The ‘waiting’ clusters were exposed to diffusion of treatment effects, as the interventions were widely publicised on the Patient Safety First website from the beginning of the study, and the original Michigan-Keystone project had received widespread attention. ICUs in ‘waiting’ clusters may also have engaged in ‘compensatory rivalry’,45 and increased their efforts to reduce CVC-BSIs while waiting to join the programme. It is also possible that the reduction in reported rates of infections may to some extent have been an artefact of reporting behaviours, since data were collected and reported by ICUs themselves and may have been influenced by perceptions of external scrutiny and performance management.46 How far any trend in reported infection rates may reflect changes in reporting behaviour over time is not easy to establish. A further limitation of our study was the absence of measures of adoption of the interventions and compliance with best practice. Several studies have reported an association between higher compliance and lower infection rates,47–49 but data completeness and the methods chosen for compliance monitoring are rarely described in detail, and the literature on hand hygiene demonstrates poor correlation between self-reported and observed compliance.50–52
The data verification sub-study provides some reassurance of validity in relation to reporting behaviours, but also demonstrates considerable variability in local practices in relation to CVC use and intensity of sampling blood for culture. Variability in surveillance techniques is well recognised and substantially alters reported infection rates.25 The survey of generic infection control practices (not compliance with the technical interventions) demonstrates wide variation, including the level of interaction between intensive care physicians and microbiologists. These factors make direct comparison between ICUs challenging. Harmonisation of practice would reduce the risk of confounding, and could bring additional benefits in reducing nosocomial infection rates.
Despite the difficulties of identifying specific programme effects, it is unlikely that the contribution of large-scale programmes such as Matching Michigan to the ‘rising tide’ is trivial. Such programmes may have a particular role in raising awareness, increasing the intensity of focus and stimulating managerial support for professional activities. Feedback of infection rates may have promoted more reliable provision of and adherence to the well known technical aspects of infection prevention for CVCs. Understanding more precisely how such programmes work remains an important task, since such understanding is likely to avoid inappropriate and ineffective interventions, optimise delivery and improve effectiveness.53 This is especially important when elements of programme design vary from the original: Matching Michigan was not exactly the same as the original Michigan-Keystone project. Differences included amendments to some of the programme materials to ensure contextual relevance; definitions of CVC-BSIs were specified more precisely; and the programme was directed by a government agency with advisory clinician input, not as a clinician-led collaborative. Contextual variability was also evident: Matching Michigan was, unlike Michigan-Keystone, implemented following extensive prior national efforts to improve practice, in a national health system in which intensive care specialists direct infection management with input from microbiology, as opposed to this being the domain of independent infection control practitioners.
It is encouraging that reported rates of pre-ICU and ICU-acquired CVC-BSIs showed reductions over the course of Matching Michigan. Reduced rates of infection will deliver health gains for patients and benefits for health systems. The apparent trend for a reduction in CVC-BSIs acquired before ICU admission should not encourage complacency, however,54 since in the absence of a denominator, conclusions cannot be drawn about rates of infection and quality of care. CVC use in non-ICU locations requires the same intensity of focus as it has received in the ICU.55–60 A national clinician-directed system for sustained continuous CVC-BSI benchmarking, such as those in Scotland61 and Wales,62 would ensure continued attention to CVC-BSIs, and could provide a platform for monitoring other healthcare-associated infections with linkage to patient outcomes.
This study adds to the science of improvement by using a quasi-experimental design that reveals the significance of underlying secular trends but does not rule out the possibility that the programme itself was implicated in that trend. Future studies should use robust mixed-methods research methodologies to clarify causal mechanisms underpinning quality improvement interventions, and to identify those most likely to promote more reliable delivery of best practice throughout the healthcare system, as well as promoting clinician ownership.63 To this end, a separate, independent ethnographic study of culture and behaviour in relation to CVC-BSIs in England was conducted at the same time as Matching Michigan and may provide insights that will promote such understanding.
Supplementary Material
Appendix
Core group: Peter Hibbert, Annette Richardson, Jeanette Beer, Sharon Jefferson, Sarah Goode, Catherine Ede, Tracy Abrusci, Gowri Sivakumaran, Bruce Warner, Julian Bion, Kevin Gunning, Geoff Bellingan, Mark Patten, Jane Cassidy, Ginny Edwards, Jane Eddleston, Adam Fraise, Keith Young.
Analytical, statistical and methodological support: Jill Altman, Martin McCutcheon, Sarah Leong, David Harrison, Peter Pronovost, Christine Goeschel.
External reference group: Helen Stirton (British Association of Critical Care Nurses), Brian Duerden (Department of Health), Andrew Pearson and Jennie Wilson (Health Protection Agency), Kathy Rowan (Intensive Care National Audit and Research Centre), Carl Waldmann (Intensive Care Society), Adam Fraise (Hospital Infection Society), Martin Kiernan (Infection Prevention Society), Steve Page and Emma Nunez (Strategic Health Authorities), Roddy O'Donnell (Paediatric Intensive Care Society), Barry Williams (Patient/User Representative), Stuart Stevenson (Patient Safety Champion), Sue Goulding (Patient Safety Action Team representative), Rachel Binks (Royal College of Nursing Critical Care Forum), Ravi Mahajan (Royal College of Anaesthetists), Simon Mackenzie (Scottish Intensive Care Society), Edward Kelley and Iciar Larizgoitia (World Health Organisation), Louise Teare (Director of Infection Prevention & Control, Bromfield Hospital), Chris Hancock (National Leadership and Innovation Agency for Healthcare).
Local project leads in each collaborating ICU: Addenbrooke's Hospital: Andrew Johnston, Rowan Burnstein, Samir Latifi, Ingrid Jackson, Pamela Bushen, Deborah White, Jumoke Sule, Nicholas Brown, Basil Matta. Airedale General Hospital: Alwyn Kotze, Karen Price, Alison Charlesworth, Bridget Fletcher. Alder Hey Children's Hospital: Steve Kerr, Andy Darbyshire, Pauline Bradshaw, Sian Snelling. Arrowe Park Hospital: Conor McGrath, Graham Ledgerton, Debbie Kretzer, Lesley Metcalfe. Ashford & St Peters Hospital: Melinda Brazier, Denise Hallett, Ingrid Johnson, Ian White, Valerie Howell, Tina Thomas. Barnet Hospital: Andy Cohen, Tony Wolff, Richard Harrison, Liz Stewart, Terina Riches. Barnsley Hospital: Jyothi Rao, Ken Inweregbu, Ye Myint, Tracey Smith. Basildon University Hospital: David Lowe, Yasser Shouman, Claire Winterflodd, Sian Morgan, Gayle Matthews, Maggie Rogers. Basingstoke & North Hampshire Hospital: David Niblett, Sandy Kirk, Fran Bertasius, Nicki Hutchinson. Birmingham Children's Hospital: Jeff Martin, Helen Winmill, Ursula Nusgen, Michelle McLoughlin. Blackpool Victoria Hospital: Nigel Randall, Palanikumar Saravanan, Lise Cross, Sue Howart, Liane Moorhouse, Paul Kelsey. Bristol Royal Hospital for Children: Peter Murphy, Tim Gould, Kathryn Hole, Katie Sweet, Louise Jenkins, Martin Williams, Jonathan Sheffield. Broomfield Hospital: Dilshan Arawwawala, Christine Mitchell-Inwang, Louise Teare, David Blainey. Calderdale Royal Hospital: Michael Howard, Johanna Turner, Helen Thomson. Castle Hill Hospital: J Elton, K Jessop, R Owen-Smith, L Hartley, E Henderson, C Hibbert. Central Middlesex Hospital: Jacek Borkowski, Nasia Ghani, Fiona Coogan, Elizabeth Robb. Charing Cross Hospital: Adrian Steele, Gary Mendoza, Clare Johnston, Alison Holmes. Chase Farm Hospital: Nicholas MaCartney. Chelsea & Westminster Hospital: Jason Tatlock, Jane Marie Hamil, Berge Azadian. Cheltenham General Hospital: Mark Haslam, Liz Bruce, Robert Jackson, Andrew Seaton. Chesterfield Royal Hospital: Charlie Cooper, Jayne Tague, Diane Simpson, Alfonzo Tramontano. City Hospital: Jonathan Hulme, Janka Webb, Beryl Oppenheim, Donal O'Donoghue. Colchester General Hospital: Mohamed Ramali Conquest District General Hospital: Kate Murray, Christa Wood, Barry Phillips, Harry Walmsley. Cumberland Infirmary: Simon Jones,Susan Brown, Manjula Meda, Sandy Brown. Darent Valley Hospital: Mike Protopapas, Tara Laybourne, Peter Orsman, Jenny Kay, Phillipa Wakefield, Helen Bradshaw. Darlington Memorial Hospital: Dominic Errington, Iain Veitch, Joanne Todd, Robert Aitken. Derriford Hospital: Andrew Tillyard, Peter Robbins, Carol Pollard, Judith Frame, Charlotte Kendall, Claire Haill, Alex Mayor. Dewsbury & District Hospital: Hugh Obeirne, Carol Wood, Wendy Smith, Tracey McErlain. Diana Princess of Wales Hospital: Annang Asumang, Mrs Amanda Holmes, Vivien Duncanson, Jane Partridge. Doncaster Royal Infirmary: David Wood, Louise Lowry, Christine Hoy, Lee Cutler. East Surrey Hospital: Mary Sexton. Eastbourne District General Hospital: Rhiana Edwards, Mandy Chequers, Barry Phillips, Harry Walmsley. Epsom Hospital: Stanislaw Jankowski, Sheila Obee, Amita Sharma. Evelina Children's Hospital: Ian Murdoch, Louise Dewsbury. Fairfield General Hospital: Ravi Marthi, Vivk Yadav, Linda Reynolds, Reeta Burman. Freeman Hospital: Jane Cassidy, Jon Walton, Kevin Brenan, Angela Johnson, Debbie Lawson, Lorna Charlton, Alistair Gascoigne, Kate Gould, Timothy Walls. Frenchay Hospital: Ian Thomas, Rachael Smith, Kim Jacobson, Christopher Burton. Friarage Hospital: Andrew Barrington, Krish Srikanth, Paul Buckley, Caroline Morley, Julie Barlow, Kolanu Prasad. Frimley Park Hospital: William Jewsbury, Justin Woods, Nupur Goel, Edward Palfrey. Furness General Hospital: David Highley, Lynne Wyre, Kim Wilson, Jackie Holt. George Eliot Hospital: Vivek Poongavanam, Marian Foster, James Clayton, Nigel Kee. Gloucestershire Royal Hospital: Chris Roberts, Jim Stone, Liz Bruce, Nicky Giles. Good Hope Hospital: Maung Kyi. Great Ormond Street Hospital, Central London: Ann Karimova, Christine Pierce, Annabel Linger, Clare Paley, Yvette Harding, James Soothill, Robert Evans. Guy's & St Thomas’ Hospitals: Duncan Wyncoll, Kath Daly, David Tucker, Eileen Sills. Hammersmith Hospital: Stephen Brett, Steve Thoresen. Harefield Hospital: Chris Meadows, Sunny Kaul, Peter Doyle, Pat Cattini, Robert Craig. Harrogate District Hospital: David Earl, Chris Gill, Kevin Kerr, Anne Lawson. Heartlands Hospital: Jock Melbeck, Sarah Quinton, Itisha Gupta, Roger Stedman. Hereford County Hospital: Anne Miles, Gillian Hill, Alison Budd. Hillingdon Hospital: Andrew Thorniley, Sohan Bissoonauth, Pippa Dorney-Kingdom, Arup Ghose, Marie Batey. Hinchingbrooke Hospital: Rizwan Hasan, Corin Prunty, Andreas Karas, Richard Dickenson. Homerton University Hospital: Robert Ghosh, Tina Stubbs, Alleyna Claxton, Pauline Brown. Horton General Hospital: Jillian Hewitt-Gray, Sharon Buchanan, Dayle Kinch. Huddersfield Royal Infirmary: Julie O'Riordan, Janice Carter. Hull Royal Infirmary: J Elton, I Smith, P Cole, C Turner, E Henderson, C Hibbert. James Paget Hospital: Katherine Kite, Lynn Everett, Linda Hawtin, Nick Coveney. John Radcliffe Hospital: Stuart McKechnie, Alison Shefler, Derrick Crook, Quentin Ainsworth, Shane George, Mhairi Speirs, Lorna Gale, Matthew Gasson, Jane Woollard, Jill Titchell, Sarah Malone, Rochelle Lay, Simon Wells, Lisa Butcher, Lily O'Connor. Kent & Sussex Hospitals: Lee Baldwin, Debbie Spinner. Kettering General Hospital: Phil Watt, June Thomas, Pam Howe, Liz Libiszewski. King's College Hospital, Denmark Hill: Stephanie Strachan, Jennifer Caguioa, Amanda Fife, Erika Grobler. King's Mill Hospital: Lisa Milligan, Valerie Whitaker, Win Maung, Andy Taylor. Leeds General Infirmary: Phil Jackson, Simon Whitely, Tim Palfreman, Sanjay Agrawal, Dorothy Kitching, Jennifer Asker, Juliette Cosgrove, Innes Reid, John Roche. Leicester Royal Infirmary and Leicester General: Kate Wilkins, Sue Davey, Moira Durbridge. Leighton Hospital: Susan Gilby, Sian Axon, Tracy Schiavone, Tracy Bullock. Lincoln County Hospital: Alan Liddle, Matthew Dolling, Sarah Southall, Charmian Hutson, Adam Wolverson. Queen Elizabeth II and Lister Hospitals: Pin Patel, Wendy Collier, Helen O'Connor, Sue Greenslade, Amanda Radford. Liverpool Heart & Chest Hospital: Nigel Scawn, Sandra Roberts, Nicola Best, Glenn Russell. Luton & Dunstable Hospital: Mark Patten, Karen Cairnduff, Rohinton Mulla, Elaine Hide. Macclesfield District General Hospital: John Hunter, Heather Cooper, Simon Cartiledge, John Wilbraham. Maidstone District General Hospital: Angus Turner, Wendy Lepke-Brown, Flo Panel-Coates. Manchester Royal Infirmary: Jane Eddleston, Niall O'Keeffe, Anne Tighe, John Logan, John Moore, Andrew Dodgson, Gill Heaton. Manor Hospital: Chris Newson, Katrina Hajdu, David Jones, Mike Browne. Medway Maritime Hospital: David Simpson, Ravi Singh, Kate Gray, Linda Dempster, Gulzar Mufti. Milton Keynes Hospital: Hamid Manji, Mali Bharamgoudar, Jane Adderley, Angela Legate, Robert Heavisides. Musgrove Park Hospital: Richard Innes, Carolyn Panton, Maggie Bradfield, Cecil Blumgart. New Cross Hospital: Simon Hester, Susan Rowlands, Joanne Ellison, David Loughton. Newcastle General Hospital: Barbara Fulton, Huw McConnell, Iain Johnstone, Catherine Coulter, Claire Riddell, Lesley Scott, Muhammad Raza. Norfolk & Norwich University Hospital: Tim Leary, Jill Roberts, Ngozi Elumogo, Judith Richards, Krishna Sethia. North Devon District Hospital: Andrew Walder, Lynsey Phillips, David Richards, Carolyn Mills. North Manchester General Hospital: Andrew MacKillop, Sally Murphy. North Middlesex Hospital: Mohan Sivarajaratnam, Vikki Howarth, Ian Hossain, Narayan Rao. North Tyneside General Hospital: Andrew McHutchon, Sue Ewart, Cathi Lang. Northampton General Hospital: Richard Marsh, Mary Burt, Anthony Bentley, William Duguid. Northern General Hospital: Steve Webber, Claire McEwan, Mike Heap, Des Breen. Northwick Park Hospital: Gary Wares, Irene Porter. Nottingham University Hospital: Julian Skoyles, Adam March, Asrar Rashid, Mallory Mercer, Helen Lushpenko-Brown, Sue Mager, Tim Boswell, Jill Wilson, Stephen Fowlie, Peter Homa, Dave Sperry, Anne Illsley. Papworth Hospital: Stephen Webb, Maura Screaton, Mari Ylivinkka, Josie Rudman. Peterborough District Hospital: Balraj Appadu, Lorna Clinton, David Enoch, Chris Wilkinson. Pilgrim Hospital: Andrew Norton, Karen Latham, Wendy Creasey, Paul Dunning. Poole General Hospital: Henrik Reschreiter, Kenneth Power, Amanda Saltmarsh, Susan Sutherland. Princess Alexandra Hospital: Rajnish Saha. Princess Royal Hospital: Ian Littlejohn, Russell Hedley, Owen Boyd, Angela Jenkinson, Clare Hebditch. Queen Alexandra Hospital: John Knighton, Matthew Richardson, Caroline Mitchell, Ursula Ward. Queen Elizabeth Hospital: Lyn Swanson, Martin Wood, Janet Thompson, Shirley Richardson. Queen Elizabeth Hospital, Birmingham: Brian Pouchet, Kaye England, Sandeep Walia, Tessa Oelofse, Tomasz Torlinski, Naomi Cole, John Fletcher, Nicki Dickson, Sam Wise, Adam Fraise, Pauline Jumaa, David Burbridge, Kevin Bolger, Kay Fawcett, Morag Jackson. Queen Elizabeth Hospital Woolwich: Peter Roberts, Sam Platt, Susan Bragman. Queen Mary's Hospital, Sidcup: Chris Palin, Sharon Paminter, Tracey Cooper. Queen Victoria Hospital, East Grinstead: Ken Sim, Nicola Heneghan, Amanda Parker, Alison Munday Queen's Hospital: Rajesh Jain, Jitendra Garg, Nicola Dearson, Corrine Cameron-Watson, Stephen Peter Burgess. Queen's Hospital Burton Upon Trent: Paul Stewart, Jackie Jones, Steve Harding, Jonathan Sheldon. Rochdale Infirmary: Anil Gupta, Helen Barrow, Claire Chadwick, Marian Carroll. Rotherham District General Hospital: Gerry Lynch, Derek Bainbridge, Sarah Cooper, Andrew Jackson, Chris Hughes. Royal Albert Edward Infirmary: Rafat Saad, Julie Barrett, Rob Nelson, Gill Harris. Royal Berkshire Hospital: Chris Danbury, Anne Savage, Linda Hosie, Nilangi Virgincar, Nigel Davies. Royal Blackburn Hospital: David Watson, Jeanette Ryde, Ade Rotowa, Catharina Schram. Royal Bolton Hospital: Ajmal Eusuf, Mel McNulty, Rizwan Khan, Maria Sinfield. Royal Bournemouth General Hospital: Martin Schuster-Bruce, Steve Morris, Bill Gransden, Tony Virgincar. Royal Brompton Hospital: Eva Zizkova. Royal Cornwall Hospital, Treliske: Cate Powell, Louise Dickinson, Joe Teape, Carol Richards. Royal Derby Hospital: Naresh Nandwani, Gill Ogden, Cathy Bratt, Em Wilkinson-Brice. Royal Hallamshire Hospital: Arthur Goldsmith, Jo Murray, Victoria West, Sue Dailly, Paula Shobbrook. Royal Lancaster Infirmary: David Highley, Lynne Wyre, Kim Wilson, Jackie Holt. Royal Manchester Children's Hospital: Peter-Marc Forune, Anne Stanton, Katie McCall. Royal Preston Hospital: Craig Spencer, Denise Brooks, Sharon Hallam, Tom Owen, David Orr, Claire Horsfield, Sue Reed, Ian Donaldson. Royal Shrewsbury Hospital: Alastair Windsor, Debbie Millington, Patricia O'Neill. Royal Surrey Country Hospital: Mike Carraretto, Margaret Eaton, Christopher Tibbs, Susan Lewis. Royal Sussex County Hospital: Andew Hill, Marco Maccario, Julie Lloyd, Jackie Portsmouth, Karen Wright, Matthew Fletcher. Royal Victoria Infirmary: Ian Clement, Lisa Squires, Manjusha Narayanan. Russells Hall Hospital: Michael Reay, Sarah Raybould, Denise McMahon. Salford Royal Hospital: Murad Ghrew, Angela Neumann, Samantha Westwell, Ann Trail, Steve Waldek. Sandwell Hospital: Jana Bellin. Scarborough General Hospital: Jo Jaidev, Sylvia Wrigglesworth, Sue Marquis, Mark Andrews. Scunthorpe General Hospital: Jerry Thomas. Sheffield Children's Hospital: Jeff Perring, Liz Murch, Derek Burke. South Tyneside District Hospital: Christopher Muench, Lorraine Spence, Carole Clark, David Shilton. Southampton General Hospital: Mike Grocott, Iain Macintosh, Laura Armstrong, Melanie Griffiths, Julie Brooks, Sarah Jeremiah, Judy Gillow. Southend Hospital: Blanca Boira, Teresa Sage, Stephen Barret, John Gilham. Southmead Hospital: Jasmeet Soar, Deborah Munro, Mariann Charlton. Southport & Formby District General Hospital: Sue Pieri-Davies, Michael Vangikar, Joyce Jordan, Martin Kiernan, Liz Yates. St George's Hospital, Tooting: Andrew Rhodes, Linda Murdoch, Deborah Dawson, Carol Kennelly, Peter Riley, Mike Bailey. St Helier Hospital: Larry Mulleague, Emma Conroy, Caroline Betts, Richard Varhegyi, Steve Hyer. St James's University Hospital: Stuart Murdoch, Karen Ledgard, Innes Reid, Juliette Cosgrove. St Mary's Hospital: Mehringise Cooper, Sonia Broadby. Stafford Hospital: John Hawkins, Christine Dooley, Debra Adams. Stepping Hill Hospital: Karen Szarfenberg, Sengottiyan Chandrasekaran. Sunderland Hospital: Alistair I Roy, Gillian Ferguson, Julie McHugh, Les Boobis. The Christie: Phil Haji-Michael, Angela Hayes, Oonagh McGugan. The Great Western Hospital: Chris Beeby, Liz Jaffray, Hilary Munube, Ruth McCarthy. The Ipswich Hospital: Andy Kong, Angela Statham, Tina Johnson, Peter Donaldson. The James Cook University Hospital: Fiona Hampton, Nicola Cree, Maria Jones, Chris Harper, Clare White, David McCaffrey, Mike Bramble, Tricia Hart. The Princess Royal Hospital: David Christmas, Stephanie Young, Carol Woods, Debbie Snooke, Steve Evans. The Queen Elizabeth Hospital: John Gibson, Katherine Wong, Lynne Liebowitz, Geoff Hunnam. The Royal London Hospital: Marie Healey, Suzanne Daniels, Michael Millar, Charles Gutteridge. The Royal Marsden Hospital: Dr Timothy Wigmore, Mr Rob Loveland, Ms Jennifer Watson, Ms Rebecca Martirani, Shelley Dolan. The Royal Oldham Hospital: Chithambaram Veerappan, Oliver Robinson. The Whittington Hospital: Andrew Badacsonyi, Breege Gilbride, Julie Andrews, Deborah Wheeler, Siobhan Harrington. Trafford General Hospital: John Barnes, Elaine Deay, Wayne Goddard, Shirley Smith. University College Hospital: Viki Mitchell, Deborah Smyth, Mary Azarcon, Geoff Bellingan. University Hospital, Coventry: Andrew Phillips, Julius Asante-Siaw, Elaine Clarke, Karen Bond, Tracey Fenwick, Kate Prevc, Ann-Marie Cannaby. University Hospital Aintree: Christopher Grant, Sharon Smith, Rick Catlin, Gary Francis. University Hospital Lewisham: Marthin Mostert, Sally Rowe, Debbie Flaxman, Claire Champion. University Hospital of Hartlepool: Sue Smith, Julie Olsen, Vijay Gupta, Louise Legg. University Hospital of North Tees: Vijay Gupta, Andrea Mockler, Julie Olsen, Sue Smith. Walton Centre: Chris Whitehead, Carole Scott, Phil Kane, Karen Dawber. Wansbeck Hospital: John Laurenson, Elizabeth Carr, Tamsin Oswald, David Evans. Warrington Hospital: Jerome McCann, Ellis Clarke, Andrew Sargent, Kathryn Holbourn. Warwick Hospital: Ian Purcell, Christine Georgeu, Steve Mather. Watford General Hospital: Thomas Stambach, Sarah Laferby, Frances Stratford, Russell Harrison. West Cumberland Hospital: Fiona Graham, Jackie Fox, Clive Graham. West Middlesex University Hospital: Amandeep Gupta, Jose Tomas, Elaine Danns. West Suffolk Hospital: Michael Palmer, James Whatling, Sue Partridge, Nichole Day. Wexham Park Hospital: Helen Challand, Lucy Everett. Whiston Hospital: Francis Andrews, Paul Jeanrenaud, Kim Sims, Josephine Keward, Mike Lynch. Worcestershire Royal Hospital: Gareth Sellors, Shelly Goodyear, Jane Stockley, Steve Graystone. Worthing Hospital: Ryck Albertyn, Janice Bates, Phillip Barnes. Wycombe and Stoke Mandeville Hospital: Richard Bunsell, Ann Ashworth, Jean O'Driscoll, Graziano Luzzi. Wythenshawe Hospital: Andrew Bentley, Gary Brear, Jane Clayton, Hayley Hardiman, AnneMarie Aziz, Meryl Graves, Amanda Bailey. Yeovil District Hospital: Jeremy Reid, Mark Robinson, Rachael Grey, Susan Jones. York Hospital: Rinus Pretorius, Anne Knaggs, Christine Cruise, Libby McManus.
Footnotes
Contributors: All collaborators are listed in the appendix. All authors contributed to the design and execution of the study, and all contributed to the interpretation of results.
Funding: The Department of Health provided some analytical (data processing) assistance at the end of the study. The National Patient Safety Agency was responsible for directing the study. Statistical analysis was provided by the Intensive Care National Audit and Research Centre. Statement of independence of researchers from funders: the statistical analysis and clinical interpretation of results of the study was performed independently of the Department of Health.
Competing interests: None.
Ethicas approval: The National Research Ethics Committee waived the requirement for informed patient consent on the basis that the intent was to improve uptake of established best practice care, and no patient-identifiable information would be collected centrally.
Open Access: This is an Open Access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 3.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/3.0/
References
- 1.Srinivasan A, Wise M, Bell M, et al. Centers for Disease Control and Prevention (CDC) . Vital signs: central line-associated blood stream infections—United States, 2001, 2008, and 2009. MMWR Morb Mortal Wkly Rep 2011;60:243–8 [PubMed] [Google Scholar]
- 2.O'Grady NP, Alexander M, Burns LA, et al. Healthcare Infection Control Practices Advisory Committee (HICPAC) Guidelines for the prevention of intravascular catheter-related infections. Clin Infect Dis 2011;52:e162–93 http://www.cdc.gov/hicpac/pdf/guidelines/bsi-guidelines-2011.pdf [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coopersmith CM, Rebmann TL, Zack JE, et al. Effect of an education program on decreasing catheter-related bloodstream infections in the surgical intensive care unit. Crit Care Med 2002;30:59–64 [DOI] [PubMed] [Google Scholar]
- 4.Coopersmith CM, Zack JE, Ward MR, et al. The impact of bedside behavior on catheter-related bacteremia in the intensive care unit. Arch Surg 2004;139:131–6 [DOI] [PubMed] [Google Scholar]
- 5.Warren DK, Zack JE, Mayfield JL, et al. The effect of an education program on the incidence of central venous catheter-associated bloodstream infection in a medical ICU. Chest 2004;126:1612–18 [DOI] [PubMed] [Google Scholar]
- 6.Eggimann P, Hugonnet S, Sax H, et al. Long-term reduction of vascular access-associated bloodstream infection. Ann Intern Med 2005;142:875–6 [DOI] [PubMed] [Google Scholar]
- 7.Bhutta A, Gilliam C, Honeycutt M, et al. Reduction of bloodstream infections associated with catheters in paediatric intensive care unit: stepwise approach. BMJ 2007;334:362–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lobo RD, Levin AS, Gomes LM, et al. Impact of an educational program and policy changes on decreasing catheter-associated bloodstream infections in a medical ICU in Brazil. Am J Infect Control 2005;33:83–7 [DOI] [PubMed] [Google Scholar]
- 9.Rosenthal VD, Guzman S, Pezzotto SM, et al. Effect of an infection control program using education and performance feedback. Am J Infect Control 2003;31:405–9 [DOI] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention (CDC) Reduction in central line-associated bloodstream infections among patients in ICUs in Pennsylvania April 2001–March 2005. MMWR Morb Mortal Wkly Rep 2005;54:1013–16 [PubMed] [Google Scholar]
- 11.Warren DK, Cosgrove SE, Diekema DJ, et al. Prevention Epicenter Program A multicenter intervention to prevent catheter-associated bloodstream infections. Infect Cont Hosp Epidemiol 2006;27:662–9 [DOI] [PubMed] [Google Scholar]
- 12.Costello JM, Morrow DF, Graham DA, et al. Systematic intervention to reduce central line–associated bloodstream infection rates in a pediatric cardiac ICU. Pediatrics 2008;121:915–23 [DOI] [PubMed] [Google Scholar]
- 13.Pronovost P, Needham D, Berenholtz S, et al. An intervention to decrease catheter-related bloodstream infections in the ICU. NEJM 2006;355:2725–32 [DOI] [PubMed] [Google Scholar]
- 14.On the CUSP: stop BSI Manuals and toolkits. http://www.onthecuspstophai.org/on-the-cuspstop-bsi/toolkits-and-resources/ accessed August 29 2012
- 15.Pronovost PJ, Goeschel CA, Colantuoni E, et al. Sustaining reductions in catheter related bloodstream infections in Michigan intensive care units: observational study. BMJ 2010;340:c309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lipitz-Snyderman A, Steinwachs D, Needham DM, et al. Impact of a statewide intensive care unit quality improvement initiative on hospital mortality and length of stay: retrospective comparative analysis. BMJ 2011;342:d219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Department of Health High Quality Care for All. NHS Next Stage Review Final Report. 2008. http://www.dh.gov.uk/prod_consum_dh/groups/dh_digitalassets/@dh/@en/documents/digitalasset/dh_085828.pdf (accessed 21 Aug 2012)
- 18.Department of Health Central Venous Catheter Care Bundle. 2010. http://hcai.dh.gov.uk/files/2011/03/2011–03-14-HII-Central-Venous-Catheter-Care-Bundle-FINAL.pdf
- 19.Department of Health High Impact Interventions. 2005, updated 2010. http://hcai.dh.gov.uk/whatdoido/high-impact-interventions (accessed 29 Aug 2012)
- 20.Health Foundation Safer Patients Initiative. http://www.health.org.uk/areas-of-work/programmes/safer-patients-initiative/ (accessed 21 Aug 2012)
- 21.Patient Safety First. http://www.patientsafetyfirst.nhs.uk/content.aspx?path=/ (accessed 21 Aug 2012)
- 22.Lin MY, Hota B, Khan YM, et al. CDC Prevention Epicenter Program Quality of traditional surveillance for public reporting of nosocomial bloodstream infection rates. JAMA 2010;304:2035–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Backman LA, Melchreit R, Rodriguez R. Validation of the surveillance and reporting of central line-associated bloodstream infection data to a state health department. Am J Infect Control 2010;38:832–8 [DOI] [PubMed] [Google Scholar]
- 24.Tomlinson D, Mermel LA, Ethier MC, et al. Defining bloodstream infections related to central venous catheters in patients with cancer: a systematic review. Clin Infect Dis 2011;53:697–710 [DOI] [PubMed] [Google Scholar]
- 25.Niedner MF, 2008 National Association of Children's Hospitals and Related Institutions Pediatric Intensive Care Unit Patient Care FOCUS Group The harder you look, the more you find: catheter-associated bloodstream infection surveillance variability. Am J Infect Control 2010;38:585–95 [DOI] [PubMed] [Google Scholar]
- 26.Hospital In Europe Link for Infection Control through Surveillance (HELICS) Surveillance of Nosocomial Infections in Intensive Care Units (2004) Protocol Version 6.1. Project commissioned by the EC/DG SANCO/F/4. Agreement reference no. VS/1999/5235 (99CVF4-0125). http://helics.univ-lyon1.fr/protocols/icu_protocol.pdf (accessed 21 Aug 2012)
- 27.O'Grady NP, Alexander M, Burns LA, et al. Healthcare Infection Control Practices Advisory Committee (HICPAC) Guidelines for the prevention of intravascular catheter-related infections. MMWR Morb Mortal Wkly Rep 2002;51:1–26 [Google Scholar]
- 28.National Nosocomial Infections Surveillance System Data summary 1992–2004. Am J Infect Control 2004;32:470–85 doi:10.1016/j.ajic.2004.10.001 [DOI] [PubMed] [Google Scholar]
- 29.Rey C, Alvarez F, De-La-Rua V, et al. Intervention to reduce catheter-related bloodstream infections in a pediatric intensive care unit. Intensive Care Med 2011;37:678–85 [DOI] [PubMed] [Google Scholar]
- 30.Li S, Bizzarro MJ. Prevention of central line associated bloodstream infections in critical care units. Curr Opin Pediatr 2011;23:85–90 [DOI] [PubMed] [Google Scholar]
- 31.Miller MR, Griswold M, Harris JM, II, et al. Decreasing PICU catheter-associated bloodstream infections: NACHRI's quality transformation efforts. Pediatrics 2010;125:206–13 [DOI] [PubMed] [Google Scholar]
- 32.Mok Q, Gilbert R. Interventions to reduce central venous catheter-associated infections in children: which ones are beneficial? Intensive Care Med 2011;37:566–8 [DOI] [PubMed] [Google Scholar]
- 33.Lakshminarayan K, Borbas C, McLaughlin B, et al. A cluster-randomized trial to improve stroke care in hospitals. Neurology 2010;74:1634–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bradley EH, Nallamothu BK, Herrin J, et al. National efforts to improve door-to-balloon time: results from the Door-to-Balloon Alliance. J Am Coll Cardiol 2009;54:2423–9 [DOI] [PubMed] [Google Scholar]
- 35.Benning A, Dixon-Woods M, Nwulu U, et al. Multiple component patient safety intervention in English hospitals: controlled evaluation of second phase. BMJ 2011;342:d199 doi: 10.1136/bmj.d199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fontela PS, Platt RW, Rocher I, et al. Epidemiology of central line-associated bloodstream infections in Quebec intensive care units: A 6-year review. Am J Infect Control 2011;40:221–6 [DOI] [PubMed] [Google Scholar]
- 37.Liang SY, Marschall J, for the Centers for Disease Control and Prevention (CDC) Vital signs: central line-associated blood stream infections—United States, 2001, 2008, and 2009. MMWR Morb Mortal Wkly Rep 2011;60:243–8 [PubMed] [Google Scholar]
- 38.Health Protection Agency Summary Points on Methicillin Resistant Staphylococcus aureus (MRSA) Bacteraemia. http://www.hpa.org.uk/webw/HPAweb&HPAwebStandard/HPAweb_C/1259151891722 (accessed 24 Jun 2012)
- 39.Brown C, Hofer T, Johal A, et al. An epistemology of patient safety research: a framework for study design and interpretation. Part 2. Study design. Qual Saf Health Care 2008;17:163–9 doi:10.1136/qshc.2007.023648 [DOI] [PubMed] [Google Scholar]
- 40.Curtis JR, Nielsen EL, Treece PD, et al. Effect of a quality-improvement intervention on end-of-life care in the intensive care unit: a randomized trial. Am J Respir Crit Care Med 2011;183:348–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Krein SL, Kowalski CP, Hofer TP, et al. Preventing hospital-acquired infections: a national survey of practices reported by U.S. Hospitals in 2005 and 2009. J Gen Intern Med 2012;27:773–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Scales DC, Dainty K, Hales B, et al. A multifaceted intervention for quality improvement in a network of intensive care units: a cluster randomized trial. JAMA 2011;305:363–72 [DOI] [PubMed] [Google Scholar]
- 43.Jeong HJ, Pham JC, Kim M, et al. Major cultural-compatibility complex: considerations on cross-cultural dissemination of patient safety programmes. BMJ Qual Saf 2012;21:612–15 [DOI] [PubMed] [Google Scholar]
- 44.Raghunathan K Karthik. Checklists, safety, my culture and me (Viewpoint). BMJ Qual Saf 2012;21:617–20 doi:10.1136/bmjqs-2011-000608 [DOI] [PubMed] [Google Scholar]
- 45.Cook TD, Campbell DT. Quasi-experimentation: design and analysis issues for field settings. Chicago: McNally College Publishing Company, 1979 [Google Scholar]
- 46.Sexton DJ, Chen LF, Anderson DJ. Current definitions of central line-associated bloodstream infection: is the emperor wearing clothes? Commentary. Infect Control Hosp Epidemiol 2010;31:1286–9 [DOI] [PubMed] [Google Scholar]
- 47.Furuya EY, Dick A, Perencevich EN, et al. Central line bundle implementation in US intensive care units and impact on bloodstream infections. PLoS One 2011;6:e15452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Central Line Associated Bacteraemia in NSW Intensive Care Units (CLAB ICU) Collaborative Burrell AR, McLaws ML, Murgo M, et al. Aseptic insertion of central venous lines to reduce bacteraemia. Med J Aust 2011;194:583–7 [DOI] [PubMed] [Google Scholar]
- 49.Render ML, Hasselbeck R, Freyberg RW, et al. VA ICU Clinical Advisory Group Reduction of central line infections in Veterans Administration intensive care units: an observational cohort using a central infrastructure to support learning and improvement. BMJ Qual Saf 2011;20:725–32 [DOI] [PubMed] [Google Scholar]
- 50.Moret L, Tequi B, Lombrail P. Should self-assessment methods be used to measure compliance with handwashing recommendations? A study carried out in a French university hospital. Am J Infect Control 2004;32:384–90 [DOI] [PubMed] [Google Scholar]
- 51.O'Boyle CA, Henly SJ, Larson E. Understanding adherence to hand hygiene recommendations: the theory of planned behavior. Am J Infect Control 2001;29:352–60 [DOI] [PubMed] [Google Scholar]
- 52.Jenner EA, Fletcher BC, Watson P, et al. Discrepancy between self-reported and observed hand hygiene behaviour in healthcare professionals. J Hosp Infect 2006;63:418–22 [DOI] [PubMed] [Google Scholar]
- 53.Dixon-Woods M, Bosk CL, Aveling EL, et al. Explaining Michigan: developing an ex post theory of a quality improvement program. Milbank Q 2011;89:167–205 doi: 10.1111/j.1468-0009.2011.00625.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shapey IM, Foster MA, Whitehouse T, et al. Central venous catheter related bloodstream infections—improving post-insertion catheter care. J Hosp Infect 2009;71:117–22 doi:10.1016/j.jhin.2008.09.016 [DOI] [PubMed] [Google Scholar]
- 55.Climo M, Diekema D, Warren DK, et al. Prevalence of the use of central venous access devices within and outside of the intensive care unit: results of a survey among hospitals in the prevention epicenter program of the Centers for Disease Control and Prevention. Infect Control Hosp Epidemiol 2003;24:942–5 [DOI] [PubMed] [Google Scholar]
- 56.Marschall J. Catheter-associated bloodstream infections: looking outside of the ICU. Am J Infect Control 2008;36:S172.e5–8 [DOI] [PubMed] [Google Scholar]
- 57.Marschall J, Leone C, Jones M, et al. Catheter-associated bloodstream infections in general medical patients outside the intensive care unit: a surveillance study. Infect Control Hosp Epidemiol 2007;28:905–9 [DOI] [PubMed] [Google Scholar]
- 58.Ajenjo MC, Morley JC, Russo AJ, et al. Peripherally inserted central venous catheter-associated bloodstream infections in hospitalized adult patients. Infect Control Hosp Epidemiol 2011;32:125–30 [DOI] [PubMed] [Google Scholar]
- 59.Zingg W, Sandoz L, Inan C, et al. Hospital-wide survey of the use of central venous catheters. J Hosp Infect 2011;77:304–8 [DOI] [PubMed] [Google Scholar]
- 60.Tejedor SC, Tong D, Stein J, et al. Temporary central venous catheter utilization patterns in a large tertiary care center: tracking the ‘idle central venous catheter’. Infect Control Hosp Epidemiol 2012;33:50–7 [DOI] [PubMed] [Google Scholar]
- 61.Welsh Healthcare Associated Infection Programme. Critical Care Annual Report. Central venous catheter and ventilator associated pneumonia 2010 All Wales. 3rd August. 2011. http://www2.nphs.wales.nhs.uk:8080/WHAIPDocs.nsf/3dc04669c9e1eaa880257062003b246b/1999047f8c054649802578fe003ef85a/$FILE/All%20Wales%202010.pdf (accessed 21 Aug 2012)
- 62.Scottish Intensive Care Society Audit Group Surveillance of Healthcare Associated Infections in Scottish Intensive Care Units. August 2011. http://www.documents.hps.scot.nhs.uk/hai/sshaip/publications/icu-surveillance/icu-annual-report-2011.pdf (accessed 21 Aug 2012)
- 63.Mountford J, Shojania KG. Refocusing quality measurement to best support quality improvement: local ownership of quality measurement by clinicians. BMJ Qual Saf 2012;21:519–23 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.