Overexpressing callose synthase in Arabidopsis enlarges callose deposits during powdery mildew infection and gives complete penetration resistance to the fungi.
Abstract
A common response by plants to fungal attack is deposition of callose, a (1,3)-β-glucan polymer, in the form of cell wall thickenings called papillae, at site of wall penetration. While it has been generally believed that the papillae provide a structural barrier to slow fungal penetration, this idea has been challenged in recent studies of Arabidopsis (Arabidopsis thaliana), where fungal resistance was found to be independent of callose deposition. To the contrary, we show that callose can strongly support penetration resistance when deposited in elevated amounts at early time points of infection. We generated transgenic Arabidopsis lines that express POWDERY MILDEW RESISTANT4 (PMR4), which encodes a stress-induced callose synthase, under the control of the constitutive 35S promoter. In these lines, we detected callose synthase activity that was four times higher than that in wild-type plants 6 h post inoculation with the virulent powdery mildew Golovinomyces cichoracearum. The callose synthase activity was correlated with enlarged callose deposits and the focal accumulation of green fluorescent protein-tagged PMR4 at sites of attempted fungal penetration. We observed similar results from infection studies with the nonadapted powdery mildew Blumeria graminis f. sp. hordei. Haustoria formation was prevented in resistant transgenic lines during both types of powdery mildew infection, and neither the salicylic acid-dependent nor jasmonate-dependent pathways were induced. We present a schematic model that highlights the differences in callose deposition between the resistant transgenic lines and the susceptible wild-type plants during compatible and incompatible interactions between Arabidopsis and powdery mildew.
Land plants are exposed to a wide range of potential pathogens and, accordingly, have evolved a variety of strategies that facilitate an early and rapid recognition of pathogens and the mobilization of biochemical and structural defenses. As a result, successful infection of plants by pathogens is the exception rather than the rule (Deverall, 1977; Smith, 1978; Bailey, 1983; Thordal-Christensen, 2003). Many plant defense responses can be specific to a phylum or even a species, while others are ubiquitous. An example of the latter is callose deposition, which appears to be induced in essentially all plants following pathogen challenge. However, the importance of callose deposits in pathogen resistance is still debated, even though this defense reaction has been studied at a cellular level for at least 150 years. deBary (1863) discovered cell wall thickenings in plants, called papillae, at sites where fungal pathogens had penetrated the cell wall, and Mangin (1895) reported that these papillae generally contain callose, a (1,3)-β-glucan polymer with some (1,6)-β-glucan branches (Aspinall and Kessler, 1957). Since then, chemical analyses of papillae have identified callose as the most common constituent. Other components are mainly proteins (e.g. peroxidases and antimicrobial thionins) and phenols as well as various amounts of unclassified compounds (Aist and Williams, 1971; Mercer et al., 1974; Mims et al., 2000). In addition to microbial stress, the local deposition of callose is also induced by abiotic stress and wounding (Wheeler, 1974; Ryals et al., 1996; Jacobs et al., 2003; Mauch-Mani and Mauch, 2005).
Based on observations of a wide variety of plant-pathogen interactions, in which the plant successfully prevented pathogen colonization, papillae are thought to act as a physical barrier to slow pathogen invasion (Stone and Clarke, 1992). As an early defense response, papilla formation can contribute to the plant’s innate immunity (Jones and Dangl, 2006; Schwessinger and Ronald, 2012), which is associated with global transcriptional changes (Boller and Felix, 2009). Plant defense mechanisms that contribute to the innate immunity give the host plant time to initiate subsequent defense reactions that require gene activation and expression, such as the hypersensitive response, phytoalexin production, and the synthesis and export of pathogenesis-related proteins (Lamb and Dixon, 1997; Brown et al., 1998). However, because callose-rich papillae have also been found at sites where the pathogen has penetrated (Aist, 1976), callose deposits apparently cannot always prevent or sufficiently slow pathogen ingress and, therefore, cannot be considered a consistent and general marker for successful plant defense. This has raised the question of the extent to which callose deposition contributes to the plant’s innate immunity and, thus, penetration resistance.
The proposed function of callose as a barrier to retard invading pathogens was further challenged by studies using Arabidopsis (Arabidopsis thaliana) mutants lacking the stress-induced callose synthase POWDERY MILDEW RESISTANT4 (PMR4; also known as GLUCAN SYNTHASE-LIKE5). Although pmr4 disruption mutants did not deposit callose at sites of attempted penetration, they unexpectedly demonstrated an increased resistance to powdery mildew species, such as Golovinomyces cichoracearum (Gc) and Golovinomyces orontii (Jacobs et al., 2003; Nishimura et al., 2003). Double-mutant and microarray analyses showed that hyperactivation of the salicylic acid pathway caused the high resistance of the pmr4 mutant (Nishimura et al., 2003), which suggests the involvement of callose or the PMR4 enzyme in regulating the salicylic acid signal transduction pathway. An active role of callose in resistance to powdery mildew was further questioned in recent studies of mildew resistance locus O (mlo) mutants of barley (Hordeum vulgare). While Consonni et al. (2010) showed that PMR4-dependent callose deposition was not required for the observed mlo2-conditioned penetration resistance in Arabidopsis, this appeared not to be the case in barley, where treatment of mlo-resistant coleoptiles with a callose inhibitor resulted in reduced formation of callose-containing papillae and decreased penetration resistance to powdery mildew (Bayles et al., 1990). Callose inhibition studies with several Gramineae, including barley, wheat (Triticum aestivum), and oat (Avena sativa), revealed that penetration resistance to powdery mildew was especially suppressed in incompatible plant-fungus interactions (Zeyen et al., 2002). A decrease in penetration resistance to nonadapted powdery mildew was also shown in Arabidopsis. The penetration rate of Blumeria graminis f. sp. hordei (Bgh) in the inappropriate host Arabidopsis was significantly increased in the pmr4 mutant relative to wild-type plants (Jacobs et al., 2003). Therefore, even though the involvement of callose in plant defense has been investigated for well over a century, the specific function of callose in plant-pathogen interactions has not been elucidated.
Our studies were aimed at evaluating the regulation and role of callose synthesis during infection. Based on the findings in the pmr4 and mlo mutants, we anticipated no change, or even a decrease in penetration resistance to powdery mildew in a compatible interaction, if PMR4 is expressed under the control of the constitutive cauliflower mosaic virus promoter 35S in Arabidopsis. Moreover, we anticipated an increase in resistance in an incompatible interaction. However, the generated PMR4-overexpressing lines showed complete resistance to both the nonadapted and the virulent powdery mildew. This strong resistance not only occurred earlier during plant infection but was also more stringent than the resistance in the pmr4 (Jacobs et al., 2003; Nishimura et al., 2003) and mlo2 (Consonni et al., 2006, 2010) mutants. Here, we identified PMR4 as a defense-related gene, the overexpression and disruption of which results in increased plant resistance, although through different defense mechanisms.
RESULTS
Disease Phenotypes of Arabidopsis 35S::PMR4-GFP Lines
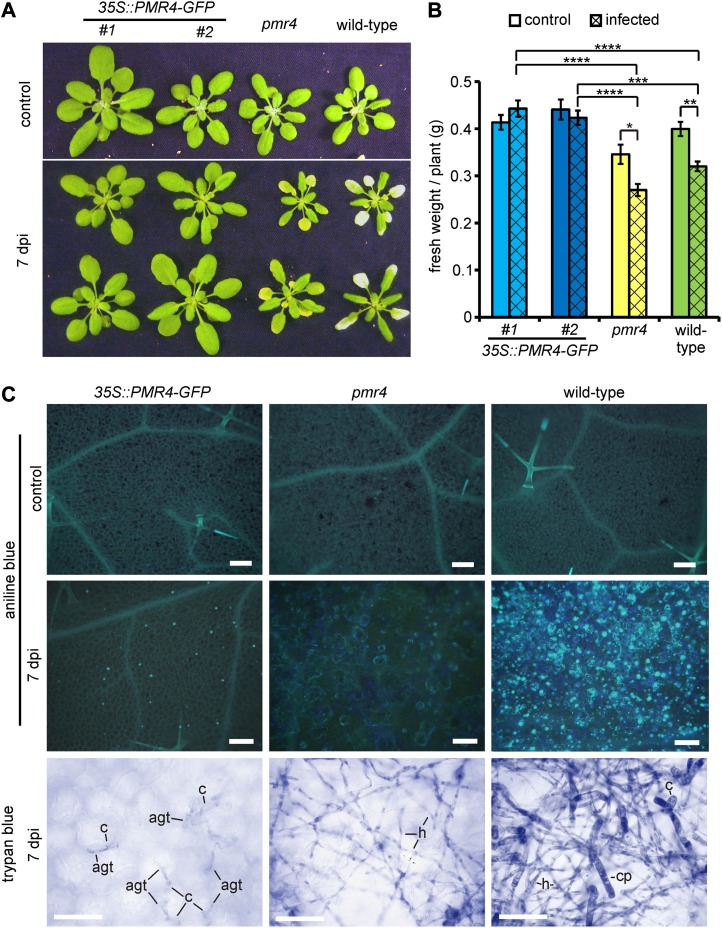
Three-week-old Arabidopsis plants were inoculated with the virulent powdery mildew Gc. At 7 d post inoculation (dpi), wild-type plants demonstrated the typical whitish powdery appearance associated with good fungal conidiation. The pmr4 mutant showed the described disease phenotype of yellow, necrotic leaves without macroscopically recognizable pathogen growth (Nishimura et al., 2003). Only 35S::PMR4-GFP leaves were free from disease symptoms (Fig. 1A). The infection did not reduce biomass production in these plants as it did in wild-type and pmr4 plants (Fig. 1B). These phenotypes were confirmed for four independent 35S::PMR4-GFP lines. Aniline blue staining allowed visualization of callose through fluorescence microscopy. The specificity of aniline blue for staining of callose under our preparative conditions was confirmed in a colocalization study with parallel immunohistochemical labeling of callose using a specific anti-callose antibody (Supplemental Fig. S1). At 7 dpi, Gc infection induced strong callose deposition not only in epidermal cells but also in underlying mesophyll cells, thereby forming connected callose patches in the wild-type plants. Only single callose spots were detected in the epidermal cells of 35S::PMR4-GFP leaves. Callose deposition did not occur in pmr4 plants. Untreated control leaves of the 35S::PMR4-GFP lines did not show aberrant or additional callose deposition relative to wild-type plants (Fig. 1C), as confirmed by immunochemical determination of the total callose amount in the control leaf tissue. The callose amount in the pmr4 mutant was significantly reduced relative to the wild type and 35S::PMR4-GFP lines (Supplemental Fig. S2). Trypan blue-stained leaves were microscopically analyzed for the density of hyphal growth. At 7 dpi, Gc formed a dense hyphal network with a relatively high number of spore-containing conidiophores on wild-type leaves. The hyphal network was less dense on pmr4 leaves but still readily observable. Conidiophore formation was almost absent on the pmr4 mutant. In contrast to wild-type and pmr4 plants, Gc did not form a hyphal network on 35S::PMR4-GFP leaves. Conidia were only able to produce short appressorial germ tubes without further growth (Fig. 1C).
Figure 1.
PMR4 overexpression confers complete powdery mildew resistance in Arabidopsis. Three-week-old 35S::PMR4-GFP, pmr4, and wild-type plants were inoculated with the virulent powdery mildew Gc. A, Infection phenotypes 7 dpi in comparison with uninfected control plants. B, Biomass determination (excluding roots) of control and infected Arabidopsis lines at 10 dpi. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 by Tukey’s test. Error bars represent se, and n ≥ 25 independent plants. A repeat experiment gave similar results. C, Localization of callose deposition by aniline blue staining (blue fluorescence in top two rows) and visualization of fungal growth by trypan blue staining (bottom row) on the rosette leaf surface at 7 dpi. agt, Appressorial germ tube; c, conidium; cp, conidiophores; h, hyphae. Bars = 50 µm.
Additional Stress-Related Phenotypes in the 35S::PMR4-GFP Lines
Infiltration of the pathogenic bacterium Pseudomonas syringae pv tomato induced strong callose deposition in both wild-type and 35S::PMR4-GFP leaves. Comparing callose deposition 1 and 3 d post infiltration, we did not observe a difference in the deposited amount of callose (Supplemental Fig. S3) or resistance (data not shown). Infiltration of the buffer solution and water only was sufficient to induce callose deposition in 35S::PMR4-GFP leaves but not in wild-type or pmr4 leaves (Supplemental Fig. S3). Similar to water infiltration, the spraying of water on the leaves induced rapid callose deposition in epidermal cells of 35S::PMR4-GFP leaves. At 1 h after spraying, ring-shaped callose deposits that traced the outer rim of the small droplets were detectable. This ring-shaped callose deposition was enhanced by spraying of the elicitor-active epitope flg22. Flg22 derives from flagellin, which is the main component of bacterial flagellum (Gómez-Gómez et al., 1999). Neither wild-type nor pmr4 mutant cells showed strong callose deposition 1 h after spraying with water or flg22 (Supplemental Fig. S4). During wounding, no differences in callose deposition between 35S::PMR4-GFP and the wild type were observed (Supplemental Fig. S5).
Callose Deposition during Early Time Points of Infection with an Adapted Powdery Mildew
The absence of a hyphal network on 35S::PMR4-GFP leaves indicated that an effective defense response was initiated during early stages of infection with Gc. Therefore, we analyzed the cell wall composition of unchallenged leaves. A detailed determination of the noncellulosic monocarbohydrate composition revealed changes in the cell wall of 35S::PMR4-GFP lines in comparison with the wild type. Fuc and Gal levels were reduced, whereas the relative amount of Glc increased by almost 60% (Supplemental Fig. S6). Before germination, powdery mildew conidia release an extracellular matrix that most likely contains cell wall-degrading enzymes and facilitates host recognition (Nielsen et al., 2000). Changes in the host cell wall composition, therefore, may affect host recognition and subsequent conidial germination. A comparison of the germination rate of the virulent powdery mildew, Gc, on 35S::PMR4-GFP, wild-type, and pmr4 leaves did not show differences (Supplemental Fig. S7A). The germination rate of the nonadapted powdery mildew, Bgh, was slightly reduced on 35S::PMR4-GFP leaves relative to wild-type and pmr4 leaves (Supplemental Fig. S7B).
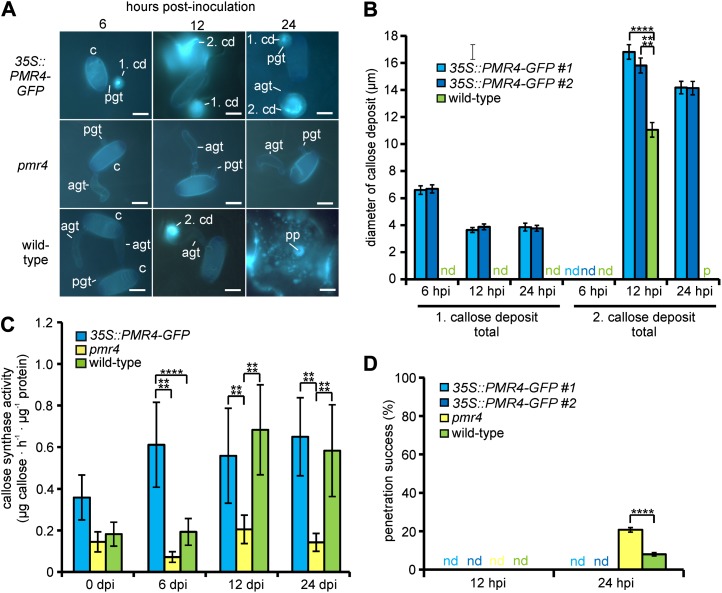
We then focused on pathogen-induced callose deposition in epidermal leaf cells at 6, 12, and 24 h post inoculation (hpi). During Gc infection, we consistently observed a callose deposit underneath the appressorial germ tube at 6 hpi and two callose deposits at 12 and 24 hpi in both 35S::PMR4-GFP and wild-type leaves (Fig. 2, A and B; Supplemental Fig. S8, A and B). However, the size and shape of the callose deposits were different.
Figure 2.
Elevated callose deposition at early time points of infection prevents adapted powdery mildew penetration in 35S::PMR4-GFP plants. Three-week-old 35S::PMR4-GFP, pmr4, and wild-type plants were inoculated with the adapted powdery mildew Gc. All tests were conducted with rosette leaves. A and B, Micrographs showing callose deposition (blue fluorescence by aniline blue staining) at sites of attempted Gc penetration at 6, 12, and 24 hpi with fungal conidia present (A) and with conidia washed off to improve visualization of deposited callose (B). agt, Appressorial germ tube; c, conidium; cd, callose deposit; ht, haustorium; pp, penetration peg; sh, secondary hyphae. Bars = 10 µm. C, Diameter of the first and second pathogen-induced callose deposits in aniline blue-stained leaves. d, Diffuse deposit (determination of diameter not possible); nd, not detectable; pp, penetration peg. *P < 0.05, ****P < 0.0001 by Tukey’s test. Error bars represent se, and n = 100 of four independent leaves. D, Relative fluorescence intensity emitted by aniline blue-stained single callose deposits, discriminating between total and core area (only in 35S::PMR4-GFP) at 6 hpi. nd, Not detectable. ****P < 0.0001 by Tukey’s test. Error bars represent se, and n = 25 independent callose deposits. E, 3D surface plots of callose deposits at 6 hpi. F, Callose synthase activity of membrane fractions at 6, 12, and 24 hpi. Unchallenged leaf membrane fractions served as controls. Activity was determined in a fluorescence-based assay detecting produced callose via the emission of callose-bound aniline blue. Values of 35S::PMR4-GFP represent the means of lines 1 and 2 in biologically independent experiments. *P < 0.05, **P < 0.01, ****P < 0.0001 by Tukey’s test. Error bars represent se, and n = 5 of six independent leaves. G, Quantification of cell entry determined by haustorium formation per conidium. nd, Not detectable. ***P < 0.001 by Tukey’s test. Error bars represent se, and n = 50 of four independent leaves. H, Quantification of secondary hyphae formation at germinated conidia 24 hpi. **P < 0.01 by Tukey’s test. Error bars represent se, and n = 50 of four independent leaves.
At 6 hpi, the diameter of the first callose deposit was 4.5 times larger in 35S::PMR4-GFP plants than in wild-type plants (Fig. 2C). The increase in size reflected a 5-fold higher fluorescence intensity (Fig. 2D) emitted by callose-adsorbed aniline blue (Currier, 1957) in the deposit and a 4-fold higher total callose synthase activity in 35S::PMR4-GFP leaf tissue relative to the wild type (Fig. 2F). The increased size of the first callose deposit in the 35S::PMR4-GFP leaf tissue was based on an additional field of callose surrounding a central callose core as visualized in a three-dimensional (3D) surface plot (Fig. 2E). The observed field of callose was not present in wild-type deposits (Fig. 2, B and E).
At 12 hpi, the first deposit in the wild-type leaf tissue showed a callose deposition pattern similar to that of 35S::PMR4-GFP, a core with a surrounding field of callose (Fig. 2B). However, this deposit was still reduced in diameter (55% of the size of the 35S::PMR4-GFP deposits; Fig. 2C). The second callose deposit at the tip of the elongated appressorial germ tube differed significantly in its shape. Nearly 90% of all deposits in the wild-type leaf had a callose-encased penetration peg, which was not present in callose deposits in 35S::PMR4-GFP leaves (Fig. 2B; Supplemental Fig. S8C).
At 24 hpi, the frequency of callose-encased penetration pegs further increased to almost 100% (Supplemental Fig. S8C) and was accompanied by a strong callose accumulation around the peg in wild-type leaves (Fig. 2B). The diameters of wild-type and 35S::PMR4-GFP secondary callose deposits were comparable (Fig. 2C). At the same time, the diameter of the first callose deposit in wild-type leaves was no longer discernible because most of the deposits became diffuse and barely detectable (Fig. 2, B and C; Supplemental Fig. S8D).
Whereas the first and second callose deposits underwent the described changes between 6 and 24 hpi in wild-type plants, the size and shape of the callose deposits in the 35S::PMR4-GFP lines were characterized by their consistency during these early stages of infection (Fig. 2B). As expected, we did not observe pathogen-induced callose deposition in the pmr4 mutant (Fig. 2A).
The penetration success of Gc was examined by the formation of haustoria (Fig. 2A), which are fungal feeding structures (Szabo and Bushnell, 2001). The normally virulent powdery mildew Gc was not able to successfully penetrate 35S::PMR4-GFP epidermal cells and to form secondary hyphae. Comparing the wild-type plant and the pmr4 mutant, the penetration success was not significantly different at 12 hpi, as it reached rates of approximately 80%. At 24 hpi, penetration success further increased in the wild-type plant to approximately 90% but was stable in pmr4 (Fig. 2G). This was correlated with the observed secondary hyphae formation, which was 90% in the wild-type plant and almost 80% in pmr4 (Fig. 2H).
Callose Deposition during Early Time Points of Nonadapted Powdery Mildew Infection
At 6 hpi with the nonadapted powdery mildew Bgh, the first callose deposit at the tip of the primary germ tube (Fig. 3A) showed a dense callose core surrounded by a field of callose in transgenic 35S::PMR4-GFP lines. These callose deposits resembled the shape of the Gc-induced deposits. However, the diameter was only one-half of that observed during Gc infection (Fig. 3B). During the progression of the Bgh infection at 12 and 24 hpi, the shape of the first callose deposit did not change but the diameter decreased by 30% (Fig. 3B). In strong contrast to 35S::PMR4-GFP plants, callose was not deposited at the primary germ tube of Bgh conidia in wild-type epidermal cells (Fig. 3, A and B; Supplemental Fig. S9A). Similar to Gc infection, the increased callose deposition of the 35S::PMR4-GFP lines at 6 hpi with Bgh reflected total callose synthase activity that was four times higher than that of the wild-type plant (Fig. 3C).
Figure 3.
Elevated callose deposition at early time points of infection prevents nonadapted powdery mildew penetration in 35S::PMR4-GFP plants. Three-week-old 35S::PMR4-GFP, pmr4, and wild-type plants were inoculated with the nonadapted powdery mildew Bgh. All tests were conducted with rosette leaves. A, Micrographs showing callose deposition (blue fluorescence after aniline blue staining) at sites of attempted Bgh penetration at 6, 12, and 24 hpi. Conidia were washed off in the wild-type, 24-hpi micrograph to improve visualization of the callose deposition and penetration peg. agt, Appressorial germ tube; c, conidium; cd, callose deposit; pgt, primary germ tube; pp, penetration peg. Bars = 10 µm. B, Diameter of the first (at primary germ tube) and second (at appressorial germ tube) callose deposits in aniline blue-stained leaves. nd, Not detectable; p, patch-like callose deposition of a whole cell (determination of diameter not possible); pp, penetration peg. ****P < 0.0001 by Tukey’s test. Error bars represent se, and n = 100 of four independent leaves. C, Callose synthase activity of membrane fractions at 6, 12, and 24 hpi. Unchallenged leaf membrane fractions served as controls. Activity was determined in a fluorescence-based assay detecting produced callose via the emission of callose-bound aniline blue. Values of 35S::PMR4-GFP represent the means of lines 1 and 2 in biologically independent experiments. ****P < 0.0001 by Tukey’s test. Error bars represent se, and n = 5 of six independent leaves. D, Quantification of nonhost cell entry determined by haustorium formation at germinated conidia. nd, Not detectable. ****P < 0.0001 by Tukey’s test. Error bars represent se, and n = 50 of four independent leaves.
At 12 hpi, the occurrence of a callose deposit at the appressorial germ tube (i.e. the so-called second callose deposit) was significantly higher in 35S::PMR4-GFP epidermal leaf cells than in wild-type epidermal leaf cells (45% and 30%, respectively; Supplemental Fig. S9B). Only wild-type secondary callose deposits showed penetration pegs (Fig. 3A), with a frequency of 80% (Supplemental Fig. S9C). The shape of the callose deposit changed from an enlarged dot-like pattern at 12 hpi to a patch-like formation at 24 hpi in wild-type leaf tissue (Fig. 3A). As previously observed during Gc infection, pathogen-induced callose deposition was absent in pmr4 during Bgh infection (Fig. 3A).
In contrast to Gc infection, the penetration success of Bgh was significantly higher in pmr4 than in wild-type plants (20% and 8%, respectively) at 24 hpi (Fig. 3D).
Transcriptional Regulation and PMR4 Localization during Powdery Mildew Infection
To analyze the regulation of pathogen-induced callose synthesis, we determined PMR4 expression in wild-type and 35S::PMR4-GFP leaf tissue. The general PMR4 expression level in unchallenged leaf tissue was approximately 2.5 times higher in 35S::PMR4-GFP tissue than in wild-type tissue (Supplemental Table S1). During Gc infection, we determined only a slight increase in PMR4 expression. PMR4 expression was 1.9-fold higher in 35S::PMR4-GFP at 12 hpi and in wild-type tissues at 24 hpi. During Bgh infection, the increase in PMR4 expression was even weaker and occurred later (Supplemental Fig. S10).
The C-terminal fusion of GFP to the callose synthase PMR4 facilitated the localization of the protein by confocal laser-scanning microscopy. In unchallenged 35S::PMR4-GFP leaves, we detected PMR4-GFP at the plant plasma membrane, which was labeled with the membrane stain FM 4-64 in epidermal cells (Fig. 4A). GFP-based fluorescence was not detected in wild-type leaves (Fig. 4A). At 6 hpi with Gc, PMR4-GFP focally accumulated in the plasma membrane underneath the appressorial germ tube at sites of attempted fungal penetration (Fig. 4, B and C). Here, PMR4-GFP coincided with localized callose deposition (Fig. 4, C–E). This area was surrounded by FM 4-64-labeled bodies (Fig. 4, D and E), which were free from aniline blue signals but may have contained PMR-GFP, as indicated in colocalization studies (Supplemental Fig. S11).
Figure 4.
Localization of the GFP-tagged callose synthase PMR4 in epidermal leaf cells. Three-week-old 35S::PMR4-GFP lines were inoculated with the virulent powdery mildew Gc (B–E), and unchallenged wild-type and 35S::PMR4-GFP plants served as controls (A). All tests were conducted with rosette leaves. Micrographs were taken by confocal laser-scanning microscopy. Green color was assigned to GFP-emitted fluorescence, red color to FM 4-64 membrane stain, and blue color to aniline blue-stained callose. A, Localization of the GFP-tagged callose synthase PMR4 at the FM 4-64-stained plasma membrane of unchallenged epidermal leaf cells of 35S::PMR4-GFP lines. No GFP-based fluorescence was seen in wild-type cells. Bars = 10 µm. B, Shadow 3D projection of germinated Gc conidium on a 35S::PMR4-GFP leaf surface at 6 hpi to visualize the position of an attempted fungal penetration site in subsequent microscopic analysis (C–E). The blue frame indicates the plane of the in silico cross section in C. Bars = 5 µm. C, In silico cross section at the site of attempted fungal penetration indicating PMR4-GFP accumulation in the plasma membrane and callose deposition at this site. Bars = 5 µm. D, Maximum-intensity 3D reconstruction at the site of attempted penetration. Shown is the view from the cytosol to the plasma membrane of the epidermal cell. Bars = 5 µm. E, Surface rendering at the site of attempted penetration. Bars = 1 µm. apt, Appressorial germ tube; c, conidium; pm, plant plasma membrane.
Regulation of the Defense-Related Secondary Metabolites Salicylic Acid and Jasmonate
Because hyperactivation of the salicylic acid pathway induces resistance in the pmr4 mutant (Nishimura et al., 2003), we wanted to know whether this plant defense response also contributes to the observed complete powdery mildew resistance in 35S::PMR4-GFP plants. We measured the gene expression of ISOCHORISMATE SYNTHASE1 (ICS1) and ENHANCED DISEASE SUSCEPTIBILITY5 (EDS5), which are both genetic markers of salicylic acid synthesis in Arabidopsis (Wildermuth et al., 2001; Nawrath et al., 2002), via quantitative PCR during Gc and Bgh infection. In accordance with the results from Nishimura et al. (2003), the absolute transcript levels of ICS1 and EDS5 in control pmr4 leaves were approximately twice as high as those in wild-type and 35S::PMR4-GFP leaves (Supplemental Table S1). During Gc infection, ICS1 and EDS5 expression continuously increased in pmr4 leaves (Fig. 5, A and B). In wild-type leaves, ICS1 expression was significantly higher only at 12 hpi (Fig. 5A). EDS5 expression was significantly induced at 12 hpi and remained at this level at 24 and 72 hpi (Fig. 5B). During Bgh infection of pmr4, ICS1 expression was similar to Gc-induced expression, as it showed a continuous increase (Fig. 5A). EDS5 reached its highest expression level at 12 hpi (Fig. 5B). In Bgh-infected wild-type leaves, ICS1 but not EDS5 expression was significantly higher than in control tissue at 12 and 24 hpi (Fig. 5A). Subsequently, ICS1 and EDS5 expression was significantly repressed at 72 hpi (Fig. 5, A and B).
Figure 5.
Expression profile of plant defense-related genes during powdery mildew infection of Arabidopsis. Three-week-old 35S::PMR4-GFP, pmr4, and wild-type plants were inoculated with the virulent powdery mildew Gc and the nonadapted powdery mildew Bgh. All tests were conducted with rosette leaves. Relative gene expression was determined by quantitative PCR. RNA was isolated from infected leaf tissue and used as template in complementary DNA generation. Gene expression at 0 hpi was used as a reference, and Actin2 expression was used for normalization. Values of 35S::PMR4-GFP represent the means of lines 1 and 2 in biologically independent experiments. *P < 0.05, **P < 0.01 by Tukey’s test. Error bars represent se, and n = 6. A, Relative expression of the salicylic acid-related gene ICS1. B, Relative expression of the salicylic acid-related gene EDS5. C, Relative expression of the jasmonate-related gene COI1. [See online article for color version of this figure.]
The transcriptional repression of salicylic acid-related genes in wild-type leaves was negatively correlated to a strong increase in CORONATINE INSENSITIVE1 (COI1) expression (Fig. 5C). The COI1 gene is responsive to jasmonate formation (Katsir et al., 2008). Jasmonate is involved in plant defense responses in incompatible plant-microbe interactions (Zimmerli et al., 2004). In pmr4, COI1 expression decreased during Bgh infection but was not altered during Gc infection (Fig. 5C).
The expression of ICS1, EDS5, and COI1 did not change during Gc or Bgh infection of 35S::PMR4-GFP leaves (Fig. 5).
DISCUSSION
The role of callose, in the form of papillae, in plant defenses during interactions with pathogens has been controversial. In incompatible interactions, results have generally indicated that callose is important for penetration resistance. The susceptibility of Arabidopsis to nonadapted fungal pathogens increased because of the inhibition or absence of callose deposition (Bayles et al., 1990; Zeyen et al., 2002; Jacobs et al., 2003). Hence, we anticipated an improved resistance to the avirulent powdery mildew Bgh in Arabidopsis lines showing increased pathogen-induced callose deposition. The complete penetration resistance of the generated 35S::PMR4-GFP lines, which indeed showed elevated early callose deposition after Bgh inoculation (Fig. 3), confirmed our assumption.
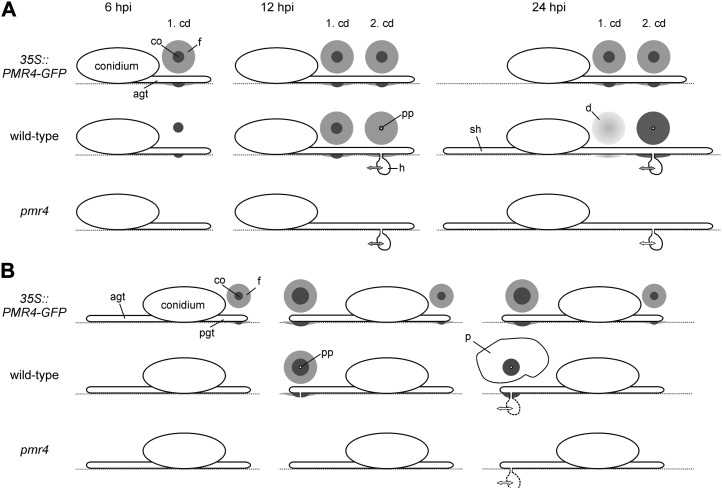
In compatible interactions, the role of callose in plant defense seemed to be dependent on the infection strategy of the pathogen. Whereas higher callose accumulation contributes to resistance against nectrophic fungal pathogens, such as Alternaria brassicicola and Plectosphaerella cucumerina (Ton and Mauch-Mani, 2004), recent results have suggested that callose deposition is irrelevant for plant resistance to adapted biotrophic powdery mildews (Consonni et al., 2010). However, our results proved that this assumption cannot be generalized. In 35S::PMR4-GFP lines, elevated amounts of callose were deposited at sites of attempted Gc penetration at early time points in the infection process. The increased callose formation provided complete resistance to the virulent, biotrophic powdery mildew Gc (Fig. 2). This clearly indicates that callose synthesis and deposition can play an active role in plant resistance to adapted powdery mildews. Complete penetration resistance to a virulent powdery mildew in Arabidopsis has only been shown for the triple knockout mutant mlo2/6/12 (Consonni et al., 2006). In contrast to the mlo triple knockout mutant, unchallenged 35S::PMR4-GFP leaves did not show spontaneous callose deposition (Fig. 1C). However, the 35S::PMR4-GFP lines revealed rapid callose formation after the leaves were sprayed with water (Supplemental Fig. S2), which reflects a previously undescribed high sensitivity to a relatively weak, external stress signal. However, external stresses, such as wounding (Supplemental Fig. S5) and bacterial infection with P. syringae (Supplemental Fig. S3), did not alter callose formation in 35S::PMR4-GFP lines relative to the wild type. Based on these results, we conclude that only distinct pathways may be affected by the changes in callose synthesis in 35S::PMR4-GFP lines, which supports recent findings regarding the complexity of the regulation of callose biosynthesis. Alterations in callose deposition were shown for different pathogen-associated molecular patterns and growth conditions (Luna et al., 2011). In order to provide an overview of the progression of callose formation in the resistant transgenic lines and wild-type plants, we summarize our results for the pathogen-induced early callose deposition and powdery mildew penetration in a comparative model (Fig. 6).
Figure 6.
Schematic overview of callose-based resistance in compatible and incompatible Arabidopsis-powdery mildew interaction. The progress of pathogen-induced callose deposition in 35S::PMR4-GFP, pmr4, and wild-type epidermal leaf cells is shown. Circles represent the shape and size of callose deposits at the indicated time points post inoculation in the compatible interaction of Arabidopsis with the powdery mildew Gc (A) and in the incompatible interaction with the powdery mildew Bgh (B). The grayscale indicates the density of deposited callose. Double-headed arrows suggest possible plant-pathogen interactions responsible for successful pathogen propagation. The coloring of double-headed arrows represents the putative level of interaction: gray, wild-type level (host); white, reduced level (resistant host). agt, Appressorial germ tube; cd, callose deposit; co, core of the callose deposit; d, diffuse callose deposit; f, field of callose; ht, haustorium; p, patch-like callose deposition; pgt, primary germ tube; pp, penetration peg; sh, secondary hyphae.
Elevated early callose deposition is likely to support penetration resistance by forming an effective physical barrier, as shown by our observations of 35S::PMR4-GFP lines. In contrast to the wild-type plant and the pmr4 mutant, powdery mildew penetration was completely absent in resistant transgenic lines. We could not detect differences between the wild-type plant and the pmr4 mutant in the initial penetration success of the virulent powdery mildew Gc (Fig. 2G), thereby confirming data from Consonni et al. (2010). Hence, callose deposition and the formation of a callose-encased penetration peg are not required for initial powdery mildew ingress and haustorium formation in a compatible interaction, which confirms previous reports regarding the development of fungal infection structures in the absence of callose formation (Jacobs et al., 2003).
Complete penetration resistance could also explain the noninduction of salicylic acid- and jasmonate-related pathways in 35S::PMR4-GFP leaves (Fig. 5). Because fungal penetration was already prevented at a very early time point of infection, an activation of subsequent defense mechanisms was not required to induce additional plant responses. Hence, hyperinduction of the salicylic acid pathway, as in the pmr4 mutant (Nishimura et al., 2003), did not contribute to powdery mildew resistance in the resistant transgenic lines. Interestingly, salicylic acid, but not jasmonate, was induced in the pmr4 mutant during infection with the nonadapted powdery mildew Bgh. The expected induction of the salicylic acid pathway in a compatible interaction and of the jasmonate pathway in an incompatible interaction (Zimmerli et al., 2004) was only confirmed for the wild-type plant. Deregulation of the jasmonate pathway in the pmr4 mutant was previously shown in the interaction with the necrotrophic pathogen A. brassicicola (Flors et al., 2008), which indicates that the callose synthase PMR4 might have not only an enzymatic function but also a regulatory role. Indeed, our data for the noncellulosic monocarbohydrate composition of the cell wall support the latter idea. Whereas the noncellulosic monocarbohydrate composition of the wild-type cell wall confirmed the results of previous leaf cell wall analyses, in which the same methods of extraction and determination were used (Jensen et al., 2008), 35S::PMR4-GFP leaf tissue showed alterations in the composition of cell wall-associated Fuc and Gal but, particularly, Glc. In contrast to its overexpression, the absence of the callose synthase PMR4 in the pmr4 mutant did not lead to alterations in cell wall composition (Supplemental Fig. S6). Because the total amount of callose was not increased in 35S::PMR4-GFP leaf tissue relative to wild-type tissue, additional (1,3)-β-glucan cannot be the origin of the increased amount of Glc. A glycan linkage analysis of hemicellulosic polymers may identify differences between 35S::PMR4-GFP and wild-type tissue and reveal a possible source of the increased relative Glc amount. By using this method, putative cell wall-related pathways that are regulated by PMR4 may be identified.
Regarding the enzymatic function of callose synthases, previous studies have proposed that callose synthase activity is not regulated by gene expression but rather by subcellular control of preexisting enzymes (Jacobs et al., 2003). Our data strongly support this idea for the stress-induced callose synthase PMR4, which is apparently regulated by posttranslational mechanisms. On the one hand, we did not detect a strong increase in pathogen-induced PMR4 transcription (Supplemental Fig. S11), thereby confirming previous reports (Jacobs et al., 2003). On the other hand, we observed GFP-tagged PMR4 at the plasma membrane of unchallenged 35S::PMR4-GFP epidermal leaf cells (Fig. 4A). In this stress-free situation, PMR4 is most likely not enzymatically active, as we did not observe additional or aberrant callose formation (Figs. 1C and 4A; Supplemental Fig. S2). After powdery mildew infection, PMR4 was translocated to the site of attempted fungal penetration, where it focally accumulated in the plasma membrane underneath the appressorial germ tube (Fig. 4, C–E). The colocalization of the GFP-tagged PMR4 with an aniline blue signal, which indicates deposited callose at the same site, suggests PMR4 activation at the site of attempted fungal penetration. We did not find any indication of the transportation of callose in multivesicular bodies to sites of papillae formation, as has been proposed for barley (Böhlenius et al., 2010). The FM 4-64-labeled body-like structures surrounding the forming callose deposit also did not show a signal for callose (Fig. 4, D and E). However, because these bodies showed a GFP signal, we concluded that they may be involved in the transport of PMR4 to the penetration site, where callose synthesis is required for structural reinforcement of the cell wall.
The general transport of PMR4 in membrane-containing bodies is also supported by a recent study from Drakakaki et al. (2012) that showed the presence of PMR4 in the SYP61 trans-Golgi network compartment via biochemical analysis. In our microscopic analysis, we only detected the PMR4-GFP signal at the plasma membrane in unchallenged leaves, underneath the appressorial germ tube, and in FM 4-64-labeled bodies at the plasma membrane. This may not exclude the involvement of the trans-Golgi network in PMR4 translocation and transport but rather supports the model of the transport of defense-related plasma membrane proteins, such as PMR4, proposed by Meyer et al. (2009). They suggested that plasma membrane proteins, which are involved in the synthesis of a structural component, including callose, the synthesis of haustorial encasements, and the synthesis of pathogen-induced papillae, are transported via multivesicular bodies and exosomes. The treatment of Arabidopsis leaves with brefeldin A, which inhibits vesicle trafficking, prior to powdery mildew infection blocked the deposition of callose at sites of fungal penetration (Nielsen et al., 2012). This indicates the involvement of multivesicular bodies in the transport of components for callose synthesis to the penetration site. In our study, we found PMR4-GFP-containing bodies around the sites of attempted penetration, which could be explained by exosome-based transport of the enzyme. At the site of attempted fungal penetration, callose synthase could then be released from putative exosomes and incorporated into the plasma membrane at the forming papillae to initiate callose synthesis. Transport via exosomes could also explain why we did not detect PMR4-GFP in bodies or vesicle-like structures in the cytosol around the site of attempted fungal penetration. These are indications of the involvement of vesicles, most likely exosomes, in PMR4 translocation to fungal penetration sites in order to form callose-containing papillae. Further microscopic analysis at early infection time points would help to further classify the already observed PMR4-GFP-containing bodies.
MATERIALS AND METHODS
Growth Conditions, Inoculations, and Cytology
Arabidopsis (Arabidopsis thaliana) wild type (Columbia), pmr4 (allele 1; Nishimura et al., 2003), and 35S::PMR4-GFP transgenic lines (this study) as well as the powdery mildews Golovinomyces cichoracearum (strain UCSC1) and Blumeria graminis f. sp. hordei (strain CR3) were cultivated as described by Stein et al. (2006). Arabidopsis inoculations (3-week-old plants were used in all experiments) and aniline blue and trypan blue staining for cytological analyses followed the protocol of Stein et al. (2006). For the bacterial infection assays, strain DC3000 of Pseudomonas syringae pv tomato (Whalen et al., 1991) was used. Cultivation and leaf infiltration followed the description by Whalen et al. (1991). A 10 mm MgCl2 solution served as a mock control in the bacterial infiltration assay. To monitor callose deposition in epidermal cells in response to a pathogen-associated molecular pattern, a 1 µm flg22 (MoBiTec) solution was sprayed on the leaf surface. Wounding experiments were performed with a needle.
Cloning and Plant Transformation
To create a PMR4 fusion to GFP under the control of the 35S promoter, 5′ (nucleotides 1–1,869 of the unspliced transcript), mid (2,030–4,049), and 3′ fragments without a stop codon (4,230–5,680) of PMR4 (At4g03550) were amplified from Arabidopsis Columbia genomic DNA; the 35S promoter was amplified from the pCAMBIA3300 vector (Cambia), and GFP was amplified from the pIGPAPA vector (Horwitz et al., 1999). All fragments contained a 20-nucleotide overhang to the adjacent fragment for sequential fusion PCR. The resulting fusion construct, 35S::PMR4-GFP (6,628 nucleotides), was cloned into pCAMBIA3300 via blunt-end ligation at the SmaI restriction site. Primer sequences are provided in Supplemental Table S2. The vector was transformed into Agrobacterium tumefaciens (strain GV3101). For Arabidopsis transformation, flowering Columbia plants were dipped into A. tumefaciens-containing infiltration medium (Cutler et al., 2000). Transformed plants were selected by application of the herbicide Basta (Bayer), to which the bar gene (phosphinothricin acetyltransferase) of the pCAMBIA vector conferred resistance.
Expression Analysis and Callose Synthase Activity
Rosette leaves were collected from uninfected and Gc- or Bgh-infected wild-type, pmr4, and 35S::PMR4-GFP plants at 6, 12, 24, and 72 hpi. Each sample represented a pool of rosette leaves from four plants. The same ground tissue was used for RNA isolation and membrane preparation. All subsequent experimental procedures required for final expression analysis and the determination of callose synthase activity followed the description of Voigt et al. (2006). Quantification of gene expression was conducted using the StepOnePlus Real-Time PCR Systems (Applied Biosystems). Primer sequences used in expression analysis are summarized in Supplemental Table S3. Callose determination in the activity assay was conducted using the Safire microplate reader (Tecan).
Confocal Microscopy and Image Analysis
Leaf samples were mounted between a microscope slide and coverslip in water. Z series were captured using the LSM 780 confocal laser-scanning microscope (Zeiss) with a Zeiss C-Apochromat 63× water-immersion objective. Callose was stained with aniline blue and membranes with FM 4-64. In the first track, aniline blue was excited at 405 nm by using a diode laser, and FM 4-64 was excited at 561 nm by using a diode-pumped solid-state laser. Emission filtering was achieved using a 472- to 490-nm bandpass filter for aniline blue and a 679- to 755-nm bandpass filter for FM 4-64. In the second track, GFP was excited at 488 nm by using an argon laser, and emission filtering was achieved using a 499- to 551-nm bandpass filter. Aniline blue and GFP signals were gathered by a highly sensitive gallium-arsenite-phosphate nondescanned photodetector (Zeiss); FM 4-64 signals were gathered by conventional photomultiplier tube detectors. Image processing, including shadow 3D projection, in silico cross section, maximum intensity 3D reconstruction, and surface rendering, was performed using integral functions of the ZEN 2010 (Zeiss) operating software.
For calculation of the aniline blue-emitted fluorescence of callose deposits, the following ImageJ tools (W.S. Rasband, National Institutes of Health; http://imagej.nih.gov/ij/) were used: “elliptical selection” to define the area of a callose deposit, “histogram list” as the reference for calculating emitted fluorescence, and “interactive 3D surface plot” to visualize the special distribution of fluorescence intensity within a callose deposit.
Statistical Analysis
Descriptive statistics including the mean and se along with the Tukey range test for multiple comparison procedures in conjunction with an ANOVA were used to determine significant differences. P < 0.05 was considered significant.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Verification of the specificity of callose staining with aniline blue by comparative immunohistochemical analysis.
Supplemental Figure S2. Callose concentration in unchallenged Arabidopsis leaves.
Supplemental Figure S3. Callose deposition in Arabidopsis leaves after infiltration of the bacterial pathogen P. syringae.
Supplemental Figure S4. Callose deposition in Arabidopsis leaves after spraying the epitope flg22 of bacterial flagellin.
Supplemental Figure S5. Callose deposition in Arabidopsis leaves after wounding.
Supplemental Figure S6. Relative noncellulosic monocarbohydrate composition of unchallenged Arabidopsis leaves.
Supplemental Figure S7. Germination rates of powdery mildew conidia on Arabidopsis leaves.
Supplemental Figure S8. Statistical analysis of callose deposits in Arabidopsis leaves after Gc infection.
Supplemental Figure S9. Statistical analysis of callose deposits in Arabidopsis leaves after B. graminis infection.
Supplemental Figure S10. Transcriptional regulation of PMR4 during early stages of powdery mildew infection.
Supplemental Figure S11. Colocalization studies of the callose synthase 35S::PMR4-GFP and FM 4-64-labeled bodies at the site of attempted powdery mildew penetration.
Supplemental Table S1. Expression of defense-related genes in unchallenged leaves of Arabidopsis 35S::PMR4-GFP and pmr4 mutant in relation to the wild type.
Supplemental Table S2. Sequences of primers used in cloning.
Supplemental Table S3. Sequences of primers used in expression analysis.
Acknowledgments
We thank Bill Underwood for his support in confocal microscopy and for providing the P. syringae strain.
Glossary
- Gc
Golovinomyces cichoracearum
- dpi
d post inoculation
- Bgh
Blumeria graminis f. sp. hordei
- hpi
h post inoculation
- 3D
three-dimensional
References
- Aist JR (1976) Papillae and related wound plugs of plant cells. In KF Baker, GA Zentmeyer, EB Cowling, eds, Annual Review of Phytopathology. Annual Reviews, Palo Alto, CA, pp 145–163 [Google Scholar]
- Aist JR, Williams PH. (1971) The cytology and kinetics of cabbage root hair penetration by Plasmodiophora brassicae. Can J Bot 49: 2023–2034 [Google Scholar]
- Aspinall GO, Kessler G (1957) The Structure of Callose from the Grape Vine. Chemistry and Industry, London [Google Scholar]
- Bailey JA (1983) Biological perspectives of host-pathogen interactions. In JA Bailey, BJ Deverall, eds, The Dynamics of Host Defence. Academic Press, Sydney, pp 1–32 [Google Scholar]
- Bayles CJ, Ghemawat MS, Aist JR. (1990) Inhibition by 2-deoxy-D-glucose of callose formation, papilla deposition, and resistance to powdery mildew in an ml-o barley mutant. Physiol Mol Plant Pathol 36: 63–72 [Google Scholar]
- Böhlenius H, Mørch SM, Godfrey D, Nielsen ME, Thordal-Christensen H. (2010) The multivesicular body-localized GTPase ARFA1b/1c is important for callose deposition and ROR2 syntaxin-dependent preinvasive basal defense in barley. Plant Cell 22: 3831–3844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boller T, Felix G. (2009) A renaissance of elicitors: perception of microbe-associated molecular patterns and danger signals by pattern-recognition receptors. Annu Rev Plant Biol 60: 379–406 [DOI] [PubMed] [Google Scholar]
- Brown I, Trethowan J, Kerry M, Mansfield J, Bolwell GP. (1998) Localization of components of the oxidative cross-linking of glycoproteins and of callose synthesis in papillae formed during the interaction between non-pathogenic strains of Xanthomonas campestris and French bean mesophyll cells. Plant J 15: 333–343 [Google Scholar]
- Consonni C, Bednarek P, Humphry M, Francocci F, Ferrari S, Harzen A, Ver Loren van Themaat E, Panstruga R. (2010) Tryptophan-derived metabolites are required for antifungal defense in the Arabidopsis mlo2 mutant. Plant Physiol 152: 1544–1561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Consonni C, Humphry ME, Hartmann HA, Livaja M, Durner J, Westphal L, Vogel J, Lipka V, Kemmerling B, Schulze-Lefert P, et al. (2006) Conserved requirement for a plant host cell protein in powdery mildew pathogenesis. Nat Genet 38: 716–720 [DOI] [PubMed] [Google Scholar]
- Currier HB. (1957) Callose substance in plant cells. Am J Bot 44: 478–488 [Google Scholar]
- Cutler SR, Ehrhardt DW, Griffitts JS, Somerville CR. (2000) Random GFP:cDNA fusions enable visualization of subcellular structures in cells of Arabidopsis at a high frequency. Proc Natl Acad Sci USA 97: 3718–3723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- deBary A. (1863) Recherches sur le développement de quelques champignons parasites. Ann Sci Nat Bot Biol Veg 20: 5–148 [Google Scholar]
- Deverall BJ (1977) Defence Mechanisms of Plants. Cambridge University Press, Cambridge, UK [Google Scholar]
- Drakakaki G, van de Ven W, Pan S, Miao Y, Wang J, Keinath NF, Weatherly B, Jiang L, Schumacher K, Hicks G, et al. (2012) Isolation and proteomic analysis of the SYP61 compartment reveal its role in exocytic trafficking in Arabidopsis. Cell Res 22: 413–424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flors V, Ton J, van Doorn R, Jakab G, García-Agustín P, Mauch-Mani B. (2008) Interplay between JA, SA and ABA signalling during basal and induced resistance against Pseudomonas syringae and Alternaria brassicicola. Plant J 54: 81–92 [DOI] [PubMed] [Google Scholar]
- Gómez-Gómez L, Felix G, Boller T. (1999) A single locus determines sensitivity to bacterial flagellin in Arabidopsis thaliana. Plant J 18: 277–284 [DOI] [PubMed] [Google Scholar]
- Horwitz BA, Sharon A, Lu SW, Ritter V, Sandrock TM, Yoder OC, Turgeon BG. (1999) A G protein alpha subunit from Cochliobolus heterostrophus involved in mating and appressorium formation. Fungal Genet Biol 26: 19–32 [DOI] [PubMed] [Google Scholar]
- Jacobs AK, Lipka V, Burton RA, Panstruga R, Strizhov N, Schulze-Lefert P, Fincher GB. (2003) An Arabidopsis callose synthase, GSL5, is required for wound and papillary callose formation. Plant Cell 15: 2503–2513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen JK, Sørensen SO, Harholt J, Geshi N, Sakuragi Y, Møller I, Zandleven J, Bernal AJ, Jensen NB, Sørensen C, et al. (2008) Identification of a xylogalacturonan xylosyltransferase involved in pectin biosynthesis in Arabidopsis. Plant Cell 20: 1289–1302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JD, Dangl JL. (2006) The plant immune system. Nature 444: 323–329 [DOI] [PubMed] [Google Scholar]
- Katsir L, Schilmiller AL, Staswick PE, He SY, Howe GA. (2008) COI1 is a critical component of a receptor for jasmonate and the bacterial virulence factor coronatine. Proc Natl Acad Sci USA 105: 7100–7105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb C, Dixon RA. (1997) The oxidative burst in plant disease resistance. Annu Rev Plant Physiol Plant Mol Biol 48: 251–275 [DOI] [PubMed] [Google Scholar]
- Luna E, Pastor V, Robert J, Flors V, Mauch-Mani B, Ton J. (2011) Callose deposition: a multifaceted plant defense response. Mol Plant Microbe Interact 24: 183–193 [DOI] [PubMed] [Google Scholar]
- Mangin L. (1895) Recherches sur les Péronosporées. Bull Soc Hist Nat Autun 8: 55–108 [Google Scholar]
- Mauch-Mani B, Mauch F. (2005) The role of abscisic acid in plant-pathogen interactions. Curr Opin Plant Biol 8: 409–414 [DOI] [PubMed] [Google Scholar]
- Mercer PC, Wood RKS, Greenwood AD. (1974) Resistance to anthracnose of French bean. Physiol Plant Pathol 4: 291–306 [Google Scholar]
- Meyer D, Pajonk S, Micali C, O’Connell R, Schulze-Lefert P. (2009) Extracellular transport and integration of plant secretory proteins into pathogen-induced cell wall compartments. Plant J 57: 986–999 [DOI] [PubMed] [Google Scholar]
- Mims CW, Sewall TC, Richardson EA. (2000) Ultrastructure of the host-pathogen relationship in Entomosporium leaf spot disease of Photinia. Int J Plant Sci 161: 291–295 [DOI] [PubMed] [Google Scholar]
- Nawrath C, Heck S, Parinthawong N, Métraux JP. (2002) EDS5, an essential component of salicylic acid-dependent signaling for disease resistance in Arabidopsis, is a member of the MATE transporter family. Plant Cell 14: 275–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen KA, Nicholson RL, Carver TLW, Kunoh H, Oliver RP. (2000) First touch: an immediate response to surface recognition in conidia of Blumeria graminis. Physiol Mol Plant Pathol 56: 63–70 [Google Scholar]
- Nielsen ME, Feechan A, Böhlenius H, Ueda T, Thordal-Christensen H. (2012) Arabidopsis ARF-GTP exchange factor, GNOM, mediates transport required for innate immunity and focal accumulation of syntaxin PEN1. Proc Natl Acad Sci USA 109: 11443–11448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura MT, Stein M, Hou BH, Vogel JP, Edwards H, Somerville SC. (2003) Loss of a callose synthase results in salicylic acid-dependent disease resistance. Science 301: 969–972 [DOI] [PubMed] [Google Scholar]
- Ryals JA, Neuenschwander UH, Willits MG, Molina A, Steiner HY, Hunt MD. (1996) Systemic acquired resistance. Plant Cell 8: 1809–1819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwessinger B, Ronald PC. (2012) Plant innate immunity: perception of conserved microbial signatures. Annu Rev Plant Biol 63: 451–482 [DOI] [PubMed] [Google Scholar]
- Smith H. (1978) Recognition and defence in plants. Nature 273: 266–268 [Google Scholar]
- Stein M, Dittgen J, Sánchez-Rodríguez C, Hou BH, Molina A, Schulze-Lefert P, Lipka V, Somerville S. (2006) Arabidopsis PEN3/PDR8, an ATP binding cassette transporter, contributes to nonhost resistance to inappropriate pathogens that enter by direct penetration. Plant Cell 18: 731–746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone BA, Clarke AE (1992) Chemistry and Biology of (1→3)-β-Glucans. La Trobe University Press, Bundoora, Australia [Google Scholar]
- Szabo LJ, Bushnell WR. (2001) Hidden robbers: the role of fungal haustoria in parasitism of plants. Proc Natl Acad Sci USA 98: 7654–7655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thordal-Christensen H. (2003) Fresh insights into processes of nonhost resistance. Curr Opin Plant Biol 6: 351–357 [DOI] [PubMed] [Google Scholar]
- Ton J, Mauch-Mani B. (2004) Beta-amino-butyric acid-induced resistance against necrotrophic pathogens is based on ABA-dependent priming for callose. Plant J 38: 119–130 [DOI] [PubMed] [Google Scholar]
- Voigt CA, Schäfer W, Salomon S. (2006) A comprehensive view on organ-specific callose synthesis in wheat (Triticum aestivum L.): glucan synthase-like gene expression, callose synthase activity, callose quantification and deposition. Plant Physiol Biochem 44: 242–247 [DOI] [PubMed] [Google Scholar]
- Whalen MC, Innes RW, Bent AF, Staskawicz BJ. (1991) Identification of Pseudomonas syringae pathogens of Arabidopsis and a bacterial locus determining avirulence on both Arabidopsis and soybean. Plant Cell 3: 49–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler H. (1974) Cell wall and plasmalemma modifications in diseased and injured plant tissue. Can J Bot 52: 1005–1009 [Google Scholar]
- Wildermuth MC, Dewdney J, Wu G, Ausubel FM. (2001) Isochorismate synthase is required to synthesize salicylic acid for plant defence. Nature 414: 562–565 [DOI] [PubMed] [Google Scholar]
- Zeyen RJ, Kruger WM, Lyngkjær MF, Carver TLW. (2002) Differential effects of D-mannose and 2-deoxy-D-glucose on attempted powdery mildew fungal infection of inappropriate and appropriate Graminea. Physiol Mol Plant Pathol 61: 315–323 [Google Scholar]
- Zimmerli L, Stein M, Lipka V, Schulze-Lefert P, Somerville S. (2004) Host and non-host pathogens elicit different jasmonate/ethylene responses in Arabidopsis. Plant J 40: 633–646 [DOI] [PubMed] [Google Scholar]