Abstract
Virulence gene expression in Staphylococcus aureus is tightly regulated by intricate networks of transcriptional regulators and two-component signal transduction systems. There is now an emerging body of evidence to suggest that the blockade of S. aureus virulence gene expression significantly attenuates infection in experimental models. In this Perspective, we will provide insights into medicinal chemistry strategies for the development of chemical reagents that have the capacity to inhibit staphylococcal virulence expression. These reagents can be broadly grouped into four categories: (1) competitive inhibitors of the accessory gene regulator (agr) quorum sensing system, (2) inhibitors of AgrA–DNA interactions, (3) RNAIII transcription inhibitors, and (4) inhibitors of the SarA family of transcriptional regulators. We discuss the potential of specific examples of antivirulence agents for the management and treatment of staphylococcal infections.
1. Introduction
The term “antibiotic agent” was initially coined by Waksman as a chemical substance “which has the capacity to inhibit the growth of and even to destroy bacteria and other micro-organisms”.1 To this day antibacterial drug development has essentially held firmly to this definition, focusing on molecules that elicit bacteriostatic or bactericidal effects. The mode of action of such conventional antibiotics largely relies on targeting essential cellular functions, such as DNA replication and protein and cell wall biosynthesis. While the success of these approaches is unquestionable, a significant limitation remains; i.e., conventional antibiotics impose immense selective pressure on bacteria. This coupled with the chronic misuse and overuse of our most potent antibiotics in modern medical and agricultural practices has fueled the rise of multiantibiotic resistant bacteria. These include methicillin resistant Staphylococcus aureus (MRSA) which constitutes a major, global public health threat.2,3 Although MRSA infections have predominantly been associated with health care settings (HA-MRSA), invasive community-acquired MRSA (CA-MRSA) strains have recently emerged that infect previously healthy individuals.4,5
Clinically significant antibiotic resistance has evolved against virtually every antibiotic deployed, while the development of new antibiotic classes has lagged far behind the requirement for new drugs.6 Many major pharmaceutical companies have withdrawn from the antibiotic discovery field mainly because of the huge economic cost of developing drugs that are likely to become rapidly obsolete through resistance. Consequently, there is an urgent need to identify novel antibacterial targets and develop new agents effective against multiresistant strains that do not rapidly succumb to resistance.
In simplistic terms, bacteria can be separated into two classes, pathogenic and nonpathogenic. Nonpathogenic bacteria derive carbon and energy from the environment, either as free-living or as host-associated commensals or symbionts.7 In contrast, pathogenic bacteria, at least transiently, may derive their carbon and energy parasitically or destructively from a host organism.7 This is accomplished through the production of diverse virulence factors that protect the pathogen from host defenses while facilitating the colonization and subsequent destruction of host cells and tissues liberating nutrients which sustain pathogen growth. In essence, virulence factors are responsible for the classical and potentially lethal symptoms of infection, such as abscesses, inflammation, and sepsis.7 This raises the question of whether virulence can be attenuated and an infection resolved if the production or action of one or more virulence factors is inhibited.8
Indeed, there is a growing body of evidence indicating that inhibiting virulence factor production can significantly attenuate infection, and thus, developing therapies to “disarm” bacteria is a promising approach to combating infection.9−13 Such an approach has a number of perceived benefits over conventional antibacterial strategies and would create an in vivo scenario that is similar to vaccination, in which the bacteria are eventually cleared by the host’s innate defenses with little to no likely impact on the normal human microbiota.14 Furthermore, in contrast to conventional antibiotic strategies inhibition of virulence factor action/production would attenuate infection via nonbactericidal pathways, and given that most virulence factors are not essential for bacterial viability, in principle, the blockade of virulence may exert less selective pressure for the generation of resistance.14 However, there have been recent examples, in laboratory settings, in which xenobiotic/chemical modulated virulence attenuation could be overcome.15
The lifestyle of pathogenic bacteria revolves around (i) locating a host, (ii) finding a colonization niche, (iii) initiating and establishing an infection, and (iv) dispersal to a new host. For a pathogen to progress from one stage to the next, changes in the sensory input that signal environmental change must be perceived and acted upon, e.g., by the induction of new gene expression.16 Such changes may result from movement from one environment to another, be due to the actions of bacteria within a given environment, or be a consequence of host responses to bacterial activity. Thus, from a prokaryotic perspective, the successful interaction of bacterial cells with mammalian host tissues depends on a coordinated response to environmental cues, such as nutrient availability, temperature, pH, and bacterial cell population density.16 It is becoming evident that inhibiting virulence gene expression and thus the ability of bacteria to adapt to the host environment offers considerable potential for attenuating infection.
In this Perspective, we will focus on virulence gene expression in S. aureus as an antibacterial target. This Gram-positive pathogen is capable of causing a diverse array of both minor and life threatening, acute, and chronic infections, including boils, pneumonia, toxemia, meningitis, endocarditis, and osteomyelitis.17,18 Virulence in S. aureus depends on a diverse range of cell-surface associated and secreted exoproducts. The former exoproduct includes fibronectin-, fibrinogen-, and immunoglobulin-cell wall binding proteins and capsular polysaccharides. Among the S. aureus secreted exotoxins are α-hemolysin, multiple enterotoxins and toxic shock syndrome toxin-1 (TSST-1), Panton–Valentine leukocidin (PVL) and the phenol soluble modulins, and multiple secreted tissue-damaging exoenzymes.17,18 While cell wall proteins are involved in promoting adherence to host tissues and aiding immune evasion, S. aureus exotoxins cause tissue damage and many function as superantigens promoting the onset of shock-like syndromes.19 Staphylococci also readily establish biofilms on host tissues and implanted medical devices, causing chronic infections that exhibit remarkable resistance to conventional antimicrobials.20 Collectively, these staphylococcal virulence determinants enable the organism to evade host defenses, adhere to cells and the tissue matrix, spread within the host, and derive energy by catabolizing host cells and tissue components.
Energetically, staphylococcal virulence factor expression is expensive and is thus tightly regulated by intricate gene regulatory networks incorporating transcriptional regulators and two-component signal transduction systems (TCSTS).21−33 Studies of S. aureus strains carrying mutations in key regulatory elements have provided significant proof-of-principle that their loss can significantly reduce virulence. For example, strains carrying perturbed or nonfunctional TCSTS, such as saeRS, srrAB, and agr mutants, display reduced virulence in mouse models of skin infection and necrotizing pneumonia.34−37 Likewise, S. aureus strains carrying mutations within transcriptional regulator genes, such as sarA, display a reduced ability to induce septic arthritis and osteomyelitis in murine models of musculoskeletal infection.38−40
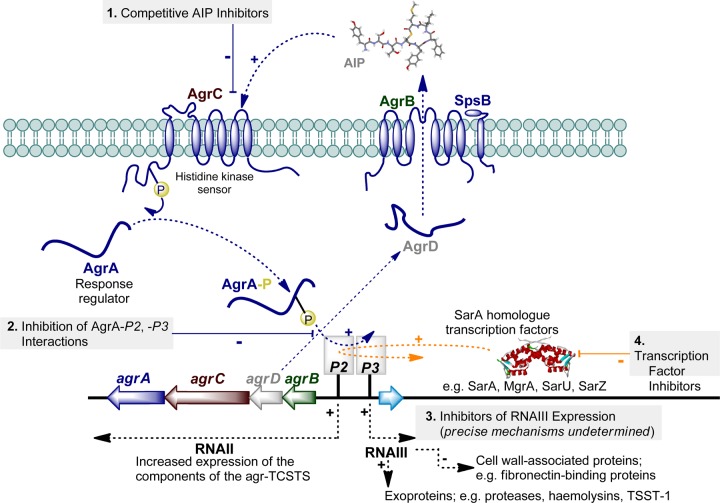
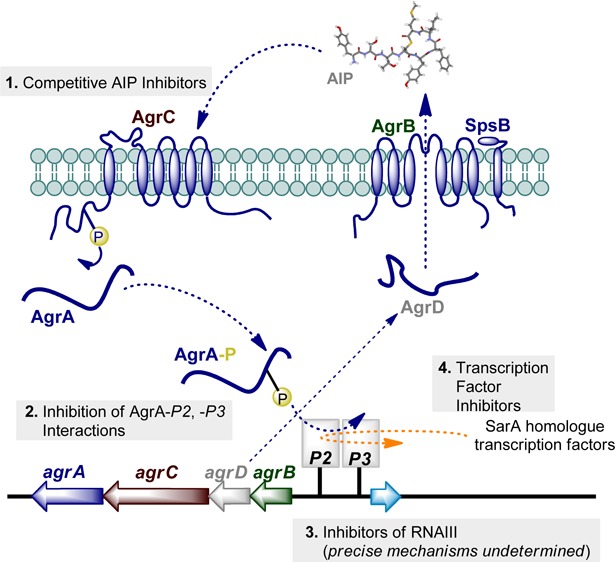
Until relatively recently, studies of S. aureus virulence mechanisms have essentially relied on genetic techniques. However, the emergence of crystal structures of key virulence regulators and the discovery of a raft of small molecules displaying virulence inhibitory activities have provided a platform to initiate medicinal chemistry programs. As outlined in Figure 1, which will be explained in greater detail throughout this article, from a medicinal chemistry perspective the most advanced strategies targeting S. aureus virulence gene regulation can be grouped into four categories of inhibitors. These inhibitors are directed toward (1) the sensor kinase AgrC, (2) the transcriptional activator AgrA–DNA, (3) RNAIII, and (4) the SarA family of transcriptional regulators.
Figure 1.
Schematic of the (a) agr system (blue) and (b) the SarA protein family (orange). (a) The agr locus is composed of two divergent transcripts called RNAII and RNAIII, driven by the P2 and P3 promoters, respectively.41−43 The RNAII transcript is an operon of four genes, agrBDCA, which encodes the core machinery of the system. AgrD, the peptide precursor to the autoinducer peptide (AIP), is processed and exported through AgrB and possibly SpsB action at the cytoplasmic membrane. AgrB catalytically functions as a cysteine endopeptidase to afford an AgrB–AgrD acyl-enzyme intermediate, which undergoes intramolecular trans-thioesterification resulting in the release/regeneration of AgrB. At the threshold concentration, AIP binds to the AgrC receptor, a membrane-bound histidine kinase. AIP binding activates the AgrC kinase, resulting in phosphorylation of the AgrA response regulator and activation of the P2 and P3 promoters.41−43 (b) The SarA protein family encompasses at least 10 transcriptional regulators that initiate intricate molecular cascades that up- or down-regulate the expression of numerous virulence systems.44,45 Activation or repression ultimately affects RNAIII expression which is considered to be the effector molecule of S. aureus virulence. Current strategies to suppress RNAIII expression can be grouped into four categories: (1) competitive inhibitors of AgrC, the agr histidine kinase receptor, (2) inhibition of AgrA–P2/P3 interactions, (3) RNAIII transcription inhibitors (precise mechanisms undetermined), and (4) transcriptional regulator inhibition.
2. Two-Component Signal Transduction Systems
Genome sequencing has revealed that there are at least 16 two-component systems in the chromosome of S. aureus.46 A full account of all TCSTS involved in S. aureus virulence falls outside the scope of this Perspective; nevertheless, an overview of a number of TCSTS known to play key roles in S. aureus pathogenesis is presented in Table 1. These TCSTS allow bacteria to adapt to environmental changes in response to various cues such as nutrient concentrations, cell population density, antibiotics, ionic strength, and membrane disturbances.
Table 1. Overview of a Number of TCSTS and Their Proposed Activators That Have Been Implicated in the Regulation of S. aureus Virulence Pathways.
| TCSTS | activators | virulence effects | reference |
|---|---|---|---|
| SaeRS | β-Lactam antibiotics or human neutrophil peptides | Implicated in the expression of 212 genes. Up-regulation results in increased expression of many virulence factors including cell wall proteins, cell-wall-associated proteins, and secreted proteins. | (21, 22) |
| ArlRS | Undetermined | Implicated to control the expression of 114 genes. Activation leads to down-regulation of transcription of some virulence genes (α-toxin, β-hemolysin, lipase, serine protease, coagulase, and protein A). Also plays a key role in autolysis which is essential in bacterial cell division and can be triggered by antibiotics or adverse physiological conditions. | (23, 24) |
| SrrAB | Reduced oxygen levels; however, other factors that reflect the oxygen level or redox state such as pH may act as the signal. | Up-regulation increases expression of colonization factors and a number of proteins involved in energy metabolism. Repression results in the up-regulation of virulence factors including TSST-1and RNAIII. | (25) |
| KdpDE | Autoinducer-2 (AI-2) and external K+ levels | High extracellular K+ levels result in reduced KdpDE expression resulting in increased expression extracellular toxins and enzymes. Reduced K+ levels activate KdpDE transcription, increasing the expression of cell wall proteins and polysaccharides, which are beneficial to colonization. | (26, 27) |
| AgrCA | Autoinducing peptides (AIPs) | Regulation of numerous metabolic, virulence, and regulatory genes through RNAIII dependent and independent mechanisms | (17, 18) |
| LytRS | Postulated to be triggered by anaerobic metabolism | Inhibits the extracellular activity of murein hydrolases. These enzymes catalyze the cleavage of specific structural components of the bacterial cell wall, aiding penicillin tolerance. Additionally activated LytR is hypothesized to induce lrgAB promoter activity which promotes cell death and lysis during biofilm development. | (30) |
| VraRS | Possible activators include glycopeptide antibiotics, e.g., vancomycin and teicoplanin. | VraRS system regulates a response to cell wall stress. Inactivation results in reduced cell wall thickness an increase susceptibility to glycopeptide antibiotics. | (31, 32) |
| GraRS | Low external salt concentrations | Activation positively regulates expression of the dlt operon. This operon encodes proteins responsible for d-alanylation. Addition of d-alanine to teichoic acids reduces the negative charge of the cell envelope, thereby influencing the binding and interaction of various compounds. d-Alanylation helps to protect from antimicrobial peptides. | (33) |
Typically TCSTS consist of two key proteins, a sensor, usually a membrane-associated histidine kinase, and a cytoplasmic response regulator that acts at the level of transcription.17 Upon activation of the sensor by the cognate signal, autophosphorylation of a His residue within the cytoplasmic kinase domain occurs followed by phosphor transfer to an Asp residue in the response regulator protein. This promotes binding of the latter to a specific DNA target sequence and leads to activation or repression of the target structural gene(s).
2.1. Accessory Gene Regulator (agr) System
At present the most extensively characterized TCSTS in S. aureus is the global regulatory system known as the accessory gene regulator (agr) which up-regulates virtually all S. aureus toxins as well as multiple exoenzymes (proteases, lipases, and nucleases) while down-regulating the expression of numerous surface protein genes.17,18 This reciprocal regulation facilitates the progression of an infection from the early stages when staphylococcal surface proteins are required to promote host tissue colonization to the later stages when exotoxins are required to combat host immune defenses alongside degradative exoenzymes that facilitate nutrient acquisition.
In contrast to other TCSTS that respond to external environmental cues, the agr system responds to a self-generated, secreted signal molecule that facilitates the coordination of gene expression at a cell population density level termed “quorum sensing”. This primitive cell-to-cell communication mechanism in S. aureus employs cyclic thiolactone peptides known as autoinducing peptides (AIPs) as quorum sensing signal molecules.42,43
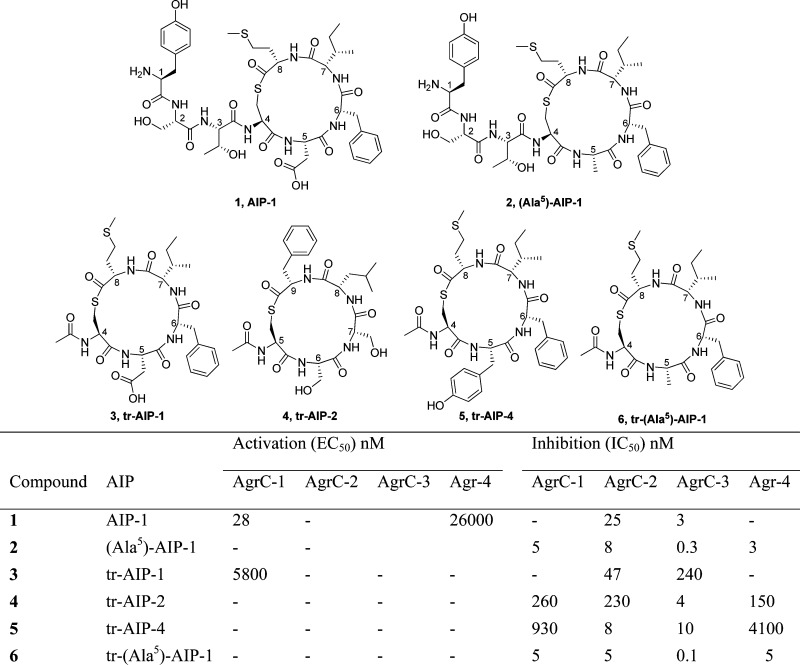
Typically, AIPs consist of seven to nine amino acids in which a central cysteine residue is covalently linked to the C-terminal amino acid carboxylate.42,43,47,48 The sequence of the AIPs is highly variable, and on the basis of AIP primary amino acid sequence, S. aureus can be subdivided into four different agr groups (I–IV). Intriguingly, most cross-group AIP–AgrC interactions are inhibitory, with AIPs activating their cognate receptors and competitively inhibiting noncognate receptors. For example, AIP-1 (compound 1, Chart 1) activates its cognate receptor AgrC-1 (EC50 ≈ 28 nM) while competitively inhibiting the noncognate receptors AgrC-2 and AgrC-3 (IC50 ≈ 25 nM and IC50 ≈ 3 nM, respectively).47
Chart 1. Structure and Biological Activities of AIP-1 (1), the Global agr-Inhibitor (Ala5)-AIP-1 (2), and Truncated Analogues tr-AIP-1 (3), tr-AIP-2 (4), tr-AIP-4 (5), and tr-(Ala5)-AIP-1 (6).
As outlined in Figure 1, the agr locus consists of two adjacent but divergent transcriptional units (RNAII and RNAIII) under the control of the P2 and P3 promoters, respectively.41 The P3 transcript, a 517-nucleotide termed RNAIII, is the effector of the agr response, initiating the production of multiple exoproduct virulence factors. The agrP2 operon consists of four genes, agrBDCA, which are required for the activation of transcription from the agrP2 and agrP3 promoters which code for the cytosolic, transmembrane, and extracellular components of this population density-sensing TCSTC.42,43 AgrD is a propeptide that is processed by AgrB to generate the AIP, which is secreted via a mechanism in which the signal peptidase SpsB has been implicated.41 AIPs binds to their cognate AgrC transmembrane receptor, which results in autophosphorylation of the cytoplasmic histidine kinase domain. Subsequent trans-phosphorylation of AgrA activates transcription from the P2 and P3 promoters, which drives the autoactivation circuitry and up-regulates production of AIP and RNAIII, respectively. Until recently RNAIII was considered to be the primary effector of the agr response, although it is now clear that the agr regulon can be divided into RNAIII-dependent and RNAIII-independent, AgrA-dependent genes.49
2.2. Competitive Inhibitors of AgrC–AIP Binding
As outlined previously, cross-group AIP–AgrC interactions are typically inhibitory with AIPs competitively inhibiting noncognate receptors (Chart 1).47 Although the precise evolutionary and physiological relevance of this cross-talk inhibition has yet to be elucidated, it offers significant therapeutic potential. Inhibition of the TCSTS by noncognate AIPs virtually abolishes the production of the enterotoxin C3, lipase, and toxic shock syndrome toxin-1. Additionally, interference of AIP signaling through the use of competing AIPs50 or AIP-sequestering antibodies51,52 reduces abscess formation in S. aureus skin and soft tissue infections. Together these studies demonstrate that competitive AIP inhibition constitutes a promising therapeutic approach for attenuating S. aureus infections.
These findings stimulated investigations directed toward the development of global inhibitors of all four S. aureus agr groups, and to this end, the AIP macrocycle has been subjected to a number of structure–activity relationship (SAR) studies.47,50,53−57 Consistent within these studies was the observation that the macrocycle is critical for AIP function while replacement of the thiolactone moiety with a lactone or lactam group virtually eliminates cognate activation but not cross-group inhibition.47,50,58,59 However, at present perhaps the most significant observation emerged from alanine-scanning of the AIP-1 scaffold which afforded the aspartate to alanine-5 (D5A) variant (compound 2, Chart 1) displaying potent inhibitory effects against all four AgrC subtypes (Chart 1).47,56 Further investigations focused on truncated analogues with tr-AIP-1 (compound 3, Chart 1), tr-AIP-2 (compound 4, Chart 1), and tr-AIP-4 (compound 5, Chart 1) which displayed potent inhibitory properties, although tr-AIP-1 still served as a weak AgrC-1 activator.47 Combining truncation and the aspartic acid to alanine mutation gave the currently accepted lead compound, N-acetylated tr-(Ala5)-AIP-1 (compound 6, Chart 1), that elicits IC50 values of ∼0.1–5 nM across all four agr systems.47
Building on the identification (Ala5)-AIP-1 as a potent global AgrC inhibitor, additional analogues were synthesized, including the aminobutyric acid analogue 7 and the 4-substituted phenoxybutyryl analogues 8 and 9.57 The rationale for the incorporation of the 4-benzylphenoxyalkanoic acid synthon was two-fold: (a) structural variants of benzoylphenol are readily accessed by the Friedel–Crafts acylation of substituted benzene using p-methoxybenzoyl chloride and (b) biaryl ketones are photoactivatable and hence the 4-benzoylphenoxyalkanoyl-derivatized AIP-1 could be used for photolabeling studies of AgrC.57 As outlined in Figure 2, the subtle replacement of a methyl with an ethyl group at the endocyclic position 5 (compound 7) increased inhibitory activity against AgrC-2 but resulted in a decreased inhibitory activity toward AgrC-1. However, the 4-benzoylphenoxybutyryl analogues (8, 9) displayed decreased potencies against both ArgC-1 and AgrC-2, indicating that the larger aromatic moiety at the exocyclic position is detrimental to activity.57
Figure 2.
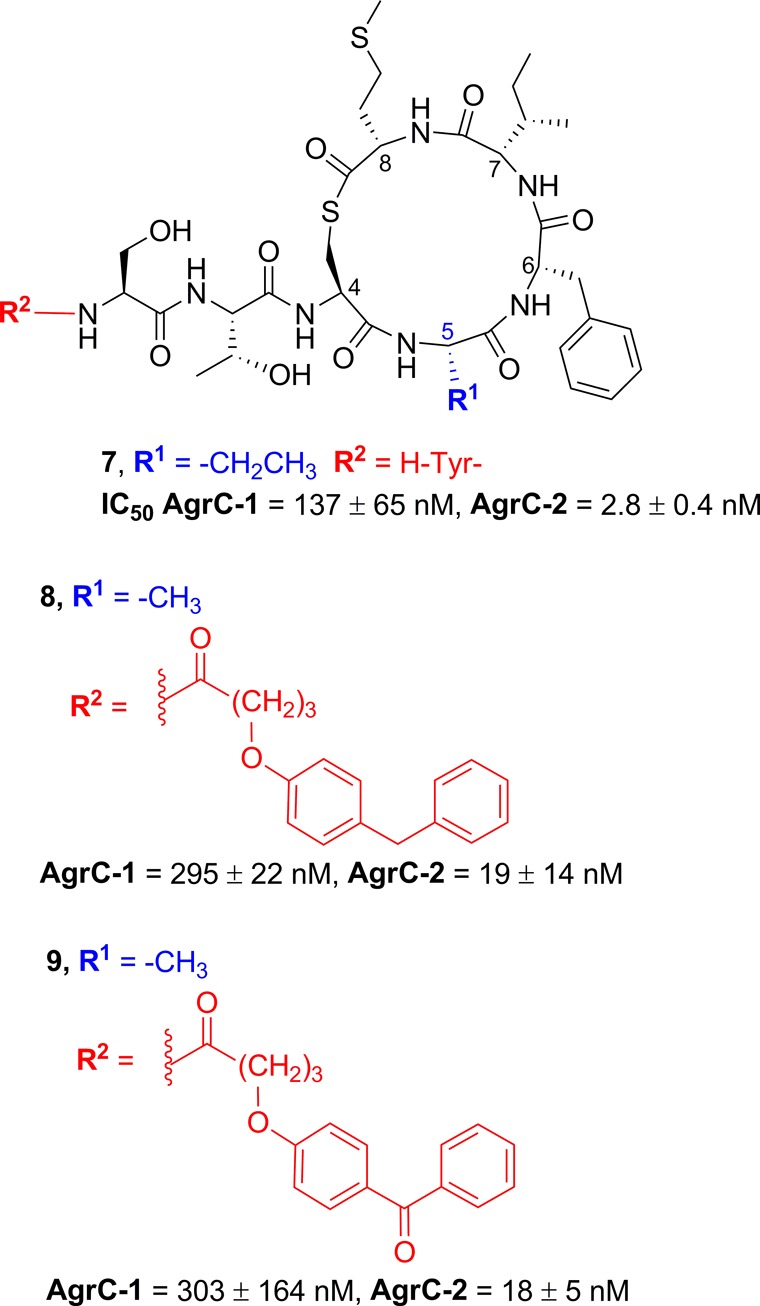
Structures of (Abu5)-AIP-1 (7) and the 4-benzoylphenoxy analogues 8 and 9 and AgrC-1 and AgrC-2 activity.
Further examination of the thiolactone scaffold focused on truncated AIP-2 analogues with glycine insertions, N-methylation scan, and alteration to the thioester linker.53 As outlined in Chart 2, N-methylation of either Ser-6 or -7 residues resulted in reduced inhibitory activities toward AgrC-1 and AgrC-2 (compounds 13 and 14). In contrast, N-methylation of the Leu-8 and Phe-9 residues (compounds 15 and 16) abolished activity against both AgrC-1 and AgrC-2. Substitution of either Ser-6 or Ser-7 (10 or 11) or both Ser residues (12) with Gly residue(s) yielded analogues with improved inhibitory activities against AgrC-1, but the later two changes marginally affected activities against AgrC-2. Unexpectedly, the Ser-6-to-Gly substitution (compound 10) resulted in a 5-fold lost of inhibitory activity against AgrC-2. Likewise, inhibition of both groups I and II AgrC receptors was substantially reduced with substitution of the two adjacent serine residues with a 5-aminopentanoyl linker (compound 17) and with replacement of the thioester bond with an amide (compound 18). Furthermore, removal of the amino group of the cysteine (compound 19) resulted in decreased activity.
Chart 2. AgrC-1 and AgrC-2 Activities of the tr-AIP-2 Analogues 10–19.
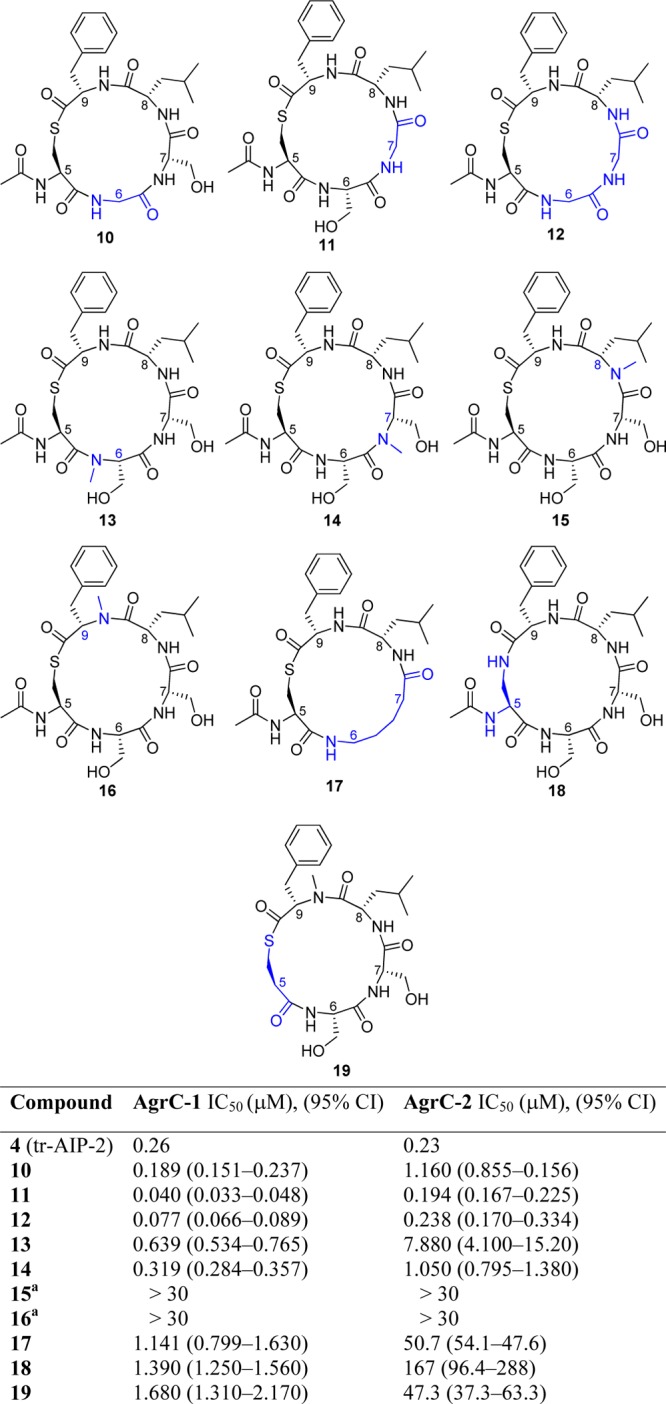
a IC50 values for peptides 15 and 16 could not be determined because of lack of inhibition up to the highest concentration tested (>30 μM).
Thus, in terms of AgrC-1 and AgrC-2 inhibition, these SAR data indicate that the cysteine and two C-terminal hydrophobic residues at endocyclic positions 7 and 8 are crucial for inhibitory activity while the remainder of the molecule appears less important (Figure 3). In the case of noncognate agr inhibition, the serine residues at endocyclic positions 5 and 6 can be replaced with an alkyl linker without dramatic loss of activity.53
Figure 3.
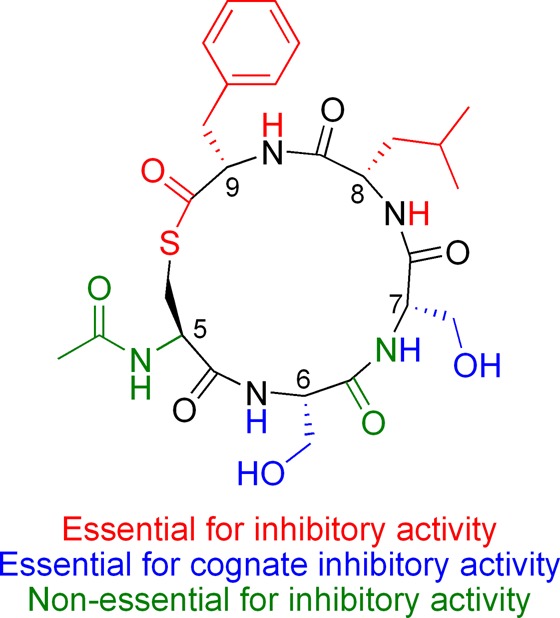
Structure of tr-AcAIP-2 color-coded, thus indicating portions of the structure that are critical for inhibition of cognate (AgrC-2) and/or noncognate (AgrC-1) receptors.53
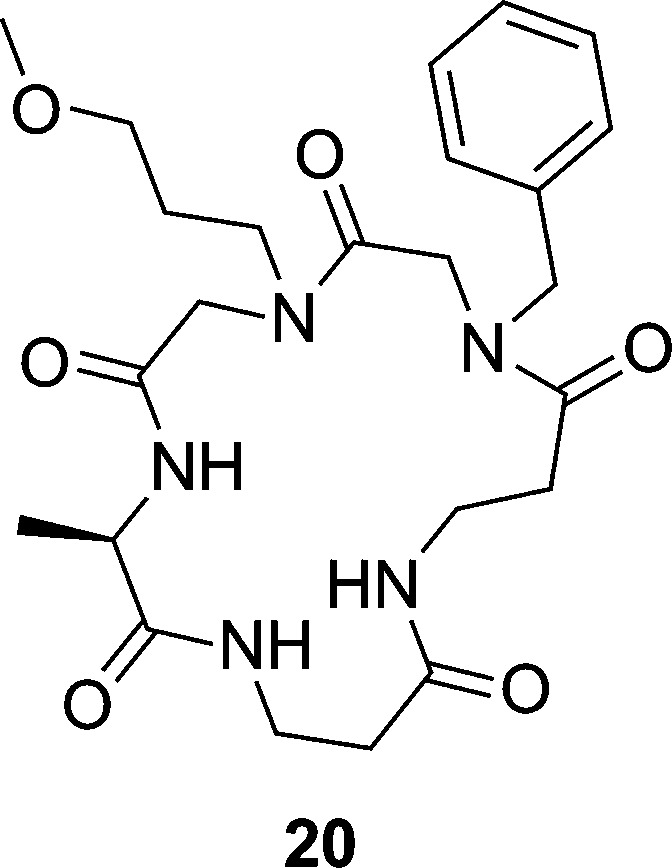
Despite their peptidic nature, the AIP analogues appear to be relatively stable in physiological conditions and are resistant to many endoproteases, including chymotrypsin, thermolysin, proteinase K, and V8 serine protease (unpublished data from our lab).42 Nevertheless, in an effort to reduce peptidic character, a series of peptoid–peptide hybrids, or peptomers, derived from the tr-AIP-1 scaffold were investigated.60 Of the 11 analogues synthesized, one analogue (compound 20, Figure 4) was shown to stimulate biofilm formation, a phenotype linked to AgrC inhibition. However, given the structural diversity and lack of activity associated with this peptomer library, no definitive SAR data could be established. Nevertheless, the peptoid scaffold does show promise for further analogue development.
Figure 4.
Structure of the tr-AIP-1 derived peptomer that was shown to stimulate biofilm formation, a phenotype typically linked to AgrC inhibition.60
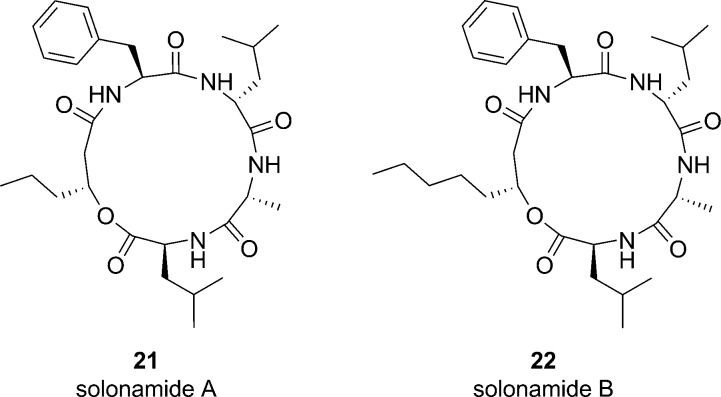
Thus, present endeavors to develop a global AgrC competitive inhibitor have focused on developing analogues based on the native S. aureus AIP structures. However, it appears that macrocyclic peptides from other bacteria may provide valuable leads. For instance, solonamide A and solonamide B (Figure 5, compounds 21 and 22, respectively), which were isolated from a marine Photobacterium, display agr inhibitory activity.61 It was speculated that the solonamides may serve as quorum sensing signals for Photobacterium, and the obvious structural similarities of with tr-AIP-2 and tr-AIP-3 suggest they may function as competitive inhibitors of the S. aureus AgrC receptor.61 As previously outlined, structural investigations of the tr-AIP-2 scaffold demonstrated that adjacent leucine and phenylalanine residues are crucial for potent AgrC competitive inhibition while substitution of the thiolactone moiety with a lactone has minimal impact on inhibitory activity;47,53,56 each of these features is present within the solonamide scaffold. Although IC50 values were not reported, Northern blot analysis confirmed the agr interfering activity of the solonamides in both S. aureus strain 8325-4 and the highly virulent CA-MRSA strain USA300.61
Figure 5.
Structures of solonamide A and solonamide B, which were isolated from a marine Photobacterium and display agr inhibitory activity. Given the structural similarities of these analogues to the tr-AIP-2 scaffold, it is postulated that they may serve as competitive inhibitors of the AgrC receptor.61
Thus, ligand-based design of competitive AgrC inhibitors has provided compelling proof-of-principle that S. aureus virulence can indeed be inhibited through chemotherapeutic intervention. A number of the macrocyclic analogues that displayed submicromolar activity against AgrC are stable against numerous endoproteases and attenuate in vivo dermonecrotic infection caused by various S. aureus strains including USA300. However, of the numerous macrocyclic peptide analogues examined, none displayed significant impact on bacterial viability and repeat exposure experiments did not induce resistance. These successes have fueled preclinical investigations of these compounds and inspired investigations of alternative pathways that could be exploited to attenuate virulence.
The existence of an additional agr-linked quorum sensing system (SQS1) in S. aureus has been proposed.62−67 However, there is considerable controversy within the field and three independent reports provide compelling evidence that the original work was flawed probably as a consequence of secondary mutations in the strains used.68−71 SQS1 was hypothesized to operate upstream of agr, controlling the activity of RNAIII via its own autoregulatory mechanism, involving an autoinducer RNAIII-activating protein (RAP), a sensor histidine kinase (SvrA), and the response regulator protein TRAP (target for RAP, encoded by traP).62−67 However, mutation of traP in a number of different S. aureus strains had no effect on either agr expression or virulence,68−70 and the original data are most likely accounted for by a nonsense mutation in agrA.
Nevertheless, the SQS1 system was reported to be inhibited by a linear peptide known as RNAIII-inhibiting peptide (RIP, sequence YSPSTNF-NH2).64,67 An in silico generated pharmacophore of RIP was screened against a library of commercially available small molecules, and a nonpeptidic RIP analogue, hamamelitannin, was discovered.72 Hamamelitannin (Figure 6) is a natural product found in the bark of Hamamelis virginiana (witch hazel). In a rat graft model, hamamelitannin prevented device-associated infections in vivo, including infections caused by methicillin-resistant S. aureus and S. epidermidis strains.72 Thus, although hamamelitannin mechanism of action has not been unequivocally delineated, the molecule may still represent a viable lead for the discovery of both virulence and biofilm formation inhibitors of S. aureus.
Figure 6.
Structure of the witch hazel derivate natural product hamamelitannin which was identified from an in silico virtual screen using a RIP based pharmacophore.72
3. AgrA as a Target
As outlined in Figure 1, RNAIII is the effector of the agr response initiating up-regulation of several exotoxins and enzymes while repressing expression of a range of bacterial cell-surface proteins. Consequently, any reduction of RNAIII transcription via perturbation of the AgrA interaction with the P2 and P3 promoters could provide an additional route to block S. aureus virulence.
AgrA is indispensable to agr P2- and P3-driven transcription,45 and while no bona fide inhibitors of AgrA–P2/P3 interactions have been reported, the recently reported cocrystallized structure of the DNA-binding domain of AgrA complexed with a DNA pentadecamer duplex has provided a potential platform for structure-based drug design endeavors.73 The crystal structure of the C-terminal DNA-binding domain, termed AgrAC (residues 137–238), indicates that three amino acid residues His-169 (blue), Asn-201 (orange), and Arg-233 (green) make specific contacts with DNA (Figure 7). Indeed, the importance of residues H169 and R233 in DNA binding was confirmed by alanine mutagenesis and subsequent isothermal titration calorimetry studies.73 Thus, small molecules specifically designed to prevent AgrA binding to DNA or that prevent AgrA activation upon phosphorylation may inhibit RNAIII expression and thus virulence factor deployment.
Figure 7.
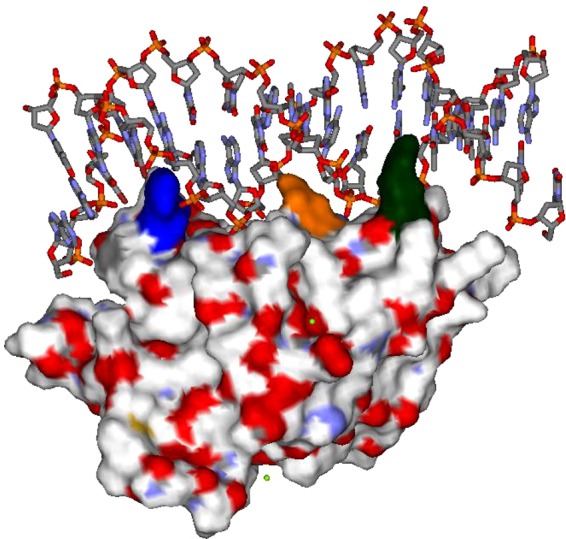
Cocrystallized structure of the C-terminal DNA-binding domain of AgrA (residues 137–238) with a DNA pentadecamer duplex (PDB accession code 3BS1). The structure indicates that three amino acid residues, H169 (blue), N201(orange), and R233 (green), make specific contacts with the DNA complex. Indeed, the importance of residues H169 and R233 in DNA binding was confirmed by alanine mutagenesis and subsequent isothermal titration calorimetry studies.73
4. Small Molecule Inhibitors of RNAIII Expression
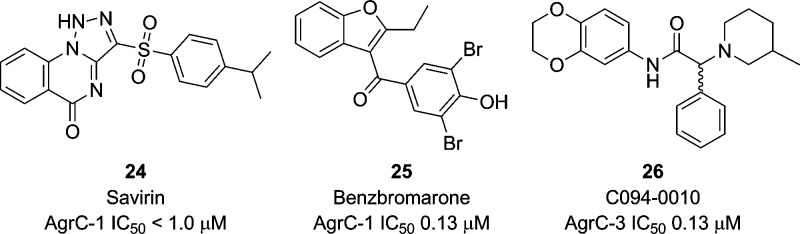
Several synthetic and natural product small molecule analogues with agr inhibitory properties including savirin (Staphylococcus aureusvirulence inhibitor) (compound 24, Figure 8), benzbromarone (compound 25, Figure 8), and a benzo-1,4-dioxane analogue (compound 26, Figure 8) have been described. These compounds were identified from extensive random screening programs, and each inhibits AIP-induced production of RNAIII transcripts and thus virulence factors such as α-hemolysin and lipase.74−76
Figure 8.
Structures and IC50 values of the small molecule agr inhibitors savirin (24), the gout drug benzbromarone (25), and C094-0010 (26) that were identified from a number of high-throughput screening program.74−76
In terms of in vivo efficacy, the most extensively examined analogue is savirin (24), and this compound was shown to prevent the development of dermonecrotic ulcers following infection with agr+ bacteria in an experimental mouse model of skin and soft tissue infection.76 Moreover, repeated exposure of S. aureus to savirin either in vivo or in vitro did not induce resistance. Although the mechanism of action of savirin has yet to be elucidated, transcriptome experiments indicate that most of the genes down-regulated in an agr positive strain on treatment with savirin were also down-regulated in the absence of agr, i.e., in an agr mutant.76 At present, the structure has not been subjected to any SAR; however, methods to rapidly produce analogues have been reported,77 thus making savirin an attractive lead.
Benzbromarone, which is traditionally utilized as a gout medication, was also found to reduce abscess formation in mouse models and provided protection against agr-II and agr-III S. aureus infections.75,76 These inhibitory effects were elicited in the S. aureus RN6390 strain as well as in NM300, which is closely related to the CA-MRSA USA300 strain.75 Once again, precise mechanism of action has not been elucidated; however, receptor binding studies indicate that benzbromarone may inhibit binding of AIP to AgrC.75 However, it also elicits more global effects on the bacteria, and in addition to inhibiting late stationary phase growth, benzbromarone also inhibits production of staphyloxanthin which is a carotenoid pigment used by the bacteria as protection against oxidants.76
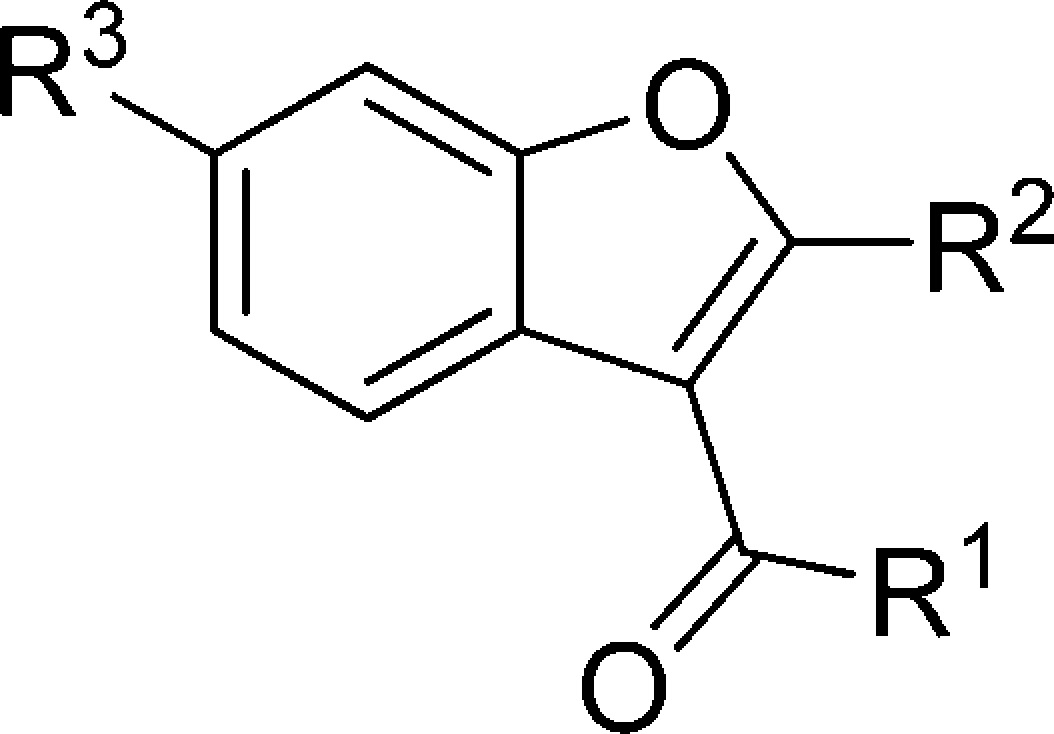
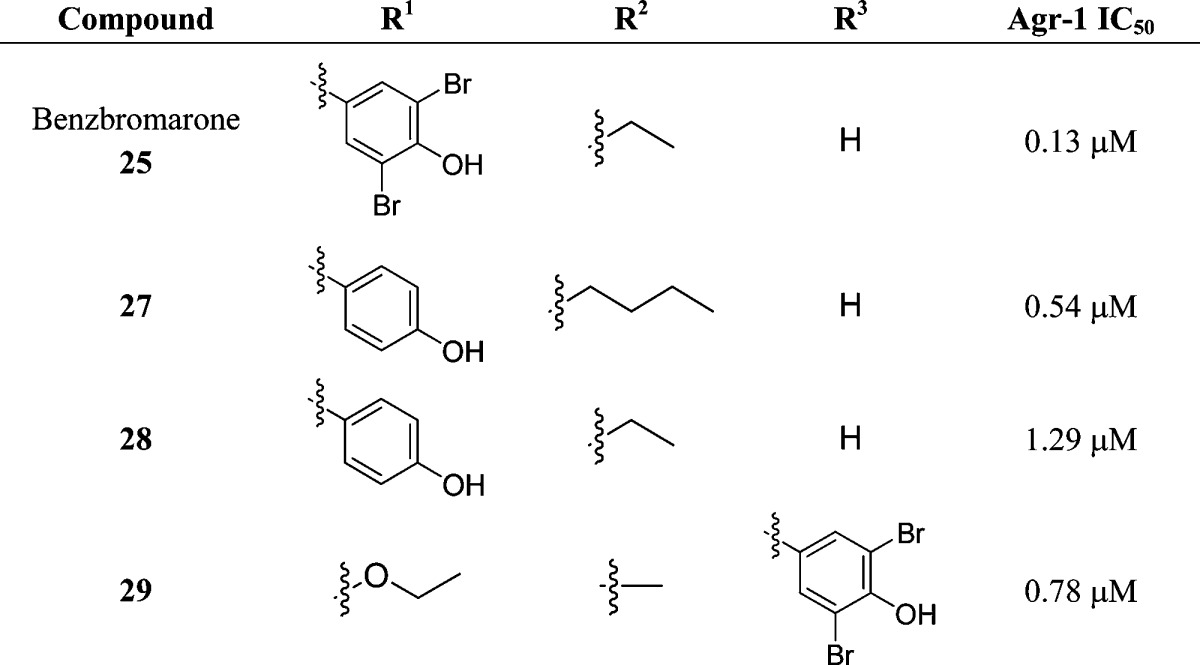
The benzbromarone scaffold has been subjected to preliminary SAR investigations; however, of the 24 analogues examined none displayed increased activity against S. aureus agr group I.75 As outlined in Table 2, alterations at R1 included a number of aryl substituents as well as the incorporation of alkyl and ester moieties. However, not all permutations of the other two regions were represented while holding R1 constant as an aryl group; for instance, compound 29 lacks an aryl group at R1 but still displays activity. Nevertheless, a p-hydroxy moiety seemed to be desirable for activity in this region with the dibromo-substituted benzbromarone maintaining its role as the best inhibitor. Alterations to R2 included alkyl and aryl substituents; however, only alkyl analogues displayed agr-I inhibitory activity. Likewise, alterations to R3 of the scaffold were detrimental to activity with benzofuran and aryl substitutions affording only one active compound, 29. Nevertheless, the activity of compound 29 is assumed to result from the inclusion of the sterically bulky dibromomethoxy moiety.
Table 2. Structure and AgrC-1 IC50 Values of the Most Active Benzbromarone Analogues Unearthed during an Initial Structure–Activity Relationship Investigation75.
An additional series of small molecules displaying inhibitory activities against agr are the 1,3-benzodioxoles and benzo-1,4-dioxanes.74 The lead compound (compound 26) inhibits RNAIII promoter activation with an IC50 in the range of 100–200 nM, and maximal effects (90% or greater inhibition) can be achieved at 12 μM in in vitro experimental systems.74 Intriguingly, the lead compound inhibits AIP-induced production of virulence factors α-hemolysin and lipase in agr group III but not group I strains of S. aureus. Receptor binding studies indicate that it does not significantly inhibit the binding of AIP to the AgrC receptor, the initial step in the bacterial quorum sensing pathway. Additionally, cell viability is unaffected.74
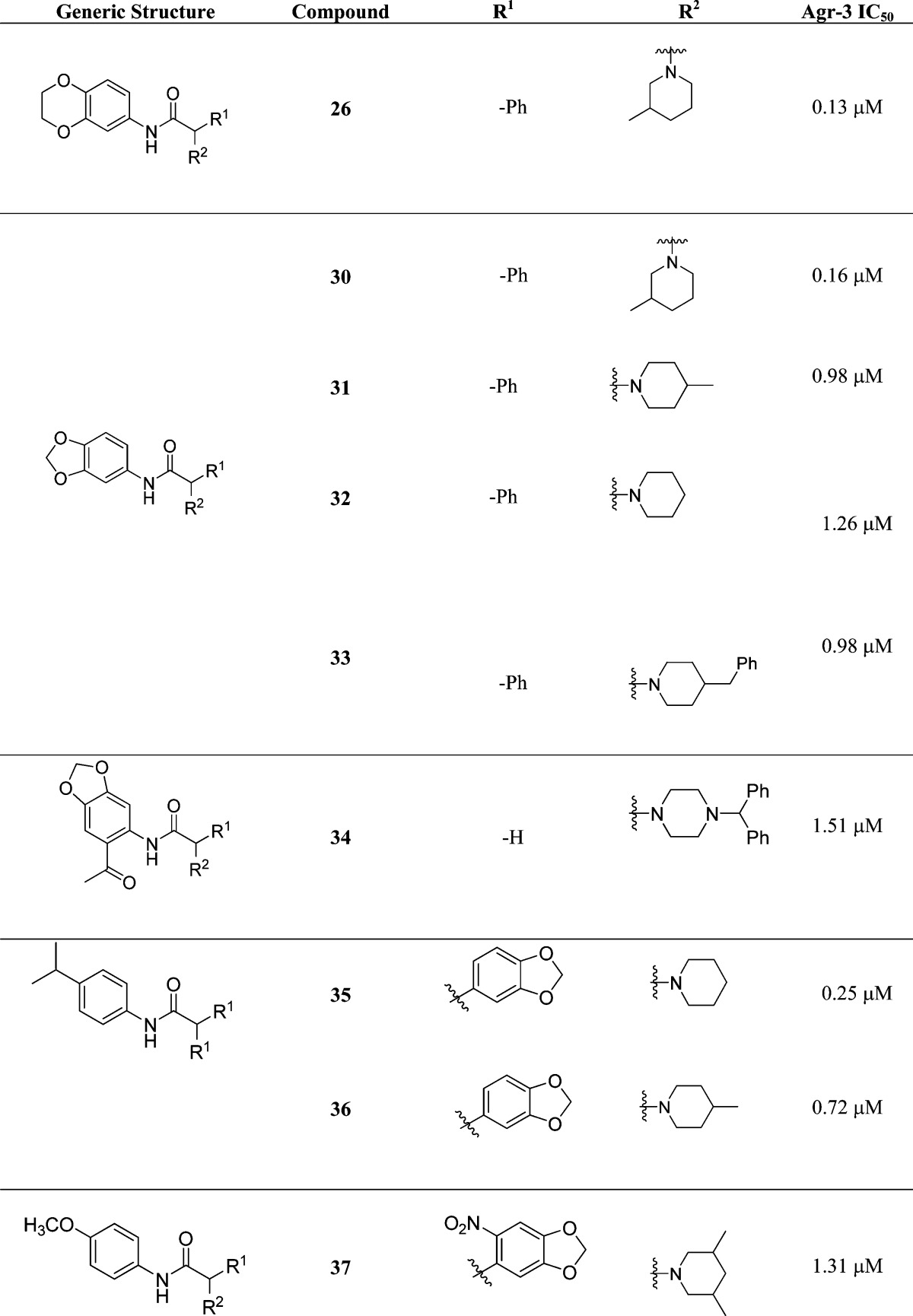
The scaffold was subjected to an extensive SAR study with a total of 44 compounds synthesized and evaluated.74 In general, the 1,3-benzodioxoles (compounds 30–33, Table 3) were the most active of the five scaffolds investigated, particularly when R1 was a simple phenyl and R2 was a piperidine moiety. Addition of heteroatoms to the piperidine moiety decreased activity with ethers, esters, ketal protections, or ketones leading to loss of inhibition. Additionally, having anything at R1 other than a simple phenyl appears to impart a detrimental effect on activity.
Table 3. Structures and AgrC-3 IC50 Values of the Most Active 1,3-Benzodioxole Analogues Unearthed during an Initial Structure–Activity Relationship Investigationa.
Intriguingly these analogues only displayed activity against S. aureus group III.74
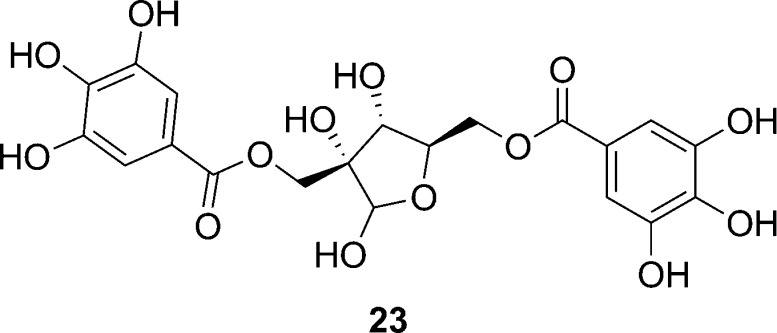
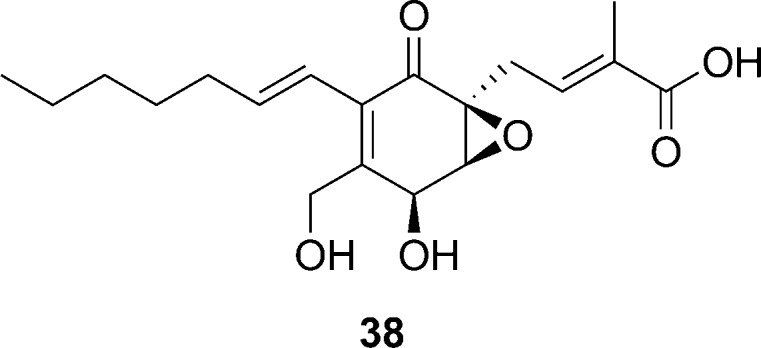
Small molecule inhibitors of RNAIII expression have also emerged from natural sources, and among these are the secondary fungal metabolite known as ambuic acid (compound 38, Figure 9). Preliminary data indicated that compound 38 inhibits AIP biosynthesis; however, even at high concentrations it was reported that the inhibitory effect is not substantial. Nevertheless, the inhibitory effect appeared sustained over a period of several hours.78 Thus, the ambuic acid scaffold offers a lead to developing indirect inhibitors of the agr TCSTS.
Figure 9.
Structure of the secondary fungal metabolite, ambuic acid.78
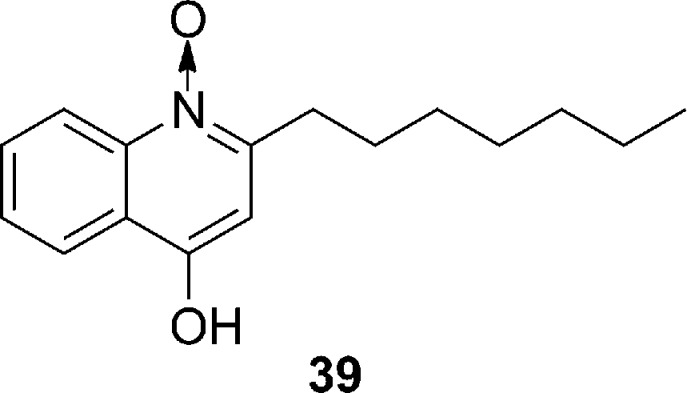
Some of the most intriguing natural compounds displaying agr inhibitory activities have emerged from investigations of mixed microbial infections. A number of these studies have focused on chronic infections within the airways of cystic fibrosis (CF) sufferers and led to the discovery that the prolonged growth of S. aureus with either Pseudomonas aeruginosa or with physiological concentrations of the P. aeruginosa exoproduct 4-hydroxy-2-heptylquinoline N-oxide (HQNO, compound 39, Figure 10) selects for typical S. aureus small-colony variants (SCVs).79 However, SCVs are well-known for aminoglycoside resistance and persistence in chronic infections, including those found in CF.79 Evidence suggests that the development of SCVs is in part due to HQNO-mediated repression of the agr system. In addition to other unknown mechanism(s) of action, HQNO apparently has the capacity to inhibit agr group I with an IC50 of 1.3 μM (unpublished data from our lab).
Figure 10.
Structure of the Pseudomonas aeruginosa alkylquinolone, 4-hydroxy-2-heptylquinoline-N-oxide, which displays inhibitory effects against the agr system.
Other studies of P. aeruginosa demonstrated that the quorum signal molecule N-(3-oxododecanoyl)-l-homoserine lactone (3-oxo-C12-HSL) (compound 40, Figure 11) also elicits inhibitory effects against agr (IC50 = 6 μM) and, at high concentrations, staphylococcal growth (100 μM).80 Indeed, exposure of S. aureus to different N-acyl l-homoserine lactones (AHLs) revealed that 3-oxosubstituted AHLs with C10 to C14 acyl chains inhibited virulence factor production and growth in a concentration-dependent manner, while short-chain AHLs had no effect.80 3-Oxo-C12-HSL inhibited the production of exotoxins and cell wall fibronectin-binding proteins but enhanced protein A expression. Although the biological mechanism by which 3-oxo-C12-HSL inhibits agr is yet to be elucidated, evidence exists that the molecule may affect SarA functionality and potentially antagonize other membrane-associated regulators, such as the sensor components of arlRS, saeRS, and srrAB.(80) Thus, although 3-oxo-C12-HSL analogues display potential as antivirulence agents, their antagonistic activity on a number of growth and metabolic pathways suggests that resistance may develop easily.
Figure 11.
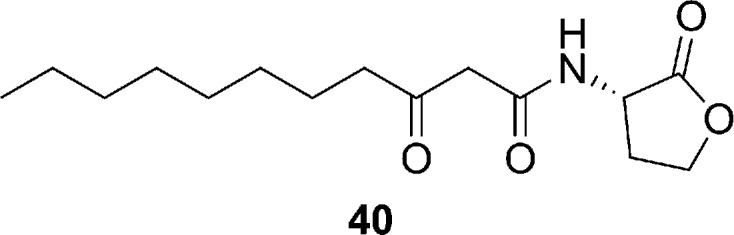
Structure of the Pseudomonas aeruginosa quorum sensing molecule 3-oxo-C12-HSL which elicits inhibitory action against agr (IC50 = 6 μM) and at high concentrations, staphylococcal growth (100 μM).80
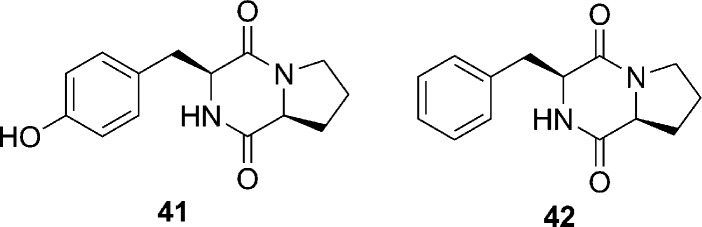
Lactobacillus reuteri RC-14, a human vaginal isolate,81 produces molecules capable of inhibiting the S. aureus agr system, and initial data indicate that this bacterium can repress the expression of TSST-1.82 TSST-1 has been associated with essentially all cases of menstruation-associated toxic shock syndrome, and evidence indicates that women with a deficiency of Lactobacillus reuteri RC-14 within the vaginal mucosa are more susceptible to this illness.83 It is believed that two active compounds involved in this interspecies communication are the cyclic dipeptides cyclo(l-Tyr-l-Pro) (compound 41, Figure 12) and cyclo(l-Phe-l-Pro) (compound 42, Figure 12).83 Although no IC50 values were reported, competition assays demonstrated that both cyclic dipeptides antagonized the AIP-mediated activation of agr, indicating that they may compete for the ligand-binding pocket on the AgrC receptor.83 Additionally, these analogues potentially interrupt other virulence regulating TCSTS, such as sarA or saeRS.83 Further investigations are required to elucidate the molecular mechanism of these dipeptides; nevertheless, their molecular scaffold is well suited to a medicinal chemistry endeavors, as these structures could be rapidly accessed and are amenable to diverse structural alterations.
Figure 12.
Structures of the Lactobacillus reuteri RC-14 derived products cyclo(l-Tyr-l-Pro) (41) and cyclo(l-Phe-l-Pro) (42).83 Although no IC50 values were reported, competition assays demonstrated that both cyclic dipeptides antagonized the AIP-mediated activation of agr, indicating that they may compete for the ligand-binding pocket on the AgrC receptor.83
Thus, distinct classes of cyclic peptides/peptoids, synthetic small molecules, natural product derivatives, and bacterial derived compounds displaying direct inhibitory activity against the agr-TCSTS or inhibition of RNAIII expression via an as yet undefined mechanism are beginning to emerge. Moreover, a number of these analogues significantly attenuate in vivo dermonecrotic infections caused by various S. aureus strains including USA300.76 Of these classes, none have displayed significant impacts on bacterial viability and a number of repeat exposure experiments did not induce resistance.76 Together these studies provide significant proof-of-concept that suppressing S. aureus toxin secretion significantly attenuates infection and imparts minimal effects on bacterial viability, thus minimizing the emergence of resistance.
There is emerging evidence that inhibiting other virulence regulating TCSTS, such as SaeRS and SrrAB, may display similar therapeutic potential,21,25,34,35 although significant work remains to be done to elucidate the precise mechanism of these TCSTS before they could be considered as useful targets for chemotherapeutic intervention. However, it is clear that TCSTS themselves are tightly controlled, and consequently such regulatory cascades may provide further opportunities for intervention.
5. Transcriptional Regulator Inhibition
Of the numerous S. aureus transcriptional regulator families, the most thoroughly investigated is the SarA protein family. Searches of a number of S. aureus genomes revealed that there are at least 10 SarA homologues, including SarR, SarS, SarT, SarU, SarV, and MgrA.44,45 These transcriptional regulators drive intricate molecular cascades that up- or down-regulate the expression of numerous virulence factors. While a complete account of all known virulence transcriptional regulators, their roles, and proposed mechanisms falls outside the scope of this Perspective, an overview of a number of SarA homologues and their proposed functions is outlined in Table 4.
Table 4. Overview of a Number of SarA Homologues and Their Proposed Functions in the Regulation of S. aureus Virulence Pathways.
| regulator | virulence effect | proposed interactions and regulations | reference |
|---|---|---|---|
| SarA | + | An activator of the agrABCD operon activating P2 transcription. SarA is also involved in agr-independent pathways via binding to conserved regions, termed Sar boxes, within the promoters of several cell-wall-associated proteins and exoproteins. | (44, 45, 87, 88) |
| SarR | – | SarR represses P2 transcription and binding of SarR to the sarA promoter represses SarA expression. | (18, 45) |
| SarS | – | Activates protein A (spa) and represses α-toxin (hla) transcription | (18, 89) |
| SarT | – | Activation of sarT results in up-regulation of sarS, thus leading to hla repression and spa activation. | (18, 44) |
| SarU | + | sarU is repressed by SarT which in turn is down-modulated by agr. Since sarU is an activator of agr expression, this will lead to amplification of the original agr signal. | (18, 44) |
| Rot | – | Represses toxin synthesis and up-regulates cell wall protein synthesis. Rot affects the transcription of 168 genes, many of which reflect an agr minus phenotype. | (90) |
| SarX | – | SarX acts as a negative regulator of agr. Furthermore, MgrA is an activator of sarX, thus implying an additional regulatory loop whereby mgrA can modulate agr expression. | (91) |
| MgrA | + | Regulates cell-wall turnover and activates the production of secreted toxins, proteases and is a regulator of autolysis. The effect of MgrA on autolysis may be mediated by SarV which is a positive regulator of several autolytic enzymes. | (92−94) |
| SarZ | + | A positive regulator of hla expression. A sarZ mutant of RN4220 had attenuated virulence in both silk worm and mouse infection models. | (18, 95) |
| SarV | – | A regulator of autolysis that is repressed by SarA and MgrA. A sarV mutant was found to be more resistant to detergent- or cell wall antibiotic-mediated lysis. | (18, 96) |
SarA was the first member identified as playing a pivotal role in the regulation of virulence genes in S. aureus. Transcriptional profiling revealed that SarA modulates either directly or indirectly at least 120 genes including up-regulation expression of extracellular proteins such as α- and β-hemolysins, TSST-1, staphylococcal enterotoxin B, and fibronectin binding protein.84 A number of studies have indicated that SarA regulates virulence via agr-dependent mechanisms, including binding to the agr-P3 promoter, and via agr-independent mechanisms including binding to the promoter regions of hla (α-hemolysin), tst (TSST-1), sec (enterotoxin C), and trxB (thioredoxin reductase).18,38,85,86
Inactivation of SarA affords strains displaying reduced virulence in several experimental staphylococcal infection models.38,40,97 Furthermore, SarA mutants display a reduced ability to induce septic arthritis and osteomyelitis in murine models of musculoskeletal infection.38−40 Although no small molecular SarA inhibitors have been reported, the current crystal structure of SarA and a number of mutagenesis studies have provided a platform for structure-based drug design methodologies.88 SarA is a typical “winged-helix” DNA binding protein with the helix–turn–helix and the winged regions proposed to interact with the major and minor grooves of target promoter DNA, respectively (Figure 13).88 Mutations of individual residues within the DNA-binding helix–turn–helix and the winged region, as well as within the metal-binding pocket, implicate basic residues Arg-84 and Arg-90 within the winged region to be critical in DNA binding, whereas acidic residues Asp-88 and Glu-89 (wing), Asp-8 and Glu-11 (metal-binding pocket), and Cys-9 are essential for SarA function.88 The presence of a sole free-cysteine within the metal-binding pocket is a ubiquitous feature of the SarA protein family, common to SarR, SarS, SarA, and MgrA.88,98−100 The cysteine residue is believed to function as an oxidation sensor or redox switch that regulates gene expression. Oxidation of the free-Cys leads to dissociation of the oxidized protein from DNA and thus inhibiting gene expression.100,101 Thus, as outlined in Figure 13, two approaches to inhibit the function of the SarA protein family exist: small molecules could be designed to bind (a) within the wing region to inhibit DNA–protein interactions or (b) within the Cys pocket, which would potentially mimic the oxidized or nonfunctional state.
Figure 13.
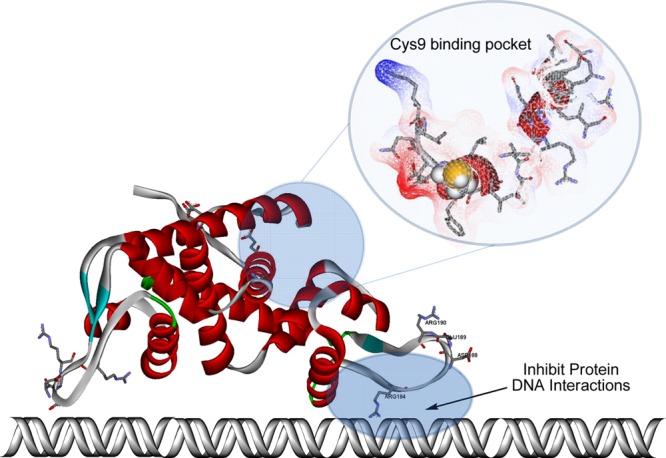
Crystal structure of the SarA protein (PDB accession code 2FRH)88 with residues of the Cys9 pocket (top right) and residues of the “wing” which are crucial for DNA binding (bottom right) highlighted. It is postulated that two approaches to inhibit the function of the SarA protein family exist: small molecules could be designed to bind (a) within the wing region to inhibit DNA–protein interactions or (b) within the Cys pocket which would potentially mimic oxidized or nonfunctional state.
The identification of a number of small molecule MgrA inhibitors clearly demonstrates that the SarA family of proteins is indeed a potential target for chemotherapeutic agents. MgrA positively affects the expression of capsular polysaccharide and nuclease while repressing expression of α-toxin, coagulase, and protein A.100 In addition to regulating virulence determinants, MgrA also represses the expression of several efflux systems such as NorA, NorB, NorC, and Tet38.6 Moreover, MgrA is known to play a critical role in S. aureus virulence, as an mgrA mutant strain exhibited 1000- to 10000-fold virulence reduction in a mouse model of infection.102 As outlined in Figure 15, the MgrA monomer possesses the typical winged helix structure consisting of eight α-helices and three β-strands and possesses the ubiquitous single cysteine redox switch (Cys-12) in the dimerization domain.101 Oxidation of this Cys residue leads to dissociation of the oxidized MgrA from the sarV promoter, thus repressing expression of a number of virulence factors.101 However, MgrA negatively regulates the expression of efflux pumps, including Tet38, NorA, NorB, and NorC, which account for bacterial resistance to multiple antibiotics, such as tetracycline, norfloxacin, and ciprofloxacin.103−106 MgrA is also shown to effect vancomycin resistance, as mutation of MgrA leads to increased resistance of the bacterium to these antibiotics.101 Thus, this could potentially limit the therapeutic potential of MgrA inhibitors in a clinical setting.
Figure 15.
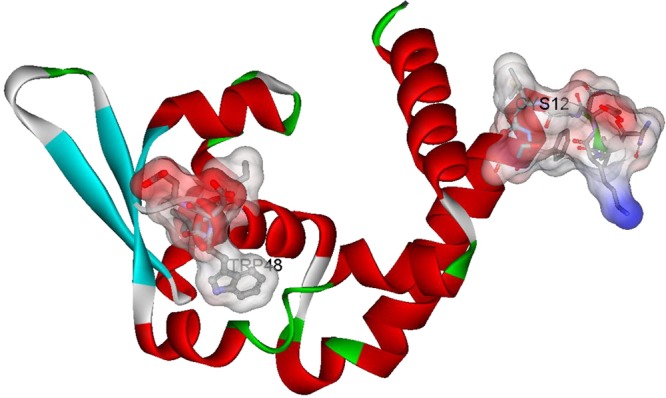
Crystal structure of a monomeric unit of MgrA (PBD accession code 2BV6)101 with residues of the Cys12 pocket (top right) and the Trp-48 pocket (middle left), in which MDSA (compound 43) is proposed to occupy, highlighted. It is postulated that three approaches to inhibit the function of the MgrA protein family exist: small molecules could be designed to bind (a) within the wing region to inhibit DNA–protein interactions or (b) within the Cys pocket which would potentially mimic oxidized or nonfunctional state or (c) the compound could be designed to occupy the Trp-48 pocket.
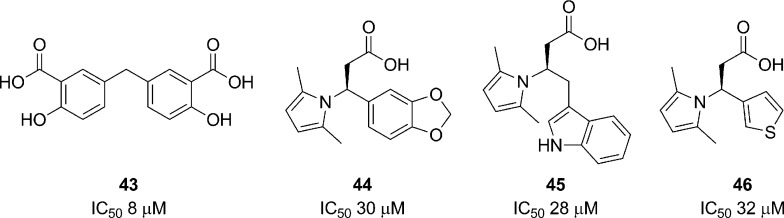
Nevertheless, a recent high-throughput screening program was conducted that identified a number of small molecule inhibitors of MgrA–DNA interactions, including 5,5-methylenedisalicylic acid (43, MDSA) and a series of 3-aryl-3-(2,5-dimethyl-1H-pyrrol-1-yl)propanoic acids 44–46 (Figure 14).100 MDSA was found to alter the transcriptional expression of a number of virulence factors including hla (α-hemolysin) and spa (protein A) while eliciting no negative impact on bacterial growth. Moreover, an esterified prodrug MDSA analogue was shown to attenuate virulence in a mouse model of infection. The mechanism by which MDSA inhibits MgrA–DNA interactions is currently unknown. However, preliminary computational docking experiments indicate that MDSA may bind around the DNA-binding lobe that is flanked by Trp-48 (Figure 15).100 Nevertheless, these compounds clearly demonstrate the therapeutic potential of virulence transcription factor inhibitors.
Figure 14.
Structure and IC50 values of 5,5-methylenedisalicylic acid (43) and the 3-aryl-3-(2,5-dimethyl-1H-pyrrol-1-yl)propanoic acids (44–46). The IC50 values are against MgrA and were obtained by a fluorescence anisotropy assay and validated by electrophoretic mobility shift assay.100
6. Conclusions and Future Directions
The rapid emergence of multiantibiotic resistant bacteria represents one of the greatest threats to human health worldwide.107 Among these superbugs, S. aureus presents one of the greatest threats with methicillin-resistant strains such as USA300, killing more Americans in 2007 (∼19 000) than emphysema, HIV/AIDS, Parkinson’s disease, and homicide combined.107 Against this backdrop the development of new classes of antibiotics is lagging. As a result, we are faced with an urgent need to better exploit the new targets that are emerging from our increased understanding of the molecular basis of bacterial pathogenicity if we are to develop novel prevention and treatment strategies. As outlined throughout this Perspective, there is an emerging body of evidence indicating that inhibiting the ability of S. aureus to produce exoproduct virulence determinants significantly attenuates infection. Consequently, developing therapies geared at “disarming” the bacterium is a promising approach for combating infections.9−13 Currently, the most advanced strategies include inhibitors of the SarA protein family and the agr-TCSTS. Given that the agr system is conserved across many different Gram positive pathogens, lessons derived from studying S. aureus will undoubtedly be applicable in the development of chemotherapeutics to treat other problematic Gram-positive pathogens, including Listeria monocytogenes and Enterococcus faecalis.108 In terms of developing clinically useful agents, much work remains. For example, most of the currently reported in vivo studies have involved coadministering virulence inhibiting compounds with bacteria, and thus, it is not clear whether such agents can effectively attenuate pre-existing infections. Further concerns relating to the propensity of agr inhibitors to stimulate biofilm formation must be investigated especially in relation to chronic S. aureus infections. This is because agr mutants form better biofilms in vitro and agr is required for biofilm dispersal.109 Since planktonic cells are more susceptible to conventional antibiotics, ironically, it has been suggested that combination therapy with AIPs could usefully be employed to disperse biofilms and restore antibiotic susceptibility.
Nevertheless the emerging palette of small molecule inhibitors of staphylococcal virulence gene expression will provide invaluable tools to further probe and manipulate virulence pathways while providing significant benefits over genetic techniques.110−112 The advantages of using small molecule inhibitors are numerous and include the following: (a) small molecules can be used in a conditional manner allowing for temporal control of a biological system; (b) small molecule perturbation of protein function is generally reversible which allows studies to be carried out on the reversibility of the system; (c) the biological effects of small molecules are generally rapid thus allowing characterization of intermediate/early responses in the system; (d) small molecule effects can be controlled by varying concentrations, thereby allowing the generation of dose–response data. Thus, compounds that are not druglike can be useful tools for temporal and dose–response studies of these systems which may not be possible by genetic manipulation.
Overall, the developments of small molecule inhibitors of staphylococcal virulence expression are still in an embryonic state. However, the proliferation of crystal structures of key virulence regulating proteins and hit compounds over the past 5 years undoubtedly means that medicinal chemists will now play an ever increasing role in developing virulence attenuation strategies. Given the current trajectory of research, it seems that there is much room for optimism that a virulence inhibiting therapeutic agent will be clinically available within the current half of the 21st century, thus providing a vital addition to our rapidly depleting antibiotic arsenal.
Acknowledgments
The work is supported by Program Grant G9219778 from the Medical Research Council, U.K., which is gratefully acknowledged.
Glossary
Abbreviations Used
- MRSA
methicillin resistant Staphylococcus aureus
- HA-MRSA
health care associated methicillin resistant S. aureus
- CA-MRSA
community-acquired methicillin resistant S. aureus
- TSST-1
toxic shock syndrome toxin-1
- PVL
Panton–Valentine leukocidin
- TCSTS
two-component signal transduction systems
- agr
accessory gene regulator
- AIP
autoinducing peptide
- CF
cystic fibrosis
- AHL
N-acyl l-homoserine lactone
Biographies
Christopher P. Gordon completed his Ph.D. studies in 2007, which focused on the design of HIV-1 integrase inhibitors, at the University of Wollongong, Australia, under the guidance Paul A. Keller. Christopher began his postdoctoral research career in Australia at the University of Newcastle under the direction of Adam McCluskey before undertaking a postdoctoral fellowship at the University of Nottingham, U.K., under the guidance of Weng Chan and Paul Williams with a primary focus of developing bacterial virulence suppressing agents. In 2012 Christopher retuned to the Newcastle as a University Fellow where he continues his research interests in developing bacterial virulence suppressing agents and antiviral agents, with a particular focus on HIV and HCV, and expanding the scope of emerging synthesis methodologies including flow reactors.
Paul Williams is Professor of Molecular Microbiology and Head of the School of Molecular Medical Sciences, University of Nottingham, U.K. He is internationally known for his research on quorum sensing and has (co)authored over 280 research and review articles, book chapters, and patents. His current research interests focus primarily on the regulation of gene expression through cell–cell communication (quorum sensing) in pathogenic bacteria with the aim of identifying new targets for novel anti-infective agents. For his quorum sensing research he was awarded the Royal Pharmaceutical Society of Great Britain Conference Science Medal (1992), the Pfizer prize in Pharmaceutical Sciences (1994), and the Society for General Microbiology Colworth Prize Lecture (2007).
Weng C. Chan is Associate Professor and Reader in Chemical Biology, University of Nottingham, U.K. He received his Ph.D. degree from the University of Nottingham in 1988, followed by postdoctoral training (1988–1992) under the guidance of Professors Barrie Bycroft and Gordon Roberts. He is a Fellow of the Royal Society of Chemistry, U.K. He has published extensively in the general area of solid-phase peptide chemistry, chemical biology, and antibacterial agents, with over 75 papers, book chapters, and patents. His research theme is focused on the development of unique chemical tools for the interrogation of Gram-positive quorum sensing systems, eukaryotic cell cycle, neurodegeneration, and stem cell differentiation.
Author Present Address
§ Chemistry Building, School of Environmental and Life Sciences, University of Newcastle, Callaghan NSW 2308, Australia.
The authors declare no competing financial interest.
References
- Waksman S. A. What is an antibiotic or an antibiotic substance?. Mycologia 1947, 39, 565–569. [PubMed] [Google Scholar]
- Chambers H. F.; DeLeo F. R. Waves of resistance: Staphylococcus aureus in the antibiotic era. Nat. Rev. Microbiol. 2009, 7, 629–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Leo F. R.; Chambers H. F. Reemergence of antibiotic-resistant Staphylococcus aureus in the genomics era. J. Clin. Invest. 2009, 119, 2464–2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klevens R. M.; Morrison M. A.; Nadle J.; Petit S.; Gershman K.; Ray S.; Harrison L. H.; Lynfield R.; Dumyati G.; Townes J. M.; Craig A. S.; Zell E. R.; Fosheim G. E.; McDougal L. K.; Carey R. B.; Fridkin S. K. Invasive methicillin-resistant Staphylococcus aureus infections in the United states. JAMA, J. Am. Med. Assoc. 2007, 298, 1763–1771. [DOI] [PubMed] [Google Scholar]
- Diep B. A.; Palazzolo-Ballance A. M.; Tattevin P.; Basuino L.; Braughton K. R.; Whitney A. R.; Chen L.; Kreiswirth B. N.; Otto M.; Deleo F. R.; Chambers H. F. Contribution of Panton–Valentine leukocidin in community-associated methicillin-resistant Staphylococcus aureus pathogenesis. PLoS One 2008, 3, e3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne D. J. Microbiology—desperately seeking new antibiotics. Science 2008, 321, 1644–1645. [DOI] [PubMed] [Google Scholar]
- Somerville G. A.; Proctor R. A. At the crossroads of bacterial metabolism and virulence factor synthesis in staphylococci. Microbiol. Mol. Biol. Rev. 2009, 73, 233–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cegelski L.; Marshall G. R.; Eldridge G. R.; Hultgren S. J. The biology and future prospects of antivirulence therapies. Nat. Rev. Microbiol. 2008, 6, 17–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams P. Quorum sensing: an emerging target for antibacterial chemotherapy?. Expert Opin. Ther. Targets 2002, 6, 257–274. [DOI] [PubMed] [Google Scholar]
- Rasmussen T. B.; Givskov M. Quorum-sensing inhibitors as anti-pathogenic drugs. Int. J. Med. Microbiol. 2006, 296, 149–161. [DOI] [PubMed] [Google Scholar]
- Costerton J. W.; Montanaro L.; Arciola C. R. Bacterial communications in implant infections: a target for an intelligence war. Int. J. Artif. Organs 2007, 30, 757–763. [DOI] [PubMed] [Google Scholar]
- Pan J.; Ren D. Quorum sensing inhibitors: a patent overview. Expert Opin. Ther. Pat. 2009, 19, 1581–1601. [DOI] [PubMed] [Google Scholar]
- Yin S.; Chang Y.; Deng S.; Wang Q.; Yu W.; Gong Q. Research progress of new antibacterial drugs that target bacterial quorum sensing systems. Yaoxue Xuebao 2011, 46, 613–621. [PubMed] [Google Scholar]
- Clatworthy A. E.; Pierson E.; Hung D. T. Targeting virulence: a new paradigm for antimicrobial therapy. Nat. Chem. Biol. 2007, 3, 541–548. [DOI] [PubMed] [Google Scholar]
- Defoirdt T.; Boon N.; Bossier P. Can bacteria evolve resistance to quorum sensing disruption?. PLoS Pathog. 2010, 6, e1000989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Withers H.; Swift S.; Williams P. Quorum sensing as an integral component of gene regulatory networks. Curr. Opin. Microbiol. 2001, 4, 186–193. [DOI] [PubMed] [Google Scholar]
- Bronner S.; Monteil H.; Prevost G. Regulation of virulence determinants in Staphylococcus aureus: complexity and applications. FEMS Microbiol. Rev. 2004, 28, 183–200. [DOI] [PubMed] [Google Scholar]
- Cheung A. L.; Bayer A. S.; Zhang G.; Gresham H.; Xiong Y.-Q. Regulation of virulence determinants in vitro and in vivo in Staphylococcus aureus. FEMS Immunol. Med. Microbiol. 2004, 40, 1–9. [DOI] [PubMed] [Google Scholar]
- Dinges M. M.; Orwin P. M.; Schlievert P. M. Exotoxins of Staphylococcus aureus. Clin. Microbiol. Rev. 2000, 13, 16–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto M. Staphylococcal biofilms. Curr. Top. Microbiol. Immunol. 2008, 322, 207–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun F.; Li C.; Jeong D.; Sohn C.; He C.; Bae T. In the Staphylococcus aureus two-component system sae, the response regulator SaeR binds to a direct repeat sequence and DNA binding requires phosphorylation by the sensor kinase SaeS. J. Bacteriol. 2010, 192, 2111–2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong D.-W.; Cho H.; Lee H.; Li C.; Garza J.; Fried M.; Bae T. Identification of the P3 promoter and distinct roles of the two promoters of the SaeRS two-component system in Staphylococcus aureus. J. Bacteriol. 2011, 193, 4672–4684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang X.; Zheng L.; Landwehr C.; Lunsford D.; Holmes D.; Ji Y. Global regulation of gene expression by ArlRS, a two-component signal transduction regulatory system of Staphylococcus aureus. J. Bacteriol. 2005, 187, 5486–5492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Memmi G.; Nair D. R.; Cheung A. Role of ArlRS in autolysis in methicillin-sensitive and methicillin-resistant Staphylococcus aureus strains. J. Bacteriol. 2012, 194, 759–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pragman A. A.; Yarwood J. M.; Tripp T. J.; Schlievert P. M. Characterization of virulence factor regulation by SrrAB, a two-component system in Staphylococcus aureus. J. Bacteriol. 2004, 186, 2430–2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue T.; You Y.; Hong D.; Sun H.; Sun B. The Staphylococcus aureus KdpDE two-component system couples extracellular K+ sensing and Agr signaling to infection programming. Infect. Immun. 2011, 79, 2154–2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L.; Xue T.; Shang F.; Sun H.; Sun B. Staphylococcus aureus AI-2 quorum sensing associates with the KdpDE two-component system to regulate capsular polysaccharide synthesis and virulence. Infect. Immun. 2010, 78, 3506–3515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto M. Staphylococcus aureus and Staphylococcus epidermidis peptide pheromones produced by the accessory gene regulator agr system. Peptides 2001, 22, 1603–1608. [DOI] [PubMed] [Google Scholar]
- Yarwood J. M.; Schlievert P. M. Quorum sensing in Staphylococcus infections. J. Clin. Invest. 2003, 112, 1620–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma-Kuinkel B. K.; Mann E. E.; Ahn J.-S.; Kuechenmeister L. J.; Dunman P. M.; Bayles K. W. The Staphylococcus aureus LytSR two-component regulatory system affects biofilm formation. J. Bacteriol. 2009, 191, 4767–4775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galbusera E.; Renzoni A.; Andrey D. O.; Monod A.; Barras C.; Tortora P.; Polissi A.; Kelley W. L. Site-specific mutation of Staphylococcus aureus VraS reveals a crucial role for the VraR-VraS sensor in the emergence of glycopeptide resistance. Antimicrob. Agents Chemother. 2011, 55, 1008–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renzoni A.; Andrey D. O.; Jousselin A.; Barras C.; Monod A.; Vaudaux P.; Lew D.; Kelley W. L. Whole genome sequencing and complete genetic analysis reveals novel pathways to glycopeptide resistance in Staphylococcus aureus. PLoS One 2011, 6, e21577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus D.; Herbert S.; Kristian S. A.; Khosravi A.; Nizet V.; Goetz F.; Peschel A. The GraRS regulatory system controls Staphylococcus aureus susceptibility to antimicrobial host defenses. BMC Microbiol. 2008, 8, 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogasch K.; Ruehmling V.; Pane-Farre J.; Hoeper D.; Weinberg C.; Fuchs S.; Schmudde M.; Broeker B. M.; Wolz C.; Hecker M.; Engelmann S. Influence of the two-component system SaeRS on global gene expression in two different Staphylococcus aureus strains. J. Bacteriol. 2006, 188, 7742–7758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pragman A. A.; Herron-Olson L.; Case L. C.; Vetter S. M.; Henke E. E.; Kapur V.; Schlievert P. M. Sequence analysis of the Staphylococcus aureus srrAB loci reveals that truncation of srrA affects growth and virulence factor expression. J. Bacteriol. 2007, 189, 7515–7519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwan W. R.; Langhorne M. H.; Ritchie H. D.; Stover C. K. Loss of hemolysin expression in Staphylococcus aureus agr mutants correlates with selective survival during mixed infections in murine abscesses and wounds. FEMS Immunol. Med. Microbiol. 2003, 38, 23–28. [DOI] [PubMed] [Google Scholar]
- Wright J. S. III; Jin R.; Novick R. P. Transient interference with staphylococcal quorum sensing blocks abscess formation. Proc. Natl. Acad. Sci. U.S.A. 2005, 102, 1691–1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blevins J. S.; Elasri M. O.; Allmendinger S. D.; Beenken K. E.; Skinner R. A.; Thomas J. R.; Smeltzer M. S. Role of sarA in the pathogenesis of Staphylococcus aureus musculoskeletal infection. Infect. Immun. 2003, 71, 516–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdelnour A.; Arvidson S.; Bremell T.; Ryden C.; Tarkowski A. The accessory gene regulator (agr) controls Staphylococcus aureus virulence in a murine arthritis model. Infect. Immun. 1993, 61, 3879–3885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson I.-M.; Bremell T.; Ryden C.; Cheung A. L.; Tarkowski A. Role of the staphylococcal accessory gene regulator (sar) in septic arthritis. Infect. Immun. 1996, 64, 4438–4443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoendel M.; Horswill A. R. Biosynthesis of peptide signals in Gram-positive bacteria. Adv. Appl. Microbiol. 2010, 71, 91–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan W. C.; Coyle B. J.; Williams P. Virulence regulation and quorum sensing in staphylococcal infections: competitive AgrC antagonists as quorum sensing inhibitors. J. Med. Chem. 2004, 47, 4633–4641. [DOI] [PubMed] [Google Scholar]
- Novick R. P.; Geisinger E. Quorum sensing in staphylococci. Annu. Rev. Genet. 2008, 42, 541–564. [DOI] [PubMed] [Google Scholar]
- Cheung A. L.; Nishina K. A.; Trotonda M. P.; Tamber S. The SarA protein family of Staphylococcus aureus. Int. J. Biochem. Cell Biol. 2008, 40, 355–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes D.; Andrey D. O.; Monod A.; Kelley W. L.; Zhang G.; Cheung A. L. Coordinated regulation by AgrA, SarA, and SarR to control agr expression in Staphylococcus aureus. J. Bacteriol. 2011, 193, 6020–6031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroda M.; Kuroda H.; Oshima T.; Takeuchi F.; Mori H.; Hiramatsu K. Two-component system VraSR positively modulates the regulation of cell-wall biosynthesis pathway in Staphylococcus aureus. Mol. Microbiol. 2003, 49, 807–821. [DOI] [PubMed] [Google Scholar]
- Lyon G. J.; Wright J. S.; Muir T. W.; Novick R. P. Key determinants of receptor activation in the agr autoinducing peptides of Staphylococcus aureus. Biochemistry 2002, 41, 10095–10104. [DOI] [PubMed] [Google Scholar]
- Thoendel M.; Horswill A. R. Identification of Staphylococcus aureus AgrD residues required for autoinducing peptide biosynthesis. J. Biol. Chem. 2009, 284, 21828–21838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Queck S. Y.; Jameson-Lee M.; Villaruz A. E.; Bach T. H. L.; Khan B. A.; Sturdevant D. E.; Ricklefs S. M.; Li M.; Otto M. RNAIII-independent target gene control by the agr quorum-sensing system: insight into the evolution of virulence regulation in Staphylococcus aureus. Mol. Cell 2008, 32, 150–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayville P.; Ji G.; Beavis R.; Yang H.; Goger M.; Novick R. P.; Muir T. W. Structure–activity analysis of synthetic autoinducing thiolactone peptides from Staphylococcus aureus responsible for virulence. Proc. Natl. Acad. Sci. U.S.A. 1999, 96, 1218–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J.; Jagasia R.; Kaufmann G. F.; Mathison J. C.; Ruiz D. I.; Moss J. A.; Meijler M. M.; Ulevitch R. J.; Janda K. D. Infection control by antibody disruption of bacterial quorum sensing signaling. Chem. Biol. 2007, 14, 1119–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchdoerfer R. N.; Garner A. L.; Flack C. E.; Mee J. M.; Horswill A. R.; Janda K. D.; Kaufmann G. F.; Wilson I. A. Structural basis for ligand recognition and discrimination of a quorum-quenching antibody. J. Biol. Chem. 2011, 286, 17351–17358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George E. A.; Novick R. P.; Muir T. W. Cyclic peptide inhibitors of staphylococcal virulence prepared by Fmoc-based thiolactone peptide synthesis. J. Am. Chem. Soc. 2008, 130, 4914–4924. [DOI] [PubMed] [Google Scholar]
- Lyon G. J.; Mayville P.; Muir T. W.; Novick R. P. Rational design of a global inhibitor of the virulence response in Staphylococcus aureus, based in part on localization of the site of inhibition to the receptor-histidine kinase, AgrC. Proc. Natl. Acad. Sci. U.S.A. 2000, 97, 13330–13335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyon G. J.; Wright J. S.; Christopoulos A.; Novick R. P.; Muir T. W. Reversible and specific extracellular antagonism of receptor-histidine kinase signaling. J. Biol. Chem. 2002, 277, 6247–6253. [DOI] [PubMed] [Google Scholar]
- McDowell P.; Affas Z.; Reynolds C.; Holden M. T. G.; Wood S. J.; Saint S.; Cockayne A.; Hill P. J.; Dodd C. E. R.; Bycroft B. W.; Chan W. C.; Williams P. Structure, activity and evolution of the group I thiolactone peptide quorum-sensing system of Staphylococcus aureus. Mol. Microbiol. 2001, 41, 503–512. [DOI] [PubMed] [Google Scholar]
- Scott R. J.; Lian L.-Y.; Muharram S. H.; Cockayne A.; Wood S. J.; Bycroft B. W.; Williams P.; Chan W. C. Side-chain-to-tail thiolactone peptide inhibitors of the staphylococcal quorum-sensing system. Bioorg. Med. Chem. Lett. 2003, 13, 2449–2453. [DOI] [PubMed] [Google Scholar]
- Ji G.; Beavis R.; Novick R. P. Bacterial interference caused by autoinducing peptide variants. Science 1997, 276, 2027–2030. [DOI] [PubMed] [Google Scholar]
- Jensen R. O.; Winzer K.; Clarke S. R.; Chan W. C.; Williams P. Differential recognition of Staphylococcus aureus quorum-sensing signals depends on both extracellular loops 1 and 2 of the transmembrane sensor AgrC. J. Mol. Biol. 2008, 381, 300–309. [DOI] [PubMed] [Google Scholar]
- Fowler S. A.; Stacy D. M.; Blackwell H. E. Design and synthesis of macrocyclic peptomers as mimics of a quorum sensing signal from Staphylococcus aureus. Org. Lett. 2008, 10, 2329–2332. [DOI] [PubMed] [Google Scholar]
- Mansson M.; Nielsen A.; Kjaerulff L.; Gotfredsen C. H.; Wietz M.; Ingmer H.; Gram L.; Larsen T. O. Inhibition of virulence gene expression in Staphylococcus aureus by novel depsipeptides from a marine Photobacterium. Mar. Drugs 2011, 9, 2537–2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balaban N.; Novick R. P. Translation of RNAIII, the Staphylococcus aureus agr regulatory RNA molecule, can be activated by a 3′-end deletion. FEMS Microbiol. Lett. 1995, 133, 155–161. [DOI] [PubMed] [Google Scholar]
- Balaban N.; Goldkorn T.; Gov Y.; Hirshberg M.; Koyfman N.; Matthews H. R.; Nhan R. T.; Singh B.; Uziel O. Regulation of Staphylococcus aureus pathogenesis via target of RNAIII-activating protein (TRAP). J. Biol. Chem. 2001, 276, 2658–2667. [DOI] [PubMed] [Google Scholar]
- Balaban N.; Goldkorn T.; Nhan R. T.; Dang L. B.; Scott S.; Ridgley R. M.; Rasooly A.; Wright S. C.; Larrick J. W.; Rasooly R.; Carlson J. R. Autoinducer of virulence as a target for vaccine and therapy against Staphylococcus aureus. Science 1998, 280, 438–440. [DOI] [PubMed] [Google Scholar]
- Korem M.; Gov Y.; Kiran M. D.; Balaban N. Transcriptional profiling of target of RNAIII-activating protein, a master regulator of staphylococcal virulence. Infect. Immun. 2005, 73, 6220–6228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korem M.; Sheoran A. S.; Gov Y.; Tzipori S.; Borovok I.; Balaban N. Characterization of RAP, a quorum sensing activator of Staphylococcus aureus. FEMS Microbiol. Lett. 2003, 223, 167–175. [DOI] [PubMed] [Google Scholar]
- Gov Y.; Bitler A.; Dell’Acqua G.; Torres J. V.; Balaban N. RNAIII inhibiting peptide (RIP), a global inhibitor of Staphylococcus aureus pathogenesis: structure and function analysis. Peptides 2001, 22, 1609–1620. [DOI] [PubMed] [Google Scholar]
- Adhikari R. P.; Arvidson S.; Novick R. P. A nonsense mutation in agrA accounts for the defect in agr expression and the avirulence of Staphylococcus aureus 8325-4 traP::kan. Infect. Immun. 2007, 75, 4534–4540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw L. N.; Jonsson I.-M.; Singh V. K.; Tarkowski A.; Stewart G. C. Inactivation of traP has no effect on the Agr quorum-sensing system or virulence of Staphylococcus aureus. Infect. Immun. 2007, 75, 4519–4527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang L. H.; Daily S. T.; Weiss E. C.; Smeltzer M. S. Mutation of traP in Staphylococcus aureus has no impact on expression of agr or biofilm formation. Infect. Immun. 2007, 75, 4528–4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick R. P.; Ross H. F.; Figueiredo A. M. S.; Abramochkin G.; Muir T. Activation and inhibition of the staphylococcal Agr system. Science 2000, 287, 391a. [Google Scholar]
- Kiran M. D.; Adikesavan N. V.; Cirioni O.; Giacometti A.; Silvestri C.; Scalise G.; Ghiselli R.; Saba V.; Orlando F.; Shoham M.; Balaban N. Discovery of a quorum-sensing inhibitor of drug-resistant staphylococcal infections by structure-based virtual screening. Mol. Pharmacol. 2008, 73, 1578–1586. [DOI] [PubMed] [Google Scholar]
- Sidote D. J.; Barbieri C. M.; Wu T.; Stock A. M. Structure of the Staphylococcus aureus AgrA LytTR domain bound to DNA reveals a beta fold with an unusual mode of binding. Structure 2008, 16, 727–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sklar L. A.; Gresham H. D.. A Small Molecule That Targets a Signaling Pathway in AIP-Dependent Bacterial Quorum Sensing of S. aureus Agr3 agr Locus Genotype. Probe Report C094-0010, Grant Number NIH 1 X01 MH078952-01; 2011; https://mli.nih.gov/mli/?dl_id=700. [Google Scholar]
- Sklar L. A.; Gresham H. D.. Small Molecule That Targets AIP Binding Interactions in AIP-Dependent Bacterial Quorum Sensing. Probe Report, Grant Number NIH 1 X01 MH078952-01; 2012; https://mli.nih.gov/mli/?dl_id=701. [Google Scholar]
- Sully E.Small Molecule Inhibitor of Staphylococcus aureus Virulence. Ph.D. Dissertation, The University of New Mexico, Albuquerque, NM, 2011; http://hdl.handle.net/1928/13173.
- Ivashchenko A. A.; Ivashchenko A. V.; Savchuk N. F.. Substituted 3-Sulphonyl-[1,2,3]triazolo[1,5-a]pyrimidines—Antagonists of Serotonin 5-HT6 Receptors and Methods for the Production Thereof. PCT Int. Appl. WO2009093934A2, 2009.
- Nakayama J.; Uemura Y.; Nishiguchi K.; Yoshimura N.; Igarashi Y.; Sonomoto K. Ambuic acid inhibits the biosynthesis of cyclic peptide quormones in Gram-positive bacteria. Antimicrob. Agents Chemother. 2009, 53, 580–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman L. R.; Deziel E.; D’Argenio D. A.; Lepine F.; Emerson J.; McNamara S.; Gibson R. L.; Ramsey B. W.; Miller S. I. Selection for Staphylococcus aureus small-colony variants due to growth in the presence of Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. U.S.A. 2006, 103, 19890–19895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qazi S.; Middleton B.; Muharram S. H.; Cockayne A.; Hill P.; O’Shea P.; Chhabra S. R.; Camara M.; Williams P. N-Acylhomoserine lactones antagonize virulence gene expression and quorum sensing in Staphylococcus aureus. Infect. Immun. 2006, 74, 910–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid G.; Beuerman D.; Heinemann C.; Bruce A. W. Probiotic Lactobacillus dose required to restore and maintain a normal vaginal flora. FEMS Immunol. Med. Microbiol. 2001, 32, 37–41. [DOI] [PubMed] [Google Scholar]
- Schlievert P. M.; Blomster D. A. Production of staphylococcal pyrogenic exotoxin type C: influence of physical and chemical factors. J. Infect. Dis. 1983, 147, 236–242. [DOI] [PubMed] [Google Scholar]
- Li J.; Wang W.; Xu S. X.; Magarvey N. A.; McCormick J. K. Lactobacillus reuteri-produced cyclic dipeptides quench agr-mediated expression of toxic shock syndrome toxin-1 in staphylococci. Proc. Natl. Acad. Sci. U.S.A. 2011, 108, 3360–3365, S3360/1–S3360/9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunman P. M.; Murphy E.; Haney S.; Palacios D.; Tucker-Kellogg G.; Wu S.; Brown E. L.; Zagursky R. J.; Shlaes D.; Projan S. J. Transcription profiling-based identification of Staphylococcus aureus genes regulated by the agr and/or sarA loci. J. Bacteriol. 2001, 183, 7341–7353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballal A.; Manna A. C. Control of thioredoxin reductase gene (trxB) transcription by SarA in Staphylococcus aureus. J. Bacteriol. 2010, 192, 336–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tormo M. A.; Marti M.; Valle J.; Manna A. C.; Cheung A. L.; Lasa I.; Penades J. R. SarA is an essential positive regulator of Staphylococcus epidermidis biofilm development. J. Bacteriol. 2005, 187, 2348–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung A. L.; Bayer M. G.; Heinrichs J. H. sar genetic determinants necessary for transcription of RNAII and RNAIII in the agr locus of Staphylococcus aureus. J. Bacteriol. 1997, 179, 3963–3971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y.; Manna A. C.; Pan C.-H.; Kriksunov I. A.; Thiel D. J.; Cheung A. L.; Zhang G. Structural and function analyses of the global regulatory protein SarA from Staphylococcus aureus. Proc. Natl. Acad. Sci. U.S.A. 2006, 103, 2392–2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegmark K.; Karlsson A.; Arvidson S. Identification and characterization of SarH1, a new global regulator of virulence gene expression in Staphylococcus aureus. Mol. Microbiol. 2000, 37, 398–409. [DOI] [PubMed] [Google Scholar]
- Said-Salim B.; Dunman P. M.; McAleese F. M.; Macapagal D.; Murphy E.; McNamara P. J.; Arvidson S.; Foster T. J.; Projan S. J.; Kreiswirth B. N. Global regulation of Staphylococcus aureus genes by Rot. J. Bacteriol. 2003, 185, 610–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manna A. C.; Cheung A. L. Expression of SarX, a negative regulator of agr and exoprotein synthesis, is activated by MgrA in Staphylococcus aureus. J. Bacteriol. 2006, 188, 4288–4299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingavale S. S.; Van Wame W.; Cheung A. L. Characterization of Rat, an autolysis regulator in Staphylococcus aureus. Mol. Microbiol. 2003, 48, 1451–1466. [DOI] [PubMed] [Google Scholar]
- Luong T. T.; Newell S. W.; Lee C. Y. Mgr, a novel global regulator in Staphylococcus aureus. J. Bacteriol. 2003, 185, 3703–3710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truong-Bolduc Q. C.; Zhang X.; Hooper D. C. Characterization of NorR protein, a multifunctional regulator of norA expression in Staphylococcus aureus. J. Bacteriol. 2003, 185, 3127–3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaito C.; Morishita D.; Matsumoto Y.; Kurokawa K.; Sekimizu K. Novel DNA binding protein SarZ contributes to virulence in Staphylococcus aureus. Mol. Microbiol. 2006, 62, 1601–1617. [DOI] [PubMed] [Google Scholar]
- Manna A. C.; Ingavale S. S.; Maloney M.; van Wamel W.; Cheung A. L. Identification of sarV (SA2062), a new transcriptional regulator, is repressed by SarA and MgrA (SA0641) and involved in the regulation of autolysis in Staphylococcus aureus. J. Bacteriol. 2004, 186, 5267–5280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung A. L.; Yeaman M. R.; Sullam P. M.; Witt M. D.; Bayer A. S. Role of the sar locus of Staphylococcus aureus in induction of endocarditis in rabbits. Infect. Immun. 1994, 62, 1719–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R.; Manna A. C.; Dai S.; Cheung A. L.; Zhang G. Crystal structure of the SarS protein from Staphylococcus aureus. J. Bacteriol. 2003, 185, 4219–4225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y.; Manna A.; Li R.; Martin W. E.; Murphy R. C.; Cheung A. L.; Zhang G. Crystal structure of the SarR protein from Staphylococcus aureus. Proc. Natl. Acad. Sci. U.S.A. 2001, 98, 6877–6882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun F.; Zhou L.; Zhao B.-C.; Deng X.; Cho H.; Yi C.; Jian X.; Song C.-X.; Luan C.-H.; Bae T.; Li Z.; He C. Targeting MgrA-mediated virulence regulation in Staphylococcus aureus. Chem. Biol. 2011, 18, 1032–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P. R.; Bae T.; Williams W. A.; Duguid E. M.; Rice P. A.; Schneewind O.; He C. An oxidation-sensing mechanism is used by the global regulator MgrA in Staphylococcus aureus. Nat. Chem. Biol. 2006, 2, 591–595. [DOI] [PubMed] [Google Scholar]
- Bae T.; Banger A. K.; Wallace A.; Glass E. M.; Aslund F.; Schneewind O.; Missiakas D. M. Staphylococcus aureus virulence genes identified by bursa aurealis mutagenesis and nematode killing. Proc. Natl. Acad. Sci. U.S.A. 2004, 101, 12312–12317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui L.; Lian J.-Q.; Neoh H.-m.; Reyes E.; Hiramatsu K. DNA microarray-based identification of genes associated with glycopeptide resistance in Staphylococcus aureus. Antimicrob. Agents Chemother. 2005, 49, 3404–3413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaatz G. W.; Thyagarajan R. V.; Seo S. M. Effect of promoter region mutations and mgrA overexpression on transcription of norA, which encodes a Staphylococcus aureus multidrug efflux transporter. Antimicrob. Agents Chemother. 2005, 49, 161–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luong T. T.; Dunman P. M.; Murphy E.; Projan S. J.; Lee C. Y. Transcription profiling of the mgrA regulon in Staphylococcus aureus. J. Bacteriol. 2006, 188, 1899–1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truong-Bolduc Q. C.; Dunman P. M.; Strahilevitz J.; Projan S. J.; Hooper D. C. MgrA is a multiple regulator of two new efflux pumps in Staphylococcus aureus. J. Bacteriol. 2005, 187, 2395–2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spellberg B.; Blaser M.; Guidos R. J.; Boucher H. W.; Bradley J. S.; Eisenstein B. I.; Gerding D.; Lynfield R.; Reller L. B.; Rex J.; Schwartz D.; Septimus E.; Tenover F. C.; Gilbert D. N. Combating antimicrobial resistance: policy recommendations to save lives. Clin. Infect. Dis. 2011, 52(Suppl. 5), S397–S428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufour P.; Jarraud S.; Vandenesch F.; Greenland T.; Novick R. P.; Bes M.; Etienne J.; Lina G. High genetic variability of the agr locus in Staphylococcus species. J. Bacteriol. 2002, 184, 1180–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiedrowski M. R.; Horswill A. R. New approaches for treating staphylococcal biofilm infections. Ann. N.Y. Acad. Sci. 2011, 1241, 104–121. [DOI] [PubMed] [Google Scholar]
- Galloway W. R. J. D.; Hodgkinson J. T.; Bowden S. D.; Welch M.; Spring D. R. Quorum sensing in Gram-negative bacteria: small molecule modulation of AHL and AI-2 quorum sensing pathways. Chem. Rev. 2011, 111, 28–67. [DOI] [PubMed] [Google Scholar]
- Geske G. D.; O’Neill J. C.; Blackwell H. E. Expanding dialogues: from natural autoinducers to non-natural analogs that modulate quorum sensing in Gram-negative bacteria. Chem. Soc. Rev. 2008, 37, 1432–1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spring D. R. Chemical genetics to chemical genomics: small molecules offer big insights. Chem. Soc. Rev. 2005, 34, 472–482. [DOI] [PubMed] [Google Scholar]