Abstract
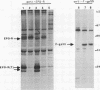
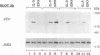
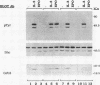
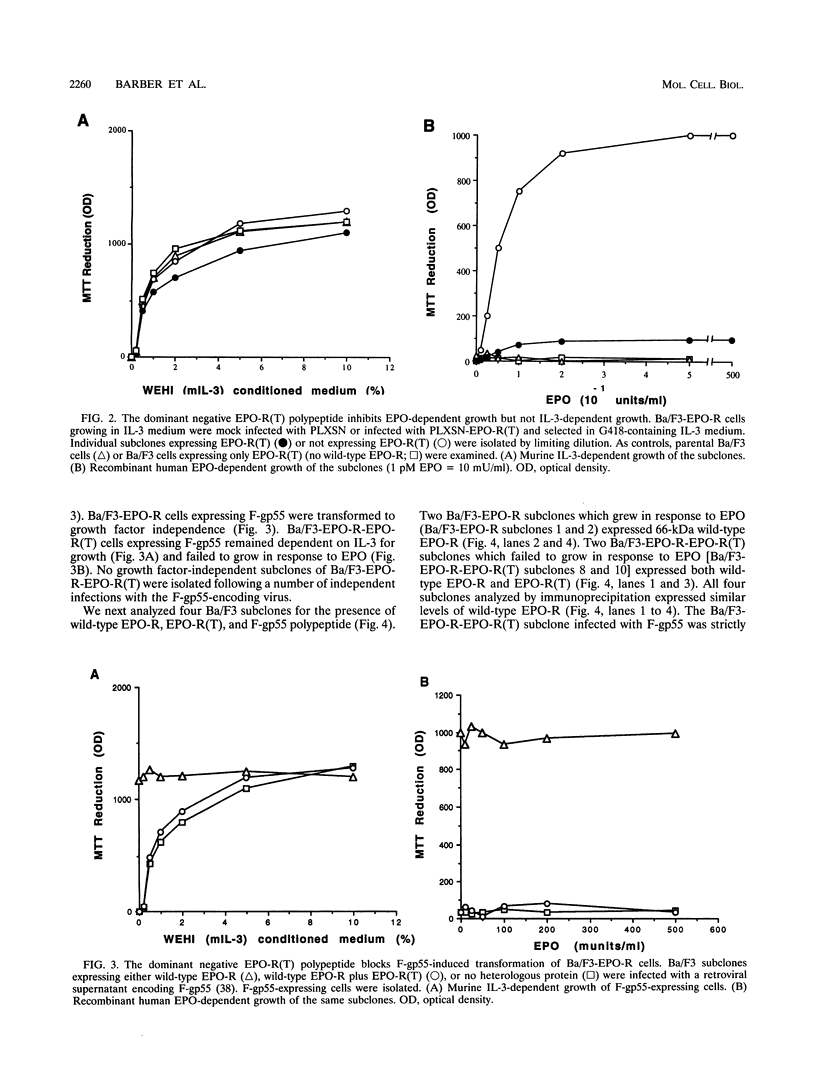
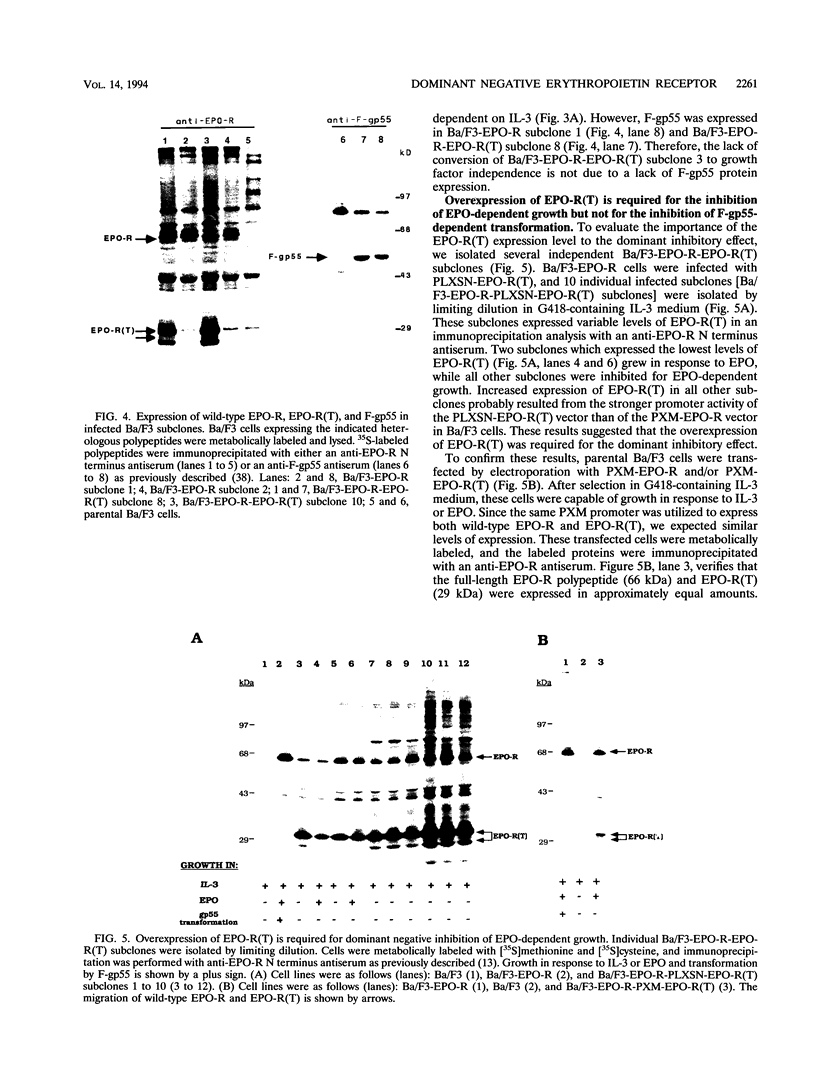
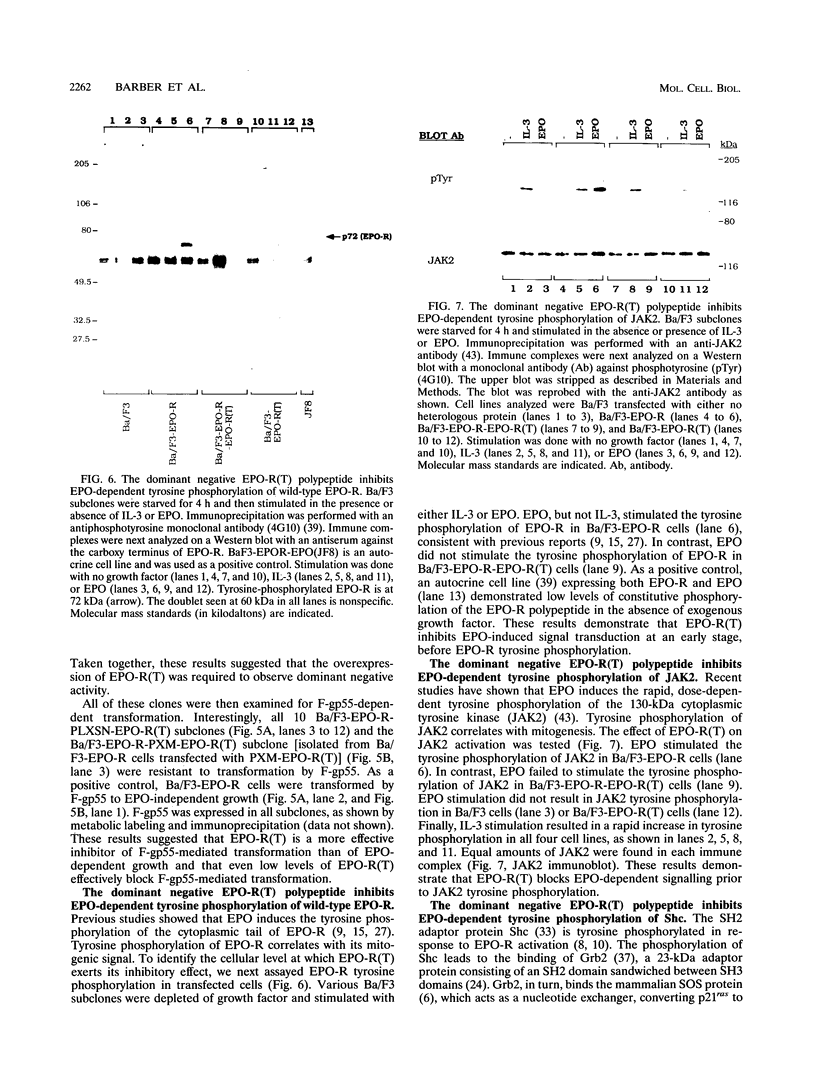
The erythropoietin receptor (EPO-R), a member of the cytokine receptor superfamily, can be activated to signal cell growth by binding either EPO or F-gp55, the Friend spleen focus-forming virus glycoprotein. Activation by F-gp55 results in constitutive EPO-R signalling and the first stage of Friend virus-induced erythroleukemia. We have generated a truncated form of the EPO-R polypeptide [EPO-R(T)] which lacks the critical cytoplasmic signal-transducing domain of the EPO-R required for EPO- or F-gp55-induced cell growth. EPO-R(T) specifically inhibited the EPO-dependent growth of EPO-R-expressing Ba/F3 cells without changing the interleukin-3-dependent growth of these cells. In addition, Ba/F3 cells that coexpressed wild-type EPO-R and EPO-R(T) were resistant to transformation by F-gp55 despite efficient expression of the F-gp55 transforming oncoprotein in infected cells. EPO-R(T) inhibited the EPO-dependent tyrosine phosphorylation of wild-type EPO-R, the tyrosine kinase (JAK2), and the SH2 adaptor protein (Shc). In conclusion, the EPO-R(T) polypeptide is a dominant negative polypeptide which specifically interferes with the early stages of EPO-R-mediated signal transduction and which prevents Friend virus transformation of erythroblasts.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amaya E., Musci T. J., Kirschner M. W. Expression of a dominant negative mutant of the FGF receptor disrupts mesoderm formation in Xenopus embryos. Cell. 1991 Jul 26;66(2):257–270. doi: 10.1016/0092-8674(91)90616-7. [DOI] [PubMed] [Google Scholar]
- Ben-David Y., Bernstein A. Friend virus-induced erythroleukemia and the multistage nature of cancer. Cell. 1991 Sep 6;66(5):831–834. doi: 10.1016/0092-8674(91)90428-2. [DOI] [PubMed] [Google Scholar]
- Brakebusch C., Nophar Y., Kemper O., Engelmann H., Wallach D. Cytoplasmic truncation of the p55 tumour necrosis factor (TNF) receptor abolishes signalling, but not induced shedding of the receptor. EMBO J. 1992 Mar;11(3):943–950. doi: 10.1002/j.1460-2075.1992.tb05133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll M. P., Spivak J. L., McMahon M., Weich N., Rapp U. R., May W. S. Erythropoietin induces Raf-1 activation and Raf-1 is required for erythropoietin-mediated proliferation. J Biol Chem. 1991 Aug 15;266(23):14964–14969. [PubMed] [Google Scholar]
- Casadevall N., Lacombe C., Muller O., Gisselbrecht S., Mayeux P. Multimeric structure of the membrane erythropoietin receptor of murine erythroleukemia cells (Friend cells). Cross-linking of erythropoietin with the spleen focus-forming virus envelope protein. J Biol Chem. 1991 Aug 25;266(24):16015–16020. [PubMed] [Google Scholar]
- Chardin P., Camonis J. H., Gale N. W., van Aelst L., Schlessinger J., Wigler M. H., Bar-Sagi D. Human Sos1: a guanine nucleotide exchange factor for Ras that binds to GRB2. Science. 1993 May 28;260(5112):1338–1343. doi: 10.1126/science.8493579. [DOI] [PubMed] [Google Scholar]
- Cunningham B. C., Ultsch M., De Vos A. M., Mulkerrin M. G., Clauser K. R., Wells J. A. Dimerization of the extracellular domain of the human growth hormone receptor by a single hormone molecule. Science. 1991 Nov 8;254(5033):821–825. doi: 10.1126/science.1948064. [DOI] [PubMed] [Google Scholar]
- Cutler R. L., Liu L., Damen J. E., Krystal G. Multiple cytokines induce the tyrosine phosphorylation of Shc and its association with Grb2 in hemopoietic cells. J Biol Chem. 1993 Oct 15;268(29):21463–21465. [PubMed] [Google Scholar]
- D'Andrea A. D., Lodish H. F., Wong G. G. Expression cloning of the murine erythropoietin receptor. Cell. 1989 Apr 21;57(2):277–285. doi: 10.1016/0092-8674(89)90965-3. [DOI] [PubMed] [Google Scholar]
- D'Andrea A. D., Yoshimura A., Youssoufian H., Zon L. I., Koo J. W., Lodish H. F. The cytoplasmic region of the erythropoietin receptor contains nonoverlapping positive and negative growth-regulatory domains. Mol Cell Biol. 1991 Apr;11(4):1980–1987. doi: 10.1128/mcb.11.4.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damen J. E., Liu L., Cutler R. L., Krystal G. Erythropoietin stimulates the tyrosine phosphorylation of Shc and its association with Grb2 and a 145-Kd tyrosine phosphorylated protein. Blood. 1993 Oct 15;82(8):2296–2303. [PubMed] [Google Scholar]
- Damen J. E., Mui A. L., Puil L., Pawson T., Krystal G. Phosphatidylinositol 3-kinase associates, via its Src homology 2 domains, with the activated erythropoietin receptor. Blood. 1993 Jun 15;81(12):3204–3210. [PubMed] [Google Scholar]
- Damen J., Mui A. L., Hughes P., Humphries K., Krystal G. Erythropoietin-induced tyrosine phosphorylations in a high erythropoietin receptor-expressing lymphoid cell line. Blood. 1992 Oct 15;80(8):1923–1932. [PubMed] [Google Scholar]
- Dusanter-Fourt I., Casadevall N., Lacombe C., Muller O., Billat C., Fischer S., Mayeux P. Erythropoietin induces the tyrosine phosphorylation of its own receptor in human erythropoietin-responsive cells. J Biol Chem. 1992 May 25;267(15):10670–10675. [PubMed] [Google Scholar]
- Ferro F. E., Jr, Kozak S. L., Hoatlin M. E., Kabat D. Cell surface site for mitogenic interaction of erythropoietin receptors with the membrane glycoprotein encoded by Friend erythroleukemia virus. J Biol Chem. 1993 Mar 15;268(8):5741–5747. [PubMed] [Google Scholar]
- Fukunaga R., Ishizaka-Ikeda E., Pan C. X., Seto Y., Nagata S. Functional domains of the granulocyte colony-stimulating factor receptor. EMBO J. 1991 Oct;10(10):2855–2865. doi: 10.1002/j.1460-2075.1991.tb07835.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanazono Y., Chiba S., Sasaki K., Mano H., Yazaki Y., Hirai H. Erythropoietin induces tyrosine phosphorylation and kinase activity of the c-fps/fes proto-oncogene product in human erythropoietin-responsive cells. Blood. 1993 Jun 15;81(12):3193–3196. [PubMed] [Google Scholar]
- Hatakeyama M., Mori H., Doi T., Taniguchi T. A restricted cytoplasmic region of IL-2 receptor beta chain is essential for growth signal transduction but not for ligand binding and internalization. Cell. 1989 Dec 1;59(5):837–845. doi: 10.1016/0092-8674(89)90607-7. [DOI] [PubMed] [Google Scholar]
- He T. C., Zhuang H., Jiang N., Waterfield M. D., Wojchowski D. M. Association of the p85 regulatory subunit of phosphatidylinositol 3-kinase with an essential erythropoietin receptor subdomain. Blood. 1993 Dec 15;82(12):3530–3538. [PubMed] [Google Scholar]
- Hoatlin M. E., Kozak S. L., Lilly F., Chakraborti A., Kozak C. A., Kabat D. Activation of erythropoietin receptors by Friend viral gp55 and by erythropoietin and down-modulation by the murine Fv-2r resistance gene. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9985–9989. doi: 10.1073/pnas.87.24.9985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashles O., Yarden Y., Fischer R., Ullrich A., Schlessinger J. A dominant negative mutation suppresses the function of normal epidermal growth factor receptors by heterodimerization. Mol Cell Biol. 1991 Mar;11(3):1454–1463. doi: 10.1128/mcb.11.3.1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J. P., D'Andrea A. D., Lodish H. F., Baltimore D. Activation of cell growth by binding of Friend spleen focus-forming virus gp55 glycoprotein to the erythropoietin receptor. Nature. 1990 Feb 22;343(6260):762–764. doi: 10.1038/343762a0. [DOI] [PubMed] [Google Scholar]
- Lowenstein E. J., Daly R. J., Batzer A. G., Li W., Margolis B., Lammers R., Ullrich A., Skolnik E. Y., Bar-Sagi D., Schlessinger J. The SH2 and SH3 domain-containing protein GRB2 links receptor tyrosine kinases to ras signaling. Cell. 1992 Aug 7;70(3):431–442. doi: 10.1016/0092-8674(92)90167-b. [DOI] [PubMed] [Google Scholar]
- Mayeux P., Dusanter-Fourt I., Muller O., Mauduit P., Sabbah M., Druker B., Vainchenker W., Fischer S., Lacombe C., Gisselbrecht S. Erythropoietin induces the association of phosphatidylinositol 3'-kinase with a tyrosine-phosphorylated protein complex containing the erythropoietin receptor. Eur J Biochem. 1993 Sep 15;216(3):821–828. doi: 10.1111/j.1432-1033.1993.tb18203.x. [DOI] [PubMed] [Google Scholar]
- Miller A. D., Rosman G. J. Improved retroviral vectors for gene transfer and expression. Biotechniques. 1989 Oct;7(9):980-2, 984-6, 989-90. [PMC free article] [PubMed] [Google Scholar]
- Miura O., Cleveland J. L., Ihle J. N. Inactivation of erythropoietin receptor function by point mutations in a region having homology with other cytokine receptors. Mol Cell Biol. 1993 Mar;13(3):1788–1795. doi: 10.1128/mcb.13.3.1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura O., D'Andrea A., Kabat D., Ihle J. N. Induction of tyrosine phosphorylation by the erythropoietin receptor correlates with mitogenesis. Mol Cell Biol. 1991 Oct;11(10):4895–4902. doi: 10.1128/mcb.11.10.4895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura O., Ihle J. N. Dimer- and oligomerization of the erythropoietin receptor by disulfide bond formation and significance of the region near the WSXWS motif in intracellular transport. Arch Biochem Biophys. 1993 Oct;306(1):200–208. doi: 10.1006/abbi.1993.1501. [DOI] [PubMed] [Google Scholar]
- Murakami M., Narazaki M., Hibi M., Yawata H., Yasukawa K., Hamaguchi M., Taga T., Kishimoto T. Critical cytoplasmic region of the interleukin 6 signal transducer gp130 is conserved in the cytokine receptor family. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11349–11353. doi: 10.1073/pnas.88.24.11349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustelin T., Burn P. Regulation of src family tyrosine kinases in lymphocytes. Trends Biochem Sci. 1993 Jun;18(6):215–220. doi: 10.1016/0968-0004(93)90192-p. [DOI] [PubMed] [Google Scholar]
- Palacios R., Steinmetz M. Il-3-dependent mouse clones that express B-220 surface antigen, contain Ig genes in germ-line configuration, and generate B lymphocytes in vivo. Cell. 1985 Jul;41(3):727–734. doi: 10.1016/s0092-8674(85)80053-2. [DOI] [PubMed] [Google Scholar]
- Pelicci G., Lanfrancone L., Grignani F., McGlade J., Cavallo F., Forni G., Nicoletti I., Grignani F., Pawson T., Pelicci P. G. A novel transforming protein (SHC) with an SH2 domain is implicated in mitogenic signal transduction. Cell. 1992 Jul 10;70(1):93–104. doi: 10.1016/0092-8674(92)90536-l. [DOI] [PubMed] [Google Scholar]
- Quelle D. E., Wojchowski D. M. Localized cytosolic domains of the erythropoietin receptor regulate growth signaling and down-modulate responsiveness to granulocyte-macrophage colony-stimulating factor. Proc Natl Acad Sci U S A. 1991 Jun 1;88(11):4801–4805. doi: 10.1073/pnas.88.11.4801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quelle F. W., Wojchowski D. M. Proliferative action of erythropoietin is associated with rapid protein tyrosine phosphorylation in responsive B6SUt.EP cells. J Biol Chem. 1991 Jan 5;266(1):609–614. [PubMed] [Google Scholar]
- Redemann N., Holzmann B., von Rüden T., Wagner E. F., Schlessinger J., Ullrich A. Anti-oncogenic activity of signalling-defective epidermal growth factor receptor mutants. Mol Cell Biol. 1992 Feb;12(2):491–498. doi: 10.1128/mcb.12.2.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozakis-Adcock M., McGlade J., Mbamalu G., Pelicci G., Daly R., Li W., Batzer A., Thomas S., Brugge J., Pelicci P. G. Association of the Shc and Grb2/Sem5 SH2-containing proteins is implicated in activation of the Ras pathway by tyrosine kinases. Nature. 1992 Dec 17;360(6405):689–692. doi: 10.1038/360689a0. [DOI] [PubMed] [Google Scholar]
- Showers M. O., DeMartino J. C., Saito Y., D'Andrea A. D. Fusion of the erythropoietin receptor and the Friend spleen focus-forming virus gp55 glycoprotein transforms a factor-dependent hematopoietic cell line. Mol Cell Biol. 1993 Feb;13(2):739–748. doi: 10.1128/mcb.13.2.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Showers M. O., Moreau J. F., Linnekin D., Druker B., D'Andrea A. D. Activation of the erythropoietin receptor by the Friend spleen focus-forming virus gp55 glycoprotein induces constitutive protein tyrosine phosphorylation. Blood. 1992 Dec 15;80(12):3070–3078. [PubMed] [Google Scholar]
- Torti M., Marti K. B., Altschuler D., Yamamoto K., Lapetina E. G. Erythropoietin induces p21ras activation and p120GAP tyrosine phosphorylation in human erythroleukemia cells. J Biol Chem. 1992 Apr 25;267(12):8293–8298. [PubMed] [Google Scholar]
- Ueno H., Colbert H., Escobedo J. A., Williams L. T. Inhibition of PDGF beta receptor signal transduction by coexpression of a truncated receptor. Science. 1991 May 10;252(5007):844–848. doi: 10.1126/science.1851331. [DOI] [PubMed] [Google Scholar]
- Watowich S. S., Yoshimura A., Longmore G. D., Hilton D. J., Yoshimura Y., Lodish H. F. Homodimerization and constitutive activation of the erythropoietin receptor. Proc Natl Acad Sci U S A. 1992 Mar 15;89(6):2140–2144. doi: 10.1073/pnas.89.6.2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witthuhn B. A., Quelle F. W., Silvennoinen O., Yi T., Tang B., Miura O., Ihle J. N. JAK2 associates with the erythropoietin receptor and is tyrosine phosphorylated and activated following stimulation with erythropoietin. Cell. 1993 Jul 30;74(2):227–236. doi: 10.1016/0092-8674(93)90414-l. [DOI] [PubMed] [Google Scholar]
- Yoshimura A., D'Andrea A. D., Lodish H. F. Friend spleen focus-forming virus glycoprotein gp55 interacts with the erythropoietin receptor in the endoplasmic reticulum and affects receptor metabolism. Proc Natl Acad Sci U S A. 1990 Jun;87(11):4139–4143. doi: 10.1073/pnas.87.11.4139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura A., Longmore G., Lodish H. F. Point mutation in the exoplasmic domain of the erythropoietin receptor resulting in hormone-independent activation and tumorigenicity. Nature. 1990 Dec 13;348(6302):647–649. doi: 10.1038/348647a0. [DOI] [PubMed] [Google Scholar]
- de Vos A. M., Ultsch M., Kossiakoff A. A. Human growth hormone and extracellular domain of its receptor: crystal structure of the complex. Science. 1992 Jan 17;255(5042):306–312. doi: 10.1126/science.1549776. [DOI] [PubMed] [Google Scholar]