Abstract
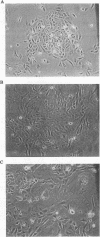
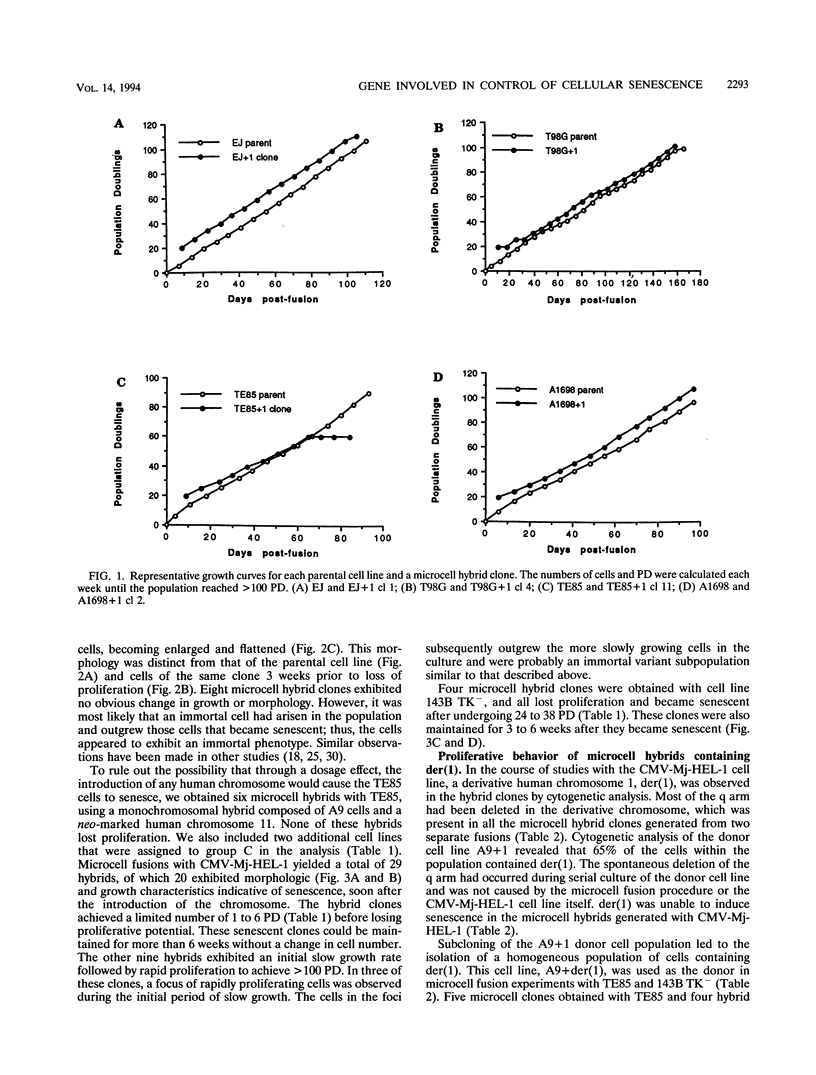
Normal cells in culture exhibit limited division potential and have been used as a model for cellular senescence. In contrast, tumor-derived or carcinogen- or virus-transformed cells are capable of indefinite division. Fusion of normal human diploid fibroblasts with immortal human cells yielded hybrids having limited life spans, indicating that cellular senescence was dominant. Fusions of various immortal human cell lines with each other led to the identification of four complementation groups for indefinite division. The purpose of this study was to determine whether human chromosome 1 could complement the recessive immortal defect of human cell lines assigned to one of the four complementation groups. Using microcell fusion, we introduced a single normal human chromosome 1 into immortal human cell lines representing the complementation groups and determined that it caused loss of proliferative potential of an osteosarcoma-derived cell line (TE85), a cytomegalovirus-transformed lung fibroblast cell line (CMV-Mj-HEL-1), and a Ki-ras(+)-transformed derivative of TE85 (143B TK-), all of which were assigned to complementation group C. This chromosome 1 caused no change in proliferative potential of cell lines representing the other complementation groups. A derivative of human chromosome 1 that had lost most of the q arm by spontaneous deletion was unable to induce senescence in any of the immortal cell lines. This finding indicates that the q arm of human chromosome 1 carries a gene or set of genes which is altered in the cell lines assigned to complementation group C and is involved in the control of cellular senescence.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alhadeff B., Velivasakis M., Siniscalco M. Simultaneous identification of chromatid replication and of human chromosomes in metaphases of man-mouse somatic cell hybrids. (With 1 color plate). Cytogenet Cell Genet. 1977;19(4):236–239. doi: 10.1159/000130814. [DOI] [PubMed] [Google Scholar]
- Barrett J. C., Oshimura M., Koi M. Role of oncogenes and tumor suppressor genes in a multistep model of carcinogenesis. Symp Fundam Cancer Res. 1986;39:45–56. [PubMed] [Google Scholar]
- Bunn C. L., Tarrant G. M. Limited lifespan in somatic cell hybrids and cybrids. Exp Cell Res. 1980 Jun;127(2):385–396. doi: 10.1016/0014-4827(80)90443-7. [DOI] [PubMed] [Google Scholar]
- Chen L. C., Dollbaum C., Smith H. S. Loss of heterozygosity on chromosome 1q in human breast cancer. Proc Natl Acad Sci U S A. 1989 Sep;86(18):7204–7207. doi: 10.1073/pnas.86.18.7204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dell'Orco R. T., Mertens J. G., Kruse P. F., Jr Doubling potential, calendar time, and donor age of human diploid cells in culture. Exp Cell Res. 1974 Mar 15;84(1):363–366. doi: 10.1016/0014-4827(74)90416-9. [DOI] [PubMed] [Google Scholar]
- Duncan E. L., Whitaker N. J., Moy E. L., Reddel R. R. Assignment of SV40-immortalized cells to more than one complementation group for immortalization. Exp Cell Res. 1993 Apr;205(2):337–344. doi: 10.1006/excr.1993.1095. [DOI] [PubMed] [Google Scholar]
- Fong C. T., Dracopoli N. C., White P. S., Merrill P. T., Griffith R. C., Housman D. E., Brodeur G. M. Loss of heterozygosity for the short arm of chromosome 1 in human neuroblastomas: correlation with N-myc amplification. Proc Natl Acad Sci U S A. 1989 May;86(10):3753–3757. doi: 10.1073/pnas.86.10.3753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAYFLICK L. THE LIMITED IN VITRO LIFETIME OF HUMAN DIPLOID CELL STRAINS. Exp Cell Res. 1965 Mar;37:614–636. doi: 10.1016/0014-4827(65)90211-9. [DOI] [PubMed] [Google Scholar]
- Killary A. M., Fournier R. E. A genetic analysis of extinction: trans-dominant loci regulate expression of liver-specific traits in hepatoma hybrid cells. Cell. 1984 Sep;38(2):523–534. doi: 10.1016/0092-8674(84)90507-5. [DOI] [PubMed] [Google Scholar]
- Klein C. B., Conway K., Wang X. W., Bhamra R. K., Lin X. H., Cohen M. D., Annab L., Barrett J. C., Costa M. Senescence of nickel-transformed cells by an X chromosome: possible epigenetic control. Science. 1991 Feb 15;251(4995):796–799. doi: 10.1126/science.1990442. [DOI] [PubMed] [Google Scholar]
- Koi M., Morita H., Shimizu M., Oshimura M. Construction of mouse A9 clones containing a single human chromosome (X/autosome translocation) via micro-cell fusion. Jpn J Cancer Res. 1989 Feb;80(2):122–125. doi: 10.1111/j.1349-7006.1989.tb02278.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew C. G., Smith B. A., Thorpe K., Wong Z., Royle N. J., Jeffreys A. J., Ponder B. A. Deletion of genes on chromosome 1 in endocrine neoplasia. Nature. 1987 Aug 6;328(6130):524–526. doi: 10.1038/328524a0. [DOI] [PubMed] [Google Scholar]
- Miller S. A., Dykes D. D., Polesky H. F. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988 Feb 11;16(3):1215–1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muggleton-Harris A. L., DeSimone D. W. Replicative potentials of various fusion products between WI-38 and SV40 transformed WI-38 cells and their components. Somatic Cell Genet. 1980 Nov;6(6):689–698. doi: 10.1007/BF01538968. [DOI] [PubMed] [Google Scholar]
- Nakamura Y., White R. Isolation and mapping of a polymorphic DNA sequence (cYNA13) on chromosome 1 [D1S74]. Nucleic Acids Res. 1988 Oct 11;16(19):9369–9369. doi: 10.1093/nar/16.19.9369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ning Y., Shay J. W., Lovell M., Taylor L., Ledbetter D. H., Pereira-Smith O. M. Tumor suppression by chromosome 11 is not due to cellular senescence. Exp Cell Res. 1991 Jan;192(1):220–226. doi: 10.1016/0014-4827(91)90179-x. [DOI] [PubMed] [Google Scholar]
- Ning Y., Weber J. L., Killary A. M., Ledbetter D. H., Smith J. R., Pereira-Smith O. M. Genetic analysis of indefinite division in human cells: evidence for a cell senescence-related gene(s) on human chromosome 4. Proc Natl Acad Sci U S A. 1991 Jul 1;88(13):5635–5639. doi: 10.1073/pnas.88.13.5635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogata T., Ayusawa D., Namba M., Takahashi E., Oshimura M., Oishi M. Chromosome 7 suppresses indefinite division of nontumorigenic immortalized human fibroblast cell lines KMST-6 and SUSM-1. Mol Cell Biol. 1993 Oct;13(10):6036–6043. doi: 10.1128/mcb.13.10.6036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paraskeva C., Finerty S., Powell S. Immortalization of a human colorectal adenoma cell line by continuous in vitro passage: possible involvement of chromosome 1 in tumour progression. Int J Cancer. 1988 Jun 15;41(6):908–912. doi: 10.1002/ijc.2910410624. [DOI] [PubMed] [Google Scholar]
- Pereira-Smith O. M., Robetorye S., Ning Y., Orson F. M. Hybrids from fusion of normal human T lymphocytes with immortal human cells exhibit limited life span. J Cell Physiol. 1990 Sep;144(3):546–549. doi: 10.1002/jcp.1041440324. [DOI] [PubMed] [Google Scholar]
- Pereira-Smith O. M., Smith J. R. Evidence for the recessive nature of cellular immortality. Science. 1983 Sep 2;221(4614):964–966. doi: 10.1126/science.6879195. [DOI] [PubMed] [Google Scholar]
- Pereira-Smith O. M., Smith J. R. Expression of SV40 T antigen in finite life-span hybrids of normal and SV40-transformed fibroblasts. Somatic Cell Genet. 1981 Jul;7(4):411–421. doi: 10.1007/BF01542986. [DOI] [PubMed] [Google Scholar]
- Pereira-Smith O. M., Smith J. R. Genetic analysis of indefinite division in human cells: identification of four complementation groups. Proc Natl Acad Sci U S A. 1988 Aug;85(16):6042–6046. doi: 10.1073/pnas.85.16.6042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira-Smith O. M., Stein G. H., Robetorye S., Meyer-Demarest S. Immortal phenotype of the HeLa variant D98 is recessive in hybrids formed with normal human fibroblasts. J Cell Physiol. 1990 May;143(2):222–225. doi: 10.1002/jcp.1041430204. [DOI] [PubMed] [Google Scholar]
- Roberts T. W., Smith J. R. The proliferative potential of chick embryo fibroblasts: population doublings vs. time in culture. Cell Biol Int Rep. 1980 Dec;4(12):1057–1063. doi: 10.1016/0309-1651(80)90042-9. [DOI] [PubMed] [Google Scholar]
- Sharma V., Litt M. Dinucleotide repeat polymorphism at the D1S117 locus. Nucleic Acids Res. 1991 Mar 11;19(5):1168–1168. doi: 10.1093/nar/19.5.1168-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugawara O., Oshimura M., Koi M., Annab L. A., Barrett J. C. Induction of cellular senescence in immortalized cells by human chromosome 1. Science. 1990 Feb 9;247(4943):707–710. doi: 10.1126/science.2300822. [DOI] [PubMed] [Google Scholar]
- Williams A. C., Harper S. J., Marshall C. J., Gill R. W., Mountford R. A., Paraskeva C. Specific cytogenetic abnormalities and k-ras mutation in two new human colorectal-adenoma-derived cell lines. Int J Cancer. 1992 Nov 11;52(5):785–790. doi: 10.1002/ijc.2910520519. [DOI] [PubMed] [Google Scholar]
- Yamada H., Horikawa I., Hashiba H., Oshimura M. Normal human chromosome 1 carries suppressor activity for various phenotypes of a Kirsten murine sarcoma virus-transformed NIH/3T3 cell line. Jpn J Cancer Res. 1990 Nov;81(11):1095–1100. doi: 10.1111/j.1349-7006.1990.tb02519.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada H., Wake N., Fujimoto S., Barrett J. C., Oshimura M. Multiple chromosomes carrying tumor suppressor activity for a uterine endometrial carcinoma cell line identified by microcell-mediated chromosome transfer. Oncogene. 1990 Aug;5(8):1141–1147. [PubMed] [Google Scholar]