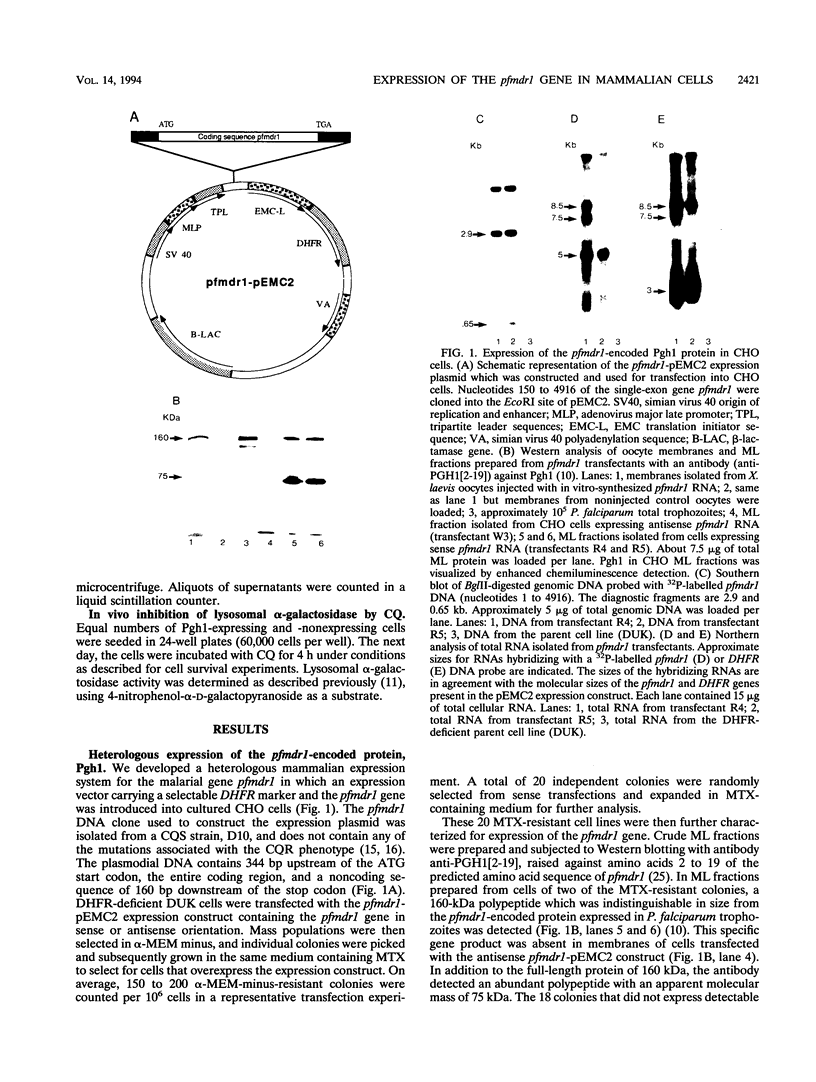
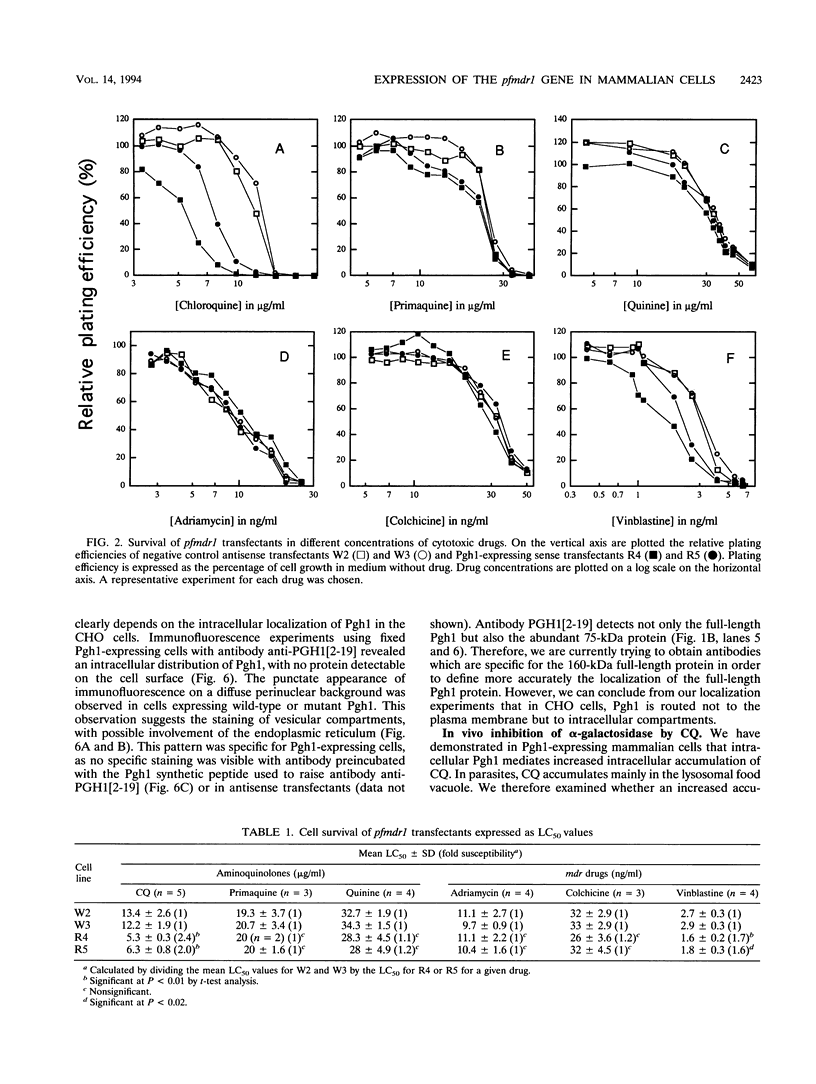
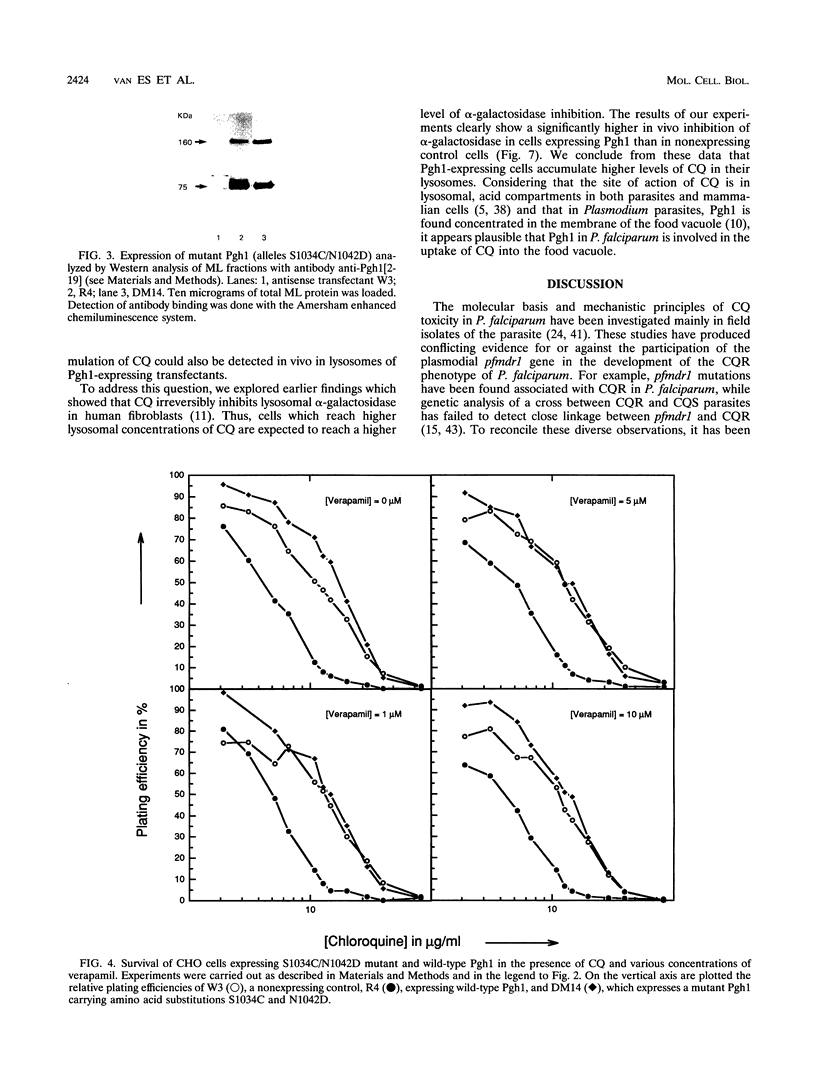
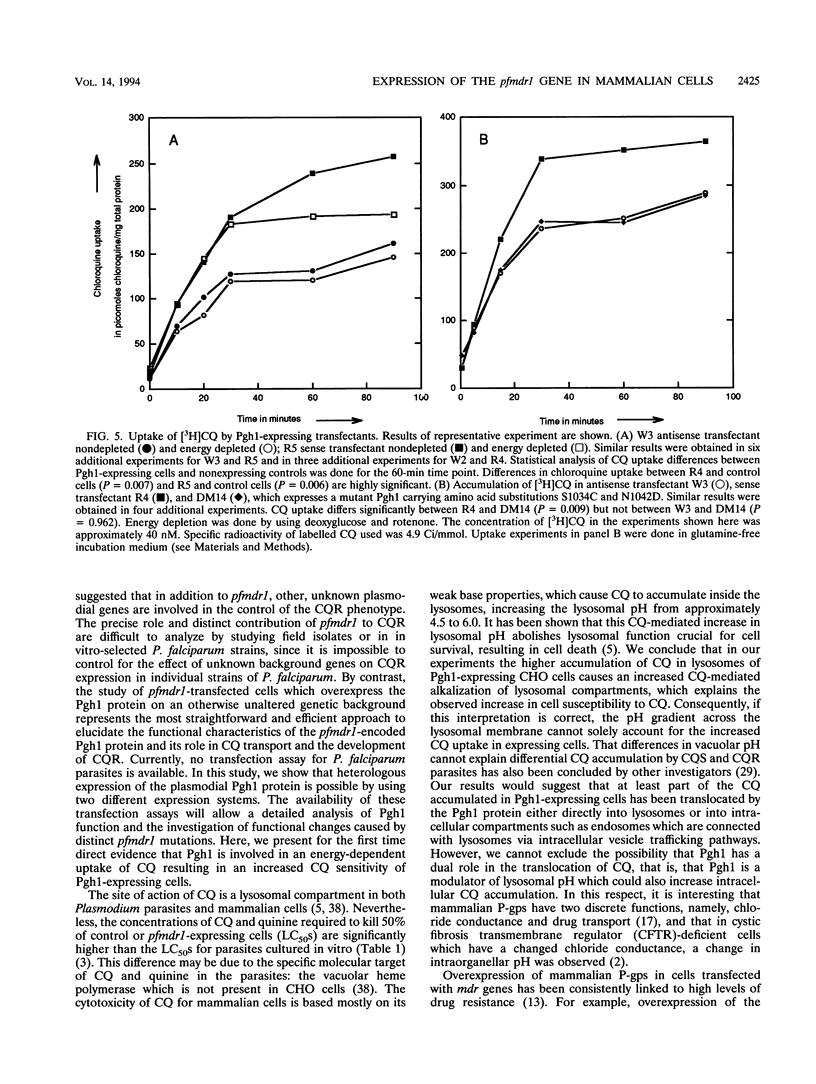
Abstract
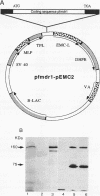
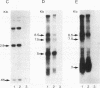
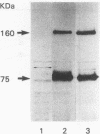
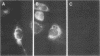
Chloroquine (CQ)-resistant (CQR) Plasmodium falciparum malaria parasites show a strong decrease in CQ accumulation in comparison with chloroquine-sensitive parasites. Controversy exists over the role of the plasmodial pfmdr1 gene in the CQR phenotype. pfmdr1 is a member of the superfamily of ATP-binding cassette transporters. Other members of this family are the mammalian multidrug resistance genes and the CFTR gene. We have expressed the pfmdr1-encoded protein, Pgh1, in CHO cells and Xenopus oocytes. CHO cells expressing the Pgh1 protein demonstrated an increased, verapamil-insensitive susceptibility to CQ. Conversely, no increase in drug susceptibility to primaquine, quinine, adriamycin, or colchicine was observed in Pgh1-expressing cells. CQ uptake experiments revealed an increased, ATP-dependent accumulation of CQ in Pgh1-expressing cells over the level in nonexpressing control cells. The increased CQ accumulation in Pgh1-expressing cells coincided with an enhanced in vivo inhibition of lysosomal alpha-galactosidase by CQ. CHO cells expressing Pgh1 carrying two of the CQR-associated Pgh1 amino acid changes (S1034C and N1042D) did not display an increased CQ sensitivity. Immunofluorescence experiments revealed an intracellular localization of both mutant and wild-type forms of Pgh1. We conclude from our results that wild-type Pgh1 protein can mediate an increased intracellular accumulation of CQ and that this function is impaired in CQR-associated mutant forms of the protein. We speculate that the Pgh1 protein plays an important role in CQ import in CQ-sensitive malaria parasites.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Awad-el-Kariem F. M., Miles M. A., Warhurst D. C. Chloroquine-resistant Plasmodium falciparum isolates from the Sudan lack two mutations in the pfmdr1 gene thought to be associated with chloroquine resistance. Trans R Soc Trop Med Hyg. 1992 Nov-Dec;86(6):587–589. doi: 10.1016/0035-9203(92)90140-8. [DOI] [PubMed] [Google Scholar]
- Barasch J., Kiss B., Prince A., Saiman L., Gruenert D., al-Awqati Q. Defective acidification of intracellular organelles in cystic fibrosis. Nature. 1991 Jul 4;352(6330):70–73. doi: 10.1038/352070a0. [DOI] [PubMed] [Google Scholar]
- Barnes D. A., Foote S. J., Galatis D., Kemp D. J., Cowman A. F. Selection for high-level chloroquine resistance results in deamplification of the pfmdr1 gene and increased sensitivity to mefloquine in Plasmodium falciparum. EMBO J. 1992 Aug;11(8):3067–3075. doi: 10.1002/j.1460-2075.1992.tb05378.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray P. G., Howells R. E., Ritchie G. Y., Ward S. A. Rapid chloroquine efflux phenotype in both chloroquine-sensitive and chloroquine-resistant Plasmodium falciparum. A correlation of chloroquine sensitivity with energy-dependent drug accumulation. Biochem Pharmacol. 1992 Oct 6;44(7):1317–1324. doi: 10.1016/0006-2952(92)90532-n. [DOI] [PubMed] [Google Scholar]
- Cain C. C., Murphy R. F. Growth inhibition of 3T3 fibroblasts by lysosomotropic amines: correlation with effects on intravesicular pH but not vacuolation. J Cell Physiol. 1986 Oct;129(1):65–70. doi: 10.1002/jcp.1041290110. [DOI] [PubMed] [Google Scholar]
- Castillo G., Vera J. C., Yang C. P., Horwitz S. B., Rosen O. M. Functional expression of murine multidrug resistance in Xenopus laevis oocytes. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4737–4741. doi: 10.1073/pnas.87.12.4737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Cornwell M. M., Pastan I., Gottesman M. M. Certain calcium channel blockers bind specifically to multidrug-resistant human KB carcinoma membrane vesicles and inhibit drug binding to P-glycoprotein. J Biol Chem. 1987 Feb 15;262(5):2166–2170. [PubMed] [Google Scholar]
- Cornwell M. M., Tsuruo T., Gottesman M. M., Pastan I. ATP-binding properties of P glycoprotein from multidrug-resistant KB cells. FASEB J. 1987 Jul;1(1):51–54. doi: 10.1096/fasebj.1.1.2886389. [DOI] [PubMed] [Google Scholar]
- De Groot P. G., Ovde Elferink R. O., Hollemans M., Strijland A., Westerveld A., Meera Khan P., Tager J. M. Inactivation by chloroquine of alpha-galactosidase in cultured human skin fibroblasts. Exp Cell Res. 1981 Dec;136(2):327–333. doi: 10.1016/0014-4827(81)90011-2. [DOI] [PubMed] [Google Scholar]
- Devault A., Gros P. Two members of the mouse mdr gene family confer multidrug resistance with overlapping but distinct drug specificities. Mol Cell Biol. 1990 Apr;10(4):1652–1663. doi: 10.1128/mcb.10.4.1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endicott J. A., Ling V. The biochemistry of P-glycoprotein-mediated multidrug resistance. Annu Rev Biochem. 1989;58:137–171. doi: 10.1146/annurev.bi.58.070189.001033. [DOI] [PubMed] [Google Scholar]
- Ferrari V., Cutler D. J. Simulation of kinetic data on the influx and efflux of chloroquine by erythrocytes infected with Plasmodium falciparum. Evidence for a drug-importer in chloroquine-sensitive strains. Biochem Pharmacol. 1991 Dec 11;42 (Suppl):S167–S179. doi: 10.1016/0006-2952(91)90407-v. [DOI] [PubMed] [Google Scholar]
- Foote S. J., Kyle D. E., Martin R. K., Oduola A. M., Forsyth K., Kemp D. J., Cowman A. F. Several alleles of the multidrug-resistance gene are closely linked to chloroquine resistance in Plasmodium falciparum. Nature. 1990 May 17;345(6272):255–258. doi: 10.1038/345255a0. [DOI] [PubMed] [Google Scholar]
- Foote S. J., Thompson J. K., Cowman A. F., Kemp D. J. Amplification of the multidrug resistance gene in some chloroquine-resistant isolates of P. falciparum. Cell. 1989 Jun 16;57(6):921–930. doi: 10.1016/0092-8674(89)90330-9. [DOI] [PubMed] [Google Scholar]
- Gill D. R., Hyde S. C., Higgins C. F., Valverde M. A., Mintenig G. M., Sepúlveda F. V. Separation of drug transport and chloride channel functions of the human multidrug resistance P-glycoprotein. Cell. 1992 Oct 2;71(1):23–32. doi: 10.1016/0092-8674(92)90263-c. [DOI] [PubMed] [Google Scholar]
- Ginsburg H., Stein W. D. Kinetic modelling of chloroquine uptake by malaria-infected erythrocytes. Assessment of the factors that may determine drug resistance. Biochem Pharmacol. 1991 May 15;41(10):1463–1470. doi: 10.1016/0006-2952(91)90562-j. [DOI] [PubMed] [Google Scholar]
- Goldberg D. E., Slater A. F. The pathway of hemoglobin degradation in malaria parasites. Parasitol Today. 1992 Aug;8(8):280–283. doi: 10.1016/0169-4758(92)90146-s. [DOI] [PubMed] [Google Scholar]
- Groen A. K., Vervoorn R. C., Wanders R. J., Van der Meer R., Tager J. M. An evaluation of the metabolite indicator method for determining the cytosolic phosphate potential in rat liver cells. Biochim Biophys Acta. 1982 Oct 11;721(2):172–177. doi: 10.1016/0167-4889(82)90065-9. [DOI] [PubMed] [Google Scholar]
- Gros P., Talbot F., Tang-Wai D., Bibi E., Kaback H. R. Lipophilic cations: a group of model substrates for the multidrug-resistance transporter. Biochemistry. 1992 Feb 25;31(7):1992–1998. doi: 10.1021/bi00122a014. [DOI] [PubMed] [Google Scholar]
- Hammond J. R., Johnstone R. M., Gros P. Enhanced efflux of [3H]vinblastine from Chinese hamster ovary cells transfected with a full-length complementary DNA clone for the mdr1 gene. Cancer Res. 1989 Jul 15;49(14):3867–3871. [PubMed] [Google Scholar]
- Higgins C. F. ABC transporters: from microorganisms to man. Annu Rev Cell Biol. 1992;8:67–113. doi: 10.1146/annurev.cb.08.110192.000435. [DOI] [PubMed] [Google Scholar]
- Karcz S. R., Galatis D., Cowman A. F. Nucleotide binding properties of a P-glycoprotein homologue from Plasmodium falciparum. Mol Biochem Parasitol. 1993 Apr;58(2):269–276. doi: 10.1016/0166-6851(93)90048-3. [DOI] [PubMed] [Google Scholar]
- Karcz S., Cowman A. F. Similarities and differences between the multidrug resistance phenotype of mammalian tumor cells and chloroquine resistance in Plasmodium falciparum. Exp Parasitol. 1991 Aug;73(2):233–240. doi: 10.1016/0014-4894(91)90027-t. [DOI] [PubMed] [Google Scholar]
- Kaufman R. J. Identification of the components necessary for adenovirus translational control and their utilization in cDNA expression vectors. Proc Natl Acad Sci U S A. 1985 Feb;82(3):689–693. doi: 10.1073/pnas.82.3.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krogstad D. J., Gluzman I. Y., Herwaldt B. L., Schlesinger P. H., Wellems T. E. Energy dependence of chloroquine accumulation and chloroquine efflux in Plasmodium falciparum. Biochem Pharmacol. 1992 Jan 9;43(1):57–62. doi: 10.1016/0006-2952(92)90661-2. [DOI] [PubMed] [Google Scholar]
- Krogstad D. J., Gluzman I. Y., Kyle D. E., Oduola A. M., Martin S. K., Milhous W. K., Schlesinger P. H. Efflux of chloroquine from Plasmodium falciparum: mechanism of chloroquine resistance. Science. 1987 Nov 27;238(4831):1283–1285. doi: 10.1126/science.3317830. [DOI] [PubMed] [Google Scholar]
- Krogstad D. J., Schlesinger P. H. The basis of antimalarial action: non-weak base effects of chloroquine on acid vesicle pH. Am J Trop Med Hyg. 1987 Mar;36(2):213–220. doi: 10.4269/ajtmh.1987.36.213. [DOI] [PubMed] [Google Scholar]
- Lemontt J. F., Azzaria M., Gros P. Increased mdr gene expression and decreased drug accumulation in multidrug-resistant human melanoma cells. Cancer Res. 1988 Nov 15;48(22):6348–6353. [PubMed] [Google Scholar]
- Martin S. K., Oduola A. M., Milhous W. K. Reversal of chloroquine resistance in Plasmodium falciparum by verapamil. Science. 1987 Feb 20;235(4791):899–901. doi: 10.1126/science.3544220. [DOI] [PubMed] [Google Scholar]
- Oude Elferink R. P., Ottenhoff R., Liefting W. G., Schoemaker B., Groen A. K., Jansen P. L. ATP-dependent efflux of GSSG and GS-conjugate from isolated rat hepatocytes. Am J Physiol. 1990 May;258(5 Pt 1):G699–G706. doi: 10.1152/ajpgi.1990.258.5.G699. [DOI] [PubMed] [Google Scholar]
- Safa A. R., Glover C. J., Meyers M. B., Biedler J. L., Felsted R. L. Vinblastine photoaffinity labeling of a high molecular weight surface membrane glycoprotein specific for multidrug-resistant cells. J Biol Chem. 1986 May 15;261(14):6137–6140. [PubMed] [Google Scholar]
- Safa A. R. Photoaffinity labeling of the multidrug-resistance-related P-glycoprotein with photoactive analogs of verapamil. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7187–7191. doi: 10.1073/pnas.85.19.7187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schurr E., Raymond M., Bell J. C., Gros P. Characterization of the multidrug resistance protein expressed in cell clones stably transfected with the mouse mdr1 cDNA. Cancer Res. 1989 May 15;49(10):2729–2733. [PubMed] [Google Scholar]
- Slater A. F., Cerami A. Inhibition by chloroquine of a novel haem polymerase enzyme activity in malaria trophozoites. Nature. 1992 Jan 9;355(6356):167–169. doi: 10.1038/355167a0. [DOI] [PubMed] [Google Scholar]
- Trager W., Jensen J. B. Human malaria parasites in continuous culture. Science. 1976 Aug 20;193(4254):673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- Veignie E., Moreau S. The mode of action of chloroquine. Non-weak base properties of 4-aminoquinolines and antimalarial effects on strains of Plasmodium. Ann Trop Med Parasitol. 1991 Apr;85(2):229–237. doi: 10.1080/00034983.1991.11812550. [DOI] [PubMed] [Google Scholar]
- Wellems T. E., Panton L. J., Gluzman I. Y., do Rosario V. E., Gwadz R. W., Walker-Jonah A., Krogstad D. J. Chloroquine resistance not linked to mdr-like genes in a Plasmodium falciparum cross. Nature. 1990 May 17;345(6272):253–255. doi: 10.1038/345253a0. [DOI] [PubMed] [Google Scholar]
- Wigler M., Pellicer A., Silverstein S., Axel R., Urlaub G., Chasin L. DNA-mediated transfer of the adenine phosphoribosyltransferase locus into mammalian cells. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1373–1376. doi: 10.1073/pnas.76.3.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson C. M., Volkman S. K., Thaithong S., Martin R. K., Kyle D. E., Milhous W. K., Wirth D. F. Amplification of pfmdr 1 associated with mefloquine and halofantrine resistance in Plasmodium falciparum from Thailand. Mol Biochem Parasitol. 1993 Jan;57(1):151–160. doi: 10.1016/0166-6851(93)90252-s. [DOI] [PubMed] [Google Scholar]
- van Es H. H., Skamene E., Schurr E. Chemotherapy of malaria: a battle against all odds? Clin Invest Med. 1993 Aug;16(4):285–293. [PubMed] [Google Scholar]