Abstract
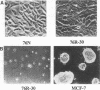
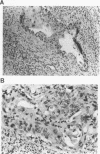
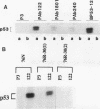
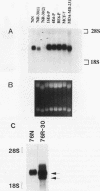
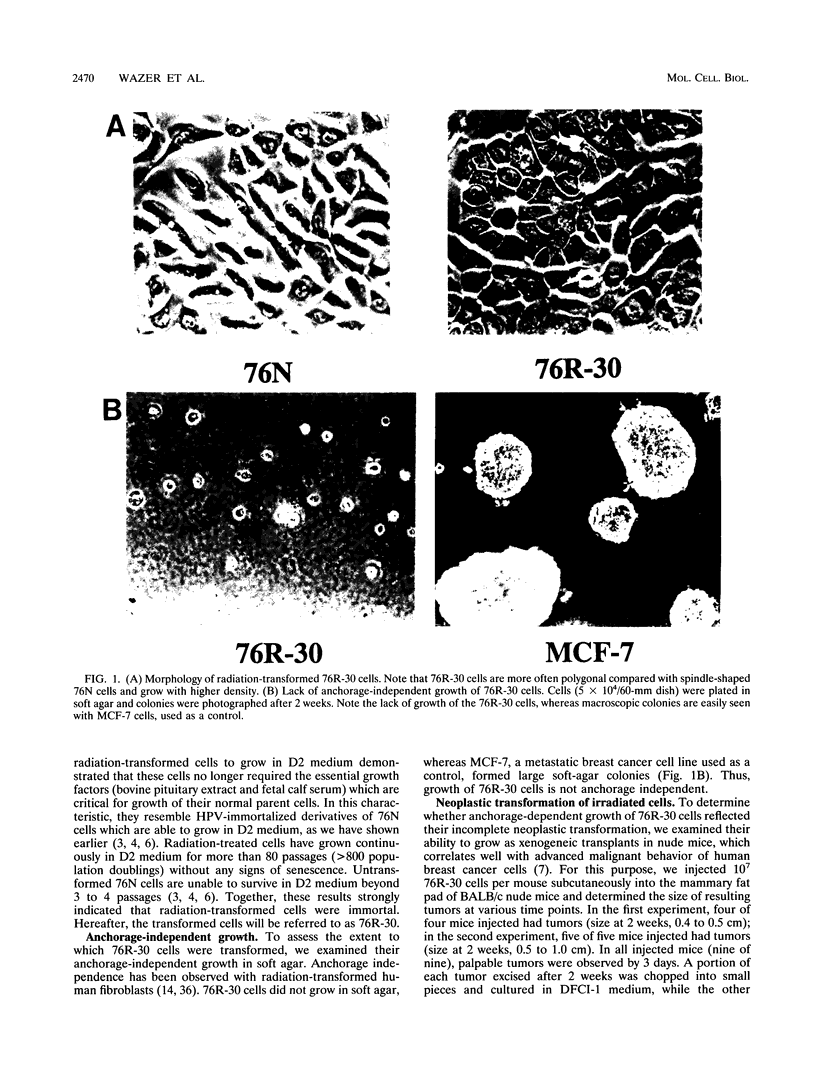
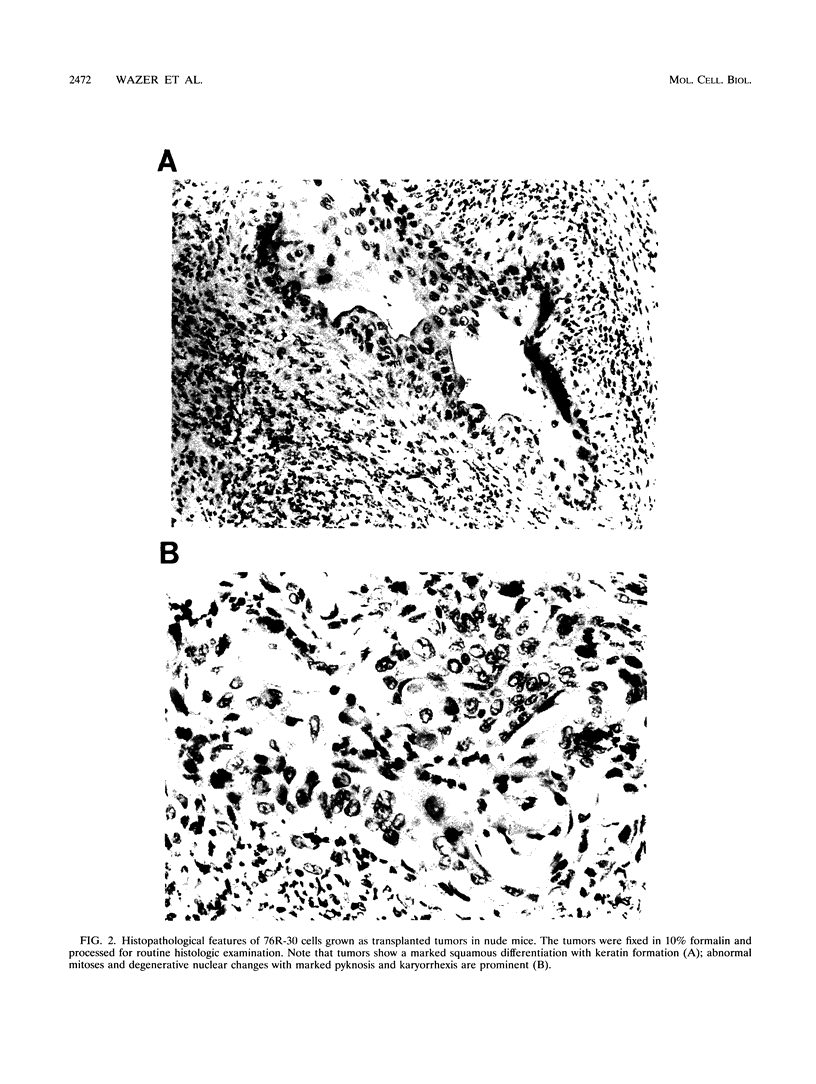
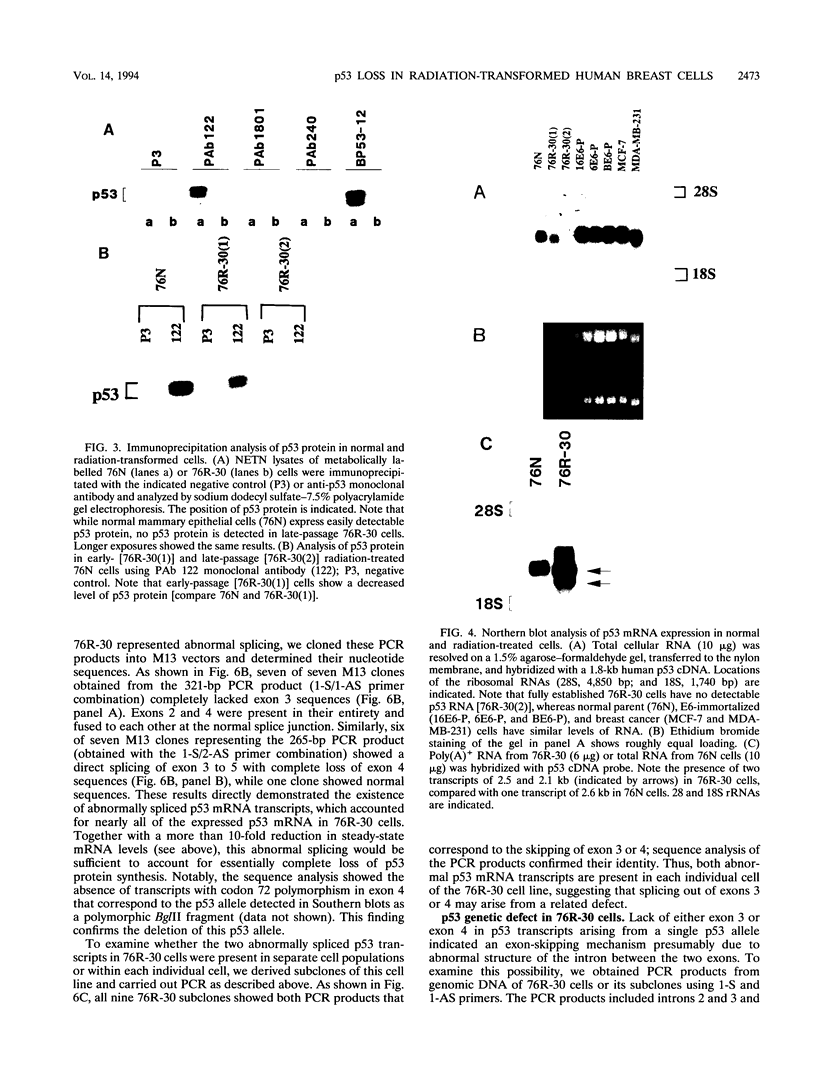
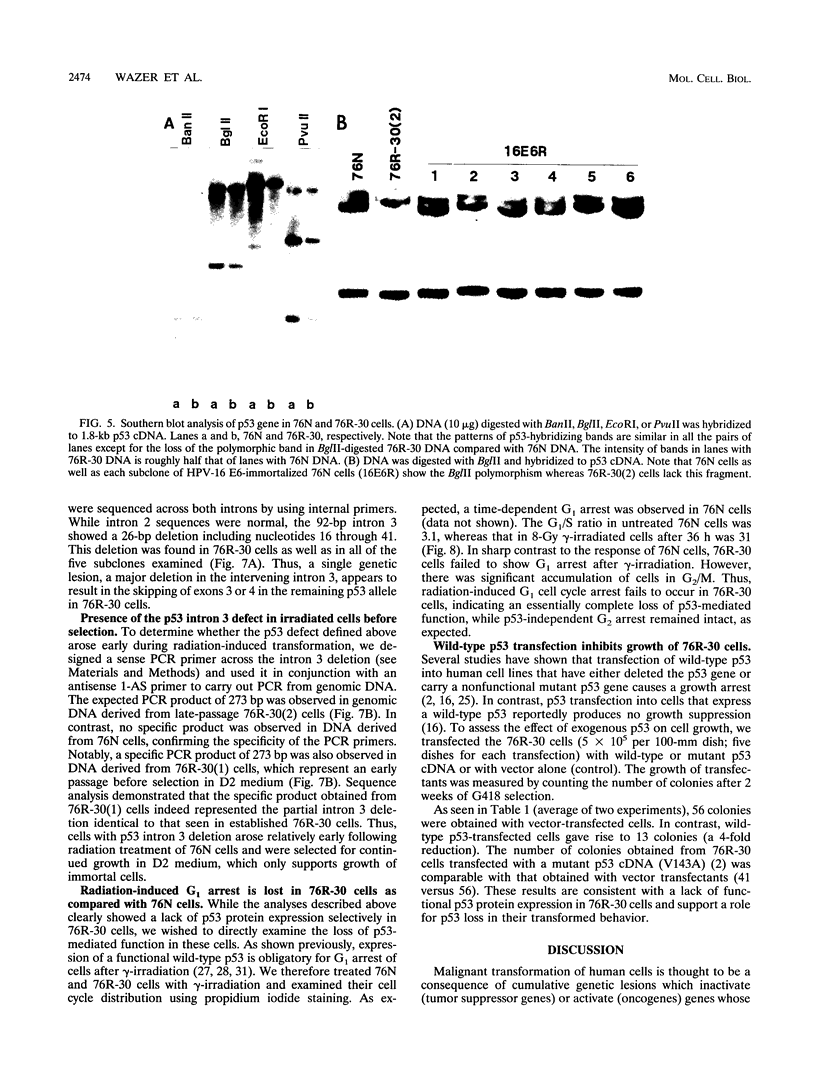
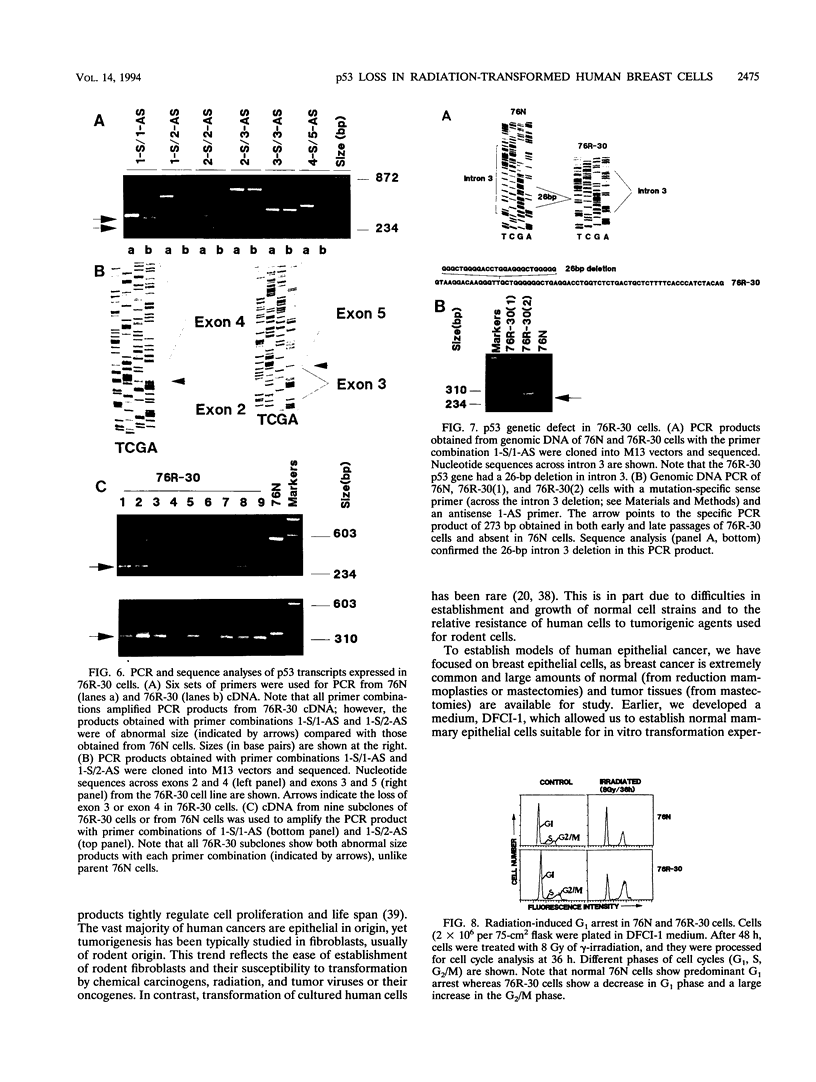
The causative factors leading to breast cancer are largely unknown. Increased incidence of breast cancer following diagnostic or therapeutic radiation suggests that radiation may contribute to mammary oncogenesis. This report describes the in vitro neoplastic transformation of a normal human mammary epithelial cell strain, 76N, by fractionated gamma-irradiation at a clinically used dose (30 Gy). The transformed cells (76R-30) were immortal, had reduced growth factor requirements, and produced tumors in nude mice. Remarkably, the 76R-30 cells completely lacked the p53 tumor suppressor protein. Loss of p53 was due to deletion of the gene on one allele and a 26-bp deletion within the third intron on the second allele which resulted in abnormal splicing out of either the third or fourth exon from the mRNA. PCR with a mutation-specific primer showed that intron 3 mutation was present in irradiated cells before selection for immortal phenotype. 76R-30 cells did not exhibit G1 arrest in response to radiation, indicating a loss of p53-mediated function. Expression of the wild-type p53 gene in 76R-30 cells led to their growth inhibition. Thus, loss of p53 protein appears to have contributed to neoplastic transformation of these cells. This unique model should facilitate analyses of molecular mechanisms of radiation-induced breast cancer and allow identification of p53-regulated cellular genes in breast cells.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker S. J., Fearon E. R., Nigro J. M., Hamilton S. R., Preisinger A. C., Jessup J. M., vanTuinen P., Ledbetter D. H., Barker D. F., Nakamura Y. Chromosome 17 deletions and p53 gene mutations in colorectal carcinomas. Science. 1989 Apr 14;244(4901):217–221. doi: 10.1126/science.2649981. [DOI] [PubMed] [Google Scholar]
- Baker S. J., Markowitz S., Fearon E. R., Willson J. K., Vogelstein B. Suppression of human colorectal carcinoma cell growth by wild-type p53. Science. 1990 Aug 24;249(4971):912–915. doi: 10.1126/science.2144057. [DOI] [PubMed] [Google Scholar]
- Band V., Dalal S., Delmolino L., Androphy E. J. Enhanced degradation of p53 protein in HPV-6 and BPV-1 E6-immortalized human mammary epithelial cells. EMBO J. 1993 May;12(5):1847–1852. doi: 10.1002/j.1460-2075.1993.tb05833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Band V., De Caprio J. A., Delmolino L., Kulesa V., Sager R. Loss of p53 protein in human papillomavirus type 16 E6-immortalized human mammary epithelial cells. J Virol. 1991 Dec;65(12):6671–6676. doi: 10.1128/jvi.65.12.6671-6676.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Band V., Sager R. Distinctive traits of normal and tumor-derived human mammary epithelial cells expressed in a medium that supports long-term growth of both cell types. Proc Natl Acad Sci U S A. 1989 Feb;86(4):1249–1253. doi: 10.1073/pnas.86.4.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Band V., Zajchowski D., Kulesa V., Sager R. Human papilloma virus DNAs immortalize normal human mammary epithelial cells and reduce their growth factor requirements. Proc Natl Acad Sci U S A. 1990 Jan;87(1):463–467. doi: 10.1073/pnas.87.1.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Band V., Zajchowski D., Stenman G., Morton C. C., Kulesa V., Connolly J., Sager R. A newly established metastatic breast tumor cell line with integrated amplified copies of ERBB2 and double minute chromosomes. Genes Chromosomes Cancer. 1989 Sep;1(1):48–58. doi: 10.1002/gcc.2870010109. [DOI] [PubMed] [Google Scholar]
- Barbacid M. ras genes. Annu Rev Biochem. 1987;56:779–827. doi: 10.1146/annurev.bi.56.070187.004023. [DOI] [PubMed] [Google Scholar]
- Bischoff J. R., Friedman P. N., Marshak D. R., Prives C., Beach D. Human p53 is phosphorylated by p60-cdc2 and cyclin B-cdc2. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4766–4770. doi: 10.1073/pnas.87.12.4766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boice J. D., Jr, Harvey E. B., Blettner M., Stovall M., Flannery J. T. Cancer in the contralateral breast after radiotherapy for breast cancer. N Engl J Med. 1992 Mar 19;326(12):781–785. doi: 10.1056/NEJM199203193261201. [DOI] [PubMed] [Google Scholar]
- Boice J. D., Jr, Preston D., Davis F. G., Monson R. R. Frequent chest X-ray fluoroscopy and breast cancer incidence among tuberculosis patients in Massachusetts. Radiat Res. 1991 Feb;125(2):214–222. [PubMed] [Google Scholar]
- Borek C. Malignant transformation in vitro: criteria, biological markers, and application in environmental screening of carcinogens. Radiat Res. 1979 Aug;79(2):209–232. [PubMed] [Google Scholar]
- Borek C. X-ray induced in vitro neoplastic transformation of human diploid cells. Nature. 1980 Feb 21;283(5749):776–778. doi: 10.1038/283776a0. [DOI] [PubMed] [Google Scholar]
- Bos J. L. ras oncogenes in human cancer: a review. Cancer Res. 1989 Sep 1;49(17):4682–4689. [PubMed] [Google Scholar]
- Bártek J., Bártková J., Vojtesek B., Stasková Z., Rejthar A., Kovarík J., Lane D. P. Patterns of expression of the p53 tumour suppressor in human breast tissues and tumours in situ and in vitro. Int J Cancer. 1990 Nov 15;46(5):839–844. doi: 10.1002/ijc.2910460515. [DOI] [PubMed] [Google Scholar]
- Casey G., Lo-Hsueh M., Lopez M. E., Vogelstein B., Stanbridge E. J. Growth suppression of human breast cancer cells by the introduction of a wild-type p53 gene. Oncogene. 1991 Oct;6(10):1791–1797. [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Crissman H. A., Steinkamp J. A. Rapid, simultaneous measurement of DNA, protein, and cell volume in single cells from large mammalian cell populations. J Cell Biol. 1973 Dec;59(3):766–771. doi: 10.1083/jcb.59.3.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delmolino L., Band H., Band V. Expression and stability of p53 protein in normal human mammary epithelial cells. Carcinogenesis. 1993 May;14(5):827–832. doi: 10.1093/carcin/14.5.827. [DOI] [PubMed] [Google Scholar]
- DiPaolo J. A. Relative difficulties in transforming human and animal cells in vitro. J Natl Cancer Inst. 1983 Jan;70(1):3–8. [PubMed] [Google Scholar]
- Funk W. D., Pak D. T., Karas R. H., Wright W. E., Shay J. W. A transcriptionally active DNA-binding site for human p53 protein complexes. Mol Cell Biol. 1992 Jun;12(6):2866–2871. doi: 10.1128/mcb.12.6.2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gannon J. V., Greaves R., Iggo R., Lane D. P. Activating mutations in p53 produce a common conformational effect. A monoclonal antibody specific for the mutant form. EMBO J. 1990 May;9(5):1595–1602. doi: 10.1002/j.1460-2075.1990.tb08279.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock S. L., Tucker M. A., Hoppe R. T. Breast cancer after treatment of Hodgkin's disease. J Natl Cancer Inst. 1993 Jan 6;85(1):25–31. doi: 10.1093/jnci/85.1.25. [DOI] [PubMed] [Google Scholar]
- Hartwell L. Defects in a cell cycle checkpoint may be responsible for the genomic instability of cancer cells. Cell. 1992 Nov 13;71(4):543–546. doi: 10.1016/0092-8674(92)90586-2. [DOI] [PubMed] [Google Scholar]
- Johnson P., Gray D., Mowat M., Benchimol S. Expression of wild-type p53 is not compatible with continued growth of p53-negative tumor cells. Mol Cell Biol. 1991 Jan;11(1):1–11. doi: 10.1128/mcb.11.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanjilal S., Pierceall W. E., Cummings K. K., Kripke M. L., Ananthaswamy H. N. High frequency of p53 mutations in ultraviolet radiation-induced murine skin tumors: evidence for strand bias and tumor heterogeneity. Cancer Res. 1993 Jul 1;53(13):2961–2964. [PubMed] [Google Scholar]
- Kastan M. B., Onyekwere O., Sidransky D., Vogelstein B., Craig R. W. Participation of p53 protein in the cellular response to DNA damage. Cancer Res. 1991 Dec 1;51(23 Pt 1):6304–6311. [PubMed] [Google Scholar]
- Kessis T. D., Slebos R. J., Nelson W. G., Kastan M. B., Plunkett B. S., Han S. M., Lorincz A. T., Hedrick L., Cho K. R. Human papillomavirus 16 E6 expression disrupts the p53-mediated cellular response to DNA damage. Proc Natl Acad Sci U S A. 1993 May 1;90(9):3988–3992. doi: 10.1073/pnas.90.9.3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krolewski B., Little J. B. Application of denaturing gradient gel blots to detect p53 mutations in X-ray-transformed mouse C3H 10T1/2 clones. Mol Carcinog. 1993;7(3):190–196. doi: 10.1002/mc.2940070309. [DOI] [PubMed] [Google Scholar]
- Kuerbitz S. J., Plunkett B. S., Walsh W. V., Kastan M. B. Wild-type p53 is a cell cycle checkpoint determinant following irradiation. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7491–7495. doi: 10.1073/pnas.89.16.7491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler G., Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975 Aug 7;256(5517):495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- Lamb P., Crawford L. Characterization of the human p53 gene. Mol Cell Biol. 1986 May;6(5):1379–1385. doi: 10.1128/mcb.6.5.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay J., Steel C. M., Elder P. A., Forrest A. P., Evans H. J. Allele loss on short arm of chromosome 17 in breast cancers. Lancet. 1988 Dec 17;2(8625):1384–1385. doi: 10.1016/s0140-6736(88)90584-3. [DOI] [PubMed] [Google Scholar]
- Malkin D., Li F. P., Strong L. C., Fraumeni J. F., Jr, Nelson C. E., Kim D. H., Kassel J., Gryka M. A., Bischoff F. Z., Tainsky M. A. Germ line p53 mutations in a familial syndrome of breast cancer, sarcomas, and other neoplasms. Science. 1990 Nov 30;250(4985):1233–1238. doi: 10.1126/science.1978757. [DOI] [PubMed] [Google Scholar]
- Mihara K., Bai L., Kano Y., Miyazaki M., Namba M. Malignant transformation of human fibroblasts previously immortalized with 60Co gamma rays. Int J Cancer. 1992 Feb 20;50(4):639–643. doi: 10.1002/ijc.2910500426. [DOI] [PubMed] [Google Scholar]
- Namba M., Nishitani K., Hyodoh F., Fukushima F., Kimoto T. Neoplastic transformation of human diploid fibroblasts (KMST-6) by treatment with 60Co gamma rays. Int J Cancer. 1985 Feb 15;35(2):275–280. doi: 10.1002/ijc.2910350221. [DOI] [PubMed] [Google Scholar]
- Runnebaum I. B., Nagarajan M., Bowman M., Soto D., Sukumar S. Mutations in p53 as potential molecular markers for human breast cancer. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10657–10661. doi: 10.1073/pnas.88.23.10657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sager R. Tumor suppressor genes: the puzzle and the promise. Science. 1989 Dec 15;246(4936):1406–1412. doi: 10.1126/science.2574499. [DOI] [PubMed] [Google Scholar]
- Srivastava S., Zou Z. Q., Pirollo K., Blattner W., Chang E. H. Germ-line transmission of a mutated p53 gene in a cancer-prone family with Li-Fraumeni syndrome. Nature. 1990 Dec 20;348(6303):747–749. doi: 10.1038/348747a0. [DOI] [PubMed] [Google Scholar]
- Stürzbecher H. W., Maimets T., Chumakov P., Brain R., Addison C., Simanis V., Rudge K., Philp R., Grimaldi M., Court W. p53 interacts with p34cdc2 in mammalian cells: implications for cell cycle control and oncogenesis. Oncogene. 1990 Jun;5(6):795–781. [PubMed] [Google Scholar]
- Tuynder M., Godfrine S., Cornelis J. J., Rommelaere J. Dose-dependent induction of resistance to terminal differentiation in x-irradiated cultures of normal human keratinocytes. Proc Natl Acad Sci U S A. 1991 Apr 1;88(7):2638–2642. doi: 10.1073/pnas.88.7.2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogelstein B., Kinzler K. W. p53 function and dysfunction. Cell. 1992 Aug 21;70(4):523–526. doi: 10.1016/0092-8674(92)90421-8. [DOI] [PubMed] [Google Scholar]
- Zajchowski D., Band V., Pauzie N., Tager A., Stampfer M., Sager R. Expression of growth factors and oncogenes in normal and tumor-derived human mammary epithelial cells. Cancer Res. 1988 Dec 15;48(24 Pt 1):7041–7047. [PubMed] [Google Scholar]