Abstract
Dopamine transporter knockout (DAT KO) mice exhibit elevated extracellular dopamine levels in brain regions that include the striatum and the nucleus accumbens, but not the prefrontal cortex. DAT KO mice model some aspects of psychiatric disorders, including schizophrenia. Smoking is more common in patients with schizophrenia, suggesting that nicotine might ameliorate aspects of the behavioral abnormalities and/or treatment side effects seen in these individuals. We report nicotine-induced normalization of effects on locomotion and prepulse inhibition of acoustic startle (PPI) in DAT KO mice that require intact serotonin 5-HT1A systems. First, we observed that the marked hyperactivity displayed by DAT KO mice was reduced by administration of nicotine. This nicotine effect was blocked by pretreatment with the non-specific nicotinic acetylcholine (nACh) receptor antagonist mecamylamine, or the 5-HT1A antagonist WAY100635. Secondly, we examined the effects of nicotine on PPI in DAT KO mice. Treatment with nicotine significantly ameliorated the PPI deficits observed in DAT KO mice. The ameliorating action of nicotine on PPI deficits in DAT KO mice was blocked by mecamylamine, the α7 nACh receptor antagonist methyllycaconitine or WAY100635, while the α4β2 nACh receptor antagonist dihydro-β-erythroidinehydrobromide (DHβE) produced only a non-significant trend toward attenuation of nicotine effects. Finally, we observed that administration of the 5-HT1A receptor agonist 8-OH-DPAT also ameliorated the deficit in PPI observed in DAT KO mice. This amelioration was antagonized by pretreatment with WAY100635. These data support the idea that nicotine might ameliorate some of the cognitive dysfunctions found in schizophrenia in a 5-HT1A-dependent fashion.
Keywords: α7 nACh receptor, α4β2 nACh receptor, 5-HT1A receptor, Schizophrenia, Locomotor activity, Prepulse inhibition
1. Introduction
Beneficial effects of nicotine and nicotinic acetylcholine (nACh) receptor agonists can be demonstrated in numerous behavioral and cognitive studies in both humans and animal models (for review see (Hall et al., 2012)). There are apparent beneficial effects of nicotine and/or nicotinic acetylcholine receptor agonists in several psychiatric disorders, including schizophrenia. The high rates of smoking in schizophrenic patients (Conklin et al., 2008; Dursun and Kutcher, 1999; Picciotto et al., 2000), have led to the suggestion that nicotine may provide self-medication for these patients, perhaps for aspects of impaired behavior and cognition characteristic of this disease and/or for unwanted effects of antipsychotic medications (Dalack et al., 1998; Kumari and Postma, 2005; Ripoll et al., 2004). Nicotinic agonists do appear to improve deficits in cognitive functioning (Potter et al., 2006; Wilens and Decker, 2007), and sensory gating (George et al., 2006; Petrovsky et al., 2010) found in schizophrenia patients.
Nicotine affects function of nACh receptors that consist of combinations of the 12 nACh receptors subunits (α2–α10 and β2–β4) (Albuquerque et al., 2009; Mihailescu and Drucker-Colin, 2000). While homomeric α7 and heteromeric α4β2 nACh receptors have been implicated in the pathophysiology of cognitive deficits in schizophrenia (Olincy et al., 2006), the exact nACh receptor subtype(s) responsible for nicotine-mediated improvement in these cognitive deficits remain to be elucidated.
Homozygous deletion of the dopamine transporter (DAT) gene in DAT knockout (DAT KO) mice produces a ten-fold increase of extracellular dopamine (DA) concentrations in the striatum measured by in vivo microdialysis (Shen et al., 2004). DAT KO mice exhibit behavioral alterations, many linked to these changes in dopaminergic function, that include hyperlocomotion (Sora et al., 1998; Gainetdinov et al., 1999b), cognitive deficits (Li et al., 2010; Morice et al., 2007), and impairments of prepulse inhibition (PPI) of the startle reflex (Arime et al., in press; Ralph et al., 2001; Yamashita et al., 2006). Pharmacological treatments that can ameliorate these deficits in DAT KO mice include psychostimulants, norepinephrine transporter (NET) blockers and DA antagonists.
Several lines of evidence suggest possible serotonergic influences on nicotine effects in DAT KO mice. In previous work examining the molecular basis of cocaine conditioned place preference, both DAT and the serotonin transporter (SERT) were found to be involved (Hall et al., 2002; Sora et al., 2001). Serotonergic mechanisms have been suggested to have locomotion-decreasing effects in DAT KO mice (Gainetdinov et al., 1999b). While the precise serotonin receptor subtypes that may underlie these differences between DAT KO and wild type (WT) mice have yet to be elucidated, treatment with 5-HT1A receptor antagonists can attenuate cocaine-induced hyperactivity in rats (Muller et al., 2002). This result suggests that 5-HT1A systems might play an important role in psychostimulant-mediated behavioral effects. Furthermore, post mortem brain tissue from schizophrenia patients displays increased numbers of prefrontal cortical 5-HT1A receptor binding sites (Burnet et al., 1997). Treatment with 5-HT1A receptor agonists can have beneficial effects on cognitive function in schizophrenia patients treated with atypical antipsychotic drugs (Sumiyoshi et al., 2007). However, we have no information about possible serotonergic involvement in the beneficial effects of nicotine or cognitive deficits in schizophrenia.
We now report effects of nicotine on hyperlocomotion and sensory gating deficits in DAT KO mice, as well as examination of the potential roles for 5-HT1A receptors in these processes using 5-HT1A receptor agonists and antagonists.
2. Materials and methods
2.1. Animals
DAT KO mice (Sora et al., 1998) were bred at the Animal Laboratory Institute of Tohoku University Graduate School of Medicine and maintained on a mixed genetic background combining C57BL/6 and 129Sv mouse strains. Offspring from heterozygote crosses were weaned at 28 days postnatal and housed in groups of two to five (segregated by sex), in a temperature- and light-controlled colony (lights on at 0800 h, lights off at 2000 h), with food and water available ad libitum. Mice were genotyped using multiplex polymerase chain reaction methods on DNA extracted from tail biopsies, as previously described (Shen et al., 2004). Behavioral testing was conducted in mice of both sexes, between 8 and 14 weeks of age. All animal experiments were performed in accordance with the Guidelines for the Care of Laboratory Animals of Tohoku University Graduate School of Medicine.
2.2. Drugs
For behavioral testing, all drugs were dissolved in physiological saline (0.9% sodium chloride), and were administered intraperitoneally in a volume of 10 ml/kg: (−)-nicotine tartrate (nicotine, Sigma-Aldrich, Japan), the non-specific nACh receptor antagonist mecamylamine hydrochloride (MCA, Sigma-Aldrich, Japan), the α4β2 nACh receptor antagonist dihydro-β-erythroidinehydrobromide (DHβE, Sigma-Aldrich, Japan), the α7 nACh receptor antagonist methyllycaconitine citrate hydrate (MLA, Sigma-Aldrich, Japan), the 5-HT1A agonist (±)-8-Hydroxy-2-(dipropylamino) tetralin hydrobromide (DPAT, Sigma-Aldrich, Japan), and the specific 5-HT1A antagonist WAY100635 maleate salt (WAY100635, Sigma-Aldrich, Japan). The pH of the nicotine solution was neutralized with sodium hydroxide. No published studies have documented the effects of acute nicotine on PPI in DAT KO mice. Hence, to determine dose ranges, we primarily consulted previous data on locomotor activity effects of nicotine in WT and DAT KO mice (Weiss et al., 2007). Since effective doses may differ between locomotion and prepulse inhibition, preliminary studies were performed. Based on the preliminary results, effective doses of each drug (described below) or dose intervals were determined.
2.3. Experimental procedures
2.3.1. Measurement of locomotor activity of WT and DAT KO mice after nicotine and/or receptor antagonist treatment
Mice were placed individually in clear plastic cages (40 × 30 × 26 cm), and locomotor activity was measured in using digital counters with passive infrared sensors (Supermex system, Muromachi Kikai, Tokyo, Japan). In nicotine-induced locomotor activity tests, WT and DAT KO mice were first habituated in the test chamber to the apparatus for 20 min and then injected with nicotine (0.3, 1 and 3 mg/kg) or saline intraperitoneally. Locomotor activity was then assessed for 60 min post-injection (N = 8–12 per treatment condition per genotype).
In experiments examining the effect of nicotine in DAT KO mice after receptor antagonists, WT and DAT KO mice were first habituated for 20 min after pretreatment with 2 mg/kg of non-specific nACh receptor antagonist mecamylamine or 1 mg/kg of specific 5-HT1A antagonist WAY100635, and then injected with nicotine (1 mg/kg) or saline. Locomotor activity was assessed for 60 min post-nicotine or saline injection (N = 8–12 per treatment condition per genotype).
2.3.2. Measurement of startle response and prepulse inhibition
Startle chambers (SR-LAB, San Diego Instruments, San Diego, CA) were used to measure the startle response. Each chamber consisted of a nonrestrictive Plexiglas cylinder mounted on a frame inside a lighted, ventilated box (35 × 35 × 47.5 cm3). Movement within the cylinder was detected by piezoelectric accelerometers attached to the cylinder’s bottom. Force detected by the accelerometer was converted into analog signals that were digitized and stored electronically. In all experiments, 65 readings were recorded at 1 ms intervals beginning at stimulus onset; the average amplitude was used to describe the acoustic startle response. A high-frequency loudspeaker inside the chamber, mounted above the cylinder, generated broadband background noise and acoustic stimuli, which were controlled by the SR-LAB software system and interface. Sound levels (dB (A) scale) and accelerometer sensitivity were calibrated routinely, as described previously (Geyer and Dulawa, 2003; Geyer and Swerdlow, 2001).
Experiments were conducted using previously reported methods (e.g., (Yamashita et al., 2006)). For acoustic startle experiments, mice were tested initially for baseline PPI and pseudo-randomly assigned to drug treatment groups based on these measurements. Experimental sessions consisted of a 5 min acclimatization period with 65 dB broadband background noise followed by PPI sessions. Sessions consisted of five different trial types: no stimulus trials (nostim); startle pulse alone, 40 ms duration at 120 dB (p120); and three prepulse + pulse trials, 20 ms duration prepulse at 68 dB (pp3), 71 dB (pp6), or 77 dB (pp12), followed by a 40 ms duration startle stimulus at 120 dB after a 100 ms delay. The nostim trial consisted of only background broadband noise. All test sessions started and concluded with six presentations of the p120 trial, while the remainder of the session consisted of 12 presentations of the p120 trial type,10 presentations of the nostim, the pp3, pp6, and pp12 trial types, in a pseudorandom order, with varying intertrial intervals (mean 15 s, range 8–23 s). Each PPI session lasted 21 min and consisted of a total of 64 trials.
The effect of nicotine on PPI was examined in WT and DAT KO mice. Nicotine (0.3 mg/kg, 1 mg/kg, or 3 mg/kg) was administered 5 min before testing to WT and DAT KO mice (N = 8–16 per treatment condition). The effects of nicotinic antagonists on the amelioration of PPI deficits in DAT KO mice by nicotine were examined by injecting DAT KO mice with the nicotinic receptor antagonists MCA (5 mg/kg or 10 mg/kg), DHβE (1 mg/kg or 2 mg/kg), or MLA (2 mg/kg or 5 mg/kg), 20 min before the nicotine treatment. Nicotine was administered 5 min before PPI testing (N = 10–15 per treatment condition). The effects of 5-HT1A antagonism on the amelioration of impaired PPI in DAT KO mice by nicotine was examined by administering the 5-HT1A antagonist WAY100635 (1 mg/kg or 3 mg/kg), 20 min before the nicotine treatment. Nicotine was administered 5 min before PPI testing (N = 14–15 per treatment condition). The effect of the 5-HT1A agonist DPAT and/or 5-HT1A antagonist WAY100635 on PPI was examined in WT and DAT KO mice. Mice of each genotype were administered saline or 3 mg/kg of WAY100635 20 min before DPAT treatment (0.3 mg/kg or 1 mg/kg DPAT, or saline), 10 min before testing (N = 10–14 per treatment condition).
2.4. Data and statistical analysis
2.4.1. Locomotor activity
Locomotor activity data were submitted to ANOVA with GENOTYPE (WT versus DAT KO), SEX and nicotine dose (NICOTINE; nicotine versus saline) as between subjects factors. In studies examining the effects of the nACh receptor antagonist, mecamylamine, or 5-HT1A receptor antagonist WAY100635, in DAT KO mice. The data were first submitted to ANOVA with GENOTYPE × SEX as between subjects factors. Subsequently, data from DAT KO mice were submitted to ANOVA with saline, mecamylamine, or WAY100635 (PRETREATMENT) and SEX as between subjects factors.
2.4.2. Prepulse inhibition
Prepulse inhibition was calculated as a percentage score for each prepulse intensity using the following equation: % PPI = 100−{(startle response for prepulse + pulse trials (pp3, pp6, or pp12))/(startle response for pulse alone trials (p120)) × 100}. The startle magnitude was calculated as the average of all pulse alone (p120) trials, excluding the first six and last six p120 trials in each session. The effects of nicotine on PPI and startle data were submitted to ANOVA with the between-subjects factors of nicotine dose (NICOTINE; nicotine versus saline), SEX and GENOTYPE (WT versus DAT KO), and the within-subjects factor of prepulse intensity (INTENSITY; pp3, pp6, and pp12). In the antagonist study, the effects of pretreatments with antagonists and NICOTINE on PPI and startle data were first submitted to ANOVA with SEX and GENOTYPE (WT versus DAT KO mice after saline treatment) as between subject factors, with the within-subjects factor of INTENSITY to confirm baseline genotypic effects. The PPI and startle data for DAT KO mice were then submitted to ANOVA with SEX and TREATMENT (saline, nicotine or nicotine in combination with one of the nACh receptor antagonists, mecamylamine, methyllycaconitine, or dihydro-β-erythroidinehydrobromide) as between subjects factors, and the within-subjects factor of INTENSITY. The effects of the 5-HT1A receptor antagonist WAY100635 were examined by ANOVA in a similar fashion. The effects of DPAT treatments on PPI and startle data were submitted to ANOVA with the between-subjects factors of DPAT treatment (DPAT), SEX and GENOTYPE, and the within-subjects factor of INTENSITY. For brevity, main effects of prepulse intensity will not be discussed because they were always significant. Where ANOVA indicated significant effects of one or more factors, Bonferroni tests of individual mean differences were conducted for post hoc analysis.
All data were presented as the mean ± SEM. Data were analyzed using the SPSS statistical package (SPSS Ver11.5J for Windows, SPSS Inc., Tokyo, Japan).
3. Results
3.1. Locomotor activity studies
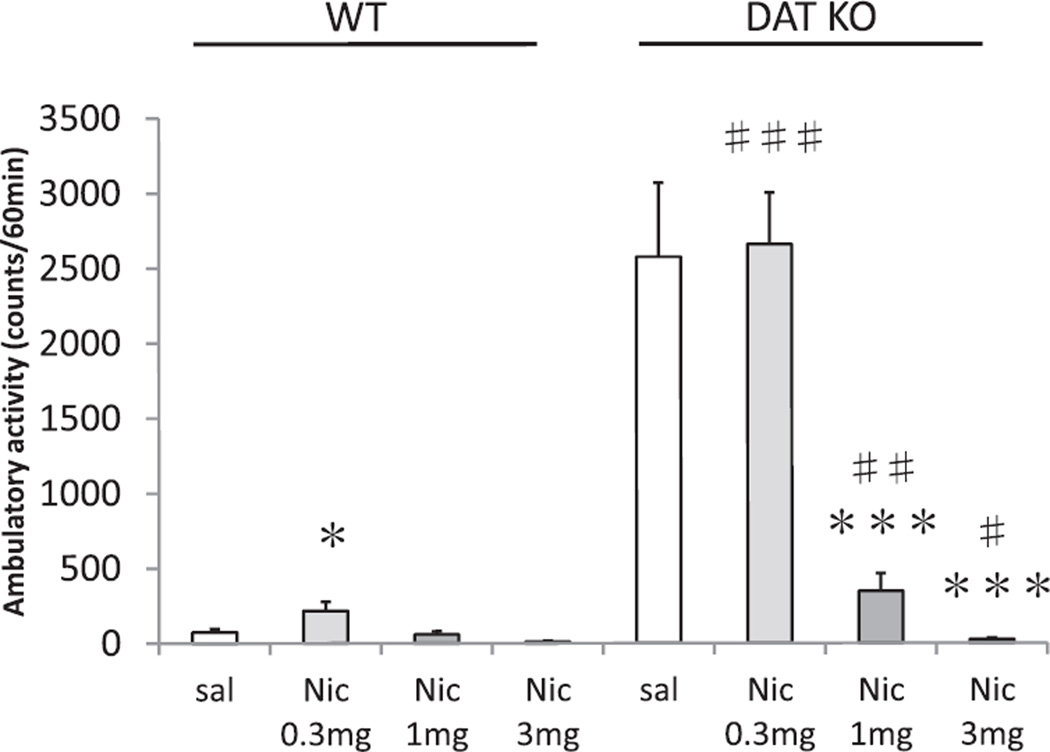
As is quite clear in Fig. 1, DAT KO mice were profoundly hyperactive compared to WT littermates, an effect that was greatly reduced by nicotine. The ANOVA revealed a significant GENOTYPE effect on locomotor activity (F(1, 73) = 108.7; p < 0.001), and a significant GENOTYPE × NICOTINE interaction on locomotor activity (F(3, 73) = 28.6; p < 0.001). Furthermore, the usual pattern of response was altered in DAT KO mice compared to WT mice. There was no significant effect of SEX nor any significant interactions with SEX. Therefore, data are presented discounting SEX. In post hoc comparisons, at the 0.3 mg/kg nicotine dose, locomotor activity of WT mice was higher than the saline treatment group (p < 0.05), but this was not observed in DAT KO mice. Treatment with only the highest dose of nicotine, 3 mg/kg nicotine, caused significant locomotor decreases in WT mice while both the 1 and 3 mg/kg doses of nicotine reduced locomotor activity (p < 0.05) in DAT KO mice. Thus, DAT KO mice were profoundly hyperactive after treatment with saline or the low dose of nicotine compared to WT mice (p < 0.001) but at the higher doses of nicotine, 1 mg/kg and 3 mg/kg nicotine treatments, activity was significantly attenuated in DAT KO mice, nearly to WT levels.
Fig. 1.
Dose-response study of nicotine-induced locomotor activity in WT and DAT KO mice. Total ambulatory activity for 60 min after administration of saline (sal), 0.3 mg/kg nicotine (Nic 0.3 mg), 1 mg/kg nicotine (Nic 1 mg), or nicotine (Nic 3 mg) were indicated. Values represent mean ± SEM. *p = 0.05, compared with WT sal; ***p < 0.001 compared with DAT KO sal; ###p < 0.001 compared with WT sal; ##p < 0.005 compared with WT nicotine 1 mg; #p < 0.05 compared with WT nicotine 3 mg. N = 8–12 per treatment condition per genotype (male N = 4–8, female N = 4–7).
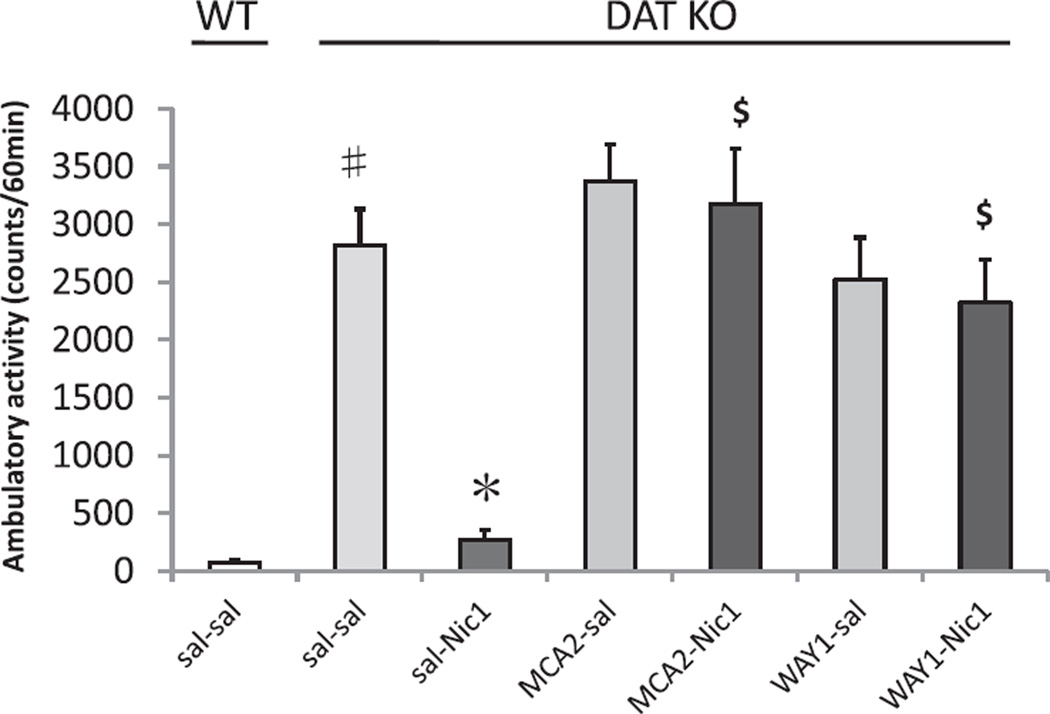
The interaction of nicotine-induced hypolocomotion in DAT KO mice with the non-specific nACh receptor antagonist mecamylamine hydrochloride (MCA) and the specific 5-HT1A antagonist WAY100635 (PRETREATMENT) were examined. Neither pretreatment with 2 mg/kg of MCA (MCA2) nor WAY100635 had significant effects on baseline DAT KO hyperactivity. However, pretreatment with either antagonist almost completely eliminated the locomotor decreasing effects of nicotine on DAT KO induced hyperlocomotion (MCA, p < 0.001 compared to DAT KO nicotine after saline pretreatment; WAY100635, p < 0.001 compared to DAT KO nicotine after saline pretreatment; Fig. 2). Again, no significant effects of SEX or significant interactions with SEX were observed, so data is presented ignoring this factor.
Fig. 2.
Effects of antagonist pretreatments on nicotine-induced locomotor activity in DAT KO mice. Total ambulatory activity for 60 min after administration of saline and 1 mg/kg nicotine (sal–Nic1) treatments significantly reduced hyperactivity compared to DAT KO treated with saline and saline (sal–sal). Pretreatment with mecamylamine 2 mg/kg (MCA2) or WAY100635 1 mg/kg (WAY1) significantly reversed the normalization of DAT hyperactivity produced by treatment with saline and 1 mg/kg nicotine (sal–Nic1). Values represent mean ± SEM. #p < 0.001 compared with WT sal–sal; *p < 0.005 compared with DAT KO sal–sal; $ p < 0.001 compared with DAT KO sal–Nic1. N = 8–12 per treatment condition (male N = 4–6, female N = 4–6).
3.2. Prepulse inhibition studies
3.2.1. The effects of nicotine on PPI in WT and DAT KO mice
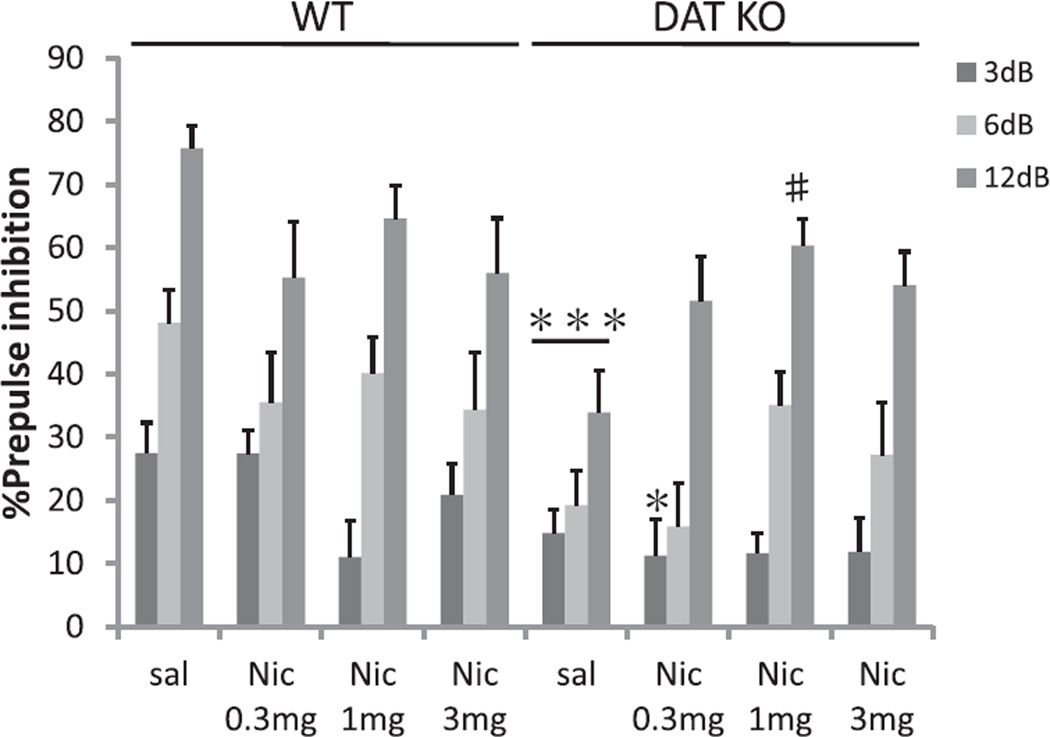
ANOVA revealed a significant GENOTYPE effect on PPI (F(1, 90) = 11.3; p < 0.005) and a significant GENOTYPE × NICOTINE interaction on PPI (F(3, 90) = 2.8; p < 0.05). There was significant main effect of NICOTINE alone on PPI (F(3, 90) = 4.1; p < 0.01). DAT KO mice displayed significantly reduced levels of PPI compared to WT mice at baseline, e.g. after saline treatment (Fig. 3). Nicotine (1 mg/kg) significantly increased PPI in DAT KO mice compared to saline treatment. Post hoc testing showed a significant difference between the saline (sal) group and the nicotine 1 mg/kg treatment (Nic1 mg) group at 12 dB prepulse intensities, for example (p < 0.005; Fig. 3). Table 1 shows the effect on startle amplitude. Startle reactivity was not significantly affected by either GENOTYPE or NICOTINE treatment (Table 1). Again, no significant effects of SEX or significant interactions with SEX were observed, so data is presented ignoring this factor.
Fig. 3.
Effects of nicotine on PPI in WT and DAT KO mice. PPI in WT and DAT KO mice after administration of saline (sal), 0.3 mg/kg nicotine (Nic 0.3 mg), 1 mg/kg nicotine (Nic 1 mg), or 3 mg/kg nicotine (Nic 3 mg); DAT KO mice treated with saline displayed significantly reduced PPI compared with WT mice. Nicotine (1 mg/kg) significantly ameriolated PPI deficits in DAT KO mice, which was significant for the pp12 dB condition. %PPI values represent mean ± SEM. *p < 0.05, compared with WT Nic 0.3 mg pp3 dB; ***p < 0.001 compared with WT sal; #p < 0.005 compared with DAT KO sal pp12 dB. N = 8–16 per treatment condition per genotype (male N = 4–7, female N = 4–9).
Table 1.
Effects of drug treatment on acoustic startle reactivity.
| Treatments | Wild type | DAT KO |
|---|---|---|
| Nicotine treatments | ||
| Saline | 101.8 ± 22.8 | 109.1 ± 17.3 |
| Nicotine 0.3 | 101.8 ± 22.8 | 121.8 ± 25.0 |
| Nicotine 1 | 106.6 ± 32.4 | 112.6 ± 24.4 |
| Nicotine 3 | 77.5 ± 22.1 | 103.8 ± 18.8 |
| Nicotine and nACh receptor antagonists treatments | ||
| sal–sal | 95.2 ± 18.2 | 78.7 ± 14.4 |
| sal–nic1 | 108.7 ± 19.4 | |
| MCA5–nic1 | 109.8 ± 18.6 | |
| MCA10–nic1 | 83.6 ± 22.8 | |
| DHβE1–nic1 | 85.1 ± 20.5 | |
| DHβE2–nic1 | 102.0 ± 21.4 | |
| MLA2–nic1 | 77.2 ± 16.3 | |
| MLA5–nic1 | 130.5 ± 29.3 | |
| Nicotine and 5-HT receptor antagonist treatments | ||
| sal–sal | 95.2 ± 18.2 | 78.7 ± 14.4 |
| sal–nic1 | 108.7 ± 19.4 | |
| WAY1–nic1 | 89.0 ± 20.3 | |
| WAY3–nic1 | 77.7 ± 15.5 | |
| 5-HT receptor agonist and antagonist treatments | ||
| sal–sal | 94.5 ± 23.3 | 61.9 ± 9.7 |
| sal–DPAT0.3 | 55.1 ± 12.2 | 51.4 ± 8.7 |
| sal–DPAT1 | 87.9 ± 22.2 | 78.2 ± 13.3 |
| WAY3–DPAT1 | 96.4 ± 21.1 | 60.3 ± 10.7 |
Values (arbitrary units) represent mean startle magnitude ± SEM. KO, dopamine transporter knockout mice. No significant main effect on acoustic startle reactivity in each treatments was found.
3.2.2. nACh receptor and 5-HT1A receptor antagonism of the effects of nicotine on PPI in DAT KO mice
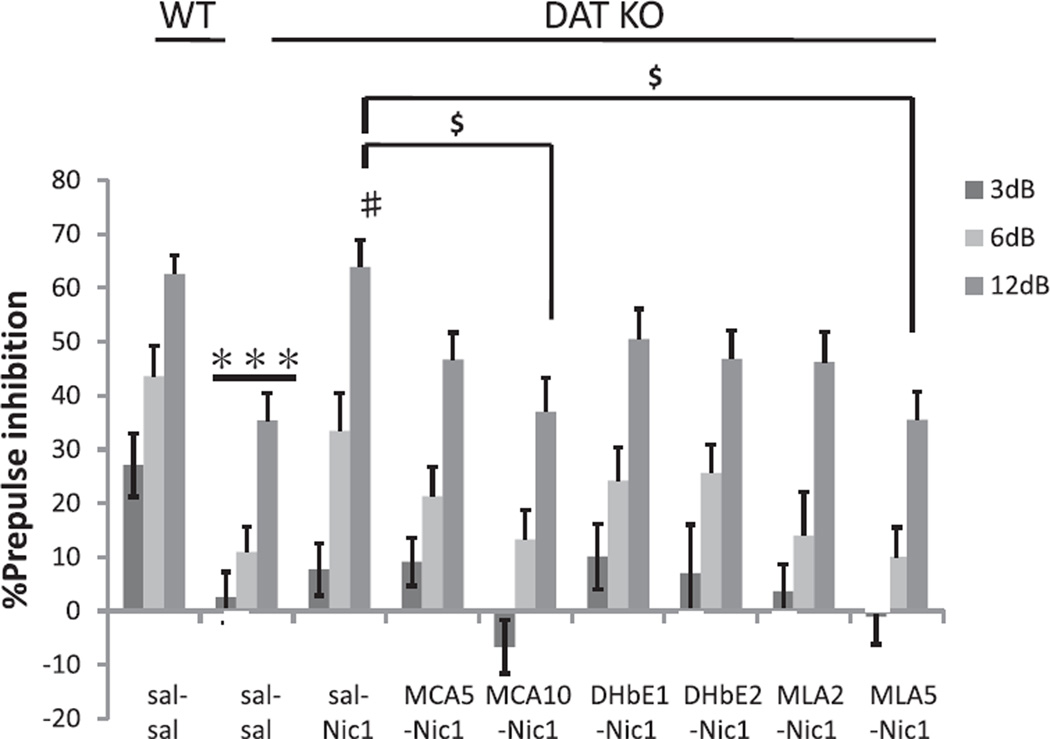
In the nicotine antagonist studies, the effects of DAT KO on PPI and the effects of nicotine on these impairments were assessed. ANOVA revealed a significant GENOTYPE effect on PPI in saline-tested wild type and DAT KO mice (F(1, 24) = 23.5, p < 0.001; Fig. 4). Again, saline-treated DAT KO mice displayed significantly reduced levels of PPI compared to similarly treated WT mice. Fig. 4 shows the effects of nicotinic receptor antagonists on the nicotine-induced amelioration of PPI deficits in DAT KO mice. ANOVA revealed a significant PRETREATMENT effect on the nicotine-induced amelioration of PPI deficits in DAT KO mice (F(7, 90) = 2.8; p < 0.05), with all antagonists reducing the effect of nicotine, although significant reductions were only observed for mecamylamine and methyllycaconitine. Post hoc tests of PRETREATMENT data showed a significant difference (p < 0.05) between the sal–Nic1 group and the MCA10–Nic1 group in DAT KO mice which at the 12 dB prepulse intensity. The selective α7 nACh receptor antagonist methyllycaconitine (MLA; 2 and 5 mg/kg) also dose-dependently antagonized the nicotine-induced PPI amelioration in DAT KO mice. Post hoc testing showed a significant difference between the nicotine 1 mg/kg/saline pretreatment (sal–Nic1) group and the nicotine 1 mg/kg methyllycaconitine pretreatment (MLA5–Nic1) group at the 12 dB prepulse intensity (p < 0.05; Fig. 4). Three of five DAT KO mice died after receiving 5 mg/kg doses of the selective α4β2 nACh receptor antagonist DhβE, therefore analysis was restricted to lower doses. 1 and 2 mg/kg DHβE treatments were tested for their antagonism of nicotine effects on PPI deficits in DAT KO mice. DHβE treatments at 1 or 2 mg/kg dose showed modest trends toward attenuation of the effects of nicotine on PPI deficits in DAT KO mice. Table 1 shows the effect on startle amplitude. Again, no significant effects of SEX or significant interactions with SEX were observed, so data is presented ignoring this factor.
Fig. 4.
Effects of nACh receptor antagonist treatments on the amelioration of PPI deficits in DAT KO mice by nicotine. Although DAT KO mice treated with saline and saline (sal–sal) displayed significantly reduced PPI compared with WT mice treated with saline and saline (sal–sal), saline and nicotine 1 mg/kg (sal–Nic1) treatments significantly reversed PPI deficits in DAT KO mice at pp12 dB. Pretreatment with mecamylamine, 10 mg/kg (MCA10) or methyllycaconitine, 5 mg/kg (MLA5) significantly antagonized the recovery of PPI by nicotine in DAT KO mice. %PPI values represent mean ± SEM. ***p < 0.001 compared with WT sal–sal; #p < 0.01 compared with DAT KO sal–sal pp12 dB; $p < 0.05 compared with DAT KO sal–Nic1 + MCA10–Nic1 group, and DAT KO sal–Nic1 + MLA5–Nic1 group. N = 11–15 per treatment condition (male N = 5–8, female N = 6–8).
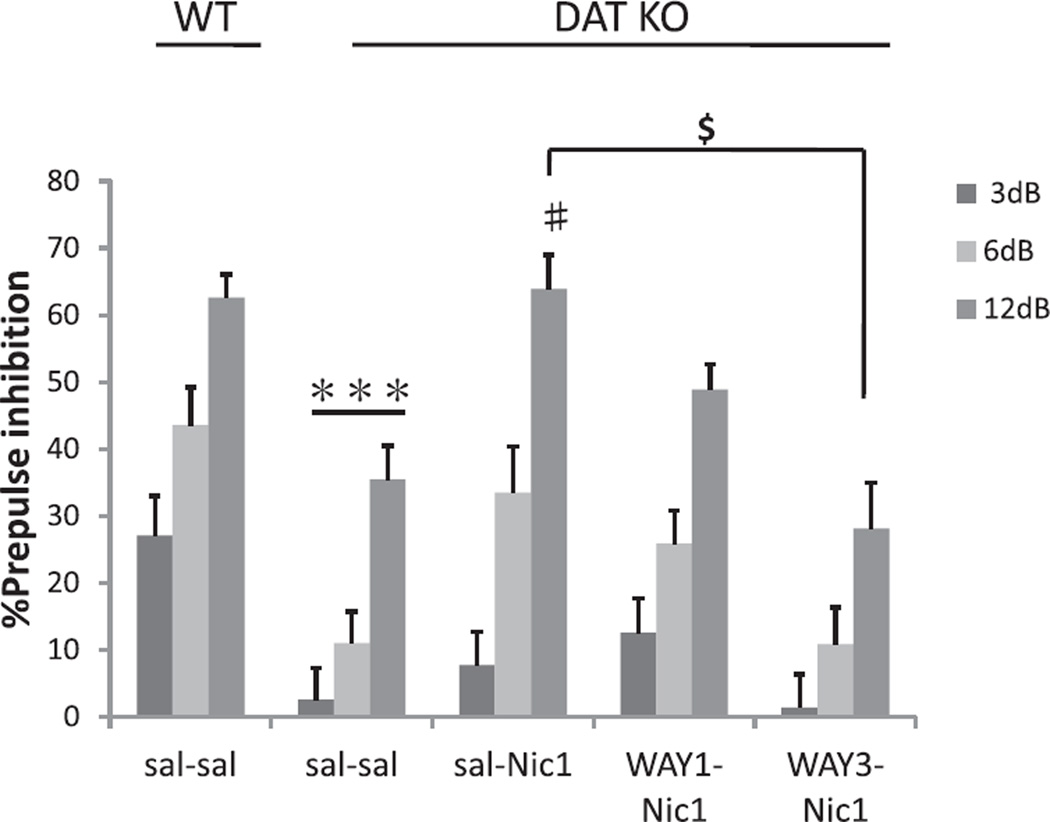
Fig. 5 shows the effects of WAY100635 on the nicotine-induced amelioration of PPI deficits in DAT KO mice. ANOVA revealed a significant PRETREATMENT (5-HT1A receptor antagonist WAY100635) effect on PPI (F(3, 54) = 6.0; p < 0.005). There was thus a reduced effect of nicotine amelioration of PPI deficits after pretreatment with the 5-HT1A receptor antagonist WAY100635 in DAT KO mice. This effect of WAY100635 (1 and 3 mg/kg) was dose-dependent. Post hoc testing showed a significant difference between the nicotine 1 mg/kg saline pretreatment (sal–Nic1) group and the nicotine 1 mg/kg WAY100635 3 mg/kg pretreatment (WAY3–Nic1) group at the 12 dB prepulse intensity (p < 0.05; Fig. 5). Table 1 shows the effect on startle amplitude, which was not significantly affected by nicotine or co-administered antagonists. Again, no significant effects of SEX or significant interactions with SEX were observed, so data is presented ignoring this factor.
Fig. 5.
Effects of a 5-HT1A receptor antagonist on the amelioration of PPI deficits in DAT KO mice by nicotine. Although DAT KO mice treated with saline and saline (sal–sal) displayed significantly reduced PPI compared with WT mice, saline and nicotine 1 mg/kg (sal–Nic1) treatments significantly reversed PPI deficits in DAT KO mice compared to saline and saline (sal–sal) treated mice at pp12 dB. Pretreatment with WAY100635 3 mg/kg (WAY3) significantly antagonized the recovery of PPI by nicotine in DAT KO mice. %PPI values represent mean ± SEM. ***p < 0.001 compared with WT sal–sal; #p < 0.01 compared with DAT KO sal–sal pp12 dB; $p < 0.05 compared with DAT KO sal–Nic1 + WAY3–Nic1 group. N = 11–15 per treatment condition (male N = 5–8, female N = 6–7).
3.2.3. The effects of DPAT on PPI in WT and DAT KO mice
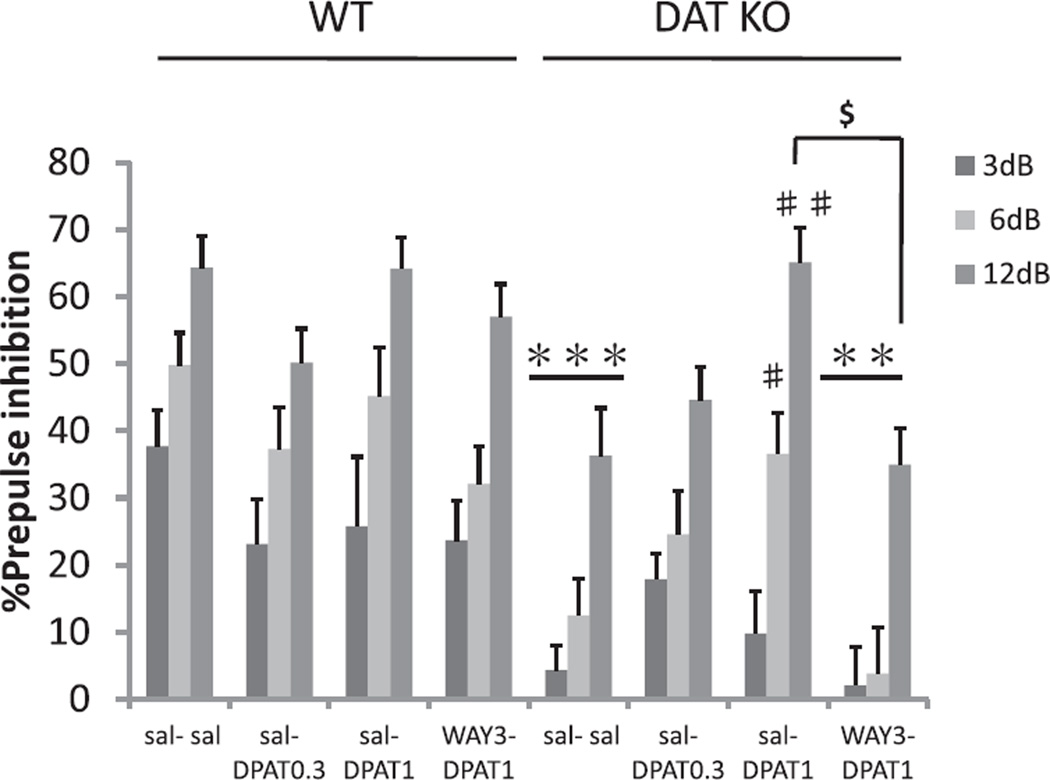
In the DPAT experiment ANOVA again revealed a significant GENOTYPE effect on PPI at baseline (F(1, 89) = 27.0; p < 0.001). DAT KO mice displayed significantly reduced levels of PPI compared to WT mice after saline treatment (Fig. 6). DPAT (1 mg/kg) significantly ameliorated impairments of PPI in DAT KO mice compared to sal–sal treatment (Fig. 6). Pretreatment with WAY100635 antagonized the amelioration of PPI deficits by DPAT in DAT KO mice. ANOVA revealed a significant effect of DPAT (F(3, 89) = 3.2; p < 0.05), and a significant GENOTYPE × DPAT interaction on PPI (F(3, 89) = 3.2; p < 0.05), reflecting normalization of PPI in DAT KO mice. Post hoc testing showed a significant difference between the DPAT1 mg/kg and saline pretreatment group (sal–DPAT1) and the DPAT1 mg/kg WAY100635 3 mg/kg pretreatment group (WAY3–DPAT1) at a prepulse intensity of 12 dB (P < 0.005; Fig. 6). Again, no significant effects of SEX or significant interactions with SEX were observed, so data is presented ignoring this factor.
Fig. 6.
The effects of a 5-HT1A receptor agonist on PPI in WT and DAT KO mice. PPI in WT and DAT KO mice after administration of saline–saline (sal–sal), saline and 0.3 mg/kg DPAT (sal–DPAT0.3), saline and 1 mg/kg DPAT (sal–DPAT1), or WAY100635 3 mg/kg–1 mg/kg DPAT (WAY3–DPAT1). sal–DPAT treatments reversed PPI deficits in DAT KO mice (sal–sal group) at pp6 dB and pp12 dB. The ameliorating action of DPAT was antagonized by pretreatment with 3 mg/kg of WAY100635. %PPI values represent mean ± SEM. ***p < 0.001 compared with WT sal–sal; **p < 0.005 compared with WT WAY3–DPAT1; #p < 0.05 compared with DAT KO sal–sal pp6 dB; ##p < 0.001 compared with DAT KO sal–sal pp12 dB; $p < 0.005 compared with DAT KO sal–DPAT1 + WAY3–DPAT1 group. N = 10–14 per treatment condition per genotype (male N = 5–8, female N = 5–7).
Table 1 shows the effect on startle amplitude. Startle reactivity was not affected by either GENOTYPE or DPAT treatment (Table 1). Again, no significant effects of SEX or significant interactions with SEX were observed, so data is presented ignoring this factor.
4. Discussion
The results from our experiments demonstrate that both of the behavioral abnormalities in DAT KO mice that are the focus of the current studies can be normalized by treatment with nicotine. We observed that both the hyperactivity and PPI deficits displayed by DAT KO mice were reduced by administration of nicotine. These effects of nicotine were attenuated by pretreatment with a α7 nACh receptor antagonist, but not a α4β2 nACh receptor antagonist, consistent with involvement of a selective subset of nACh receptors. Attenuation of nicotine effects by pretreatment with a 5-HT1A receptor antagonist, normalization of DAT KO PPI deficits following and reversal of this 5-HT1A agonist effect by pretreatment with a 5-HT1A receptor antagonist all support roles for specific serotonin systems in nicotine’s effects in DAT KO mice.
Nicotine can activate midbrain dopaminergic neurons and alter function of presynaptic striatal dopaminergic terminals (Zhou et al., 2001), altering DA release and dopaminergic control of reward and motor activity. Locomotor activity in WT mice increased significantly after a low dose of nicotine (0.3 mg/kg), while a higher dose of nicotine (1 mg/kg) did not increase locomotion, and locomotion was decreased at the highest nicotine dose. By contrast, the profound hyperactivity displayed by DAT KO mice was significantly, and substantially, reduced by administration of either 1 or 3 mg/kg nicotine. These data confirm previous observations that DAT KO mice are more sensitive than WT mice to the locomotor decreasing effects of nicotine (Weiss et al., 2007). However, it must be noted that WT mice in that study were found to have a greater reduction in locomotor activity than in the present study, which may suggest that genetic background (or other factors) alter the effects of nicotine in these two DAT mutant strains. Nonetheless, the main finding of that study was replicated in another DAT KO strain.
Nicotine, as well as the psychostimulant monoamine transporter blockers cocaine and amphetamine induced increases extracellular DA concentrations in the prefrontal cortex and nucleus accumbens of WT mice as assessed by in vivo microdialysis (Budygin et al., 2004; Carboni et al., 2001). While DAT KO mice display vastly increased basal DA levels in striatal microdialysates (Weiss et al., 2007), basal prefrontal DA levels are unchanged, as are responses to psychostimulants in the cortex and striatum (Shen et al., 2004). Monoamine transporter blockers can attenuate hyperlocomotion in DAT KO mice in ways that interact with level of habituation (e.g. compare (Gainetdinov et al., 1999a; Giros et al., 1996; Spielewoy et al., 2001)). The locomotor decreasing effects of psychostimulants were initially suggested to be associated with influences of those drugs on serotonin release (Gainetdinov et al., 1999a). The failure of DAT deletion to affect prefrontal DA function can be attributed to low DAT expression in this region, allowing much released DA to be taken up into norepinephrine terminals (Bymaster et al., 2002; Moron et al., 2002) (Fig. 7). We have thus hypothesized that attenuated hyperlocomotion and normalization of PPI deficits in DAT KO mice treated with monoamine transporter blockers could be explained by alterations in dopaminergic neurotransmission produced by NET blockade in the prefrontal cortex and consequent alterations in prefrontal–accumbens activity (Arime et al., 2011). This hypothesis was confirmed by the demonstration that direct injections of NET blockers into the prefrontal cortex normalizes PPI deficits in DAT KO mice (Arime et al., in press). Nicotine treatment also regulates DA neurotransmission in the prefrontal cortex of rats (Shearman et al., 2008). Thus, it would appear that dopaminergic neurotransmission in the prefrontal cortex regulated by nicotine treatment may contribute to the attenuated hyperlocomotion in DAT KO mice treated with nicotine.
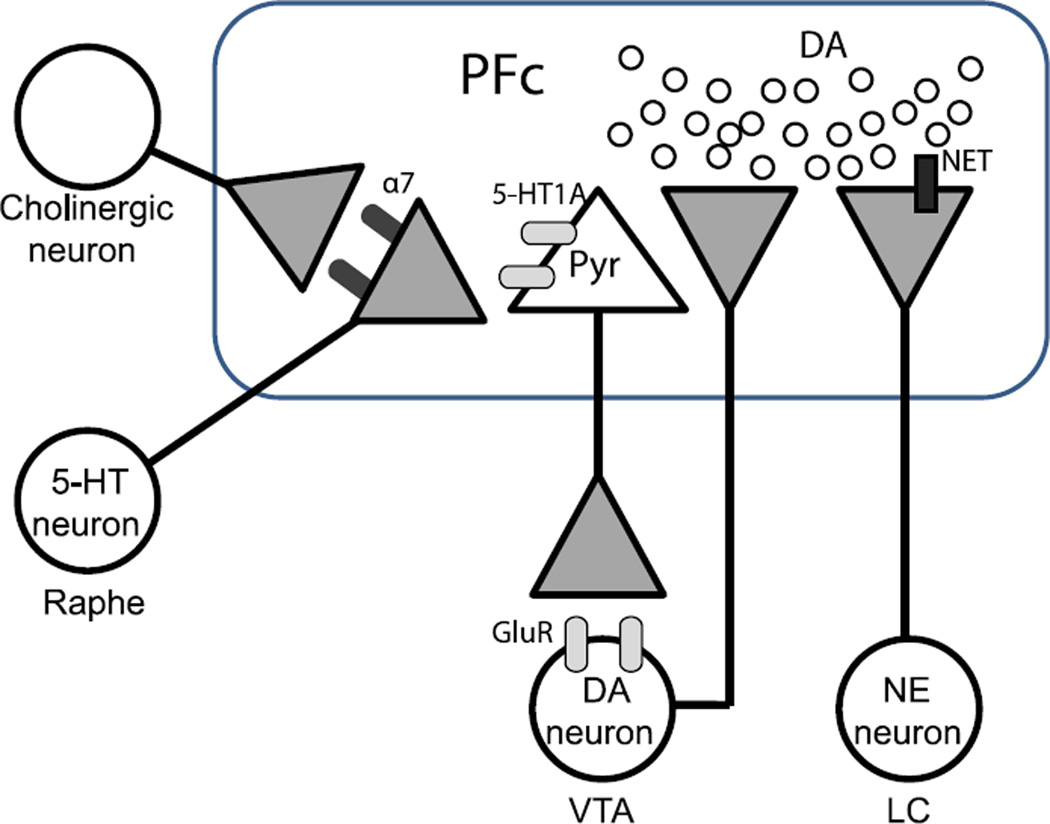
Fig. 7.
Schematic representation of the structures and neurotransmitters involved in the regulation of the nicotine–serotonin interactive dopamine release in the prefrontal cortex (PFc). Serotonin (5-HT) from Raphe nuclei (Raphe), might be released by activation of presynaptic α7 nicotinic acetylcholine (nACh) receptor in the prefrontal cortex (Soria-Fregozo et al., in press). Pyramidal neurons (Pyr) of the prefrontal cortex innervate glutamatergic neurotransmission to the ventral tegmental area (VTA) via 5-HT1A receptor may be involved in the regulation of dopamine (DA) release (Wedzony et al., 2007). Norepinephrine transporter (NET) blockers also could be explained by alterations in dopaminergic neurotransmission produced by NET blockade in the prefrontal cortex activity (Arime et al., 2011). α7; α7 nACh receptor, 5-HT1A; Serotonin1A receptor, GluR; glutamate receptor, NE; norepinephrine, LC; locus coeruleus.
To elucidate the possible mechanisms underlying the effects of nicotine on hyperlocomotion in DAT KO mice, we examined locomotion after pretreatment with nACh or serotonin receptor antagonists. The reduction of hyperlocomotion by nicotine treatment in DAT KO mice was blocked by pretreatment with 2 mg/kg of the non-specific nACh antagonist mecamylamine, confirming the involvement of nACh receptors in these effects. This finding confirmed the previous work of Weiss et al. who reported that co-administration of the α4β2 and α7 nACh receptor antagonists, DHβE and methyllycaconitine, respectively, reversed the effects of 1 mg/kg nicotine treatment in DAT KO mice (Weiss et al., 2007). Furthermore, based on the present series of experiments the effects of nicotine in DAT KO mice appear to depend on serotonergic mechanisms. Pretreatment with the selective 5-HT1A antagonist WAY100635 blocked the reduction of hyperlocomotion produced by nicotine treatment in DAT KO mice. Pretreatment with a 5-HT1A antagonist also attenuated cocaine-induced hyperactivity in rats (Muller et al., 2002). These results suggest that serotonergic mechanisms involving 5-HT1A receptors may play an important role in the reduction of locomotor activity induced by nicotine treatment in DAT KO mice. Electrophysiology studies have revealed the influence of nicotine on serotonin release from the dorsal raphe nucleus (DRN). Serotonergic DRN cells express postsynaptic functional nACh receptors (Galindo-Charles et al., 2008). Presynaptic α7 nACh receptors may modulate function of serotonergic neurons that innervate to the prefrontal cortex (Soria-Fregozo et al., in press) (Fig. 7). The majority of serotonergic DRN neurons increase their action potential firing in response to nicotine, leading to increased serotonin release (Mihailescu et al., 2002). Thus, increased release of serotonin by nicotine treatment might reduce locomotor hyperactivity and PPI deficits observed in DAT KO mice.
There are some limitations to the present study. 1 mg/kg nicotine treatment induces hypothermia in mice (Sack et al., 2005). Lower body temperature with nicotine treatment may reduce locomotion. It is conceivable that nicotine-reduced body temperature might contribute to its effects on the locomotor activity in DAT KO mice. In this study, we used mice whose genetic background combined C57BL/6 and 129Sv/J mouse strains. Although 129Sv/J mouse strain had lower locomotor activity compared with C57BL/6, mixed genetic background of C57BL/6 and 129Sv/J shows locomotor activity similar to that of C57BL/6 strains (Miner, 1997). While we regard effects of overall genetic background upon locomotion as unlikely to have substantially affected the results, a more important issue may be that WT mice do express lower locomotor activity than DAT KO mice, possibly reducing the sensitivity with which we could detect motor-suppressive effects of nicotine in WT mice.
Attempts to correlate PPI deficits with cognitive function in human studies have yielded mixed results (Powell et al., 2009). Some studies did not report positive correlations between PPI and cognitive function as measured by Wisconsin Card Sorting Task (Swerdlow et al., 2006). However, there was positive relationship between PPI and Independent Living scales (Swerdlow et al., 2006) or Cambridge Neuropsychological Test Automated Battery (CANTAB) (Giakoumaki et al., 2006). The CNTRICS (Cognitive Neuroscience measures of Treatment Response of Impaired Cognition in Schizophrenia) program also considered PPI to provide a measure of cognitive function as a specific aspect of the perceptual abnormalities seen in patients with schizophrenia (Green et al., 2009).
Nicotine and subtype specific nACh receptor agonists affect auditory gating. Reports of the magnitude and direction of these effects have been highly variable however. This variability may be due to differences in the tested dose range, selectivity of the test compound, species and strain. Nicotine has mixed effects on PPI in rat models, producing an increase in PPI at low doses. The effects of nicotine are notoriously dose-dependent for virtually all nicotine effects. Furthermore, the present results may suggest that effects of nicotine on PPI might only be observed in subjects that have impaired PPI to begin with. Nicotine had a biphasic dose effect on startle amplitude, with increases at lower doses (0.01 mg/kg) and decreases at higher doses (0.5–5.0 mg/kg SC) in rat models (Acri et al., 1994). Nicotinic acetylcholine receptor subtypes are differentially involved in the control of auditory gating. PPI was disrupted at higher doses that were sensitive to antagonism of α4β2, but not α7 nACh receptors (Schreiber et al., 2002). However, opposite effects of 5-HT1A receptor activation on PPI in rats and mice have been previously reported (Dulawa et al., 1997).
Considerable animal research has been performed with PPI. PPI can be measured by similar means in human and animals, providing translational validity to this model of sensorimotor gating deficits (Castagne et al., 2009). Although there are no humans that have a complete deficit of DAT expression, DAT KO mice nonetheless appear to model some aspects of neuropsychiatric disorders that depend on DA function. The model has predictive validity for these conditions and may produce a state of altered fronto-striatal activity that models important aspects of schizophrenia. Our experiments have further demonstrated that nicotine ameliorates PPI deficits in DAT KO mice. DAT KO mice displayed significant deficits of PPI compared to WT mice, consistent with previous reports (Ralph et al., 2001; Yamashita et al., 2006). Nicotine treatment normalized these PPI deficits in DAT KO mice. This recovery of PPI produced by nicotine was blocked by non-selective and α7 selective nACh receptor antagonists, mecamylamine, methyllycaconitine, respectively, while α4β2 nACh receptor antagonist, DHβE produced only a slight trend toward attenuation of these effects (although it must be noted that toxicity prevented testing of higher doses of α4β2 nACh receptor antagonist). The blockade of the recovery of PPI deficits by nicotine in animals treated with the α7 selective nACh receptor antagonist methyllycaconitine suggests that the recovery of PPI deficits in DAT KO mice was mediated by the α7 nACh receptor. By contrast, nACh receptor antagonists MCA (Gould et al., 2005; Schreiber et al., 2002), DHβE and MLA (Mizoguchi et al., 2009) did not affect PPI in WT mice.
The α7 nACh receptor has been previously associated with the pathophysiology of attentional deficits of schizophrenia (Olincy et al., 2006). Nicotinic α4β2 receptors also have been implicated in cognitive function, and in improvement of attentional deficits (Sarter et al., 2009). Treatments with either α7 or α4β2 nACh receptor antagonists in the ventral hippocampus impair working memory (Levin et al., 2002). Patients with schizophrenia display a P50 auditory-evoked potential gating deficit that may be related to these hippocampal α7 nACh receptors (Adler et al.,1998; Leonard et al., 1996). Furthermore, Leonard et al. reported that nicotine treatment normalizes P50 gating deficits in patients with schizophrenia (Leonard et al., 1996). An α7 nACh receptor agonist, 3-2,4 dimethoxybenzylidene anabaseine (DMXB-A) can also improve the P50 auditory-evoked potential in schizophrenia patients (Olincy et al., 2006). Our results that the recovery of PPI deficits in DAT KO mice involves the α7 nACh receptor adds to support for development of α7 nACh receptor agonists to improve cognitive function in schizophrenia and/or other attentional or cognitive disorders. A recent study reported that the atypical antipsychotics clozapine and quetiapine reversed PPI deficits in DAT KO mice (Powell et al., 2008), however these treatments do not sufficiently improve cognition in schizophrenia patients. Therapeutic studies have reported that the beneficial effects on cognitive function in schizophrenia patients treated with the 5-HT1A receptor agonist buspirone in combination with atypical antipsychotic drugs but not treatment with buspirone alone (Sumiyoshi et al., 2007). The high rate of smoking in schizophrenia patients might also be beneficial in a similar way, augmenting the effects of antipsychotic drugs co-administration.
Our findings suggest that nicotine’s actions via the α7 subunit of the nACh receptor depend on intact 5-HT1A receptor function indicates that serotonergic manipulations may provide additional avenues through which to ameliorate the behavioral impairments observed in DAT KO mice, and the pathophysiological conditions that these interesting animals may model. The effects of nicotine on PPI in DAT KO mice were blocked by WAY100635, a selective 5-HT1A receptor antagonist that did not affect PPI in WT mice (Sakaue et al., 2003; Shanahan et al., 2009). Thus, we have demonstrated that administration of the 5-HT1A receptor agonist DPAT also reversed deficits in PPI in DAT KO mice, and this effect was antagonized by pretreatment with a selective 5-HT1A antagonist. Administration of a DA D1 receptor agonist leads to normalization of prefrontal activity revealed by fMRI observations in schizophrenia patients (Mu et al., 2007). In vivo microdialysis studies reveal that 5-HT1A receptor agonists increase DA efflux in the prefrontal cortex in experimental animals (Ago et al., 2002; Sakaue et al., 2000). In addition, the atypical antipsychotics clozapine and ziprasidone, also increase DA release in the cortex of rats, and the effects are blocked by treatment with selective 5-HT1A antagonists (Rollema et al., 2000, 1997). In addition, 5-HT1A receptors are expressed on pyramidal neurons of the rat medial prefrontal cortex (Wedzony et al., 2007), so that cortical glutamatergic neurotransmission via 5-HT1A receptors may be involved in the regulation of DA release in the prefrontal cortex (Fig. 7). Our results may suggest the possibility that normalization of PPI in DAT KO mice by 5-HT1A receptor agonists is caused by dopaminergic activation in the prefrontal cortex of DAT KO mice, and consequent restoration of the balance between prefrontal and striatal DA activity, in a manner similar to the effects of NET blockers.
The cognition enhancing effect of nicotine does not appear to diminish with continued treatment. Similar to acute nicotine, the beneficial effects of chronic nicotine administration were found to be specific to working memory function (Levin et al.,1996). A better mechanistic understanding of the neural systems involved in the nicotine-induced cognitive improvement is necessary for development of nicotinic treatments of cognitive dysfunction (Bancroft and Levin, 2000).
5. Conclusion
The results from our experiments demonstrate behavioral abnormalities in DAT KO mice that are normalized by treatment with nicotine. We observed that the hyperactivity and PPI deficits displayed by DAT KO mice were reduced by administration of nicotine. These nicotine effects were attenuated by pretreatment with α7 nACh receptor antagonists, or a 5-HT1A receptor antagonist, but not a α4β2 nACh receptor antagonist. Furthermore, treatment with a 5-HT1A receptor agonist also directly ameliorated PPI deficits in DAT KO mice, and effect which was also blocked by pretreatment with a 5-HT1A receptor antagonist. These data support the idea that nicotine might ameliorate some of the cognitive dysfunctions found in schizophrenia via a mechanism that involves 5-HT1A receptors. In addition, our present study suggests that examination of the effects of 5HT1A agonists would also be potentially beneficial in the treatment of schizophrenia patients.
Acknowledgments
This work was supported by Grant from the Smoking Research Foundation, Japan, Grants-in-Aid from MECSST and Health Sciences Research Grants from MHLW, Japan, and the Global COE Program, MEXT, Japan. This work was also supported in part by funding from the Intramural Research Program of the National Institute on Drug Abuse, USA (FSH, GRU).
References
- Acri JB, Morse DE, Popke EJ, Grunberg NE. Nicotine increases sensory gating measured as inhibition of the acoustic startle reflex in rats. Psychopharmacology (Berl.) 1994;114:369–374. doi: 10.1007/BF02244861. [DOI] [PubMed] [Google Scholar]
- Adler LE, Olincy A, Waldo M, Harris JG, Griffith J, Stevens K, Flach K, Nagamoto H, Bickford P, Leonard S, Freedman R. Schizophrenia, sensory gating, and nicotinic receptors. Schizophr. Bull. 1998;24:189–202. doi: 10.1093/oxfordjournals.schbul.a033320. [DOI] [PubMed] [Google Scholar]
- Ago Y, Sakaue M, Baba A, Matsuda T. Selective reduction by isolation rearing of 5-HT1A receptor-mediated dopamine release in vivo in the frontal cortex of mice. J. Neurochem. 2002;83:353–359. doi: 10.1046/j.1471-4159.2002.01128.x. [DOI] [PubMed] [Google Scholar]
- Albuquerque EX, Pereira EF, Alkondon M, Rogers SW. Mammalian nicotinic acetylcholine receptors: from structure to function. Physiol. Rev. 2009;89:73–120. doi: 10.1152/physrev.00015.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arime Y, Kasahara Y, Hall FS, Uhl GR, Sora I. Cortico-subcortical neuromodulation involved in the amelioration of prepulse inhibition deficits in dopamine transporter knockout mice. Neuropsychopharmacology. doi: 10.1038/npp.2012.114. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arime Y, Kubo Y, Sora I. Animal models of attention-deficit/hyperactivity disorder. Biol. Pharm. Bull. 2011;34:1373–1376. doi: 10.1248/bpb.34.1373. [DOI] [PubMed] [Google Scholar]
- Bancroft A, Levin ED. Ventral hippocampal alpha4beta2 nicotinic receptors and chronic nicotine effects on memory. Neuropharmacology. 2000;39:2770–2778. doi: 10.1016/s0028-3908(00)00099-x. [DOI] [PubMed] [Google Scholar]
- Budygin EA, Brodie MS, Sotnikova TD, Mateo Y, John CE, Cyr M, Gainetdinov RR, Jones SR. Dissociation of rewarding and dopamine transporter-mediated properties of amphetamine. Proc. Natl. Acad. Sci. U. S. A. 2004;101:7781–7786. doi: 10.1073/pnas.0401418101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnet PW, Eastwood SL, Harrison PJ. [3H]WAY-100635 for 5-HT1A receptor autoradiography in human brain: a comparison with [3H]8-OH-DPAT and demonstration of increased binding in the frontal cortex in schizophrenia. Neurochem. Int. 1997;30:565–574. doi: 10.1016/s0197-0186(96)00124-6. [DOI] [PubMed] [Google Scholar]
- Bymaster FP, Katner JS, Nelson DL, Hemrick-Luecke SK, Threlkeld PG, Heiligenstein JH, Morin SM, Gehlert DR, Perry KW. Atomoxetine increases extracellular levels of norepinephrine and dopamine in prefrontal cortex of rat: a potential mechanism for efficacy in attention deficit/hyperactivity disorder. Neuropsychopharmacology. 2002;27:699–711. doi: 10.1016/S0893-133X(02)00346-9. [DOI] [PubMed] [Google Scholar]
- Carboni E, Spielewoy C, Vacca C, Nosten-Bertrand M, Giros B, Di Chiara G. Cocaine and amphetamine increase extracellular dopamine in the nucleus accumbens of mice lacking the dopamine transporter gene. J. Neurosci. 2001;21(RC141):141–144. doi: 10.1523/JNEUROSCI.21-09-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castagne V, Moser PC, Porsolt RD. Preclinical behavioral models for predicting antipsychotic activity. Adv. Pharmacol. 2009;57:381–418. doi: 10.1016/S1054-3589(08)57010-4. [DOI] [PubMed] [Google Scholar]
- Conklin CA, Robin N, Perkins KA, Salkeld RP, McClernon FJ. Proximal versus distal cues to smoke: the effects of environments on smokers’ cue-reactivity. Exp. Clin. Psychopharmacol. 2008;16:207–214. doi: 10.1037/1064-1297.16.3.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalack GW, Healy DJ, Meador-Woodruff JH. Nicotine dependence in schizophrenia: clinical phenomena and laboratory findings. Am. J. Psychiatry. 1998;155:1490–1501. doi: 10.1176/ajp.155.11.1490. [DOI] [PubMed] [Google Scholar]
- Dulawa SC, Hen R, Scearce-Levie K, Geyer MA. Serotonin1B receptor modulation of startle reactivity, habituation, and prepulse inhibition in wild-type and serotonin1B knockout mice. Psychopharmacology (Berl.) 1997;132:125–134. doi: 10.1007/s002130050328. [DOI] [PubMed] [Google Scholar]
- Dursun SM, Kutcher S. Smoking, nicotine and psychiatric disorders: evidence for therapeutic role, controversies and implications for future research. Med. Hypotheses. 1999;52:101–109. doi: 10.1054/mehy.1997.0623. [DOI] [PubMed] [Google Scholar]
- Gainetdinov RR, Jones SR, Caron MG. Functional hyperdopaminergia in dopamine transporter knock-out mice. Biol. Psychiatry. 1999a;46:303–311. doi: 10.1016/s0006-3223(99)00122-5. [DOI] [PubMed] [Google Scholar]
- Gainetdinov RR, Wetsel WC, Jones SR, Levin ED, Jaber M, Caron MG. Role of serotonin in the paradoxical calming effect of psychostimulants on hyperactivity. Science. 1999b;283:397–401. doi: 10.1126/science.283.5400.397. [DOI] [PubMed] [Google Scholar]
- Galindo-Charles L, Hernandez-Lopez S, Galarraga E, Tapia D, Bargas J, Garduno J, Frias-Dominguez C, Drucker-Colin R, Mihailescu S. Serotoninergic dorsal raphe neurons possess functional postsynaptic nicotinic acetylcholine receptors. Synapse. 2008;62:601–615. doi: 10.1002/syn.20526. [DOI] [PubMed] [Google Scholar]
- George TP, Termine A, Sacco KA, Allen TM, Reutenauer E, Vessicchio JC, Duncan EJ. A preliminary study of the effects of cigarette smoking on prepulse inhibition in schizophrenia: involvement of nicotinic receptor mechanisms. Schizophr. Res. 2006;87:307–315. doi: 10.1016/j.schres.2006.05.022. [DOI] [PubMed] [Google Scholar]
- Geyer MA, Dulawa SC. Assessment of murine startle reactivity, prepulse inhibition, and habituation. Curr. Protoc. Neurosci. 2003;Chapter 8(Unit 8):17. doi: 10.1002/0471142301.ns0817s24. [DOI] [PubMed] [Google Scholar]
- Geyer MA, Swerdlow NR. Measurement of startle response, prepulse inhibition, and habituation. Curr. Protoc. Neurosci. 2001;Chapter 8(Unit 8):7. doi: 10.1002/0471142301.ns0807s03. [DOI] [PubMed] [Google Scholar]
- Giakoumaki SG, Bitsios P, Frangou S. The level of prepulse inhibition in healthy individuals may index cortical modulation of early information processing. Brain Res. 2006;1078:168–170. doi: 10.1016/j.brainres.2006.01.056. [DOI] [PubMed] [Google Scholar]
- Giros B, Jaber M, Jones SR, Wightman RM, Caron MG. Hyperlocomotion and indifference to cocaine and amphetamine in mice lacking the dopamine transporter. Nature. 1996;379:606–612. doi: 10.1038/379606a0. [DOI] [PubMed] [Google Scholar]
- Gould TJ, Rukstalis M, Lewis MC. Atomoxetine and nicotine enhance prepulse inhibition of acoustic startle in C57BL/6 mice. Neurosci. Lett. 2005;377:85–90. doi: 10.1016/j.neulet.2004.11.073. [DOI] [PubMed] [Google Scholar]
- Green MF, Butler PD, Chen Y, Geyer MA, Silverstein S, Wynn JK, Yoon JH, Zemon V. Perception measurement in clinical trials of schizophrenia: promising paradigms from CNTRICS. Schizophr. Bull. 2009;35:163–181. doi: 10.1093/schbul/sbn156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall FS, Li XF, Sora I, Xu F, Caron M, Lesch KP, Murphy DL, Uhl GR. Cocaine mechanisms: enhanced cocaine, fluoxetine and nisoxetine place preferences following monoamine transporter deletions. Neuroscience. 2002;115:153–161. doi: 10.1016/s0306-4522(02)00379-2. [DOI] [PubMed] [Google Scholar]
- Hall FS, Markou A, Levin ED, Uhl GR. Mouse models for studying genetic influences on factors determining smoking cessation success in humans. Ann. N. Y. Acad. Sci. 2012;1248:39–70. doi: 10.1111/j.1749-6632.2011.06415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari V, Postma P. Nicotine use in schizophrenia: the self medication hypotheses. Neurosci. Biobehav. Rev. 2005;29:1021–1034. doi: 10.1016/j.neubiorev.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Leonard S, Adams C, Breese CR, Adler LE, Bickford P, Byerley W, Coon H, Griffith JM, Miller C, Myles-Worsley M, Nagamoto HT, Rollins Y, Stevens KE, Waldo M, Freedman R. Nicotinic receptor function in schizophrenia. Schizophr. Bull. 1996;22:431–445. doi: 10.1093/schbul/22.3.431. [DOI] [PubMed] [Google Scholar]
- Levin ED, Bradley A, Addy N, Sigurani N. Hippocampal alpha 7 and alpha 4 beta 2 nicotinic receptors and working memory. Neuroscience. 2002;109:757–765. doi: 10.1016/s0306-4522(01)00538-3. [DOI] [PubMed] [Google Scholar]
- Levin ED, Kim P, Meray R. Chronic nicotine working and reference memory effects in the 16-arm radial maze: interactions with D1 agonist and antagonist drugs. Psychopharmacology (Berl.) 1996;127:25–30. doi: 10.1007/BF02805971. [DOI] [PubMed] [Google Scholar]
- Li B, Arime Y, Hall FS, Uhl GR, Sora I. Impaired spatial working memory and decreased frontal cortex BDNF protein level in dopamine transporter knockout mice. Eur. J. Pharmacol. 2010;628:104–107. doi: 10.1016/j.ejphar.2009.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihailescu S, Drucker-Colin R. Nicotine, brain nicotinic receptors, and neuropsychiatric disorders. Arch. Med. Res. 2000;31:131–144. doi: 10.1016/s0188-4409(99)00087-9. [DOI] [PubMed] [Google Scholar]
- Mihailescu S, Guzman-Marin R, Dominguez Mdel C, Drucker-Colin R. Mechanisms of nicotine actions on dorsal raphe serotoninergic neurons. Eur. J. Pharmacol. 2002;452:77–82. doi: 10.1016/s0014-2999(02)02244-6. [DOI] [PubMed] [Google Scholar]
- Miner LL. Cocaine reward and locomotor activity in C57BL/6J and 129/SvJ inbred mice and their F1 cross. Pharmacol. Biochem. Behav. 1997;58:25–30. doi: 10.1016/s0091-3057(96)00465-0. [DOI] [PubMed] [Google Scholar]
- Mizoguchi H, Arai S, Koike H, Ibi D, Kamei H, Nabeshima T, Kim HC, Takuma K, Yamada K. Therapeutic potential of nicotine for methamphetamine-induced impairment of sensorimotor gating: involvement of pallidotegmental neurons. Psychopharmacology (Berl.) 2009;207:235–243. doi: 10.1007/s00213-009-1651-z. [DOI] [PubMed] [Google Scholar]
- Morice E, Billard JM, Denis C, Mathieu F, Betancur C, Epelbaum J, Giros B, Nosten-Bertrand M. Parallel loss of hippocampal LTD and cognitive flexibility in a genetic model of hyperdopaminergia. Neuropsychopharmacology. 2007;32:2108–2116. doi: 10.1038/sj.npp.1301354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moron JA, Brockington A, Wise RA, Rocha BA, Hope BT. Dopamine uptake through the norepinephrine transporter in brain regions with low levels of the dopamine transporter: evidence from knock-out mouse lines. J. Neurosci. 2002;22:389–395. doi: 10.1523/JNEUROSCI.22-02-00389.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu Q, Johnson K, Morgan PS, Grenesko EL, Molnar CE, Anderson B, Nahas Z, Kozel FA, Kose S, Knable M, Fernandes P, Nichols DE, Mailman RB, George MS. A single 20 mg dose of the full D1 dopamine agonist dihydrexidine (DAR-0100) increases prefrontal perfusion in schizophrenia. Schizophr. Res. 2007;94:332–341. doi: 10.1016/j.schres.2007.03.033. [DOI] [PubMed] [Google Scholar]
- Muller CP, De Souza Silva MA, DePalma G, Tomaz C, Carey RJ, Huston JP. The selective serotonin(1A)-receptor antagonist WAY 100635 blocks behavioral stimulating effects of cocaine but not ventral striatal dopamine increase. Behav. Brain Res. 2002;134:337–346. doi: 10.1016/s0166-4328(02)00042-6. [DOI] [PubMed] [Google Scholar]
- Olincy A, Harris JG, Johnson LL, Pender V, Kongs S, Allensworth D, Ellis J, Zerbe GO, Leonard S, Stevens KE, Stevens JO, Martin L, Adler LE, Soti F, Kem WR, Freedman R. Proof-of-concept trial of an alpha7 nicotinic agonist in schizophrenia. Arch. Gen. Psychiatry. 2006;63:630–638. doi: 10.1001/archpsyc.63.6.630. [DOI] [PubMed] [Google Scholar]
- Petrovsky N, Quednow BB, Ettinger U, Schmechtig A, Mossner R, Collier DA, Kuhn KU, Maier W, Wagner M, Kumari V. Sensorimotor gating is associated with CHRNA3 polymorphisms in schizophrenia and healthy volunteers. Neuropsychopharmacology. 2010;35:1429–1439. doi: 10.1038/npp.2010.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picciotto MR, Caldarone BJ, King SL, Zachariou V. Nicotinic receptors in the brain. Links between molecular biology and behavior. Neuropsychopharmacology. 2000;22:451–465. doi: 10.1016/S0893-133X(99)00146-3. [DOI] [PubMed] [Google Scholar]
- Potter AS, Newhouse PA, Bucci DJ. Central nicotinic cholinergic systems: a role in the cognitive dysfunction in attention-deficit/hyperactivity disorder? Behav. Brain Res. 2006;175:201–211. doi: 10.1016/j.bbr.2006.09.015. [DOI] [PubMed] [Google Scholar]
- Powell SB, Young JW, Ong JC, Caron MG, Geyer MA. Atypical anti-psychotics clozapine and quetiapine attenuate prepulse inhibition deficits in dopamine transporter knockout mice. Behav. Pharmacol. 2008;19:562–565. doi: 10.1097/FBP.0b013e32830dc110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell SB, Zhou X, Geyer MA. Prepulse inhibition and genetic mouse models of schizophrenia. Behav. Brain Res. 2009;204:282–294. doi: 10.1016/j.bbr.2009.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralph RJ, Paulus MP, Fumagalli F, Caron MG, Geyer MA. Prepulse inhibition deficits and perseverative motor patterns in dopamine transporter knock-out mice: differential effects of D1 and D2 receptor antagonists. J. Neurosci. 2001;21:305–313. doi: 10.1523/JNEUROSCI.21-01-00305.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ripoll N, Bronnec M, Bourin M. Nicotinic receptors and schizophrenia. Curr. Med. Res. Opin. 2004;20:1057–1074. doi: 10.1185/030079904125004060. [DOI] [PubMed] [Google Scholar]
- Rollema H, Lu Y, Schmidt AW, Sprouse JS, Zorn SH. 5-HT(1A) receptor activation contributes to ziprasidone-induced dopamine release in the rat prefrontal cortex. Biol. Psychiatry. 2000;48:229–237. doi: 10.1016/s0006-3223(00)00850-7. [DOI] [PubMed] [Google Scholar]
- Rollema H, Lu Y, Schmidt AW, Zorn SH. Clozapine increases dopamine release in prefrontal cortex by 5-HT1A receptor activation. Eur. J. Pharmacol. 1997;338:R3–R5. doi: 10.1016/s0014-2999(97)81951-6. [DOI] [PubMed] [Google Scholar]
- Sack R, Gochberg-Sarver A, Rozovsky U, Kedmi M, Rosner S, Orr-Urtreger A. Lower core body temperature and attenuated nicotine-induced hypothermic response in mice lacking the beta4 neuronal nicotinic acetylcholine receptor subunit. Brain Res. Bull. 2005;66:30–36. doi: 10.1016/j.brainresbull.2005.02.032. [DOI] [PubMed] [Google Scholar]
- Sakaue M, Ago Y, Baba A, Matsuda T. The 5-HT1A receptor agonist MKC-242 reverses isolation rearing-induced deficits of prepulse inhibition in mice. Psychopharmacology (Berl.) 2003;170:73–79. doi: 10.1007/s00213-003-1515-x. [DOI] [PubMed] [Google Scholar]
- Sakaue M, Somboonthum P, Nishihara B, Koyama Y, Hashimoto H, Baba A, Matsuda T. Postsynaptic 5-hydroxytryptamine(1A) receptor activation increases in vivo dopamine release in rat prefrontal cortex. Br. J. Pharmacol. 2000;129:1028–1034. doi: 10.1038/sj.bjp.0703139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarter M, Parikh V, Howe WM. nAChR agonist-induced cognition enhancement: integration of cognitive and neuronal mechanisms. Biochem. Pharmacol. 2009;78:658–667. doi: 10.1016/j.bcp.2009.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber R, Dalmus M, De Vry J. Effects of alpha 4/beta 2- and alpha 7-nicotine acetylcholine receptor agonists on prepulse inhibition of the acoustic startle response in rats and mice. Psychopharmacology (Berl.) 2002;159:248–257. doi: 10.1007/s00213-001-0927-8. [DOI] [PubMed] [Google Scholar]
- Shanahan NA, Holick Pierz KA, Masten VL, Waeber C, Ansorge M, Gingrich JA, Geyer MA, Hen R, Dulawa SC. Chronic reductions in serotonin transporter function prevent 5-HT1B-induced behavioral effects in mice. Biol. Psychiatry. 2009;65:401–408. doi: 10.1016/j.biopsych.2008.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shearman E, Fallon S, Sershen H, Lajtha A. Nicotine-induced monoamine neurotransmitter changes in the brain of young rats. Brain Res. Bull. 2008;76:626–639. doi: 10.1016/j.brainresbull.2008.03.017. [DOI] [PubMed] [Google Scholar]
- Shen HW, Hagino Y, Kobayashi H, Shinohara-Tanaka K, Ikeda K, Yamamoto H, Yamamoto T, Lesch KP, Murphy DL, Hall FS, Uhl GR, Sora I. Regional differences in extracellular dopamine and serotonin assessed by in vivo microdialysis in mice lacking dopamine and/or serotonin transporters. Neuropsychopharmacology. 2004;29:1790–1799. doi: 10.1038/sj.npp.1300476. [DOI] [PubMed] [Google Scholar]
- Sora I, Hall FS, Andrews AM, Itokawa M, Li XF, Wei HB, Wichems C, Lesch KP, Murphy DL, Uhl GR. Molecular mechanisms of cocaine reward: combined dopamine and serotonin transporter knockouts eliminate cocaine place preference. Proc. Natl. Acad. Sci. U. S. A. 2001;98:5300–5305. doi: 10.1073/pnas.091039298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sora I, Wichems C, Takahashi N, Li XF, Zeng Z, Revay R, Lesch KP, Murphy DL, Uhl GR. Cocaine reward models: conditioned place preference can be established in dopamine- and in serotonin-transporter knockout mice. Proc. Natl. Acad. Sci. U. S. A. 1998;95:7699–7704. doi: 10.1073/pnas.95.13.7699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soria-Fregozo CF-S, Pérez-Vega ME, Feria-Velasco MI, Feria-Velasco A. 5-HT denervation of the adult rat prefrontal cortex induces changes in the expression of α4 and α7 nicotinic acetylcholine receptor subtypes. Neurologia. doi: 10.1016/j.nrl.2012.04.002. in press. [DOI] [PubMed] [Google Scholar]
- Spielewoy C, Biala G, Roubert C, Hamon M, Betancur C, Giros B. Hypolocomotor effects of acute and daily d-amphetamine in mice lacking the dopamine transporter. Psychopharmacology (Berl.) 2001;159:2–9. doi: 10.1007/s002130100901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumiyoshi T, Park S, Jayathilake K, Roy A, Ertugrul A, Meltzer HY. Effect of buspirone, a serotonin1A partial agonist, on cognitive function in schizophrenia: a randomized, double-blind, placebo-controlled study. Schizophr. Res. 2007;95:158–168. doi: 10.1016/j.schres.2007.06.008. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Light GA, Cadenhead KS, Sprock J, Hsieh MH, Braff DL. Startle gating deficits in a large cohort of patients with schizophrenia: relationship to medications, symptoms, neurocognition, and level of function. Arch. Gen. Psychiatry. 2006;63:1325–1335. doi: 10.1001/archpsyc.63.12.1325. [DOI] [PubMed] [Google Scholar]
- Wedzony K, Chocyk A, Kolasiewicz W, Mackowiak M. Glutamatergic neurons of rat medial prefrontal cortex innervating the ventral tegmental area are positive for serotonin 5-HT1A receptor protein. J. Physiol. Pharmacol. 2007;58:611–624. [PubMed] [Google Scholar]
- Weiss S, Tzavara ET, Davis RJ, Nomikos GG, Michael McIntosh J, Giros B, Martres MP. Functional alterations of nicotinic neurotransmission in dopamine transporter knock-out mice. Neuropharmacology. 2007;52:1496–1508. doi: 10.1016/j.neuropharm.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Wilens TE, Decker MW. Neuronal nicotinic receptor agonists for the treatment of attention-deficit/hyperactivity disorder: focus on cognition. Biochem. Pharmacol. 2007;74:1212–1223. doi: 10.1016/j.bcp.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita M, Fukushima S, Shen HW, Hall FS, Uhl GR, Numachi Y, Kobayashi H, Sora I. Norepinephrine transporter blockade can normalize the prepulse inhibition deficits found in dopamine transporter knockout mice. Neuropsychopharmacology. 2006;31:2132–2139. doi: 10.1038/sj.npp.1301009. [DOI] [PubMed] [Google Scholar]
- Zhou FM, Liang Y, Dani JA. Endogenous nicotinic cholinergic activity regulates dopamine release in the striatum. Nat. Neurosci. 2001;4:1224–1229. doi: 10.1038/nn769. [DOI] [PubMed] [Google Scholar]