Abstract
The annual worldwide burden of the preventable disease cervical cancer is over 530,000 new cases and 275,000 deaths, with the majority occurring in low- and middle-income countries (LMICs), where cervical cancer screening and early treatment are uncommon. Widely used in high-income countries, Pap smear (cytology-based) screening is expensive and challenging for implementation in LMICs, where lower-cost, effective alternatives such as visual inspection with acetic acid (VIA) and rapid human papillomavirus (HPV)-based screening tests offer promise for scaling up prevention services. Integrating HPV screening with VIA in “screen-and-treat-or-refer”’ programs offers the dual benefits of HPV screening to maximize detection and using VIA to triage for advanced lesions/cancer, as well as a pelvic exam to address other gynecologic issues. A major issue in LMICs is co-infection with human immunodeficiency virus (HIV) and HPV, which further increases the risk for cervical cancer and marks a population with perhaps the greatest need of cervical cancer prevention. Public-private partnerships to enhance the availability of cervical cancer prevention services within HIV/AIDS care delivery platforms through initiatives such as Pink Ribbon Red Ribbon® present an historic opportunity to expand cervical cancer screening in LMICs.
Introduction
Cervical cancer is a preventable malignancy, yet every year over 530,000 women are diagnosed with and over 275,000 women die from the disease worldwide (1). The distribution of cases and deaths is heavily weighted towards low- and middle-income countries (LMICs), which have 86% of the global cases and 88% of the total deaths (2). High-income countries have effectively integrated Pap smear–based cervical cancer screening services into both medical and public health services and have achieved reasonably high coverage rates, effectively reducing incidence and mortality over the past seven decades (3). The expanding use of effective prophylactic vaccines for preventing infection with human papillomavirus (HPV) types 16 and 18, common etiologic agents for cervical cancer, offers even greater promise for eventual elimination of cervical cancer as a major public health problem (4, 5). Yet, continued high rates of cervical cancer in LMICs point to the failure to bring sustainable prevention programs up to a substantial scale in these countries. This gap between scientific, clinical, and public health discovery and the implementation of service delivery showcases a significant global public health failure.
Unrealizable Promise of Cervical Cytology in LMICs
George Papanicolaou invented a simple technique (cervical cytology or Pap smear) for early detection of cervical cancer by collection, smearing, and microscopic observation of desquamative cells of the cervix in 1928; the Pap smear became highly popular in higher-income nations in the 1940s (6). Cervical cytology was soon refined and adopted as a routine part of preventive care, saving millions of women’s lives (7). On its face, a cytology program seems simple, yet it has multiple infrastructural and resource requirements, along with the need for awareness in the population, trained cytology technicians, and cytopathologists. With a critical lack of resources for health in general and of commitment to preventive health for women in particular, most LMICs do not have the current capacity to sustain cytology-based cervical cancer prevention programs (8). Even in venues with functioning health-care systems, there are multiple operational factors that inhibit quality, including the follow-up challenges of multiple visits for screening and later post-diagnosis therapy, inefficient recall and referral systems, inadequate resources for screening and treatment, and competing priorities in the healthcare system. Effective cervical cancer control is uncommon in resource-limited settings (9).
Suitable Screening and Prevention Technologies for LMICs
The continued high incidence of cervical cancer across LMICs has prompted the development, evaluation, and adoption of innovative approaches for improving sustainable prevention efforts (Table 1). Visual inspection with acetic acid (VIA) is readily mastered by non-physician providers and has been extensively studied as an alternative screening approach to the Pap smear (10–12). VIA gives immediate results and can be linked to cryotherapy in a relatively low-cost single-visit “see-and-treat” approach. Cryotherapy-based treatment of eligible VIA-positive lesions has been shown to be safe, feasible, acceptable, and effective in treating appropriate precancerous lesions (13, 14). Patients with cryotherapy-ineligible VIA-positive precancerous lesions and visually apparent frank invasive cervical cancer can be referred to hospitals offering excisional methods for diagnosis and treatment such as loop electrosurgical excision procedure (LEEP) and hysterectomy; even if advanced cancer management by surgery and chemoradiation is unavailable, many cervical cancer cases can be prevented or remediated at early stages (15, 16). More aptly called “see-and-treat-or-refer,” this cost-effective paradigm represents a pragmatic innovation for rapidly scaling up cervical cancer prevention services in LMICs.
Table 1.
Comparison of operational aspects of currently available cervical cancer screening tests
| Operational aspect | Pap smear (cytology) | VIA | Low-cost HPV tests |
|---|---|---|---|
| Cost | Moderate to high ($10–$25/test) | Low (< $5/test) | Low (< $8/test) |
| Provider | Cytotechnologist and cytopathologist (physician) | Nurses or mid-level providers | Lab technician |
| Training requirements | Substantial | Relatively modest | Relatively modest |
| Quality assurance | Substantial need for ensuring quality | Significant need for ensuring quality | Minimal quality assurance for processing samples |
| Technology ownership/copyright | Open source/public domain | Open source/public domain | Proprietary technology |
| Automation in results | Not possible | Not possible | Automated readout in some/not all formats |
| Range of sensitivity of single test | 60%–80% | 50%–80% | 80%–95% |
| Range of specificity of single test | 85%–95% | 70%–80% | 50%–70% |
| Minimum number of visits | 2 | 1 | 1 or 2 |
| Linking screening and treatment | Not possible in same visit | Possible in same visit (“see-and-treat”) | Possible in same visit with high-volume screening approach |
| Home-based/ self testing | Not possible | Not possible | Possible |
| Inter-observer variation | Significant | Significant | Minimal |
| Reproducibility | Limited, but possible with digital imaging of slides | Limited, possible with digital cervicography | Easily achievable |
| Evidence of effectiveness | Declining rates in developed countries since 1940s | Results from cross-sectional studies and randomized trials | Results from cross-sectional studies and randomized trials |
| Clinical limitations | Sample collection on slide may be inadequate or improperly stained | Limited use in post-menopausal women and endocervical lesions | Not all detected HPV infections are clinically significant; not available widely in 2011 |
| Other ancillary benefits | Can detect other infections on smears | Can detect other gynecological abnormalities during pelvic exam | Sample can be stored for testing by other molecular markers |
Visualization of lesions is not the only screening alternative to Pap smears. HPV can be detected in cervical sampling by performing a pelvic examination or through patient self-collection. HPV testing offers the most biologically compelling method of screening since virtually all cervical cancers result from chronic, persistent HPV infection (17). In comparison with other screening methods, HPV screening was superior in helping reduce both the incidence and mortality of cervical cancer in a large community-based randomized trial (18). With the ongoing development of low-cost, rapid molecular-assay technologies for HPV that are robust for field operations (19, 20), HPV-based screening has the promise to become a frontline method for cervical cancer screening to maximize detection and expand access across LMICs. Integrating HPV testing with VIA-based “see-and-treat-or-refer” platforms can combine the high accuracy of HPV DNA testing with the same-visit benefit of triage by VIA-based screening (21–23). With innovative public-private partnerships, it can be expected that HPV screening tests would be cheap enough for widespread deployment in low-income nations.
Limited resources for disease prevention require LMIC policy makers and health care providers to evaluate the utility of individual disease control efforts through the lens of cost effectiveness (24). It is encouraging that cervical cancer prevention programs using VIA and HPV testing have “incremental cost-effectiveness ratios” (ratio of the difference between the cost of an intervention and that of the next best strategy to the change in effects due to the intervention) well below the globally accepted definitions for public health interventions judged as cost effective by public health agencies; these programs may even save money in many specific settings where costs of the illness and its treatment exceed costs of prevention (25–27). Combining HPV vaccination with cervical cancer screening can further maximize the cost effectiveness of prevention strategies for both current and future generations of at-risk populations (27, 28).
Cervical Cancer Prevention in HIV-infected Women
The human immunodeficiency virus (HIV)/acquired immunodeficiency syndrome (AIDS) epidemic has led to a historic health burden in LMICs, particularly in sub-Saharan Africa. The incidence, progression, and recurrence of cervical precancerous lesions are higher in HIV-infected than HIV-uninfected women. Before combination antiretroviral therapy (cART) became widely available, HIV-infected women did not live long enough for precancerous lesions to progress to cervical cancer (29). Given that cART has a limited or no impact on reducing cervical cancer rates (30), HIV-infected women who live longer on cART are at an increased risk of persistent HPV infection and cervical precancer progressing to cervical cancer. This scenario reminds HIV clinical service providers of the futility of a “surgery that is a success” even though the patient died.
Still, the natural course of cervical neoplastic disease in the context of HIV infection is not yet fully elucidated, particularly in relation to varying levels of immunosuppression and regimens of cART. And at a local level, HIV-HPV coinfection involves a complex interplay of pro-inflammatory cytokines and enzymatic pathways that leads to enhanced inflammatory responses in the cervicovaginal milieu (31–34). In this issue of the journal, Fitzgerald and colleagues (35) present their pilot, hypothesis-generating study suggesting that HIV is associated with increased levels of cervical cyclooxygenase-2 (COX-2) and elevated systemic prostaglandin E2 (PGE2) levels. Since PGE2 can modulate chronic inflammation–mediated carcinogenesis (36), if its elevation in HIV-positive women is confirmed in larger, prospective studies, it might serve as a useful biomarker to predict the progression of persistent HPV infection to cancer in the context of both local and systemic immunosuppression in HIV-infected women. A role for anti-inflammatory drugs, including commonly used non-steroidal anti inflammatory drugs (NSAIDs), might be conceivable for prevention of inflammation-mediated cervical cancer risk, as has been demonstrated particularly in the colorectum (37). Indeed, exogenous factors influencing cervical inflammation—e.g., intrauterine devices (38), other hormonal methods for contraception (39), concurrent sexually transmitted and other infections (40), and local inflammatory changes with use of vaginal microbicides and other topical treatments (41–43)—continue to be important for understanding why only a fraction of HPV infections persist and progress to cervical cancer. Although beyond the scope of this review, recently reported observational studies have shown an increased risk of HIV acquisition linked to HPV infection (44); might HPV be a risk factor for HIV acquisition through its local immunomodulatory impact, along with its microvascular and cervical tissue physical changes (45)?
Inflammation may also be a key factor in influencing the variable response to cryotherapy-based treatment of cervical lesions in HIV-infected women (46, 47). It is possible that cryotherapy would enhance the potential for sexual transmission of HIV by HIV-infected women, given the bleeding and inflammation caused by the procedure. Similarly, HIV acquisition by at-risk uninfected women undergoing cryotherapy may be more likely because of breached integrity of the cervicovaginal mucosae. Although recently suggested to be less likely than previously assumed (48), such risk may be influenced by local PGE2-mediated inflammatory responses. Further research is needed to evaluate this risk, and if an association with PGE2 is established, local or systemic treatment by anti-inflammatory agents may augment advice about temporary abstinence and use of condoms in preventing sexual transmission or acquisition of HIV after cryotherapy.
The study by Fitzgerald and colleagues (35) is hypothesis-generating and highlights an important consideration for implementing screening strategies, particularly for HIV-infected women. Cervical cancer screening strategies that use HPV-based screening via self-collection as the first-line approach can reduce the burden of pelvic examinations for women who test HPV negative since the high negative predictive value of HPV DNA testing provides reassurance of safety against current risk for cervical cancer (49). However, an undesirable side effect of this approach is that HPV-negative women end up not receiving a detailed pelvic examination in the context of cervical cancer screening. Since resource limitations often preclude follow-up visits, this “once-in-a-lifetime” self collection–based approach for HPV-based screening, while saving costs and resources for HPV testing, may in fact represent a missed opportunity for evaluating risk for other cervical conditions in HPV-negative women, including evaluation of cervical inflammation. Therefore, we believe that it is important to emphasize the need for at least one pelvic exam when offering cervical cancer screening services for HIV-infected women, ideally accompanied by evaluation for other gynecologic conditions (including local inflammation and infection-associated changes) and appropriate treatment for sexually transmitted infections. Only a small minority of women has been screened even once in a lifetime in lower-income nations; it is therefore vital to emphasize the importance of a pelvic examination as part of routine reproductive health care services for all women and especially for those with immunosuppressive illnesses.
Integration of Cervical Cancer Prevention Programs with HIV/AIDS Care
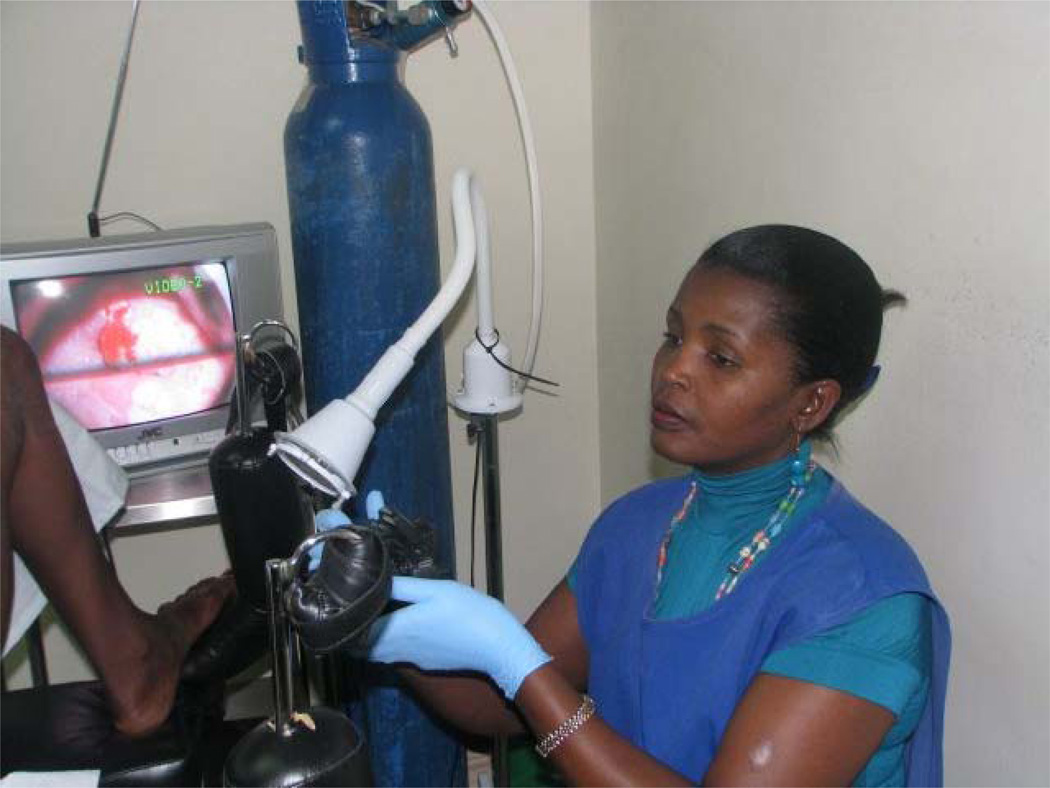
Cervical cancer prevention with a woman-centric approach is amenable to effective integration with other public health programs being implemented in LMICs (15, 50, 51). Since the advent of the President’s Emergency Plan for AIDS Relief (PEPFAR) in 2003, the global community has experienced a major surge in funding for prevention and treatment of HIV/AIDS and other infectious diseases in LMICs. The clinical infrastructures being created or expanded through PEPFAR, the Global Fund to Fight AIDS, Tuberculosis and Malaria, and the World Bank offer historic opportunities to integrate cervical cancer screening services with expanded HIV screening and care infrastructures. As described by the U.S. State Department, the September 2011 launch of the Pink Ribbon Red Ribbon® campaign is expanding “the availability of vital cervical cancer screening and treatment—especially for high-risk HIV-positive women—and also promot[ing] breast cancer education” (http://www.state.gov/r/pa/prs/ps/2011/09/172244.htm). This initiative was inspired by the success of PEPFAR-supported implementation initiatives such as our Cervical Cancer Prevention Program in Zambia (CCPPZ; refs. 15, 52). Now a routine part of public sector services, we are effectively delivering cervical cancer prevention services to HIV-infected and other at-risk women with nurses (Fig. 1) as frontline care providers (16, 53). CCPPZ has also pioneered the use of digital cervicographic adjunct to routine VIA screening, thereby achieving efficiencies in quality assurance and provider retraining, as well as providing an opportunity for bedside patient education and feedback (53). Furthermore, digital cervicography has allowed internet and cell-phone–based clinical consultations at a distance between nurses in peripheral clinics and gynecologists located centrally, thereby allowing efficient utilization of health care manpower through a “hub and spoke” model (54). Thousands of precancerous lesions and hundreds of cancers have been treated (and deaths prevented) in this program that has now screened more than 65,000 women over five years (15).
Figure 1.
A nurse conducts a cervical cancer screening examination in a public sector clinic operated by CCPPZ in Lusaka, Zambia. Nurses use digital cervicography–aided VIA to provide immediate screening results and offer same-visit cryotherapy for eligible VIA-positive women, or they refer advanced lesions to a central hospital for further care.
Can HPV vaccine be helpful in HIV-infected women? Little is known about the utility of HPV vaccine in HIV-infected women, but arguments are compelling that women who are not infected with HPV types 16 or 18 may benefit from vaccination even if they are not in the lower-age target group of virginal girls (55). Many women globally are infected with oncogenic strains of HPV but not yet with types 16 or 18, suggesting that vaccine protection from the HPV types would be helpful (56–58). Of course, multivalent vaccines now in development should protect against even more oncogenic HPV types of relevance to women in LMICs. Combination prevention efforts are being advocated for HIV control in LMICs (59–64). Can we afford to do less with combining HPV vaccination and HPV screening/treatment together to maximize long-term and immediate impact (65)?
Concluding Remarks
We are confident that affordable screening for cervical cancer is feasible and effective in LMICs (5, 11, 15, 16, 52, 53). Along with the Pink Ribbon Red Ribbon® campaign organizers, we believe it is an opportune time for expanding cancer prevention initiatives nested within ongoing public health programs in LMICs. Vertical programs, like HIV screening, also should be broadened to include cancer screening. While immunosuppressed women are of special concern, mitigation of risk through pragmatic clinical prevention services, including HPV vaccine, can be expanded to reach a wide swath of at-risk women in an implementation catchment area. A concerted push by key stakeholders—clinicians, public health professionals, researchers, politicians, policy makers, and women themselves—is needed to build on this momentum and dramatically enhance cost-effective cervical cancer screening and treatment to prevent unnecessary deaths of women in LMICs.
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 3.Forouzanfar MH, Foreman KJ, Delossantos AM, et al. Breast and cervical cancer in 187 countries between 1980 and 2010: a systematic analysis. Lancet. 2011;378:1461–1484. doi: 10.1016/S0140-6736(11)61351-2. [DOI] [PubMed] [Google Scholar]
- 4.Kane MA. Preventing cancer with vaccines: Progress in the global control of cancer. Cancer Prev Res (Phila) 2012;5 doi: 10.1158/1940-6207.CAPR-11-0533. XX-XX (to be paginated). [DOI] [PubMed] [Google Scholar]
- 5.Lowy DR, Schiller JT. Reducing HPV-associated Cancer Globally. Cancer Prev Res (Phila) 2012;5 doi: 10.1158/1940-6207.CAPR-11-0542. XX-XX (to be paginated). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Papanicolaou GN, Traut HF. The diagnostic value of vaginal smears in carcinoma of the uterus. 1941. Arch Pathol Lab Med. 1997;121:211–224. [PubMed] [Google Scholar]
- 7.Kitchener HC, Castle PE, Cox JT. Chapter 7: Achievements and limitations of cervical cytology screening. Vaccine. 2006;24(Suppl 3) doi: 10.1016/j.vaccine.2006.05.113. S3/63-70. [DOI] [PubMed] [Google Scholar]
- 8.Sankaranarayanan R, Budukh AM, Rajkumar R. Effective screening programmes for cervical cancer in low- and middle-income developing countries. Bull World Health Organ. 2001;79:954–962. [PMC free article] [PubMed] [Google Scholar]
- 9.Gakidou E, Nordhagen S, Obermeyer Z. Coverage of cervical cancer screening in 57 countries: low average levels and large inequalities. PLoS Med. 2008;5:132. doi: 10.1371/journal.pmed.0050132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sauvaget C, Fayette JM, Muwonge R, Wesley R, Sankaranarayanan R. Accuracy of visual inspection with acetic acid for cervical cancer screening. Int J Gynaecol Obstet. 2011;113:14–24. doi: 10.1016/j.ijgo.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 11.Sahasrabuddhe VV, Bhosale RA, Kavatkar AN, et al. Comparison of visual inspection with acetic acid and cervical cytology to detect high-grade cervical neoplasia among HIV-infected women in India. Int J Cancer. 2012;130:234–240. doi: 10.1002/ijc.25971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sankaranarayanan R, Nessa A, Esmy PO, Dangou JM. Visual inspection methods for cervical cancer prevention. Best Pract Res Clin Obstet Gynaecol. 2011 doi: 10.1016/j.bpobgyn.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 13.Sankaranarayanan R, Esmy PO, Rajkumar R, et al. Effect of visual screening on cervical cancer incidence and mortality in Tamil Nadu, India: a cluster-randomised trial. Lancet. 2007;370:398–406. doi: 10.1016/S0140-6736(07)61195-7. [DOI] [PubMed] [Google Scholar]
- 14.Sankaranarayanan R, Rajkumar R, Esmy PO, et al. Effectiveness, safety and acceptability of 'see and treat' with cryotherapy by nurses in a cervical screening study in India. Br J Cancer. 2007;96:738–743. doi: 10.1038/sj.bjc.6603633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mwanahamuntu MH, Sahasrabuddhe VV, Kapambwe S, et al. Advancing cervical cancer prevention initiatives in resource-constrained settings: insights from the Cervical Cancer Prevention Program in Zambia. PLoS Med. 2011;8:e1001032. doi: 10.1371/journal.pmed.1001032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pfaendler KS, Mwanahamuntu MH, Sahasrabuddhe VV, Mudenda V, Stringer JS, Parham GP. Management of cryotherapy-ineligible women in a "screen-and-treat" cervical cancer prevention program targeting HIV-infected women in Zambia: lessons from the field. Gynecol Oncol. 2008;110:402–407. doi: 10.1016/j.ygyno.2008.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schiffman M, Wentzensen N, Wacholder S, Kinney W, Gage JC, Castle PE. Human papillomavirus testing in the prevention of cervical cancer. J Natl Cancer Inst. 2011;103:368–383. doi: 10.1093/jnci/djq562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sankaranarayanan R, Nene BM, Shastri SS, et al. HPV screening for cervical cancer in rural India. N Engl J Med. 2009;360:1385–1394. doi: 10.1056/NEJMoa0808516. [DOI] [PubMed] [Google Scholar]
- 19.Qiao YL, Sellors JW, Eder PS, et al. A new HPV-DNA test for cervical-cancer screening in developing regions: a cross-sectional study of clinical accuracy in rural China. Lancet Oncol. 2008;9:929–936. doi: 10.1016/S1470-2045(08)70210-9. [DOI] [PubMed] [Google Scholar]
- 20.Yang HP, Walmer DK, Merisier D, et al. A pilot analytic study of a research-level, lower-cost human papillomavirus 16, 18, and 45 test. J Virol Methods. 2011;176:112–114. doi: 10.1016/j.jviromet.2011.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wiwanitkit V. Screening for cervical cancer: which common technique is the most cost-effective choice? Asian Pac J Cancer Prev. 2009;10:531–532. [PubMed] [Google Scholar]
- 22.Shastri SS, Dinshaw K, Amin G, et al. Concurrent evaluation of visual, cytological and HPV testing as screening methods for the early detection of cervical neoplasia in Mumbai, India. Bull World Health Organ. 2005;83:186–194. [PMC free article] [PubMed] [Google Scholar]
- 23.Cuzick J, Arbyn M, Sankaranarayanan R, et al. Overview of human papillomavirus-based and other novel options for cervical cancer screening in developed and developing countries. Vaccine. 2008;26(Suppl 10):K29–K41. doi: 10.1016/j.vaccine.2008.06.019. [DOI] [PubMed] [Google Scholar]
- 24.Goldhaber-Fiebert JD, Denny LA, De Souza M, Kuhn L, Goldie SJ. Program spending to increase adherence: South African cervical cancer screening. PLoS One. 2009;4:e5691. doi: 10.1371/journal.pone.0005691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goldie SJ, Gaffikin L, Goldhaber-Fiebert JD, et al. Cost-effectiveness of cervical-cancer screening in five developing countries. N Engl J Med. 2005;353:2158–2168. doi: 10.1056/NEJMsa044278. [DOI] [PubMed] [Google Scholar]
- 26.Vanni T, Luz PM, Grinsztejn B, et al. Cervical cancer screening among HIV-infected women: An economic evaluation in a middle-income country. Int J Cancer. 2011 doi: 10.1002/ijc.26472. [DOI] [PubMed] [Google Scholar]
- 27.Diaz M, Kim JJ, Albero G, et al. Health and economic impact of HPV 16 and 18 vaccination and cervical cancer screening in India. Br J Cancer. 2008;99:230–238. doi: 10.1038/sj.bjc.6604462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Campos NG, Kim JJ, Castle PE, et al. Health and economic impact of HPV 16/18 vaccination and cervical cancer screening in Eastern Africa. Int J Cancer. 2011 doi: 10.1002/ijc.26269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Franceschi S, Jaffe H. Cervical cancer screening of women living with HIV infection: a must in the era of antiretroviral therapy. Clin Infect Dis. 2007;45:510–513. doi: 10.1086/520022. [DOI] [PubMed] [Google Scholar]
- 30.Bratcher LF, Sahasrabuddhe VV. The impact of antiretroviral therapy on HPV and cervical intraepithelial neoplasia: current evidence and directions for future research. Infect Agent Cancer. 2010;5:8. doi: 10.1186/1750-9378-5-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Behbahani H, Walther-Jallow L, Klareskog E, et al. Proinflammatory and type 1 cytokine expression in cervical mucosa during HIV-1 and human papillomavirus infection. J Acquir Immune Defic Syndr. 2007;45:9–19. doi: 10.1097/QAI.0b013e3180415da7. [DOI] [PubMed] [Google Scholar]
- 32.Mkhize NN, Gumbi PP, Liebenberg LJ, et al. Persistence of genital tract T cell responses in HIV-infected women on highly active antiretroviral therapy. J Virol. 2010;84:10765–10772. doi: 10.1128/JVI.00518-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Henning TR, Kissinger P, Lacour N, Meyaski-Schluter M, Clark R, Amedee AM. Elevated cervical white blood cell infiltrate is associated with genital HIV detection in a longitudinal cohort of antiretroviral therapy-adherent women. J Infect Dis. 2010;202:1543–1552. doi: 10.1086/656720. [DOI] [PubMed] [Google Scholar]
- 34.Hirbod T, Nilsson J, Andersson S, et al. Upregulation of interferon-alpha and RANTES in the cervix of HIV-1-seronegative women with high-risk behavior. J Acquir Immune Defic Syndr. 2006;43:137–143. doi: 10.1097/01.qai.0000229016.85192.60. [DOI] [PubMed] [Google Scholar]
- 35.Fitzgerald DW, Bezak K, Ocheretina O, et al. The Effect of HIV and HPV Co-infection on Cervical COX-2 Expression and Systemic Prostaglandin E2 Levels. Cancer Prev Res (Phila) 2012;5 doi: 10.1158/1940-6207.CAPR-11-0496. XX-XX (to be paginated). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Greenhough A, Smartt HJ, Moore AE, et al. The COX-2/PGE2 pathway: key roles in the hallmarks of cancer and adaptation to the tumour microenvironment. Carcinogenesis. 2009;30:377–386. doi: 10.1093/carcin/bgp014. [DOI] [PubMed] [Google Scholar]
- 37.Chan AT. Aspirin and familial adenomatous polyposis: coming full circle. Cancer Prev Res (Phila) 2011;4:623–627. doi: 10.1158/1940-6207.CAPR-11-0157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Castellsague X, Diaz M, Vaccarella S, et al. Intrauterine device use, cervical infection with human papillomavirus, and risk of cervical cancer: a pooled analysis of 26 epidemiological studies. Lancet Oncol. 2011;12:1023–1031. doi: 10.1016/S1470-2045(11)70223-6. [DOI] [PubMed] [Google Scholar]
- 39.Marks M, Gravitt PE, Gupta SB, et al. Combined oral contraceptive use increases HPV persistence but not new HPV detection in a cohort of women from Thailand. J Infect Dis. 2011;204:1505–1513. doi: 10.1093/infdis/jir560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Al-Daraji WI, Smith JH. Infection and cervical neoplasia: facts and fiction. Int J Clin Exp Pathol. 2009;2:48–64. [PMC free article] [PubMed] [Google Scholar]
- 41.Zehbe I, Richard C, Lee KF, Campbell M, Hampson L, Hampson IN. Lopinavir shows greater specificity than zinc finger ejecting compounds as a potential treatment for human papillomavirus-related lesions. Antiviral Res. 2011;91:161–166. doi: 10.1016/j.antiviral.2011.05.016. [DOI] [PubMed] [Google Scholar]
- 42.Roberts JN, Kines RC, Katki HA, Lowy DR, Schiller JT. Effect of Pap smear collection and carrageenan on cervicovaginal human papillomavirus-16 infection in a rhesus macaque model. J Natl Cancer Inst. 2011;103:737–743. doi: 10.1093/jnci/djr061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Van Pachterbeke C, Bucella D, Rozenberg S, et al. Topical treatment of CIN 2+ by cidofovir: results of a phase II, double-blind, prospective, placebo-controlled study. Gynecol Oncol. 2009;115:69–74. doi: 10.1016/j.ygyno.2009.06.042. [DOI] [PubMed] [Google Scholar]
- 44.Auvert B, Marais D, Lissouba P, Zarca K, Ramjee G, Williamson AL. High-risk human papillomavirus is associated with HIV acquisition among South African female sex workers. Infect Dis Obstet Gynecol. 2011;2011:692012. doi: 10.1155/2011/692012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.van der Loeff MF, Nyitray AG, Giuliano AR. HPV vaccination to prevent HIV infection: time for randomized controlled trials. Sex Transm Dis. 2011;38:640–643. doi: 10.1097/OLQ.0b013e31820bca01. [DOI] [PubMed] [Google Scholar]
- 46.Taylor S, Wang C, Wright TC, Denny L, Tsai WY, Kuhn L. Reduced acquisition and reactivation of human papillomavirus infections among older women treated with cryotherapy: results from a randomized trial in South Africa. BMC Med. 2010;8:40. doi: 10.1186/1741-7015-8-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tate DR, Anderson RJ. Recrudescence of cervical dysplasia among women who are infected with the human immunodeficiency virus: a case-control analysis. Am J Obstet Gynecol. 2002;186:880–882. doi: 10.1067/mob.2002.123607. [DOI] [PubMed] [Google Scholar]
- 48.Chung MH, McKenzie KP, Richardson BA, et al. Cervical HIV-1 RNA shedding after cryotherapy among HIV-positive women with cervical intraepithelial neoplasia stage 2 or 3. AIDS. 2011;25:1915–1919. doi: 10.1097/QAD.0b013e32834a3654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gage JC, Castle PE. Preventing cervical cancer globally by acting locally: if not now, when? J Natl Cancer Inst. 2010;102:1524–1527. doi: 10.1093/jnci/djq382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Teguete I, Muwonge R, Traore C, Dolo A, Bayo S, Sankaranarayanan R. Can visual cervical screening be sustained in routine health services? Experience from Mali, Africa. BJOG. 2011 doi: 10.1111/j.1471-0528.2011.03122.x. [DOI] [PubMed] [Google Scholar]
- 51.Mutyaba T, Mirembe F, Sandin S, Weiderpass E. Evaluation of 'see-see and treat' strategy and role of HIV on cervical cancer prevention in Uganda. Reprod Health. 2010;7:4. doi: 10.1186/1742-4755-7-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Parham GP, Mwanahamuntu MH, Sahasrabuddhe VV, et al. Implementation of cervical cancer prevention services for HIV-infected women in Zambia: measuring program effectiveness. HIV Therapy. 2010;4:713–722. doi: 10.2217/hiv.10.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mwanahamuntu MH, Sahasrabuddhe VV, Pfaendler KS, et al. Implementation of 'see-and-treat' cervical cancer prevention services linked to HIV care in Zambia. AIDS. 2009;23:N1–N5. doi: 10.1097/QAD.0b013e3283236e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Parham GP, Mwanahamuntu MH, Pfaendler KS, et al. eC3--a modern telecommunications matrix for cervical cancer prevention in Zambia. J Low Genit Tract Dis. 2010;14:167–173. doi: 10.1097/LGT.0b013e3181cd6d5e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Castellsague X, Schneider A, Kaufmann AM, Bosch FX. HPV vaccination against cervical cancer in women above 25 years of age: key considerations and current perspectives. Gynecol Oncol. 2009;115:S15–S23. doi: 10.1016/j.ygyno.2009.09.021. [DOI] [PubMed] [Google Scholar]
- 56.Castellsague X, Diaz M, de Sanjose S, et al. Worldwide human papillomavirus etiology of cervical adenocarcinoma and its cofactors: implications for screening and prevention. J Natl Cancer Inst. 2006;98:303–315. doi: 10.1093/jnci/djj067. [DOI] [PubMed] [Google Scholar]
- 57.Sahasrabuddhe VV, Mwanahamuntu MH, Vermund SH, et al. Prevalence and distribution of HPV genotypes among HIV-infected women in Zambia. Br J Cancer. 2007;96:1480–1483. doi: 10.1038/sj.bjc.6603737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bollen LJ, Chuachoowong R, Kilmarx PH, et al. Human papillomavirus (HPV) detection among human immunodeficiency virus-infected pregnant Thai women: implications for future HPV immunization. Sex Transm Dis. 2006;33:259–264. doi: 10.1097/01.olq.0000187208.94655.34. [DOI] [PubMed] [Google Scholar]
- 59.Rotheram-Borus MJ, Swendeman D, Chovnick G. The past, present, and future of HIV prevention: integrating behavioral, biomedical, and structural intervention strategies for the next generation of HIV prevention. Annu Rev Clin Psychol. 2009;5:143–167. doi: 10.1146/annurev.clinpsy.032408.153530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Buchbinder S. HIV epidemiology, testing strategies, and prevention interventions. Top HIV Med. 2010;18:38–44. [PubMed] [Google Scholar]
- 61.Kurth AE, Celum C, Baeten JM, Vermund SH, Wasserheit JN. Combination HIV prevention: significance, challenges, and opportunities. Curr HIV/AIDS Rep. 2011;8:62–72. doi: 10.1007/s11904-010-0063-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Matovu JK. Preventing HIV transmission in married and cohabiting HIV-discordant couples in sub-Saharan Africa through combination prevention. Curr HIV Res. 2010;8:430–440. doi: 10.2174/157016210793499303. [DOI] [PubMed] [Google Scholar]
- 63.Burns DN, Dieffenbach CW, Vermund SH. Rethinking prevention of HIV type 1 infection. Clin Infect Dis. 2010;51:725–731. doi: 10.1086/655889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vermund SH, Allen KL, Karim QA. HIV-prevention science at a crossroads: advances in reducing sexual risk. Curr Opin HIV AIDS. 2009;4:266–273. doi: 10.1097/COH.0b013e32832c91dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stanley M. Human papillomavirus vaccines versus cervical cancer screening. Clin Oncol (R Coll Radiol) 2008;20:388–394. doi: 10.1016/j.clon.2008.04.006. [DOI] [PubMed] [Google Scholar]