Abstract
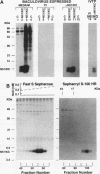
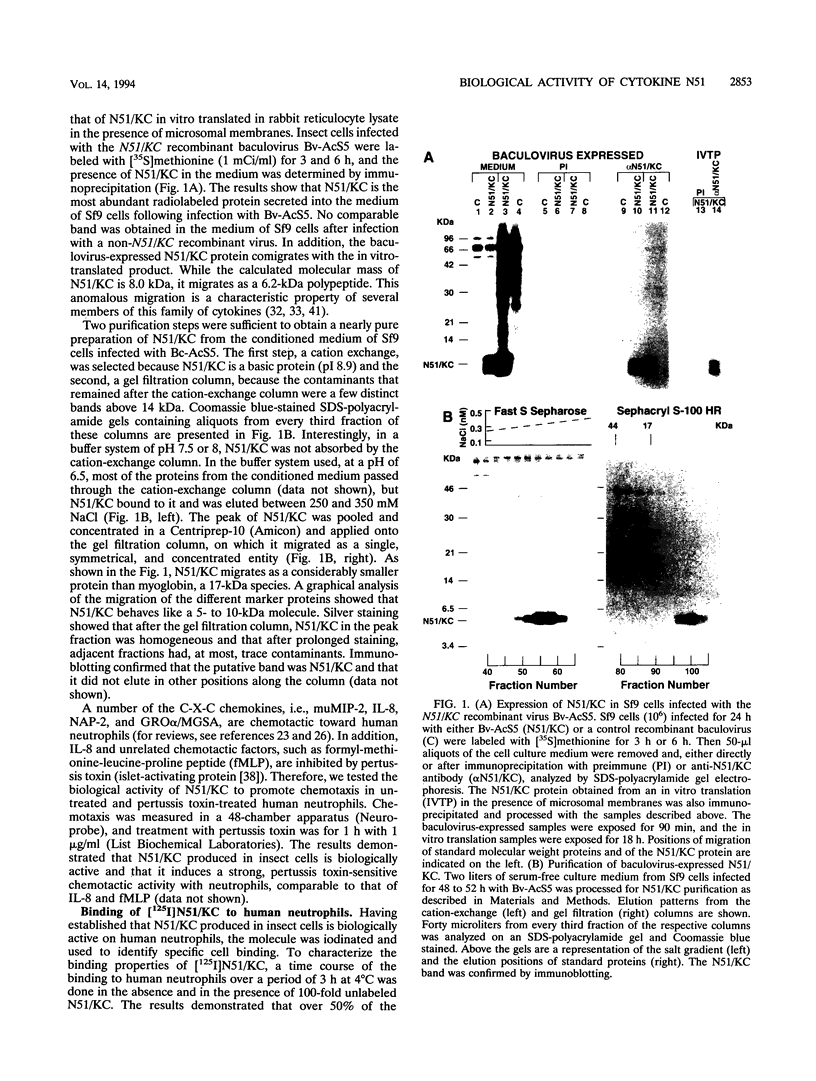
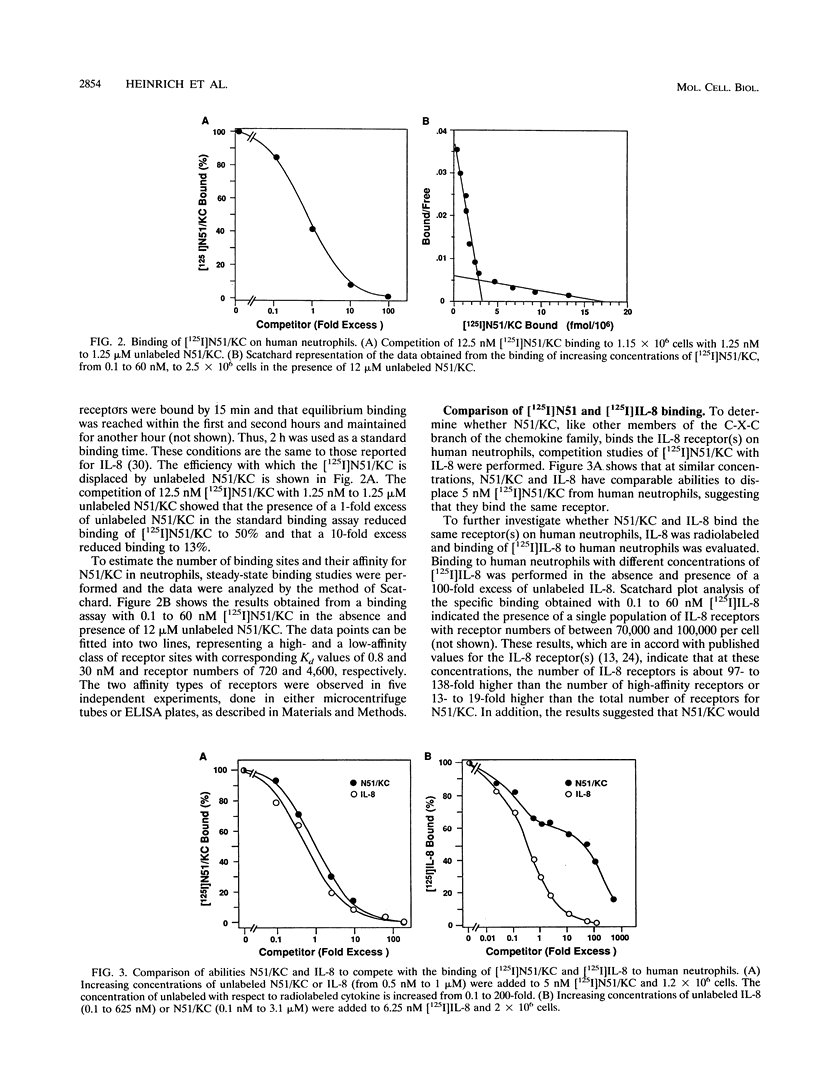
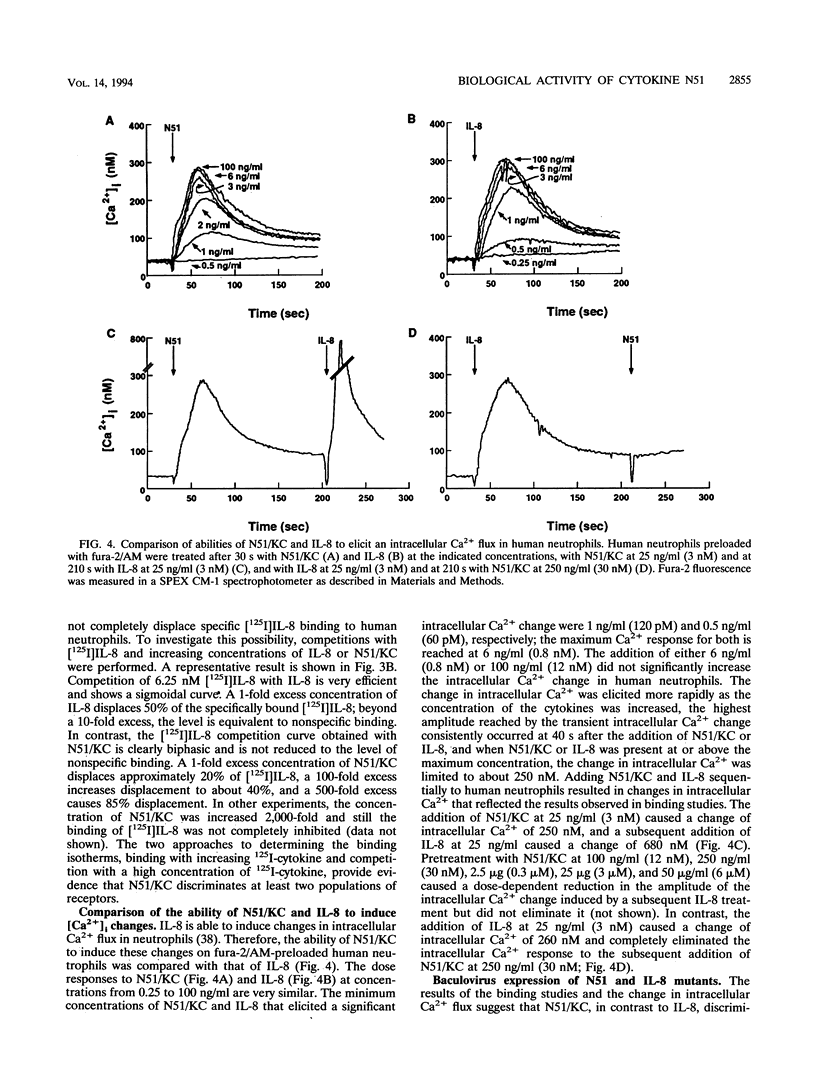
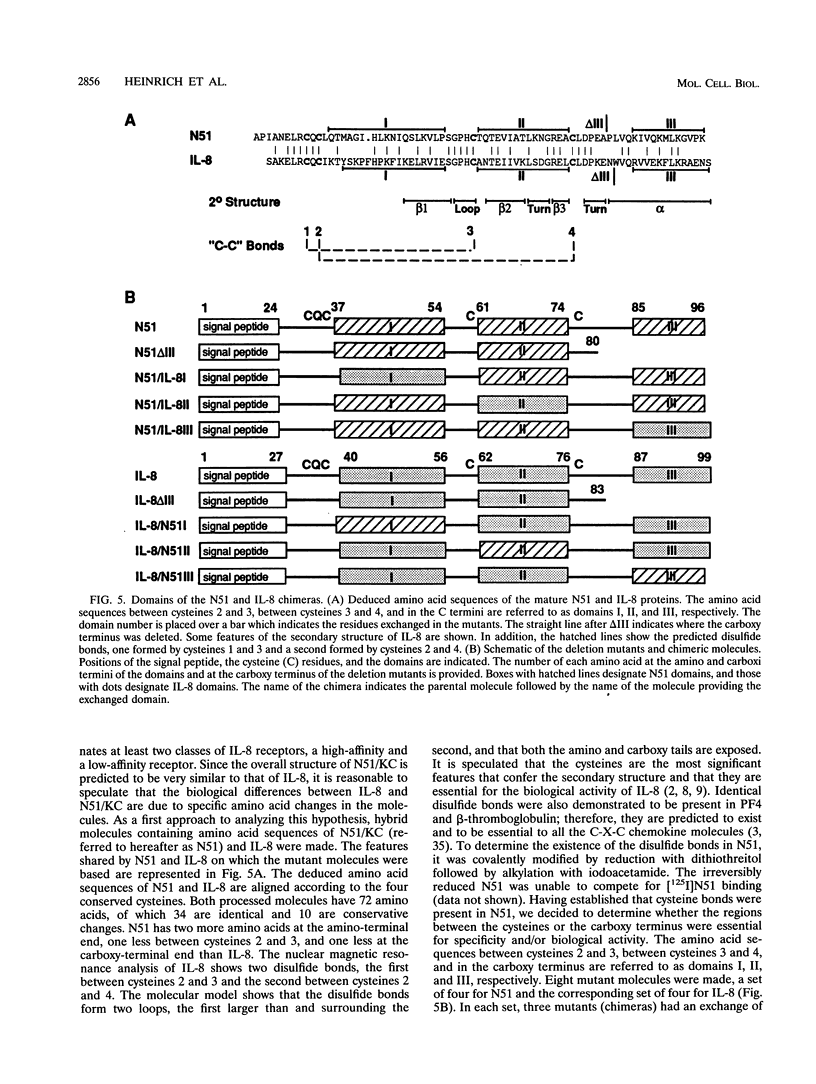
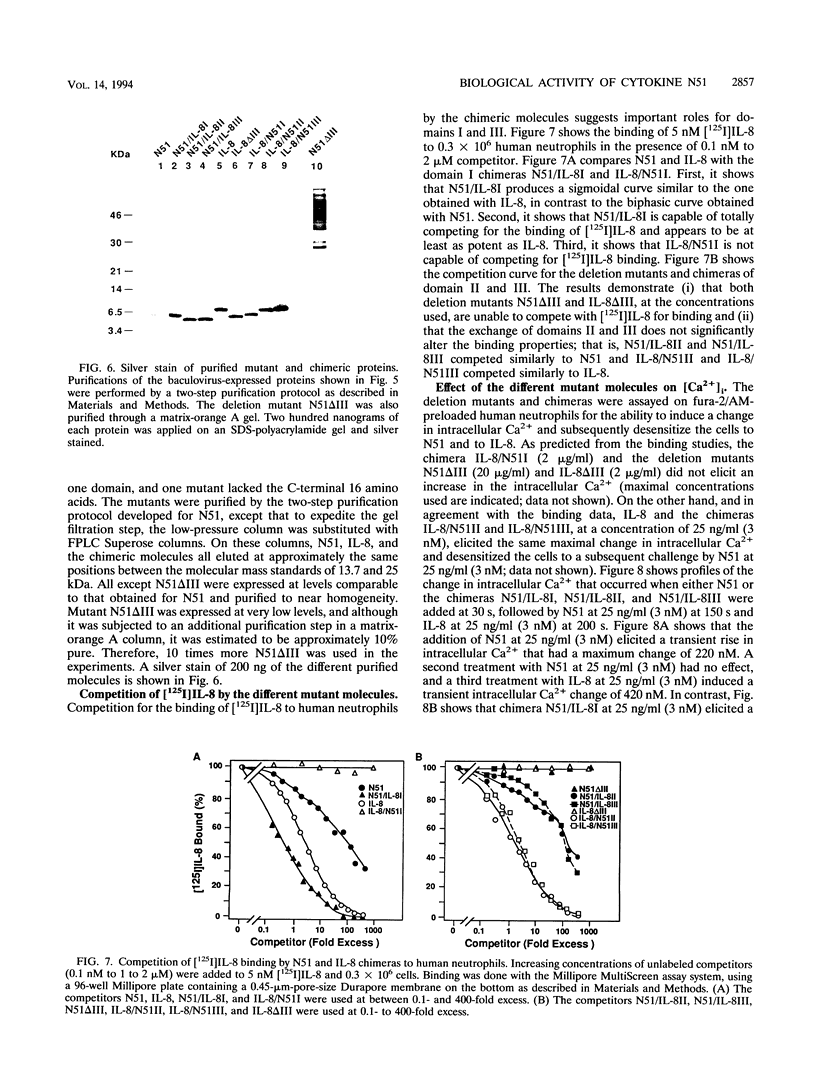
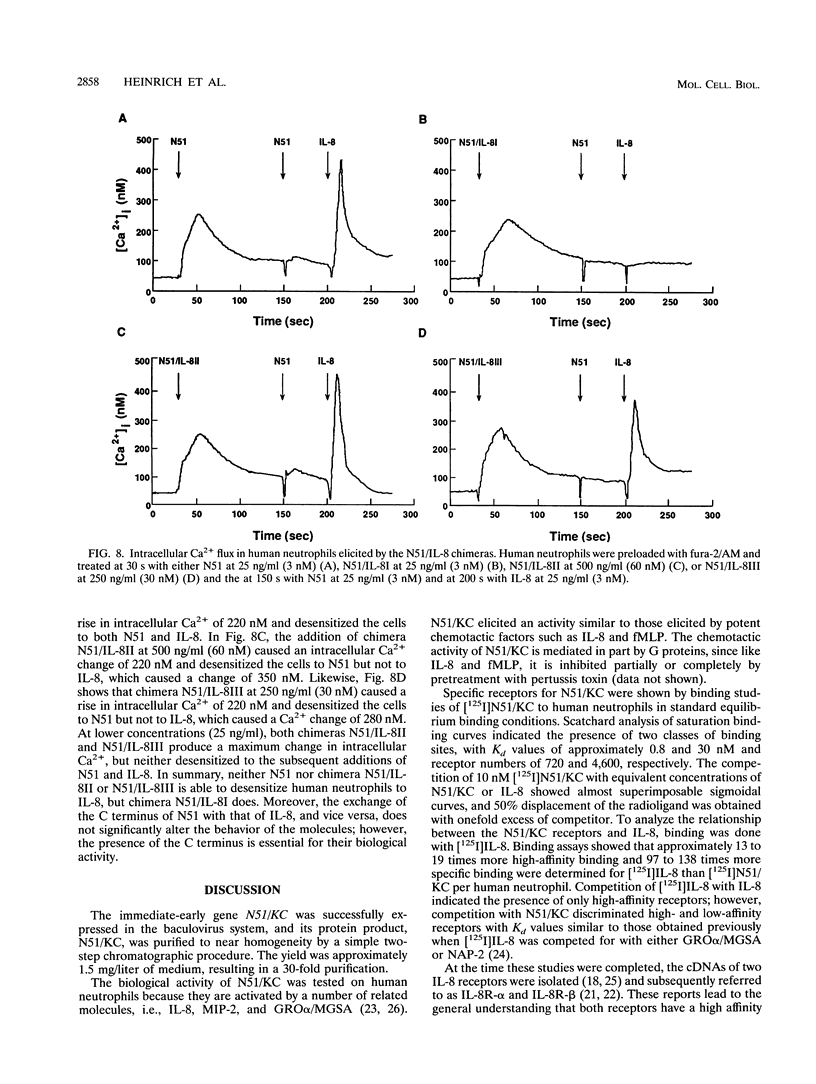
The immediate-early gene N51/KC encodes a protein which following expression in the baculovirus system and purification to apparent homogeneity is able to induce chemotaxis and intracellular Ca2+ flux, to compete for 125I-labeled interleukin-8 (IL-8) binding, and upon iodination, to bind specifically to human neutrophils. The activity of N51/KC can be distinguished from that of IL-8 by a number of criteria. First, at equivalent concentrations, the specific binding of [125I]N51/KC to human neutrophils is about 10 times less than that of [125I]IL-8. Second, the competition studies of [125I]IL-8 with IL-8 define a single class of high-affinity receptors, while the presence of both a high- and a low-affinity class of receptors is defined by N51/KC. Third, although the changes in intracellular Ca2+ of fura-2/AM-preloaded human neutrophils elicited by N51/KC and IL-8 are similar, pretreatment of the cells with N51/KC did not result in a loss of response to a subsequent treatment with IL-8; in contrast, treatment with IL-8 did result in the subsequent desensitization to N51/KC. To further characterize N51/KC, mutants and hybrids of N51/KC and IL-8 were produced and analyzed for the ability to compete for [125I]IL-8 binding and elicit intracellular Ca2+ changes in human neutrophils. Two important observations came from these studies. First, the N51/IL-8I hybrid in which the N51/KC sequence between cysteines 2 and 3 (or first disulfide bond) is replaced by the corresponding sequence in IL-8 shows IL-8-like properties, indicating that this region is important for specific receptor recognition. Second, the N51 delta III and IL-8 delta III C-terminus deletion mutants were biologically inactive, but the hybrid molecules N51/IL-8III and IL-8/N51III, in which the C termini were exchanged, had biological activities similar to that of the wild-type molecules, demonstrating that the presence of the C terminus is essential for the biological activity of these chemokines but does not confer receptor specificity.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almendral J. M., Sommer D., Macdonald-Bravo H., Burckhardt J., Perera J., Bravo R. Complexity of the early genetic response to growth factors in mouse fibroblasts. Mol Cell Biol. 1988 May;8(5):2140–2148. doi: 10.1128/mcb.8.5.2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin E. T., Weber I. T., St Charles R., Xuan J. C., Appella E., Yamada M., Matsushima K., Edwards B. F., Clore G. M., Gronenborn A. M. Crystal structure of interleukin 8: symbiosis of NMR and crystallography. Proc Natl Acad Sci U S A. 1991 Jan 15;88(2):502–506. doi: 10.1073/pnas.88.2.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begg G. S., Pepper D. S., Chesterman C. N., Morgan F. J. Complete covalent structure of human beta-thromboglobulin. Biochemistry. 1978 May 2;17(9):1739–1744. doi: 10.1021/bi00602a024. [DOI] [PubMed] [Google Scholar]
- Bolton A. E., Hunter W. M. The labelling of proteins to high specific radioactivities by conjugation to a 125I-containing acylating agent. Biochem J. 1973 Jul;133(3):529–539. doi: 10.1042/bj1330529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark-Lewis I., Dewald B., Geiser T., Moser B., Baggiolini M. Platelet factor 4 binds to interleukin 8 receptors and activates neutrophils when its N terminus is modified with Glu-Leu-Arg. Proc Natl Acad Sci U S A. 1993 Apr 15;90(8):3574–3577. doi: 10.1073/pnas.90.8.3574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark-Lewis I., Schumacher C., Baggiolini M., Moser B. Structure-activity relationships of interleukin-8 determined using chemically synthesized analogs. Critical role of NH2-terminal residues and evidence for uncoupling of neutrophil chemotaxis, exocytosis, and receptor binding activities. J Biol Chem. 1991 Dec 5;266(34):23128–23134. [PubMed] [Google Scholar]
- Clore G. M., Appella E., Yamada M., Matsushima K., Gronenborn A. M. Determination of the secondary structure of interleukin-8 by nuclear magnetic resonance spectroscopy. J Biol Chem. 1989 Nov 15;264(32):18907–18911. [PubMed] [Google Scholar]
- Clore G. M., Appella E., Yamada M., Matsushima K., Gronenborn A. M. Three-dimensional structure of interleukin 8 in solution. Biochemistry. 1990 Feb 20;29(7):1689–1696. doi: 10.1021/bi00459a004. [DOI] [PubMed] [Google Scholar]
- Cochran B. H., Reffel A. C., Stiles C. D. Molecular cloning of gene sequences regulated by platelet-derived growth factor. Cell. 1983 Jul;33(3):939–947. doi: 10.1016/0092-8674(83)90037-5. [DOI] [PubMed] [Google Scholar]
- Farber J. M. A macrophage mRNA selectively induced by gamma-interferon encodes a member of the platelet factor 4 family of cytokines. Proc Natl Acad Sci U S A. 1990 Jul;87(14):5238–5242. doi: 10.1073/pnas.87.14.5238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiser T., Dewald B., Ehrengruber M. U., Clark-Lewis I., Baggiolini M. The interleukin-8-related chemotactic cytokines GRO alpha, GRO beta, and GRO gamma activate human neutrophil and basophil leukocytes. J Biol Chem. 1993 Jul 25;268(21):15419–15424. [PubMed] [Google Scholar]
- Grob P. M., David E., Warren T. C., DeLeon R. P., Farina P. R., Homon C. A. Characterization of a receptor for human monocyte-derived neutrophil chemotactic factor/interleukin-8. J Biol Chem. 1990 May 15;265(14):8311–8316. [PubMed] [Google Scholar]
- Haskill S., Peace A., Morris J., Sporn S. A., Anisowicz A., Lee S. W., Smith T., Martin G., Ralph P., Sager R. Identification of three related human GRO genes encoding cytokine functions. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7732–7736. doi: 10.1073/pnas.87.19.7732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrich J. N., Ryseck R. P., Macdonald-Bravo H., Bravo R. The product of a novel growth factor-activated gene, fic, is a biologically active "C-C"-type cytokine. Mol Cell Biol. 1993 Apr;13(4):2020–2030. doi: 10.1128/mcb.13.4.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes W. E., Lee J., Kuang W. J., Rice G. C., Wood W. I. Structure and functional expression of a human interleukin-8 receptor. Science. 1991 Sep 13;253(5025):1278–1280. doi: 10.1126/science.1840701. [DOI] [PubMed] [Google Scholar]
- Horuk R., Yansura D. G., Reilly D., Spencer S., Bourell J., Henzel W., Rice G., Unemori E. Purification, receptor binding analysis, and biological characterization of human melanoma growth stimulating activity (MGSA). Evidence for a novel MGSA receptor. J Biol Chem. 1993 Jan 5;268(1):541–546. [PubMed] [Google Scholar]
- Hébert C. A., Chuntharapai A., Smith M., Colby T., Kim J., Horuk R. Partial functional mapping of the human interleukin-8 type A receptor. Identification of a major ligand binding domain. J Biol Chem. 1993 Sep 5;268(25):18549–18553. [PubMed] [Google Scholar]
- Hébert C. A., Vitangcol R. V., Baker J. B. Scanning mutagenesis of interleukin-8 identifies a cluster of residues required for receptor binding. J Biol Chem. 1991 Oct 5;266(28):18989–18994. [PubMed] [Google Scholar]
- Iida N., Grotendorst G. R. Cloning and sequencing of a new gro transcript from activated human monocytes: expression in leukocytes and wound tissue. Mol Cell Biol. 1990 Oct;10(10):5596–5599. doi: 10.1128/mcb.10.10.5596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaRosa G. J., Thomas K. M., Kaufmann M. E., Mark R., White M., Taylor L., Gray G., Witt D., Navarro J. Amino terminus of the interleukin-8 receptor is a major determinant of receptor subtype specificity. J Biol Chem. 1992 Dec 15;267(35):25402–25406. [PubMed] [Google Scholar]
- Lee J., Horuk R., Rice G. C., Bennett G. L., Camerato T., Wood W. I. Characterization of two high affinity human interleukin-8 receptors. J Biol Chem. 1992 Aug 15;267(23):16283–16287. [PubMed] [Google Scholar]
- Miller M. D., Krangel M. S. Biology and biochemistry of the chemokines: a family of chemotactic and inflammatory cytokines. Crit Rev Immunol. 1992;12(1-2):17–46. [PubMed] [Google Scholar]
- Moser B., Schumacher C., von Tscharner V., Clark-Lewis I., Baggiolini M. Neutrophil-activating peptide 2 and gro/melanoma growth-stimulatory activity interact with neutrophil-activating peptide 1/interleukin 8 receptors on human neutrophils. J Biol Chem. 1991 Jun 5;266(16):10666–10671. [PubMed] [Google Scholar]
- Murphy P. M., Tiffany H. L. Cloning of complementary DNA encoding a functional human interleukin-8 receptor. Science. 1991 Sep 13;253(5025):1280–1283. doi: 10.1126/science.1891716. [DOI] [PubMed] [Google Scholar]
- Oppenheim J. J., Zachariae C. O., Mukaida N., Matsushima K. Properties of the novel proinflammatory supergene "intercrine" cytokine family. Annu Rev Immunol. 1991;9:617–648. doi: 10.1146/annurev.iy.09.040191.003153. [DOI] [PubMed] [Google Scholar]
- Oquendo P., Alberta J., Wen D. Z., Graycar J. L., Derynck R., Stiles C. D. The platelet-derived growth factor-inducible KC gene encodes a secretory protein related to platelet alpha-granule proteins. J Biol Chem. 1989 Mar 5;264(7):4133–4137. [PubMed] [Google Scholar]
- Richmond A., Balentien E., Thomas H. G., Flaggs G., Barton D. E., Spiess J., Bordoni R., Francke U., Derynck R. Molecular characterization and chromosomal mapping of melanoma growth stimulatory activity, a growth factor structurally related to beta-thromboglobulin. EMBO J. 1988 Jul;7(7):2025–2033. doi: 10.1002/j.1460-2075.1988.tb03042.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryseck R. P., MacDonald-Bravo H., Mattei M. G., Bravo R. Cloning and sequence of a secretory protein induced by growth factors in mouse fibroblasts. Exp Cell Res. 1989 Jan;180(1):266–275. doi: 10.1016/0014-4827(89)90230-9. [DOI] [PubMed] [Google Scholar]
- Samanta A. K., Oppenheim J. J., Matsushima K. Identification and characterization of specific receptors for monocyte-derived neutrophil chemotactic factor (MDNCF) on human neutrophils. J Exp Med. 1989 Mar 1;169(3):1185–1189. doi: 10.1084/jem.169.3.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnitzel W., Garbeis B., Monschein U., Besemer J. Neutrophil activating peptide-2 binds with two affinities to receptor(s) on human neutrophils. Biochem Biophys Res Commun. 1991 Oct 15;180(1):301–307. doi: 10.1016/s0006-291x(05)81292-6. [DOI] [PubMed] [Google Scholar]
- Schröder J. M., Persoon N. L., Christophers E. Lipopolysaccharide-stimulated human monocytes secrete, apart from neutrophil-activating peptide 1/interleukin 8, a second neutrophil-activating protein. NH2-terminal amino acid sequence identity with melanoma growth stimulatory activity. J Exp Med. 1990 Apr 1;171(4):1091–1100. doi: 10.1084/jem.171.4.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schröder J. M., Sticherling M., Henneicke H. H., Preissner W. C., Christophers E. IL-1 alpha or tumor necrosis factor-alpha stimulate release of three NAP-1/IL-8-related neutrophil chemotactic proteins in human dermal fibroblasts. J Immunol. 1990 Mar 15;144(6):2223–2232. [PubMed] [Google Scholar]
- Schumacher C., Clark-Lewis I., Baggiolini M., Moser B. High- and low-affinity binding of GRO alpha and neutrophil-activating peptide 2 to interleukin 8 receptors on human neutrophils. Proc Natl Acad Sci U S A. 1992 Nov 1;89(21):10542–10546. doi: 10.1073/pnas.89.21.10542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Charles R., Walz D. A., Edwards B. F. The three-dimensional structure of bovine platelet factor 4 at 3.0-A resolution. J Biol Chem. 1989 Feb 5;264(4):2092–2099. [PubMed] [Google Scholar]
- Tekamp-Olson P., Gallegos C., Bauer D., McClain J., Sherry B., Fabre M., van Deventer S., Cerami A. Cloning and characterization of cDNAs for murine macrophage inflammatory protein 2 and its human homologues. J Exp Med. 1990 Sep 1;172(3):911–919. doi: 10.1084/jem.172.3.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thelen M., Peveri P., Kernen P., von Tscharner V., Walz A., Baggiolini M. Mechanism of neutrophil activation by NAF, a novel monocyte-derived peptide agonist. FASEB J. 1988 Aug;2(11):2702–2706. [PubMed] [Google Scholar]
- Unemori E. N., Amento E. P., Bauer E. A., Horuk R. Melanoma growth-stimulatory activity/GRO decreases collagen expression by human fibroblasts. Regulation by C-X-C but not C-C cytokines. J Biol Chem. 1993 Jan 15;268(2):1338–1342. [PubMed] [Google Scholar]
- Wen D. Z., Rowland A., Derynck R. Expression and secretion of gro/MGSA by stimulated human endothelial cells. EMBO J. 1989 Jun;8(6):1761–1766. doi: 10.1002/j.1460-2075.1989.tb03569.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolpe S. D., Sherry B., Juers D., Davatelis G., Yurt R. W., Cerami A. Identification and characterization of macrophage inflammatory protein 2. Proc Natl Acad Sci U S A. 1989 Jan;86(2):612–616. doi: 10.1073/pnas.86.2.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray W., Boulikas T., Wray V. P., Hancock R. Silver staining of proteins in polyacrylamide gels. Anal Biochem. 1981 Nov 15;118(1):197–203. doi: 10.1016/0003-2697(81)90179-2. [DOI] [PubMed] [Google Scholar]
- Zucker M. B., Katz I. R., Thorbecke G. J., Milot D. C., Holt J. Immunoregulatory activity of peptides related to platelet factor 4. Proc Natl Acad Sci U S A. 1989 Oct;86(19):7571–7574. doi: 10.1073/pnas.86.19.7571. [DOI] [PMC free article] [PubMed] [Google Scholar]