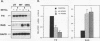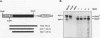Abstract
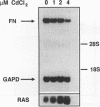
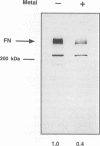
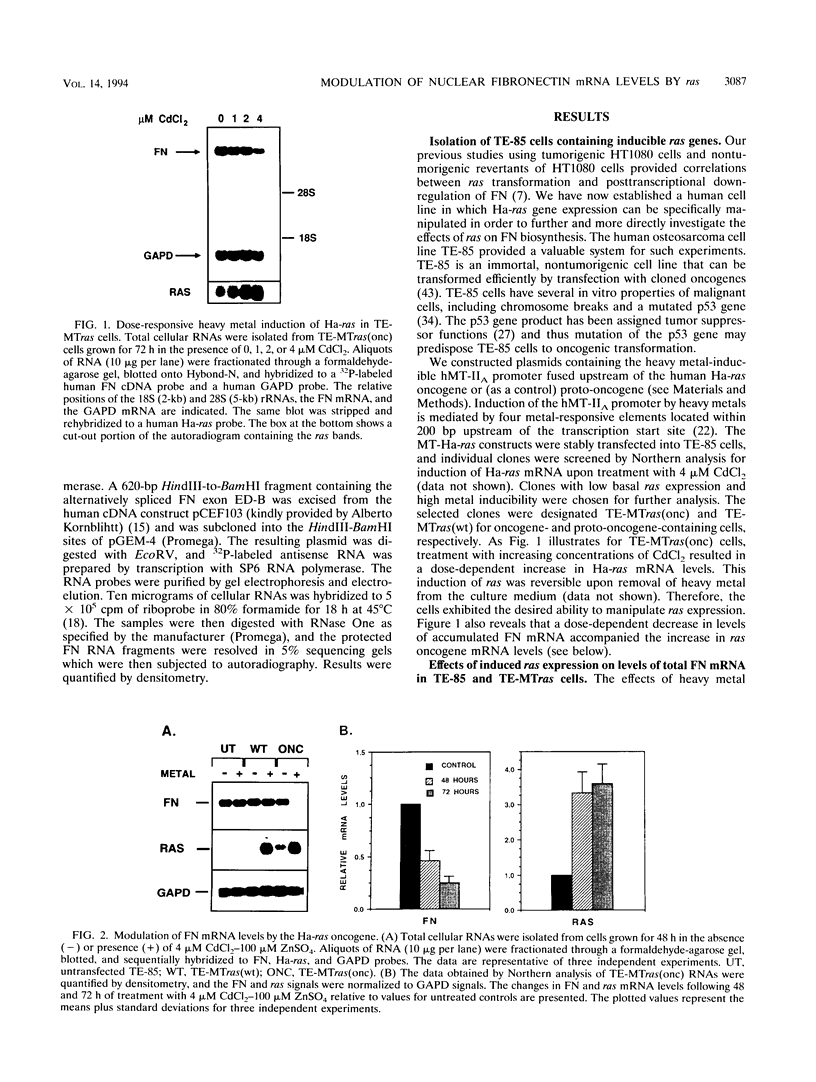
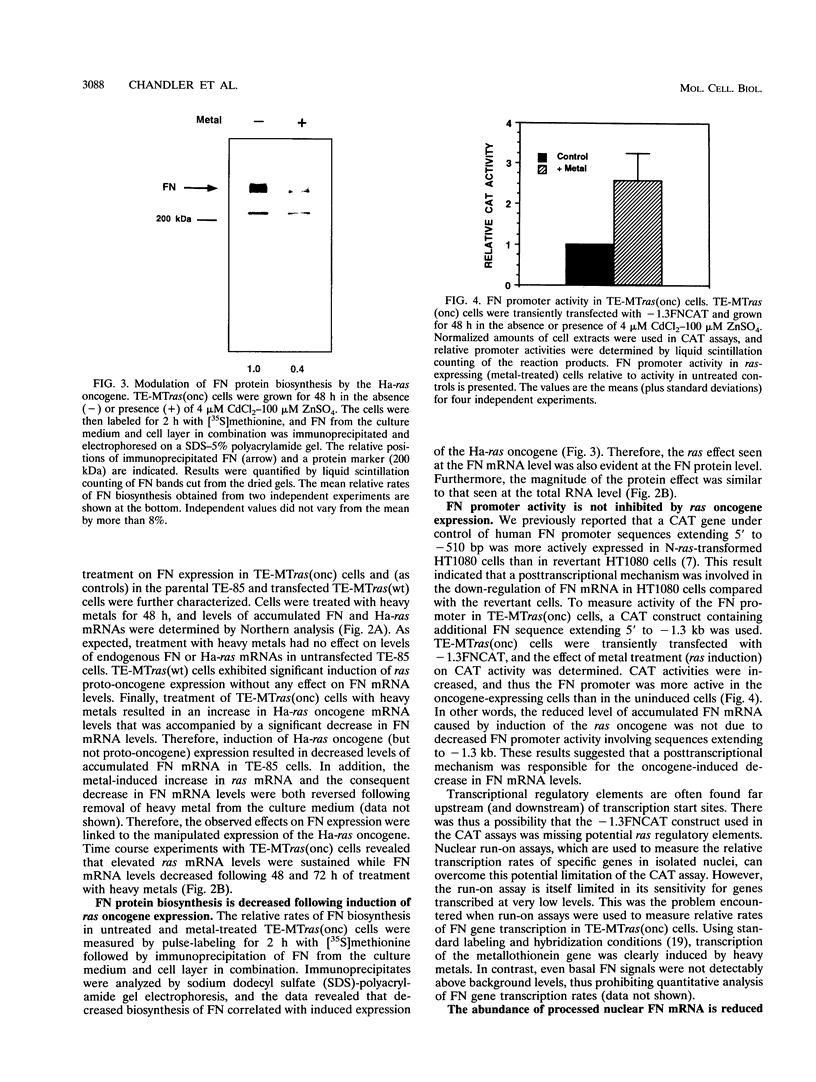
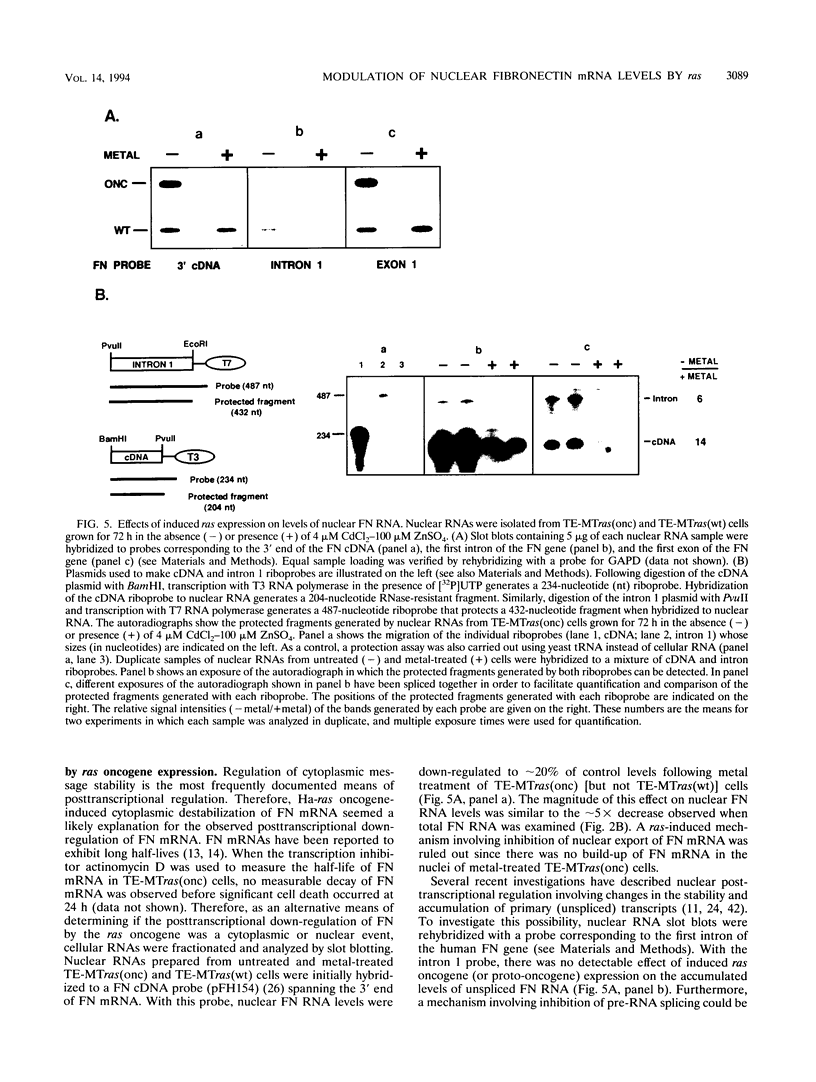
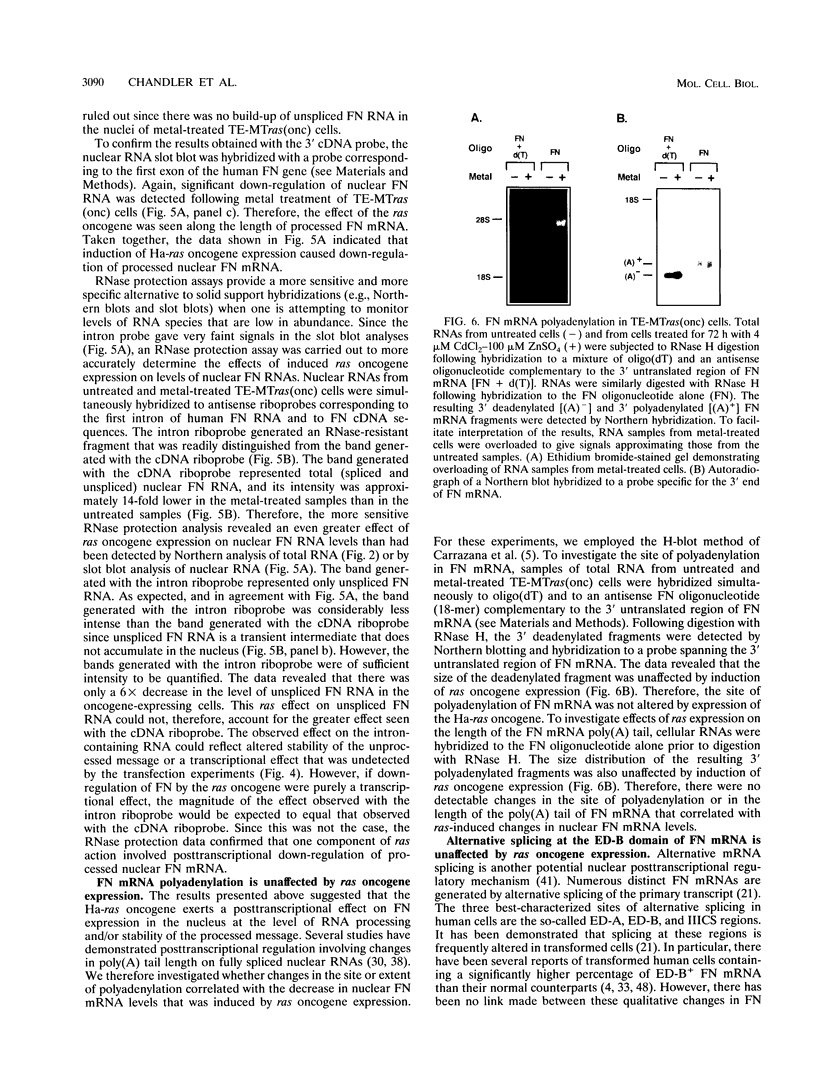
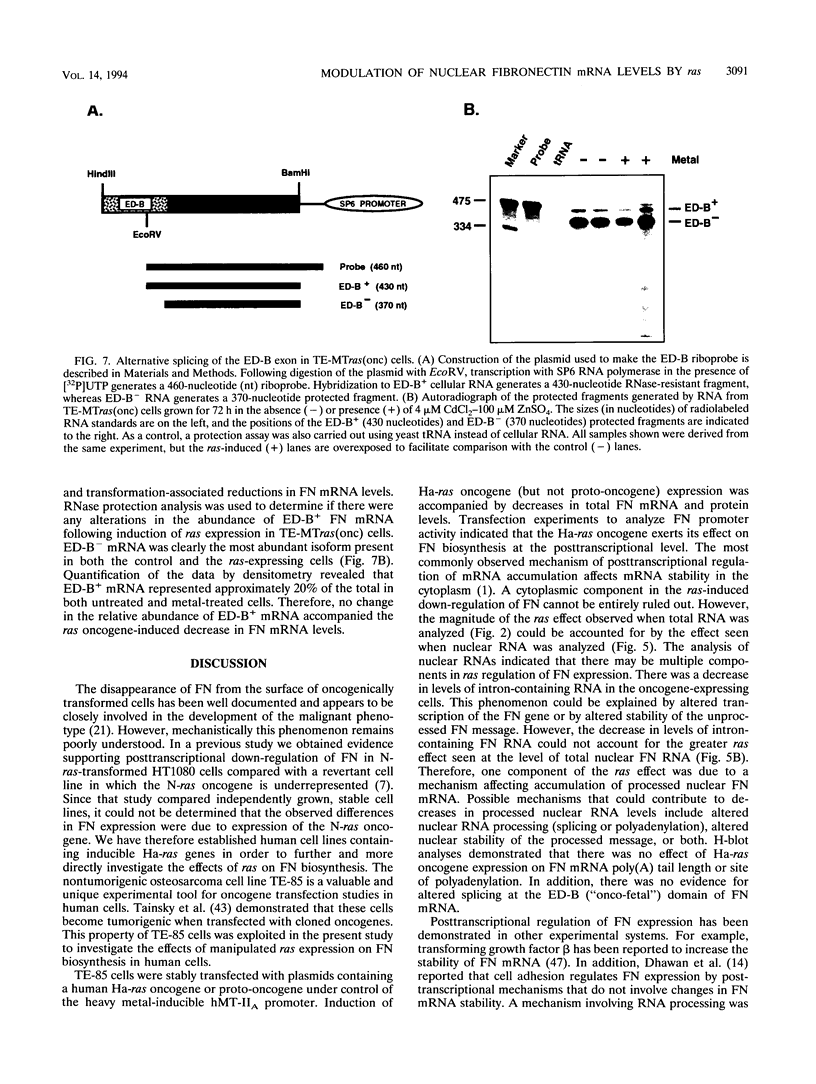
Although loss of cell surface fibronectin (FN) is a hallmark of many oncogenically transformed cells, the mechanisms responsible for this phenomenon remain poorly understood. The present study utilized the nontumorigenic human osteosarcoma cell line TE-85 to investigate the effects of induced Ha-ras oncogene expression on FN biosynthesis. TE-85 cells were stably transfected with metallothionein-Ha-ras fusion genes, and the effects of metal-induced ras expression on FN biosynthesis were determined. Induction of the ras oncogene, but not proto-oncogene, was accompanied by a decrease in total FN mRNA and protein levels. Transfection experiments indicated that these oncogene effects were not due to reduced FN promoter activity, suggesting that a posttranscriptional mechanism was involved. The most common mechanism of posttranscriptional regulation affects cytoplasmic mRNA stability. However, in this study the down-regulation of FN was identified as a nuclear event. A component of the ras effect was due to a mechanism affecting accumulation of processed nuclear FN RNA. Mechanisms that would generate such an effect include altered RNA processing and altered stability of the processed message in the nucleus. There was no effect of ras on FN mRNA poly(A) tail length or site of polyadenylation. There was also no evidence for altered splicing at the ED-B domain of FN mRNA. This demonstration of nuclear posttranscriptional down-regulation of FN by the Ha-ras oncogene identifies a new level at which ras oncoproteins can regulate gene expression and thus contribute to development of the malignant phenotype.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bollag G., McCormick F. Regulators and effectors of ras proteins. Annu Rev Cell Biol. 1991;7:601–632. doi: 10.1146/annurev.cb.07.110191.003125. [DOI] [PubMed] [Google Scholar]
- Capon D. J., Chen E. Y., Levinson A. D., Seeburg P. H., Goeddel D. V. Complete nucleotide sequences of the T24 human bladder carcinoma oncogene and its normal homologue. Nature. 1983 Mar 3;302(5903):33–37. doi: 10.1038/302033a0. [DOI] [PubMed] [Google Scholar]
- Carnemolla B., Balza E., Siri A., Zardi L., Nicotra M. R., Bigotti A., Natali P. G. A tumor-associated fibronectin isoform generated by alternative splicing of messenger RNA precursors. J Cell Biol. 1989 Mar;108(3):1139–1148. doi: 10.1083/jcb.108.3.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrazana E. J., Pasieka K. B., Majzoub J. A. The vasopressin mRNA poly(A) tract is unusually long and increases during stimulation of vasopressin gene expression in vivo. Mol Cell Biol. 1988 Jun;8(6):2267–2274. doi: 10.1128/mcb.8.6.2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarty S., Jan Y., Levine A., McClenic B., Varani J. Fibronectin/laminin and their receptors in aberrant growth control in FR3T3 cells transformed by Ha-ras oncogene and epidermal growth factor gene. Int J Cancer. 1989 Aug 15;44(2):325–331. doi: 10.1002/ijc.2910440223. [DOI] [PubMed] [Google Scholar]
- Chandler L. A., Bourgeois S. Posttranscriptional down-regulation of fibronectin in N-ras-transformed cells. Cell Growth Differ. 1991 Aug;2(8):379–384. [PubMed] [Google Scholar]
- Cheng J., Maquat L. E. Nonsense codons can reduce the abundance of nuclear mRNA without affecting the abundance of pre-mRNA or the half-life of cytoplasmic mRNA. Mol Cell Biol. 1993 Mar;13(3):1892–1902. doi: 10.1128/mcb.13.3.1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin K. V., Ueda K., Pastan I., Gottesman M. M. Modulation of activity of the promoter of the human MDR1 gene by Ras and p53. Science. 1992 Jan 24;255(5043):459–462. doi: 10.1126/science.1346476. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Cohen R. B., Boal T. R., Safer B. Increased eIF-2 alpha expression in mitogen-activated primary T lymphocytes. EMBO J. 1990 Dec;9(12):3831–3837. doi: 10.1002/j.1460-2075.1990.tb07601.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean D. C., Bowlus C. L., Bourgeois S. Cloning and analysis of the promotor region of the human fibronectin gene. Proc Natl Acad Sci U S A. 1987 Apr;84(7):1876–1880. doi: 10.1073/pnas.84.7.1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean D. C., Newby R. F., Bourgeois S. Regulation of fibronectin biosynthesis by dexamethasone, transforming growth factor beta, and cAMP in human cell lines. J Cell Biol. 1988 Jun;106(6):2159–2170. doi: 10.1083/jcb.106.6.2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhawan J., Lichtler A. C., Rowe D. W., Farmer S. R. Cell adhesion regulates pro-alpha 1(I) collagen mRNA stability and transcription in mouse fibroblasts. J Biol Chem. 1991 May 5;266(13):8470–8475. [PubMed] [Google Scholar]
- Dufour S., Gutman A., Bois F., Lamb N., Thiery J. P., Kornblihtt A. R. Generation of full-length cDNA recombinant vectors for the transient expression of human fibronectin in mammalian cell lines. Exp Cell Res. 1991 Apr;193(2):331–338. doi: 10.1016/0014-4827(91)90104-3. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Geiser A. G., Kim S. J., Roberts A. B., Sporn M. B. Characterization of the mouse transforming growth factor-beta 1 promoter and activation by the Ha-ras oncogene. Mol Cell Biol. 1991 Jan;11(1):84–92. doi: 10.1128/mcb.11.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hölttä E., Sistonen L., Alitalo K. The mechanisms of ornithine decarboxylase deregulation in c-Ha-ras oncogene-transformed NIH 3T3 cells. J Biol Chem. 1988 Mar 25;263(9):4500–4507. [PubMed] [Google Scholar]
- Karin M., Haslinger A., Heguy A., Dietlin T., Cooke T. Metal-responsive elements act as positive modulators of human metallothionein-IIA enhancer activity. Mol Cell Biol. 1987 Feb;7(2):606–613. doi: 10.1128/mcb.7.2.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kedar P. S., Lowy D. R., Widen S. G., Wilson S. H. Transfected human beta-polymerase promoter contains a ras-responsive element. Mol Cell Biol. 1990 Jul;10(7):3852–3856. doi: 10.1128/mcb.10.7.3852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiledjian M., Kadesch T. Post-transcriptional regulation of the human liver/bone/kidney alkaline phosphatase gene. J Biol Chem. 1991 Mar 5;266(7):4207–4213. [PubMed] [Google Scholar]
- Kornblihtt A. R., Vibe-Pedersen K., Baralle F. E. Human fibronectin: molecular cloning evidence for two mRNA species differing by an internal segment coding for a structural domain. EMBO J. 1984 Jan;3(1):221–226. doi: 10.1002/j.1460-2075.1984.tb01787.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornblihtt A. R., Vibe-Pedersen K., Baralle F. E. Isolation and characterization of cDNA clones for human and bovine fibronectins. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3218–3222. doi: 10.1073/pnas.80.11.3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine A. J., Momand J., Finlay C. A. The p53 tumour suppressor gene. Nature. 1991 Jun 6;351(6326):453–456. doi: 10.1038/351453a0. [DOI] [PubMed] [Google Scholar]
- Li Y. C., Lieberman M. W. Two genes associated with liver cancer are regulated by different mechanisms in rasT24 transformed liver epithelial cells. Oncogene. 1989 Jun;4(6):795–798. [PubMed] [Google Scholar]
- McAllister R. M., Gardner M. B., Greene A. E., Bradt C., Nichols W. W., Landing B. H. Cultivation in vitro of cells derived from a human osteosarcoma. Cancer. 1971 Feb;27(2):397–402. doi: 10.1002/1097-0142(197102)27:2<397::aid-cncr2820270224>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Murphy D., Pardy K., Seah V., Carter D. Posttranscriptional regulation of rat growth hormone gene expression: increased message stability and nuclear polyadenylation accompany thyroid hormone depletion. Mol Cell Biol. 1992 Jun;12(6):2624–2632. doi: 10.1128/mcb.12.6.2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima T., Nakamura T., Tsunoda S., Nakada S., Oda K. E1A-responsive elements for repression of rat fibronectin gene transcription. Mol Cell Biol. 1992 Jun;12(6):2837–2846. doi: 10.1128/mcb.12.6.2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen R. D., Ostrowski M. C. Transcriptional activation of a conserved sequence element by ras requires a nuclear factor distinct from c-fos or c-jun. Proc Natl Acad Sci U S A. 1990 May;87(10):3866–3870. doi: 10.1073/pnas.87.10.3866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyama F., Hirohashi S., Shimosato Y., Titani K., Sekiguchi K. Oncodevelopmental regulation of the alternative splicing of fibronectin pre-messenger RNA in human lung tissues. Cancer Res. 1990 Feb 15;50(4):1075–1078. [PubMed] [Google Scholar]
- Romano J. W., Ehrhart J. C., Duthu A., Kim C. M., Appella E., May P. Identification and characterization of a p53 gene mutation in a human osteosarcoma cell line. Oncogene. 1989 Dec;4(12):1483–1488. [PubMed] [Google Scholar]
- Santos E., Tronick S. R., Aaronson S. A., Pulciani S., Barbacid M. T24 human bladder carcinoma oncogene is an activated form of the normal human homologue of BALB- and Harvey-MSV transforming genes. Nature. 1982 Jul 22;298(5872):343–347. doi: 10.1038/298343a0. [DOI] [PubMed] [Google Scholar]
- Schalken J. A., Ebeling S. B., Isaacs J. T., Treiger B., Bussemakers M. J., de Jong M. E., Van de Ven W. J. Down modulation of fibronectin messenger RNA in metastasizing rat prostatic cancer cells revealed by differential hybridization analysis. Cancer Res. 1988 Apr 15;48(8):2042–2046. [PubMed] [Google Scholar]
- Seed B., Sheen J. Y. A simple phase-extraction assay for chloramphenicol acyltransferase activity. Gene. 1988 Jul 30;67(2):271–277. doi: 10.1016/0378-1119(88)90403-9. [DOI] [PubMed] [Google Scholar]
- Shiels B. R., Northemann W., Gehring M. R., Fey G. H. Modified nuclear processing of alpha 1-acid glycoprotein RNA during inflammation. J Biol Chem. 1987 Sep 15;262(26):12826–12831. [PubMed] [Google Scholar]
- Sistonen L., Keski-Oja J., Ulmanen I., Hölttä E., Wikgren B. J., Alitalo K. Dose effects of transfected c-Ha-rasVal 12 oncogene in transformed cell clones. Exp Cell Res. 1987 Feb;168(2):518–530. doi: 10.1016/0014-4827(87)90024-3. [DOI] [PubMed] [Google Scholar]
- Slack J. L., Parker M. I., Robinson V. R., Bornstein P. Regulation of collagen I gene expression by ras. Mol Cell Biol. 1992 Oct;12(10):4714–4723. doi: 10.1128/mcb.12.10.4714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. W., Patton J. G., Nadal-Ginard B. Alternative splicing in the control of gene expression. Annu Rev Genet. 1989;23:527–577. doi: 10.1146/annurev.ge.23.120189.002523. [DOI] [PubMed] [Google Scholar]
- Song M. K., Dozin B., Grieco D., Rall J. E., Nikodem V. M. Transcriptional activation and stabilization of malic enzyme mRNA precursor by thyroid hormone. J Biol Chem. 1988 Dec 5;263(34):17970–17974. [PubMed] [Google Scholar]
- Tainsky M. A., Shamanski F. L., Blair D., Vande Woude G. Human recipient cell for oncogene transfection studies. Mol Cell Biol. 1987 Mar;7(3):1280–1284. doi: 10.1128/mcb.7.3.1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tso J. Y., Sun X. H., Kao T. H., Reece K. S., Wu R. Isolation and characterization of rat and human glyceraldehyde-3-phosphate dehydrogenase cDNAs: genomic complexity and molecular evolution of the gene. Nucleic Acids Res. 1985 Apr 11;13(7):2485–2502. doi: 10.1093/nar/13.7.2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyagi J. S., Hirano H., Merlino G. T., Pastan I. Transcriptional control of the fibronectin gene in chick embryo fibroblasts transformed by Rous sarcoma virus. J Biol Chem. 1983 May 10;258(9):5787–5793. [PubMed] [Google Scholar]
- Wigler M., Silverstein S., Lee L. S., Pellicer A., Cheng Y. c., Axel R. Transfer of purified herpes virus thymidine kinase gene to cultured mouse cells. Cell. 1977 May;11(1):223–232. doi: 10.1016/0092-8674(77)90333-6. [DOI] [PubMed] [Google Scholar]
- Wrana J. L., Overall C. M., Sodek J. Regulation of the expression of a secreted acidic protein rich in cysteine (SPARC) in human fibroblasts by transforming growth factor beta. Comparison of transcriptional and post-transcriptional control with fibronectin and type I collagen. Eur J Biochem. 1991 Apr 23;197(2):519–528. doi: 10.1111/j.1432-1033.1991.tb15940.x. [DOI] [PubMed] [Google Scholar]
- Zardi L., Carnemolla B., Siri A., Petersen T. E., Paolella G., Sebastio G., Baralle F. E. Transformed human cells produce a new fibronectin isoform by preferential alternative splicing of a previously unobserved exon. EMBO J. 1987 Aug;6(8):2337–2342. doi: 10.1002/j.1460-2075.1987.tb02509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]