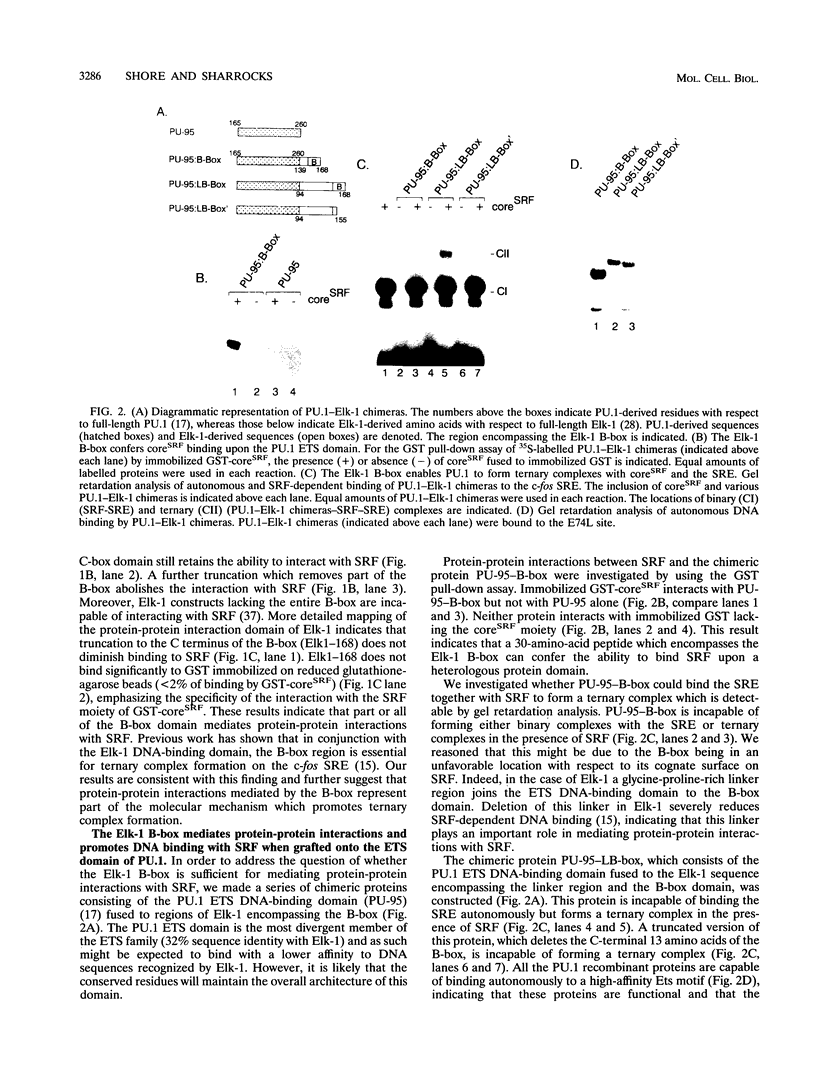
Abstract
Transcriptional induction of the c-fos gene in response to epidermal growth factor stimulation is mediated in part by a ternary nucleoprotein complex within the promoter consisting of serum response factor (SRF), p62TCF/Elk-1 and the serum response element (SRE). Both SRF and p62TCF/Elk-1 contact the DNA and bind in a cooperative manner to the SRE. In this study, we demonstrate that SRF and Elk-1 interact directly in the absence of the SRE. A 30-amino-acid peptide from Elk-1 (B-box) is both necessary and sufficient to mediate protein-protein contacts with SRF. Moreover, the Elk-1 B-box is necessary to enable SRF-dependent binding of an alternative ETS domain (from the transcription factor PU.1) to the c-fos SRE. Mutations in either the Elk-1 B-box or the C-terminal half of the SRF DNA-binding domain (coreSRF) which show reduced ability to form ternary complexes also show greatly reduced protein-protein interactions in the absence of the SRE. Our results clearly demonstrate that direct protein-protein interactions between the transcription factors Elk-1 and SRF, in addition to DNA contacts, contribute to the formation of a ternary complex on the c-fos SRE. We discuss the wider applicability of our results in describing specific protein-protein interactions between short well-defined transcription factor domains.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bordo D., Argos P. Suggestions for "safe" residue substitutions in site-directed mutagenesis. J Mol Biol. 1991 Feb 20;217(4):721–729. doi: 10.1016/0022-2836(91)90528-e. [DOI] [PubMed] [Google Scholar]
- Brown T. A., McKnight S. L. Specificities of protein-protein and protein-DNA interaction of GABP alpha and two newly defined ets-related proteins. Genes Dev. 1992 Dec;6(12B):2502–2512. doi: 10.1101/gad.6.12b.2502. [DOI] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Prediction of the secondary structure of proteins from their amino acid sequence. Adv Enzymol Relat Areas Mol Biol. 1978;47:45–148. doi: 10.1002/9780470122921.ch2. [DOI] [PubMed] [Google Scholar]
- Dalton S., Treisman R. Characterization of SAP-1, a protein recruited by serum response factor to the c-fos serum response element. Cell. 1992 Feb 7;68(3):597–612. doi: 10.1016/0092-8674(92)90194-h. [DOI] [PubMed] [Google Scholar]
- Garnier J., Osguthorpe D. J., Robson B. Analysis of the accuracy and implications of simple methods for predicting the secondary structure of globular proteins. J Mol Biol. 1978 Mar 25;120(1):97–120. doi: 10.1016/0022-2836(78)90297-8. [DOI] [PubMed] [Google Scholar]
- Gille H., Sharrocks A. D., Shaw P. E. Phosphorylation of transcription factor p62TCF by MAP kinase stimulates ternary complex formation at c-fos promoter. Nature. 1992 Jul 30;358(6385):414–417. doi: 10.1038/358414a0. [DOI] [PubMed] [Google Scholar]
- Graham R., Gilman M. Distinct protein targets for signals acting at the c-fos serum response element. Science. 1991 Jan 11;251(4990):189–192. doi: 10.1126/science.1898992. [DOI] [PubMed] [Google Scholar]
- Gustafson T. A., Taylor A., Kedes L. DNA bending is induced by a transcription factor that interacts with the human c-FOS and alpha-actin promoters. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2162–2166. doi: 10.1073/pnas.86.7.2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha I., Roberts S., Maldonado E., Sun X., Kim L. U., Green M., Reinberg D. Multiple functional domains of human transcription factor IIB: distinct interactions with two general transcription factors and RNA polymerase II. Genes Dev. 1993 Jun;7(6):1021–1032. doi: 10.1101/gad.7.6.1021. [DOI] [PubMed] [Google Scholar]
- Hagemeier C., Bannister A. J., Cook A., Kouzarides T. The activation domain of transcription factor PU.1 binds the retinoblastoma (RB) protein and the transcription factor TFIID in vitro: RB shows sequence similarity to TFIID and TFIIB. Proc Natl Acad Sci U S A. 1993 Feb 15;90(4):1580–1584. doi: 10.1073/pnas.90.4.1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera R. E., Shaw P. E., Nordheim A. Occupation of the c-fos serum response element in vivo by a multi-protein complex is unaltered by growth factor induction. Nature. 1989 Jul 6;340(6228):68–70. doi: 10.1038/340068a0. [DOI] [PubMed] [Google Scholar]
- Hill C. S., Marais R., John S., Wynne J., Dalton S., Treisman R. Functional analysis of a growth factor-responsive transcription factor complex. Cell. 1993 Apr 23;73(2):395–406. doi: 10.1016/0092-8674(93)90238-l. [DOI] [PubMed] [Google Scholar]
- Horwitz B. H., DiMaio D. Saturation mutagenesis using mixed oligonucleotides and M13 templates containing uracil. Methods Enzymol. 1990;185:599–611. doi: 10.1016/0076-6879(90)85047-r. [DOI] [PubMed] [Google Scholar]
- Janknecht R., Nordheim A. Elk-1 protein domains required for direct and SRF-assisted DNA-binding. Nucleic Acids Res. 1992 Jul 11;20(13):3317–3324. doi: 10.1093/nar/20.13.3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karim F. D., Urness L. D., Thummel C. S., Klemsz M. J., McKercher S. R., Celada A., Van Beveren C., Maki R. A., Gunther C. V., Nye J. A. The ETS-domain: a new DNA-binding motif that recognizes a purine-rich core DNA sequence. Genes Dev. 1990 Sep;4(9):1451–1453. doi: 10.1101/gad.4.9.1451. [DOI] [PubMed] [Google Scholar]
- Klemsz M. J., McKercher S. R., Celada A., Van Beveren C., Maki R. A. The macrophage and B cell-specific transcription factor PU.1 is related to the ets oncogene. Cell. 1990 Apr 6;61(1):113–124. doi: 10.1016/0092-8674(90)90219-5. [DOI] [PubMed] [Google Scholar]
- König H. Cell-type specific multiprotein complex formation over the c-fos serum response element in vivo: ternary complex formation is not required for the induction of c-fos. Nucleic Acids Res. 1991 Jul 11;19(13):3607–3611. doi: 10.1093/nar/19.13.3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landt O., Grunert H. P., Hahn U. A general method for rapid site-directed mutagenesis using the polymerase chain reaction. Gene. 1990 Nov 30;96(1):125–128. doi: 10.1016/0378-1119(90)90351-q. [DOI] [PubMed] [Google Scholar]
- Maddox J. Towards synthetic self-replication. Nature. 1991 Dec 5;354(6352):351–351. doi: 10.1038/354351a0. [DOI] [PubMed] [Google Scholar]
- Malik R. K., Roe M. W., Blackshear P. J. Epidermal growth factor and other mitogens induce binding of a protein complex to the c-fos serum response element in human astrocytoma and other cells. J Biol Chem. 1991 May 5;266(13):8576–8582. [PubMed] [Google Scholar]
- Marais R., Wynne J., Treisman R. The SRF accessory protein Elk-1 contains a growth factor-regulated transcriptional activation domain. Cell. 1993 Apr 23;73(2):381–393. doi: 10.1016/0092-8674(93)90237-k. [DOI] [PubMed] [Google Scholar]
- Morrison H. G., Desrosiers R. C. A PCR-based strategy for extensive mutagenesis of a target DNA sequence. Biotechniques. 1993 Mar;14(3):454–457. [PubMed] [Google Scholar]
- Mueller C. G., Nordheim A. A protein domain conserved between yeast MCM1 and human SRF directs ternary complex formation. EMBO J. 1991 Dec;10(13):4219–4229. doi: 10.1002/j.1460-2075.1991.tb05000.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollock R., Treisman R. Human SRF-related proteins: DNA-binding properties and potential regulatory targets. Genes Dev. 1991 Dec;5(12A):2327–2341. doi: 10.1101/gad.5.12a.2327. [DOI] [PubMed] [Google Scholar]
- Pongubala J. M., Van Beveren C., Nagulapalli S., Klemsz M. J., McKercher S. R., Maki R. A., Atchison M. L. Effect of PU.1 phosphorylation on interaction with NF-EM5 and transcriptional activation. Science. 1993 Mar 12;259(5101):1622–1625. doi: 10.1126/science.8456286. [DOI] [PubMed] [Google Scholar]
- Pontius B. W. Close encounters: why unstructured, polymeric domains can increase rates of specific macromolecular association. Trends Biochem Sci. 1993 May;18(5):181–186. doi: 10.1016/0968-0004(93)90111-y. [DOI] [PubMed] [Google Scholar]
- Prywes R., Roeder R. G. Purification of the c-fos enhancer-binding protein. Mol Cell Biol. 1987 Oct;7(10):3482–3489. doi: 10.1128/mcb.7.10.3482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao V. N., Huebner K., Isobe M., ar-Rushdi A., Croce C. M., Reddy E. S. elk, tissue-specific ets-related genes on chromosomes X and 14 near translocation breakpoints. Science. 1989 Apr 7;244(4900):66–70. doi: 10.1126/science.2539641. [DOI] [PubMed] [Google Scholar]
- Schröter H., Mueller C. G., Meese K., Nordheim A. Synergism in ternary complex formation between the dimeric glycoprotein p67SRF, polypeptide p62TCF and the c-fos serum response element. EMBO J. 1990 Apr;9(4):1123–1130. doi: 10.1002/j.1460-2075.1990.tb08218.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharrocks A. D., Gille H., Shaw P. E. Identification of amino acids essential for DNA binding and dimerization in p67SRF: implications for a novel DNA-binding motif. Mol Cell Biol. 1993 Jan;13(1):123–132. doi: 10.1128/mcb.13.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharrocks A. D., Shaw P. E. Improved primer design for PCR-based, site-directed mutagenesis. Nucleic Acids Res. 1992 Mar 11;20(5):1147–1147. doi: 10.1093/nar/20.5.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharrocks A. D., von Hesler F., Shaw P. E. The identification of elements determining the different DNA binding specificities of the MADS box proteins p67SRF and RSRFC4. Nucleic Acids Res. 1993 Jan 25;21(2):215–221. doi: 10.1093/nar/21.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P. E., Schröter H., Nordheim A. The ability of a ternary complex to form over the serum response element correlates with serum inducibility of the human c-fos promoter. Cell. 1989 Feb 24;56(4):563–572. doi: 10.1016/0092-8674(89)90579-5. [DOI] [PubMed] [Google Scholar]
- Shaw P. E. Ternary complex formation over the c-fos serum response element: p62TCF exhibits dual component specificity with contacts to DNA and an extended structure in the DNA-binding domain of p67SRF. EMBO J. 1992 Aug;11(8):3011–3019. doi: 10.1002/j.1460-2075.1992.tb05371.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. B., Johnson K. S. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene. 1988 Jul 15;67(1):31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- Treisman R., Marais R., Wynne J. Spatial flexibility in ternary complexes between SRF and its accessory proteins. EMBO J. 1992 Dec;11(12):4631–4640. doi: 10.1002/j.1460-2075.1992.tb05565.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treisman R. The SRE: a growth factor responsive transcriptional regulator. Semin Cancer Biol. 1990 Feb;1(1):47–58. [PubMed] [Google Scholar]
- Treisman R. The serum response element. Trends Biochem Sci. 1992 Oct;17(10):423–426. doi: 10.1016/0968-0004(92)90013-y. [DOI] [PubMed] [Google Scholar]
- Vershon A. K., Johnson A. D. A short, disordered protein region mediates interactions between the homeodomain of the yeast alpha 2 protein and the MCM1 protein. Cell. 1993 Jan 15;72(1):105–112. doi: 10.1016/0092-8674(93)90054-t. [DOI] [PubMed] [Google Scholar]
- Wang C. Y., Petryniak B., Thompson C. B., Kaelin W. G., Leiden J. M. Regulation of the Ets-related transcription factor Elf-1 by binding to the retinoblastoma protein. Science. 1993 May 28;260(5112):1330–1335. doi: 10.1126/science.8493578. [DOI] [PubMed] [Google Scholar]
- Wasylyk B., Hahn S. L., Giovane A. The Ets family of transcription factors. Eur J Biochem. 1993 Jan 15;211(1-2):7–18. doi: 10.1007/978-3-642-78757-7_2. [DOI] [PubMed] [Google Scholar]
- Yuan Y. O., Stroke I. L., Fields S. Coupling of cell identity to signal response in yeast: interaction between the alpha 1 and STE12 proteins. Genes Dev. 1993 Aug;7(8):1584–1597. doi: 10.1101/gad.7.8.1584. [DOI] [PubMed] [Google Scholar]
- Zinck R., Hipskind R. A., Pingoud V., Nordheim A. c-fos transcriptional activation and repression correlate temporally with the phosphorylation status of TCF. EMBO J. 1993 Jun;12(6):2377–2387. doi: 10.1002/j.1460-2075.1993.tb05892.x. [DOI] [PMC free article] [PubMed] [Google Scholar]