Abstract
Background
Sensitization to food antigen may occur through cutaneous exposure.
Objective
Test the hypothesis that epicutaneous (EC) sensitization with food antigen predisposes to IgE-mediated anaphylaxis upon oral allergen challenge.
Methods
BALB/c mice were EC sensitized by repeated application of ovalbumin (OVA) to tape-stripped skin over 7 weeks, or orally immunized with OVA and cholera toxin (CT) weekly for 8 weeks, then orally challenged with OVA. Body temperature was monitored and serum mouse mast cell protease 1 (mMCP-1) level was determined following challenge. Tissue mast cells (MCs) were examined by chloroacetate esterase (CAE) staining. Serum OVA-specific IgE and IgG1 antibodies, and cytokines in supernatants of OVA-stimulated splenocytes, were measured by ELISA. Serum interleukin-4 (IL-4) levels were measured using an in vivo cytokine capture assay (IVCCA).
Results
EC sensitized mice exhibited expansion of connective tissue MC in the jejunum, increased serum IL-4 levels, and systemic anaphylaxis following oral challenge, as evidenced by decreased body temperature and increased serum mMCP-1 level. Intestinal MC expansion and anaphylaxis were IgE-dependent, as they did not occur in EC sensitized IgE−/− mice. Mice orally immunized with OVA+CT failed to increase serum IL-4 levels, expand their intestinal MCs, or develop anaphylaxis following oral challenge, despite OVA-specific IgE levels and splenocyte cytokine production in response to OVA stimulation, which were comparable to those of EC sensitized mice.
Conclusion
EC sensitized mice, but not mice orally immunized with antigen+CT, develop expansion of intestinal MCs and IgE-mediated anaphylaxis following single oral antigen challenge. IgE is necessary but not sufficient for food anaphylaxis, and MC expansion in the gut may play an important role in the development of anaphylaxis.
Clinical Implications
The skin may be an important route of sensitization to food antigens. Avoidance of cutaneous sensitization may prevent the development of food anaphylaxis.
Keywords: Food allergy, epicutaneous sensitization, IgE, mast cells, anaphylaxis
INTRODUCTION
Anaphylaxis to food results from IgE-mediated sensitivity to a food allergen. However, IgE antibodies to foods can exist in individuals who can ingest the foods without the experiencing anaphylaxis, 1 suggesting that factors other than IgE may be required. In many cases, allergic reactions to foods occur upon the first known ingestion, suggesting that routes other than the oral one may be important in sensitization. Epidemiologic data suggests that sensitization to peanut protein may occur in children through the application of peanut oil to inflamed skin, 2 consistent with the skin being an important route of allergen sensitization.
Altered skin barrier function in patients with AD is thought to promote cutaneous sensitization to environmental antigens including food proteins, potentially leading to the development of food allergies. Little is known about how to prevent the development of food allergy in atopic patients, and presently there is no cure for it. Current therapy relies on allergen avoidance and treatment of severe reactions with epinephrine. We have used a mouse model of allergic skin inflammation with many features of AD 3, 4 to demonstrate that EC sensitization, but not oral immunization, with the food antigen OVA results in IgE-dependent expansion of intestinal MCs and IgE-mediated anaphylaxis following oral challenge.
METHODS
Mice
BALB/c mice were purchased from Charles River Laboratories (Wilmington, MA). IgE−/− mice on a BALB/c background were previously reported. 5 All mice were housed in a specific pathogen-free environment and fed an OVA-free diet. All procedures were performed in accordance with the Animal Care and Use Committee of Boston Children’s Hospital.
Epicutaneous sensitization and oral immunization
EC sensitization of mice was performed as previously described. 3 Each mouse had a total of three one-week exposures to OVA (grade V; Sigma, St. Louis, MO) applied as a patch to tape stripped skin, separated by two-week rest intervals.
Oral immunization of mice was performed as previously described. 6 Briefly, 4–6 week old mice were enterally (subsequently referred to as orally) immunized by gavage once a week for seven weeks with 5 mg OVA and 10 µg CT (azide free; List Biological Laboratories, Inc, Campbell, CA) in 150 µl normal saline or placebo (10 µg CT alone in 150 µl normal saline), using a ball-ended mouse feeding needle.
Induction and measurement of systemic anaphylaxis
At week 7 (EC sensitization model) or week 8 (oral immunization model), mice received a bolus oral challenge with 100 mg OVA or intravenous challenge with 100 µg OVA. Temperature changes were measured using DAS-6006 Smart Probe and transponders (Biomedic Data Systems, Seaford, DE) injected subcutaneously. Mice were sacrificed at 60 minutes following challenge to collect serum and harvest tissues.
Serum antibody measurement
OVA-specific IgG1 and IgE levels were determined by ELISA as previously described. 3
In vitro cytokine production and proliferation assay
Spleen single cell suspensions were cultured at 2 × 106/ml in the presence of OVA (200 µg/ml) for 96 hours as described previously. 7 Cytokine secretion in supernatants was measured by ELISA per the manufacturer’s instructions (IL-4 and IFN-γ, eBioscience; IL-13, R&D Systems, Minneapolis, MN). Splenocyte proliferation was measured by [3H] incorporation after 72 hours of culture.
Serum mMCP-1 levels
mMCP-1 concentrations were measured in serum collected 1 day before and 60 minutes following oral challenge by ELISA per the manufacturer’s instructions (eBioscience).
Histologic analysis of mast cells
Tissue specimens were fixed in 4% paraformaldehyde, embedded in glycomethyacrylate, and sections were stained with chloroacetate esterase (CAE) for quantification of MCs, as previously described. 8 Tissue sections were examined by investigators who were blinded to the identities of the samples. MCs were counted in 10 high-power fields (HPFs) at a magnification of 400×.
In vivo cytokine capture assay (IVCCA) assay for IL-4
IVCCA assay for IL-4 was performed as previously described. 9 Briefly, mice were intravenously (i.v.) injected with 10 µg biotin-anti-IL-4 mAb (BVD6-24G2, eBioscience) and bled 16 hours later. Serum IL-4 levels were determined by ELISA.
IL-4 mRNA expression in mesenteric lymph nodes (MLN)
Total RNA was extracted from homogenized MLN with RNAqueous extraction kit (Ambion Inc). cDNA was generated with iScript cDNA synthesis kit (Bio-rad Laboratory). Quantitative real-time PCR was done using Taqman Gene Expression Assay, universal PCR master mix and the ABI Prism 7300 sequence detection system (Applied Biosystems). Fold induction of IL-4 gene expression was calculated using the comparative method for relative quantitation by normalization to the internal control β2-microglobulin, as described previously.6
Statistical analysis
Statistical significance was determined using GraphPad Prism, version 4.0a (Graphpad Software, La Jolla, CA). Statistical differences were calculated by Student’s t-test (between two groups) and 2-way ANOVA (between curves). A P-value of <0.05 was considered statistically significant.
RESULTS
EC sensitization with OVA results in anaphylaxis following oral antigen challenge
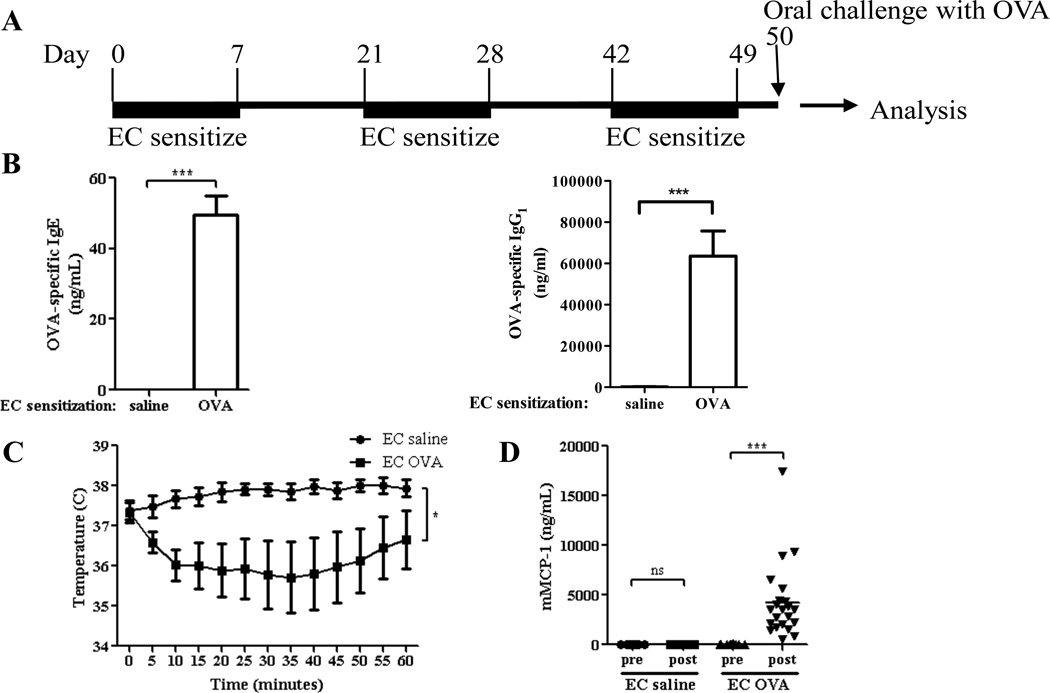
WT BALB/c mice were subjected to three cycles of EC sensitization with OVA or saline as control, then orally challenged with OVA the day after the last cycle of sensitization (Fig. 1A). EC sensitization with OVA resulted in the generation of OVA specific IgE and IgG1 antibodies (Fig. 1B), as previously published. 3, 10 Following oral challenge, mice EC sensitized with OVA exhibited a significant decrease in core body temperature (Fig. 1C), and significantly elevated serum levels of the β-chymase mMCP-1expressed in mucosal MCs 8, 11, 12 compared to controls EC sensitized with saline (Fig. 1D). Oral challenge of EC sensitized mice with saline caused no detectable changes in core body temperature or mMCP-1 levels (data not shown). WT BALB/c mice EC sensitized with 100 µg peanut extract over 7 weeks, then orally challenged with 100 mg peanut flour, also exhibited a significant drop in temperature and an increase in serum mMCP-1 levels (Fig. E1). These results indicate that cutaneous introduction of antigen promotes anaphylaxis and MC degranulation following oral antigen challenge.
Figure 1. EC sensitization with OVA results in anaphylaxis following oral OVA challenge.
A. Experimental protocol. B. OVA specific IgE and IgG1 levels. C. Core body temperature after challenge. D. Serum mMCP-1 levels pre and 60 min post challenge. n=8–20 mice/group. Columns and bars represent mean and SEM. * P <0.05, *** P <0.001. ns = not significant.
Anaphylaxis in EC sensitized mice is IgE-dependent
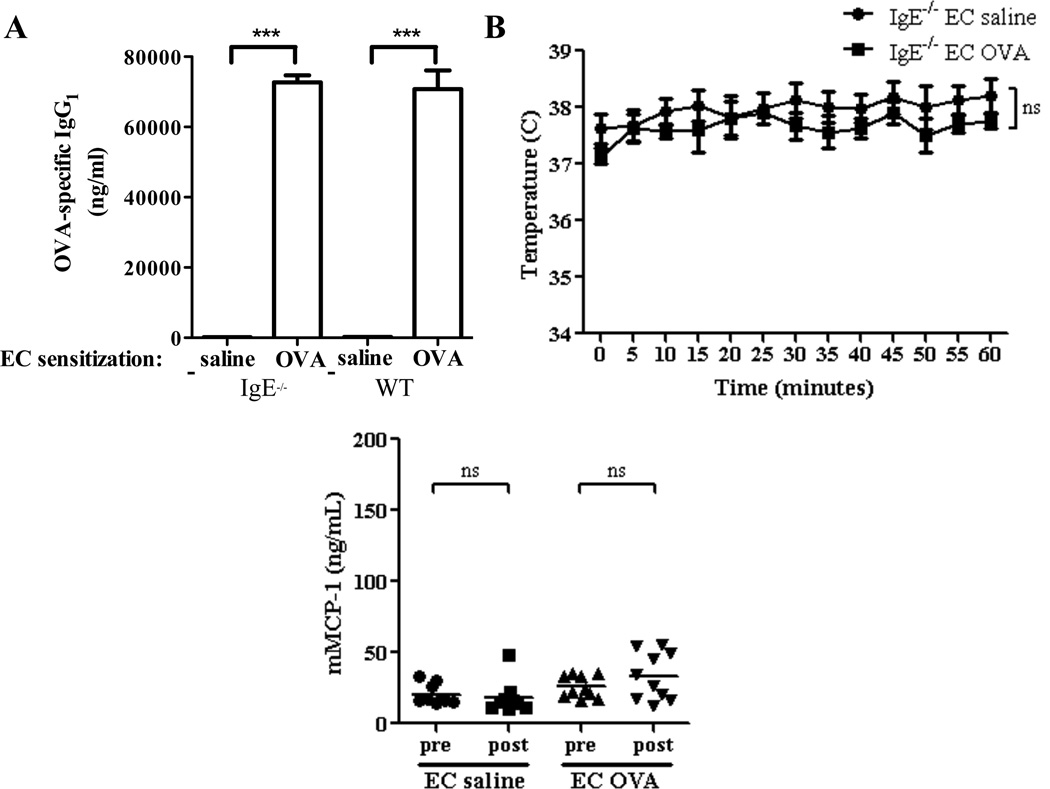
Both IgE and IgG1 antibodies can mediate anaphylaxis in mice. 12 We used IgE−/− mice to examine the role of IgE in our model. Following EC sensitization with OVA, IgE−/− mice exhibited comparable levels of OVA-specific IgG1 compared to WT mice (Fig. 2A), but no detectable OVA-specific IgE (data not shown). IgE−/− mice EC sensitized with OVA failed to decrease their body temperature (Fig. 2B) or increase serum mMCP-1 levels (Fig. 2C) following oral OVA challenge. These results suggest that IgE is necessary for the development of anaphylaxis in our model.
Figure 2. Anaphylaxis following oral OVA challenge in EC sensitized mice is IgE-dependent.
A. OVA specific IgG1 in EC sensitized IgE−/− mice and WT controls. B. Core body temperature after oral OVA challenge. C. Serum mMCP-1 level pre and 60 min post challenge. n=4–10 mice/group. Columns and bars represent mean and SEM. *** P <0.001. ns = not significant.
Oral immunization with OVA+CT does not result in anaphylaxis to oral challenge, despite eliciting an IgE antibody response comparable to that elicited by EC sensitization
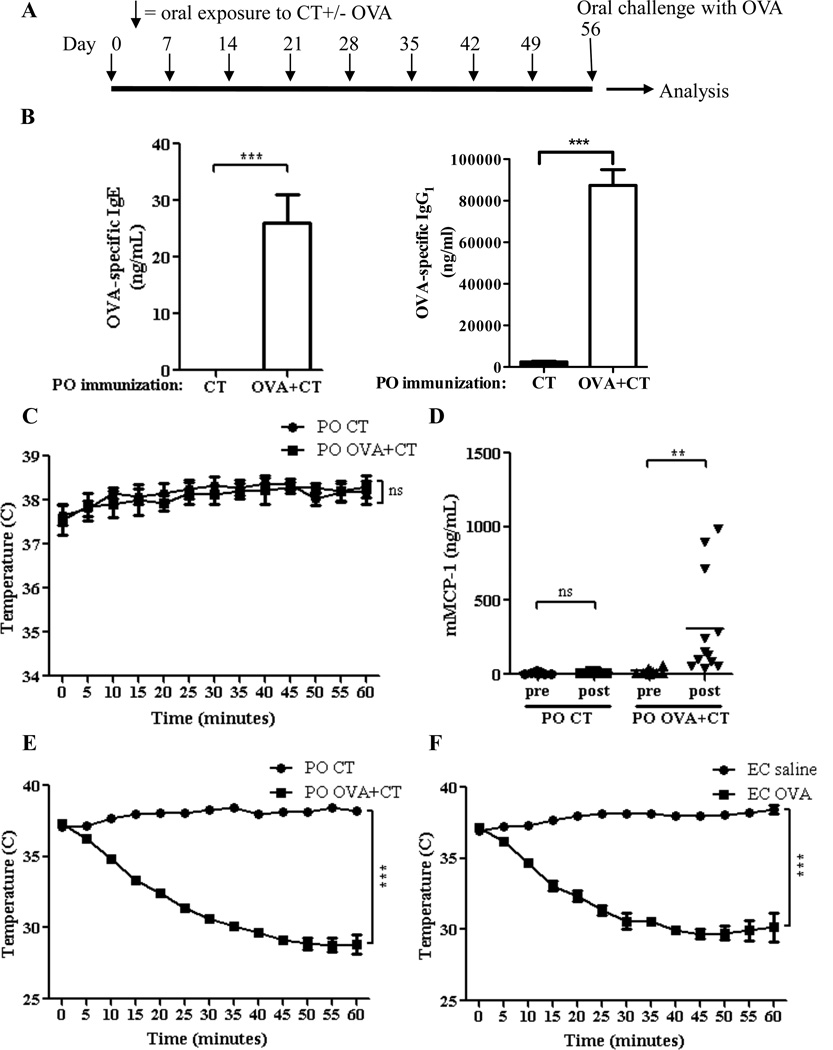
We have previously shown that oral immunization with OVA plus the mucosal adjuvant CT (OVA+CT) elicits a robust Th2 dominated response to OVA with IgG1 and IgE antibodies. 6 We used this model to investigate whether a robust IgE antibody is sufficient for oral anaphylaxis. WT BALB/c mice were orally immunized with OVA+CT or CT alone, as a negative control, by weekly gavage for 8 weeks, then orally challenged with OVA (Fig. 3A). Oral immunization with OVA+CT elicited detectable serum levels of OVA-specific IgE and IgG1which were in the same range as those elicited by EC sensitization with OVA (Fig. 3B). Unlike EC sensitized mice, orally immunized mice failed to decrease their body temperature following antigen challenge (Fig. 3C). Serum levels of mMCP-1 modestly increased post-challenge in mice orally immunized with OVA+CT (Fig. 3D), but were significantly lower than in mice EC sensitized with OVA (mean 310.7 ng/mL and 4, 214.9 ng/mL, respectively, P= 0.001). In contrast to their resistance to oral anaphylaxis, mice orally immunized with OVA+CT readily underwent anaphylaxis to intravenous administration of OVA antigen, as evidenced by a marked drop in body temperature (Fig. 3E). This decrease was comparable to that observed following intravenous challenge of mice EC sensitized with OVA (Fig. 3F). This result indicates that resistance of orally immunized mice to oral anaphylaxis is not due to a generalized inability to undergo anaphylaxis. These results indicate that IgE antibody is sufficient to cause oral anaphylaxis.
Figure 3. Response of orally immunized mice to antigen challenge.
A. Experimental protocol. B. OVA-specific IgE and IgG1. C. Core body temperature after oral challenge. D. Serum mMCP-1. E, F. Core body temperature after i.v. challenge. n/group= 10–12 in A-D and 4 in E, F. Columns and bars: mean and SEM. ** P<0.01, *** P <0.001. ns = not significant.
Th2 cytokines have been shown to play an important role in anaphylaxis, in part by promoting MC expansion and activation. Splenocytes from orally immunized and EC sensitized mice proliferated to a comparable extent in response to in vitro stimulation with OVA (Fig. E2A) and secreted comparable amounts of the Th2 cytokines IL-4 and IL-13, as well as the Th1 cytokine IFN-γ, which reciprocally regulates the effect of IL-4 on mast cells 13 (Fig. E2B–D). Furthermore, mRNA expression in the jejunum for IL-4, IL-13 and IFN-γ was comparable in mice EC sensitized with OVA and mice orally immunized with OVA+CT (data not shown).
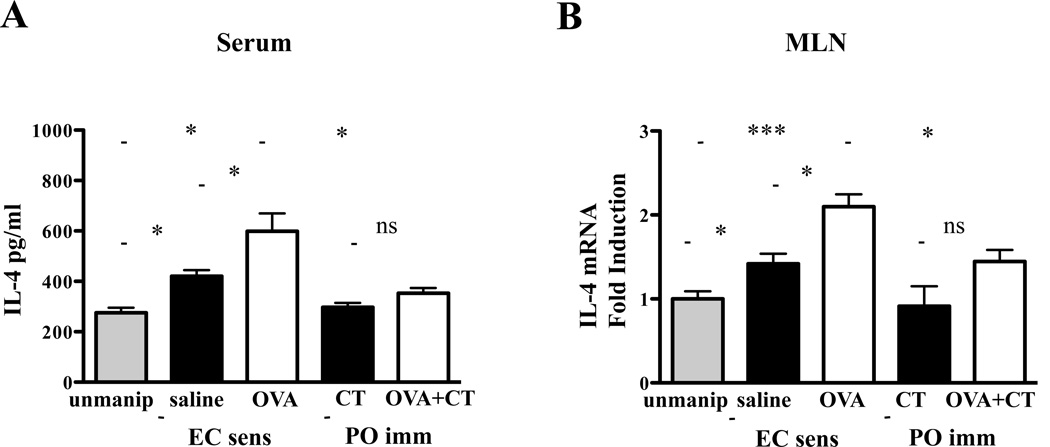
Non-T-cells, including basophils and various populations of innate lymphoid helper cells, secrete Type-2 cytokines. 14, 15 To assess IL-4 systemic output in vivo, we examined IL-4 serum levels and IL-4 mRNA expression in mesenteric lymph nodes (MLN). Serum IL-4 levels were significantly higher in mice EC sensitized with OVA than in mice EC sensitized with saline (Fig. 4A). In addition, serum levels of IL-4 were significantly higher in mice EC sensitized with saline than in unmanipulated controls. In contrast, there was no significant increase in circulating IL-4 levels in mice orally sensitized with OVA+CT or saline+CT. compared to unmanipulated controls. IL-4 mRNA levels were significantly higher in MLN of mice EC sensitized with OVA compared to mice EC sensitized with saline (Fig. 4B). In addition, IL-4 mRNA levels were significantly higher in MLN from mice EC sensitized with saline than in MLN from unmanipulated mice. These results suggest EC sensitization is associated with increased systemic output of IL-4 in vivo.
Figure 4. Increased IL-4 serum levels in EC sensitized mice.
A. Serum levels of IL-4 determined by IVCCA. B. IL-4 mRNA levels in MLN determined by quantitative PCR. Columns and bars represent mean and SEM.* P <0.05, *** P<0.001. ns = not significant.
EC sensitization induces MC expansion in the jejunum
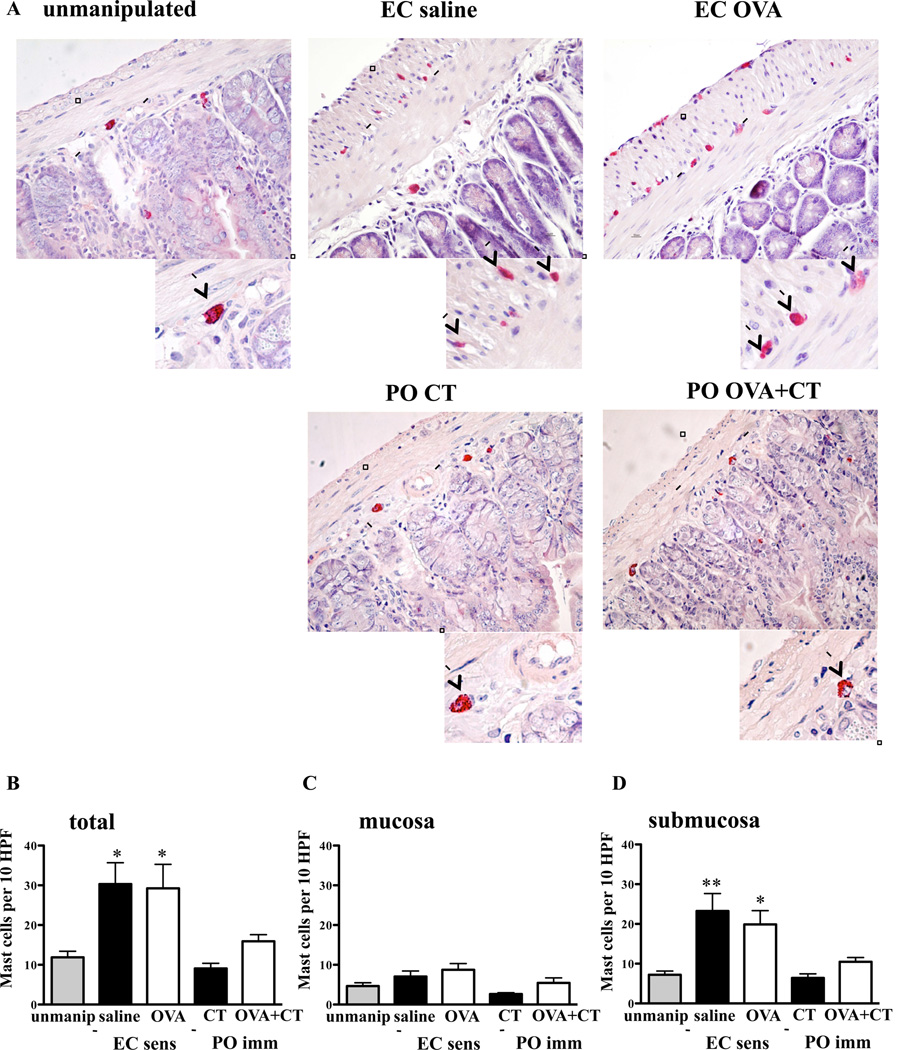
MCs play an important role in IgE mediated anaphylaxis. 12 We counted the number of MCs in jejunum of BALB/c mice at the end of the 7-week EC sensitization period and in unmanipulated BALB/c controls, by staining with CAE, an enzyme expressed in both mucosal and submucosal MCs. 16 We detected a robust and significant increase in the number of MCs in the jejunum of mice EC sensitized with OVA compared to unmanipulated controls (Fig. 5A,B). Unexpectedly, mice EC sensitized with saline also exhibited a robust increase in the number of jejunal MCs. There was no detectable increase in jejunal MCs in mice orally immunized with OVA+CT or CT alone (Fig. 5A,B). The expansion in MC numbers in the jejunum of EC sensitized mice occurred predominantly in the submucosa. Analysis of MC numbers in CAE stained sections revealed a modest, but not significant, 1.5–1.8 fold increase in the numbers of mucosal MCs in EC sensitized mice (Fig. 5C). In contrast, there was a significant 2.7–3.2 fold increase in the numbers of submucosal connective tissue MCs (CTMCs) in these mice (Fig. 5D). CTMCs in EC sensitized mice did not express mMCP-1 (data not shown), suggesting that systemic activation of mucosal MCs in various tissues was responsible for the increased serum levels of mMCP-1 following oral challenge.
Figure 5. Expansion of submucosal MCs in the jejunum of EC sensitized mice.
A. CAE staining of jejunum from EC sensitized and orally immunized mice. Magnification 200X, Inset 400X. B–D. Total (B), mucosal (C), and submucosal (D) MCs in jejunum of EC sensitized, orally immunized, and unmanipulated mice. n= 5–10 mice/group. Columns and bars: mean and SEM. Statistical significance was calculated relative to unmanipulated group. * P <0.05, ** P <0.01.
The increase in intestinal MCs in EC sensitized mice was selective. Consistent with previous reports, EC sensitization with OVA, but not saline, resulted in a 2.4 fold increase in MC numbers at the site of OVA sensitization 3 (Fig. E3A). However, there was no significant increase in the number of MCs in the ears, spleens, tracheas, or lungs (Fig. E3B–E). These results indicate that repeated tape-stripping of the skin, with or without application of antigen, causes selective expansion of intestinal submucosal MCs.
The comparable intestinal MC expansion in mice EC sensitized with saline vs. OVA prompted us to examine whether CTMC expansion in the gut was sufficient for IgE dependent oral anaphylaxis. Intravenous (i.v.) administration on day-1 before challenge of 4 µg of anti-OVA-IgE per mouse to mice EC sensitized with saline did not result in anaphylaxis in response to oral challenge with OVA (Fig. E4). In contrast, it resulted in anaphylaxis following i.v. challenge with OVA (Fig. E4), indicating that the anti-OVA-IgE we used was functional. These results suggest that factors other than the number of intestinal MCs are also important for the development of oral anaphylaxis.
Expansion of intestinal MCs is IgE-dependent
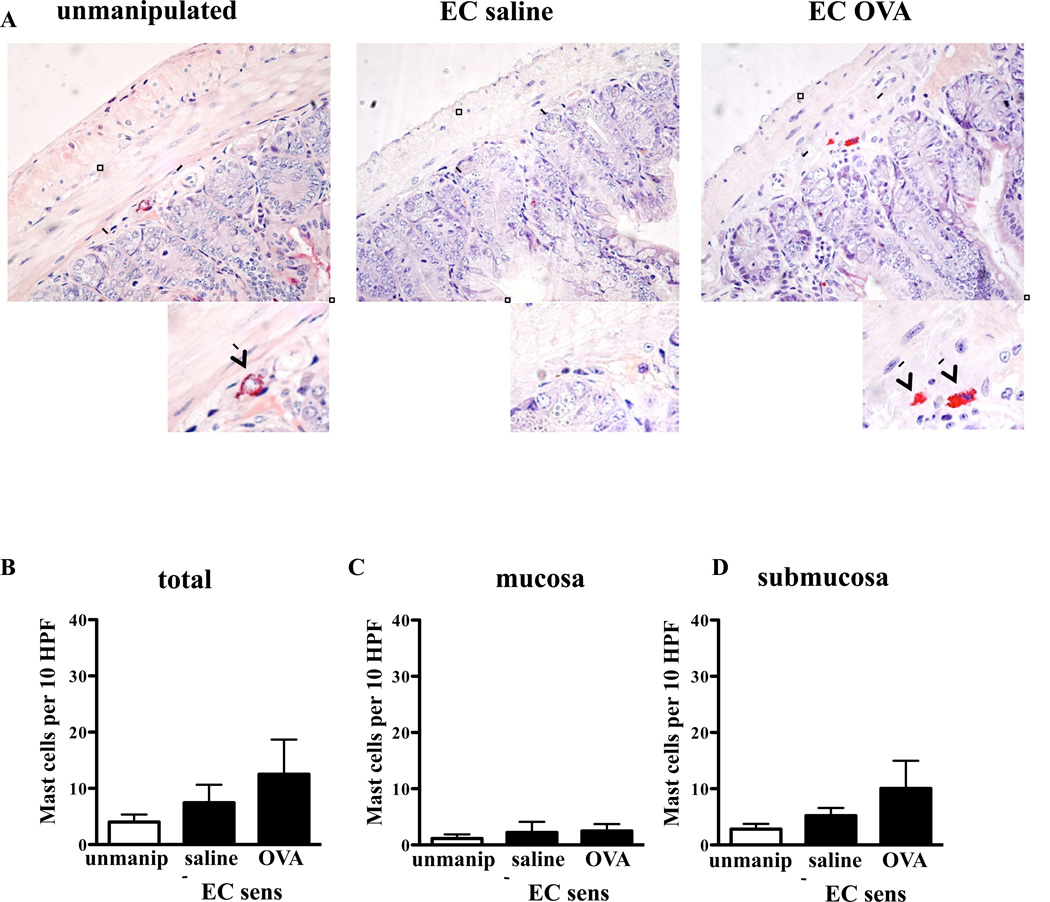
IgE plays an important role in MC homeostasis, and has been shown to drive MC expansion and sustain the survival of mature MCs. 17 Histologic examination of the jejunum from IgE−/− mice EC sensitized with OVA or saline revealed no significant increase in the numbers of MCs compared to unmanipulated IgE−/− controls (Fig. 6). These results indicate that IgE plays an essential role in driving the expansion of intestinal MCs in mice subjected to repeated tape-stripping of the skin, with or without application of antigen.
Figure 6. Expansion of intestinal MCs in EC sensitized mice is IgE-dependent.
Quantitation of MCs in CAE stained jejunal tissue sections from EC sensitized IgE−/− mice and unmanipulated IgE−/− controls, n= 5 mice/group. Columns and bars represent mean and SEM.
DISCUSSION
We have demonstrated that EC sensitization with the food antigen OVA results in IgE-mediated anaphylaxis following oral antigen challenge and IgE-dependent expansion of intestinal MCs. These findings support the hypothesis that cutaneous sensitization to food allergens plays an important role in the development of food allergy in humans, and that IgE and intestinal MCs are critical to this pathology.
Mice EC sensitized by repeated application of OVA to tape-stripped skin underwent anaphylaxis upon oral food challenge as evidenced by decreased body temperature and increased serum levels of mMCP-1, indicative of mucosal MC degranulation. The development of oral anaphylaxis in EC sensitized mice is in agreement with the results of Hsieh et al. 10, who reported that oral challenge of mice EC sensitized with OVA according to our protocol resulted in anaphylaxis with an increase in symptom score and serum histamine levels.
Tape-stripping, which can be considered as a surrogate for scratching, disrupts the normal skin barrier to antigens and elicits a local cutaneous inflammatory response.18 Thus, our data is likely relevant to the potential cutaneous sensitization of children with AD, a disease characterized by scratching of dry skin with a disrupted barrier, high incidence of food specific IgE antibodies, and high prevalence of food allergies. 19, 20 The observation that loss-of-function variants in the filaggrin gene are a significant risk for peanut allergy, 21 together with the known role of filaggrin in maintaining an intact skin barrier, 22 further supports the relevance of our model to food allergy. In contrast to EC sensitization of tape stripped skin, antigen uptake via intact skin downregulates the allergen-specific response in previously sensitized mice. 23
The fact that EC sensitized mice anaphylaxed to a single oral challenge in an IgE-dependent manner makes our model highly relevant to human IgE-mediated food anaphylaxis. In previous reports of IgE mediated anaphylaxis in WT mice, 24, 25 the mice were i.p. immunized, and systemic anaphylaxis, evidenced by a temperature drop, was observed in only one of these reports, and required multiple oral challenges. We previously reported IgE-dependent anaphylaxis in orally immunized IL-4Rα F709Y chain mutant mice in response to a single oral challenge. The corresponding mutation in the human IL-4Rα F709Y chain has not been described.
In contrast to EC sensitization, oral immunization with OVA+CT failed to elicit anaphylaxis in response to food challenge. This is in agreement with several published studies on oral immunization with a variety of antigens that include OVA, and peanut antigens using CT as an adjuvant. 26–28 However, other groups have reported anaphylaxis following oral challenge of mice orally immunized with OVA+CT.29, 30 Differences in the doses and schedules of immunization, and in the intestinal flora may be important in determining whether oral immunization leads to anaphylaxis. 31–33 It has been reported that antimicrobial regimens promote anaphylaxis following oral immunization, possibly by modulating TLR signaling by microbially derived TLR ligands or by altering cytokine production by T cells and the activity of Treg cells, 34, 35 which can suppress MC mediator release. 36 Nevertheless, under certain conditions of immunizations, CT could act both as an adjuvant and a suppressor of anaphylaxis, possibly by promoting Treg cells.37–39
The failure of oral immunization to result in anaphylaxis to oral challenge despite induction of OVA specific IgE antibody levels, which were comparable to those induced by EC sensitization, clearly indicates that although IgE is necessary, it is not sufficient for the development of food anaphylaxis. It is well established that many individuals, including some patients with AD, have circulating IgE antibodies to foods but demonstrate no clinical evidence of food allergy. 20 We have ruled out differences between the orally immunized mice and EC sensitized mice in antigen-driven T cell production of IL-4, which promotes IgE-mediated anaphylaxis. 40 However, circulating IL-4 levels, and IL-4 mRNA expression in MLN, were significantly elevated in EC sensitized mice, but not in orally immunized mice, suggesting that elevated systemic IL-4 output, which was associated with intestinal CTMC expansion, may have played an important role in the oral anaphylaxis of EC sensitized mice. Immunohistochemistry studies did not reveal differences in the numbers of Foxp3+ Treg cells in the gut, and Foxp3 mRNA analysis revealed comparable levels in the jejunum of EC sensitized and orally immunized mice (data not shown). However, further studies are needed to assess the generation of OVA specific Treg cells in the two immunization protocols.
MCs are important in food anaphylaxis. MC depletion by treatment with a c-kit monoclonal antibody24 and treatment with the MC stabilizer cromolyn41 ameliorate food anaphylaxis in mice. A major difference between EC sensitized and orally immunized mice was the marked expansion in CTMCs in the jejunum of EC sensitized mice, which was not detected in their orally immunized counterparts. The lack of MC expansion in the gut of orally immunized mice may explain their resistance to anaphylaxis.
MC expansion in EC sensitized mice involved predominantly the submucosal MCs. Furthermore, except for a modest 2.4 fold expansion of MCs in OVA sensitized skin sites, there was no evidence of MC expansion in several other organs of EC sensitized mice. In a recent study from our group, 28 mice carrying a gain of function mutation in the IL-4Rα chain (Y709F) were found to be susceptible to develop food allergy after oral immunization and had a modest expansion of MCs, which appeared to involve predominantly the mucosal MCs and to be IgE independent. 28 The differential requirement for IgE in intestinal MC expansion in the two models points to important differences. These may include differences in responsiveness to IL-4 and routes of antigen sensitization. The observation that FcεRI promotes survival of CTMCs but not mucosal MCs in vitro 42 supports our finding that IgE is necessary for CTMC expansion.
Understanding the factors that drive the expansion of intestinal MCs in EC sensitized mice is of critical importance in understanding food allergy. IgE is clearly one of these factors, as intestinal MCs failed to expand in EC sensitized IgE−/− mice. However, IgE was not sufficient for the expansion of intestinal MCs in mice orally immunized with OVA+CT. IL-4 has a well-established known role in MC homeostasis.43, 44 Its elevation in the serum and MLN of EC sensitized mice suggests that it may play a role in the intestinal expansion of CTMCs in these mice.
Given the lack of MC expansion in other organs except the skin, MC expansion in the gut of EC sensitized mice may be mediated by cells that traffic from skin to gut, which could include dendritic cells and T cells. 6, 45 We previously reported that mechanical injury caused by tape stripping induces TSLP production in the skin, and that DCs derived from skin injured by tape stripping are programmed by TSLP to drive a Th2 response and migrate to MLN. 46 TSLP levels were elevated levels in EC sensitized skin (Fig. E5), and may have contributed to the MC expansion in the intestine of EC sensitized mice. The precise nature of the cells and the factors that mediate the skin to gut crosstalk that results in gut MC expansion remains to be determined.
The finding that MCs underwent expansion in the jejunum of mice EC sensitized with saline was unexpected. It could have resulted from the skin inflammation caused by tape-stripping, which may have released cytokines and cells that promoted MC expansion in the gut or/and from sensitization to environmental antigens (i.e. pathogens, proteins in mouse diet) absorbed through tape-stripped skin. Experiments with germ free mice and mice maintained on elemental diets should help distinguish between these possibilities.
During oral antigen challenge, antigen traffics across the intestinal epithelium in two phases. 47 In phase 1, antigen transport occurs via the transcellular route in a MC-independent manner and is likely mediated by CD23-dependent IgE-mediated uptake. 48, 49 In phase 2, which occurs several minutes later, massive antigen transport occurs via the transcellular route, and this is dependent on FcεRI-mediated activation of MCs. Degranulating MCs secrete a variety of mediators that can enhance intestinal permeability which would promote systemic anaphylaxis. These include mMCP-1, which originates from mucosal MCs and mMCP-4 which is derived from submucosal CTMCs. 33 Increased intestinal MC expansion has been reported in adults with food allergy 50 and could underlie the increased intestinal permeability in these patients. 33, 47
Our findings demonstrate that introduction of food antigen through the skin causes expansion of intestinal MCs and predisposes to IgE-mediated anaphylaxis. Prevention of cutaneous sensitization by allergen avoidance and aggressive skin barrier protection and therapies targeted at limiting the expansion and activation of intestinal MCs may protect against food allergies.
Acknowledgments
Funding: This work was supported by NIH grants AR-047417 and R01-AI083516. L.M.B. was supported by training grant T32-AI-007512 and the 2010 AAAAI/Food Allergy Initiative Howard Gittis Memorial Third/Fourth Year Fellowship/Junior Faculty Research Award. M.K.O. was supported by the Harvard Digestive Disease Center, NIH Grant P30 DK34845, and by the Boston Children's Hospital Faculty Career Development Fellowship/Eleanor and Miles Shore Program for Scholars in Medicine at Harvard Medical School. M.F.G was 24 supported by NIAID: RO1- AI083516. H.C.O. was supported by NIH NIAID R56 AI100889-01 and EPA Grant 83482501.
Abbreviations
- AD
atopic dermatitis
- CAE
chloroacetate esterase
- CT
cholera toxin
- CTMC
connective tissue mast cell
- EC
epicutaneous
- IL
interleukin
- i.p.
intraperitoneal(ly)
- i.v.
intravenous(ly)
- IVCCA
In vivo cytokine capture assay
- MC
mast cell
- mMCP-1
mouse mast cell protease 1
- MLN
mesenteric lymph node
- MMC
mucosal mast cell
- OVA
ovalbumin
- WT
wild-type
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Hill DJ, Heine RG, Hosking CS, Brown J, Thiele L, Allen KJ, et al. IgE food sensitization in infants with eczema attending a dermatology department. J Pediatr. 2007;151:359–363. doi: 10.1016/j.jpeds.2007.04.070. [DOI] [PubMed] [Google Scholar]
- 2.Lack G, Fox D, Northstone K, Golding J. Factors associated with the development of peanut allergy in childhood. N Engl J Med. 2003;348:977–985. doi: 10.1056/NEJMoa013536. [DOI] [PubMed] [Google Scholar]
- 3.Spergel JM, Mizoguchi E, Brewer JP, Martin TR, Bhan AK, Geha RS. Epicutaneous sensitization with protein antigen induces localized allergic dermatitis and hyperresponsiveness to methacholine after single exposure to aerosolized antigen in mice. J Clin Invest. 1998;101:1614–1622. doi: 10.1172/JCI1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jin H, He R, Oyoshi M, Geha RS. Animal models of atopic dermatitis. J Invest Dermatol. 2009;129:31–40. doi: 10.1038/jid.2008.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oettgen HC, Martin TR, Wynshaw-Boris A, Deng C, Drazen JM, Leder P. Active anaphylaxis in IgE-deficient mice. Nature. 1994;370:367–370. doi: 10.1038/370367a0. [DOI] [PubMed] [Google Scholar]
- 6.Oyoshi MK, Elkhal A, Scott JE, Wurbel MA, Hornick JL, Campbell JJ, et al. Epicutaneous challenge of orally immunized mice redirects antigen-specific gut-homing T cells to the skin. J Clin Invest. 2011;121:2210–2220. doi: 10.1172/JCI43586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jin H, Kumar L, Mathias C, Zurakowski D, Oettgen H, Gorelik L, et al. Toll-like receptor 2 is important for the T(H)1 response to cutaneous sensitization. J Allergy Clin Immunol. 2009;123:875–882. e1. doi: 10.1016/j.jaci.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xing W, Austen KF, Gurish MF, Jones TG. Protease phenotype of constitutive connective tissue and of induced mucosal mast cells in mice is regulated by the tissue. Proc Natl Acad Sci U S A. 2011;108:14210–14215. doi: 10.1073/pnas.1111048108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Finkelman F, Morris S, Orekhova T, Sehy D. The in vivo cytokine capture assay for measurement of cytokine production in the mouse. Current protocols in immunology / edited by John E. Coligan … [et al.] 2003 doi: 10.1002/0471142735.im0628s54. Chapter 6:Unit 6 28. [DOI] [PubMed] [Google Scholar]
- 10.Hsieh KY, Tsai CC, Wu CH, Lin RH. Epicutaneous exposure to protein antigen and food allergy. Clin Exp Allergy. 2003;33:1067–1075. doi: 10.1046/j.1365-2222.2003.01724.x. [DOI] [PubMed] [Google Scholar]
- 11.Andersson MK, Pemberton AD, Miller HR, Hellman L. Extended cleavage specificity of mMCP-1, the major mucosal mast cell protease in mouse-high specificity indicates high substrate selectivity. Mol Immunol. 2008;45:2548–2558. doi: 10.1016/j.molimm.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 12.Finkelman FD. Anaphylaxis: lessons from mouse models. J Allergy Clin Immunol. 2007;120:506–515. doi: 10.1016/j.jaci.2007.07.033. quiz 16-7. [DOI] [PubMed] [Google Scholar]
- 13.Deschoolmeester ML, Eastmond NC, Dearman RJ, Kimber I, Basketter DA, Coleman JW. Reciprocal effects of interleukin-4 and interferon-gamma on immunoglobulin E-mediated mast cell degranulation: a role for nitric oxide but not peroxynitrite or cyclic guanosine monophosphate. Immunology. 1999;96:138–144. doi: 10.1046/j.1365-2567.1999.00662.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Neill DR, Wong SH, Bellosi A, Flynn RJ, Daly M, Langford TK, et al. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature. 2010;464:1367–1370. doi: 10.1038/nature08900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oliphant CJ, Barlow JL, McKenzie AN. Insights into the initiation of type 2 immune responses. Immunology. 2011;134:378–385. doi: 10.1111/j.1365-2567.2011.03499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Friend DS, Ghildyal N, Austen KF, Gurish MF, Matsumoto R, Stevens RL. Mast cells that reside at different locations in the jejunum of mice infected with Trichinella spiralis exhibit sequential changes in their granule ultrastructure and chymase phenotype. J Cell Biol. 1996;135:279–290. doi: 10.1083/jcb.135.1.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mathias CB, Freyschmidt EJ, Caplan B, Jones T, Poddighe D, Xing W, et al. IgE influences the number and function of mature mast cells, but not progenitor recruitment in allergic pulmonary inflammation. J Immunol. 2009;182:2416–2424. doi: 10.4049/jimmunol.0801569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wood LC, Stalder AK, Liou A, Campbell IL, Grunfeld C, Elias PM, et al. Barrier disruption increases gene expression of cytokines and the 55 kD TNF receptor in murine skin. Exp Dermatol. 1997;6:98–104. doi: 10.1111/j.1600-0625.1997.tb00154.x. [DOI] [PubMed] [Google Scholar]
- 19.Eigenmann PA, Sicherer SH, Borkowski TA, Cohen BA, Sampson HA. Prevalence of IgE-mediated food allergy among children with atopic dermatitis. Pediatrics. 1998;101:E8. doi: 10.1542/peds.101.3.e8. [DOI] [PubMed] [Google Scholar]
- 20.Boyce JA, Assa'ad A, Burks AW, Jones SM, Sampson HA, Wood RA, et al. Guidelines for the diagnosis and management of food allergy in the United States: report of the NIAID-sponsored expert panel. J Allergy Clin Immunol. 2010;126:S1–S58. doi: 10.1016/j.jaci.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brown SJ, Asai Y, Cordell HJ, Campbell LE, Zhao Y, Liao H, et al. Loss-of-function variants in the filaggrin gene are a significant risk factor for peanut allergy. J Allergy Clin Immunol. 2011;127:661–667. doi: 10.1016/j.jaci.2011.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O'Regan GM, Irvine AD. The role of filaggrin in the atopic diathesis. Clinical and experimental allergy : journal of the British Society for Allergy and Clinical Immunology. 2010;40:965–972. doi: 10.1111/j.1365-2222.2010.03522.x. [DOI] [PubMed] [Google Scholar]
- 23.Dioszeghy V, Mondoulet L, Dhelft V, Ligouis M, Puteaux E, Benhamou PH, et al. Epicutaneous immunotherapy results in rapid allergen uptake by dendritic cells through intact skin and downregulates the allergen-specific response in sensitized mice. J Immunol. 2011;186:5629–5637. doi: 10.4049/jimmunol.1003134. [DOI] [PubMed] [Google Scholar]
- 24.Brandt EB, Strait RT, Hershko D, Wang Q, Muntel EE, Scribner TA, et al. Mast cells are required for experimental oral allergen-induced diarrhea. J Clin Invest. 2003;112:1666–1677. doi: 10.1172/JCI19785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Osterfeld H, Ahrens R, Strait R, Finkelman FD, Renauld JC, Hogan SP. Differential roles for the IL-9/IL-9 receptor alpha-chain pathway in systemic and oral antigen-induced anaphylaxis. J Allergy Clin Immunol. 2010;125:469–476. e2. doi: 10.1016/j.jaci.2009.09.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bashir ME, Louie S, Shi HN, Nagler-Anderson C. Toll-like receptor 4 signaling by intestinal microbes influences susceptibility to food allergy. J Immunol. 2004;172:6978–6987. doi: 10.4049/jimmunol.172.11.6978. [DOI] [PubMed] [Google Scholar]
- 27.Berin MC, Zheng Y, Domaradzki M, Li XM, Sampson HA. Role of TLR4 in allergic sensitization to food proteins in mice. Allergy. 2006;61:64–71. doi: 10.1111/j.1398-9995.2006.01012.x. [DOI] [PubMed] [Google Scholar]
- 28.Mathias CB, Hobson SA, Garcia-Lloret M, Lawson G, Poddighe D, Freyschmidt EJ, et al. IgE-mediated systemic anaphylaxis and impaired tolerance to food antigens in mice with enhanced IL-4 receptor signaling. J Allergy Clin Immunol. 2011;127:795–805. e1–e6. doi: 10.1016/j.jaci.2010.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Perrier C, Thierry AC, Mercenier A, Corthesy B. Allergen-specific antibody and cytokine responses, mast cell reactivity and intestinal permeability upon oral challenge of sensitized and tolerized mice. Clinical and experimental allergy : journal of the British Society for Allergy and Clinical Immunology. 2010;40:153–162. doi: 10.1111/j.1365-2222.2009.03329.x. [DOI] [PubMed] [Google Scholar]
- 30.Ganeshan K, Neilsen CV, Hadsaitong A, Schleimer RP, Luo X, Bryce PJ. Impairing oral tolerance promotes allergy and anaphylaxis: a new murine food allergy model. J Allergy Clin Immunol. 2009;123:231–238. e4. doi: 10.1016/j.jaci.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Du Toit G, Katz Y, Sasieni P, Mesher D, Maleki SJ, Fisher HR, et al. Early consumption of peanuts in infancy is associated with a low prevalence of peanut allergy. J Allergy Clin Immunol. 2008;122:984–991. doi: 10.1016/j.jaci.2008.08.039. [DOI] [PubMed] [Google Scholar]
- 32.Diesner SC, Knittelfelder R, Krishnamurthy D, Pali-Scholl I, Gajdzik L, Jensen-Jarolim E, et al. Dose-dependent food allergy induction against ovalbumin under acid-suppression: a murine food allergy model. Immunol Lett. 2008;121:45–51. doi: 10.1016/j.imlet.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Groschwitz KR, Hogan SP. Intestinal barrier function: molecular regulation and disease pathogenesis. J Allergy Clin Immunol. 2009;124:3–20. doi: 10.1016/j.jaci.2009.05.038. quiz 1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Prioult G, Nagler-Anderson C. Mucosal immunity and allergic responses: lack of regulation and/or lack of microbial stimulation? Immunol Rev. 2005;206:204–218. doi: 10.1111/j.0105-2896.2005.00277.x. [DOI] [PubMed] [Google Scholar]
- 35.Atarashi K, Tanoue T, Shima T, Imaoka A, Kuwahara T, Momose Y, et al. Induction of colonic regulatory T cells by indigenous Clostridium species. Science. 2011;331:337–341. doi: 10.1126/science.1198469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gri G, Piconese S, Frossi B, Manfroi V, Merluzzi S, Tripodo C, et al. CD4+CD25+ regulatory T cells suppress mast cell degranulation and allergic responses through OX40-OX40L interaction. Immunity. 2008;29:771–781. doi: 10.1016/j.immuni.2008.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sun JB, Cuburu N, Blomquist M, Li BL, Czerkinsky C, Holmgren J. Sublingual tolerance induction with antigen conjugated to cholera toxin B subunit induces Foxp3+CD25+CD4+ regulatory T cells and suppresses delayed-type hypersensitivity reactions. Scand J Immunol. 2006;64:251–259. doi: 10.1111/j.1365-3083.2006.01823.x. [DOI] [PubMed] [Google Scholar]
- 38.Sun JB, Czerkinsky C, Holmgren J. Sublingual 'oral tolerance' induction with antigen conjugated to cholera toxin B subunit generates regulatory T cells that induce apoptosis and depletion of effector T cells. Scand J Immunol. 2007;66:278–286. doi: 10.1111/j.1365-3083.2007.01975.x. [DOI] [PubMed] [Google Scholar]
- 39.Sun JB, Raghavan S, Sjoling A, Lundin S, Holmgren J. Oral tolerance induction with antigen conjugated to cholera toxin B subunit generates both Foxp3+CD25+ and Foxp3-CD25- CD4+ regulatory T cells. J Immunol. 2006;177:7634–7644. doi: 10.4049/jimmunol.177.11.7634. [DOI] [PubMed] [Google Scholar]
- 40.Strait RT, Morris SC, Smiley K, Urban JF, Jr, Finkelman FD. IL-4 exacerbates anaphylaxis. J Immunol. 2003;170:3835–3842. doi: 10.4049/jimmunol.170.7.3835. [DOI] [PubMed] [Google Scholar]
- 41.Forbes EE, Groschwitz K, Abonia JP, Brandt EB, Cohen E, Blanchard C, et al. IL-9- and mast cell-mediated intestinal permeability predisposes to oral antigen hypersensitivity. J Exp Med. 2008;205:897–913. doi: 10.1084/jem.20071046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ekoff M, Strasser A, Nilsson G. FcepsilonRI aggregation promotes survival of connective tissue-like mast cells but not mucosal-like mast cells. J Immunol. 2007;178:4177–4183. doi: 10.4049/jimmunol.178.7.4177. [DOI] [PubMed] [Google Scholar]
- 43.Lorentz A, Wilke M, Sellge G, Worthmann H, Klempnauer J, Manns MP, et al. IL-4-induced priming of human intestinal mast cells for enhanced survival and Th2 cytokine generation is reversible and associated with increased activity of ERK1/2 and c-Fos. J Immunol. 2005;174:6751–6756. doi: 10.4049/jimmunol.174.11.6751. [DOI] [PubMed] [Google Scholar]
- 44.Shelburne CP, Ryan JJ. The role of Th2 cytokines in mast cell homeostasis. Immunol Rev. 2001;179:82–93. doi: 10.1034/j.1600-065x.2001.790109.x. [DOI] [PubMed] [Google Scholar]
- 45.Hammerschmidt SI, Friedrichsen M, Boelter J, Lyszkiewicz M, Kremmer E, Pabst O, et al. Retinoic acid induces homing of protective T and B cells to the gut after subcutaneous immunization in mice. J Clin Invest. 2011;121:3051–3061. doi: 10.1172/JCI44262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Oyoshi MK, Larson RP, Ziegler SF, Geha RS. Mechanical injury polarizes skin dendritic cells to elicit a T(H)2 response by inducing cutaneous thymic stromal lymphopoietin expression. J Allergy Clin Immunol. 2010;126:976–984. 84, e1–e5. doi: 10.1016/j.jaci.2010.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Perrier C, Corthesy B. Gut permeability and food allergies. Clinical and experimental allergy : journal of the British Society for Allergy and Clinical Immunology. 2011;41:20–28. doi: 10.1111/j.1365-2222.2010.03639.x. [DOI] [PubMed] [Google Scholar]
- 48.Li H, Nowak-Wegrzyn A, Charlop-Powers Z, Shreffler W, Chehade M, Thomas S, et al. Transcytosis of IgE-antigen complexes by CD23a in human intestinal epithelial cells and its role in food allergy. Gastroenterology. 2006;131:47–58. doi: 10.1053/j.gastro.2006.03.044. [DOI] [PubMed] [Google Scholar]
- 49.Bevilacqua C, Montagnac G, Benmerah A, Candalh C, Brousse N, Cerf-Bensussan N, et al. Food allergens are protected from degradation during CD23-mediated transepithelial transport. Int Arch Allergy Immunol. 2004;135:108–116. doi: 10.1159/000080653. [DOI] [PubMed] [Google Scholar]
- 50.Chen X, Song CH, Liu ZQ, Feng BS, Zheng PY, Li P, et al. Intestinal epithelial cells express galectin-9 in patients with food allergy that plays a critical role in sustaining allergic status in mouse intestine. Allergy. 2011;66:1038–1046. doi: 10.1111/j.1398-9995.2011.02585.x. [DOI] [PubMed] [Google Scholar]