Abstract
A therapeutic goal in the treatment of certain CNS diseases, including multiple sclerosis, amyotrophic lateral sclerosis, and Parkinson disease, is to down-regulate inflammatory pathways. Inflammatory molecules produced by microglia are responsible for removal of damaged neurons, but can cause collateral damage to normal neurons located close to defective neurons. Although estrogen can inactivate microglia and inhibit the recruitment of T cells and macrophages into the CNS, there is controversy regarding which of the two estrogen receptors (ERs), ERα or ERβ, mediates the beneficial effects in microglia. In this study, we found that ERβ, but not ERα, is expressed in microglia. Using the experimental autoimmune encephalomyelitis (EAE) model in SJL/J mice, we evaluated the benefit of an ERβ agonist as a modulator of neuroinflammation. Treatment of EAE mice with LY3201, a selective ERβ agonist provided by Eli Lilly, resulted in marked reduction of activated microglia in the spinal cord. LY3201 down-regulated the nuclear transcription factor NF-κB, as well as the NF-κB–induced gene inducible nitric oxide synthase in microglia and CD3+ T cells. In addition, LY3201 inhibited T-cell reactivity through regulation of indoleamine-2,3-dioxygenase. In the EAE model, treatment with LY3201 decreased mortality in the first 2 wk after disease onset, and also reduced the severity of symptoms in mice surviving for 4 wk. Our data show that ERβ-selective agonists, by modulating the immune system in both microglia and T cells, offer promise as a useful class of drugs for treating degenerative diseases of the CNS.
Keywords: glia, nuclear receptor
Activated microglia provide a therapeutic target in the treatment of neuroinflammatory diseases, including multiple sclerosis (MS), amyotrophic lateral sclerosis, and Parkinson disease. MS is the most common inflammatory demyelinating disease of the CNS (1), affecting approximately two million people worldwide (2). MS is subdivided into three main clinical subgroups based on the temporal course of the disease: relapsing-remitting MS (RR-MS), secondary progressive MS, and primary progressive MS (3). Current therapeutic agents target mainly autoimmune and inflammatory aspects of the disease (4–8). Most of these agents are used only to treat RR-MS, and some have shown efficacy in decreasing the relapse rate in RR-MS and time to progression; however, they cannot cure MS, and treatments for secondary progressive MS and primary progressive MS are still being sought.
Immunocompetent, macrophage-like cells residing in the CNS, microglia play essential roles in the maintenance of homeostasis and responses to infection and injury (9–11). Under physiological conditions, microglia are maintained in a quiescent state and constantly survey the surrounding environment through their ramified processes, which give them their characteristic shape (12). Once microglia sense danger, they become activated (13); on activation, they release a number of potentially neurotoxic substances, including cytokines such as TNFα, reactive oxygen species, and nitric oxide (NO) synthesized by inducible nitric oxide synthase (iNOS). Activated microglia are thought to be an important contributor to tissue damage in MS and experimental autoimmune encephalomyelitis (EAE), an animal model of MS (14). In EAE, microglia accumulate in areas of demyelination, and a reduction in the number of either macrophages or microglia results in marked attenuation of symptoms (15).
There remain differences of opinion among laboratories as to whether ERα or ERβ is responsible for the anti-inflammatory effects of estrogen on microglia (16). Saijo et al. (17) demonstrated that at the mRNA level, ERβ was much more abundant than ERα. Furthermore, synthetic ERβ-specific ligands (indazole-Br and indazole-Cl) repressed inflammatory response in microglia in vitro and prevented EAE in mice (17). ER ligands, through ERα or ERβ, can inhibit NF-κB transcriptional activity (18) and significantly inhibit inflammatory responses. Activation of ERβ decreases NO production and iNOS expression in response to LPS stimulation of BV-2 microglia in which only ERβ is expressed (19). However, Vegeto et al. (20) identified ERα as the receptor modulating microglial activity.
NF-κB, an ubiquitous transcription factor, plays an important role in controlling the expression of genes involved in immunity, inflammation, cell proliferation, and apoptosis (21, 22), including iNOS (23). Agents that decrease activation of NF-κB can provide protection against EAE (24, 25). Under pathological conditions, activated NF-κB and iNOS expression are increased (26). The NO produced through the activity of iNOS increases free radicals, which are involved in the pathophysiology of several human diseases (27). Reduction of NO synthesis can prevent the development of EAE (28) suggesting an important role of NO in the onset of MS.
In the present study, we used an ERβ-selective agonist (LY3201) in the EAE mouse model to demonstrate that LY3201 can inactivate microglia and invading T cells through reducing expression of NF-κB and iNOS.
Results
ERβ Is the Sole ER Expressed in Microglia.
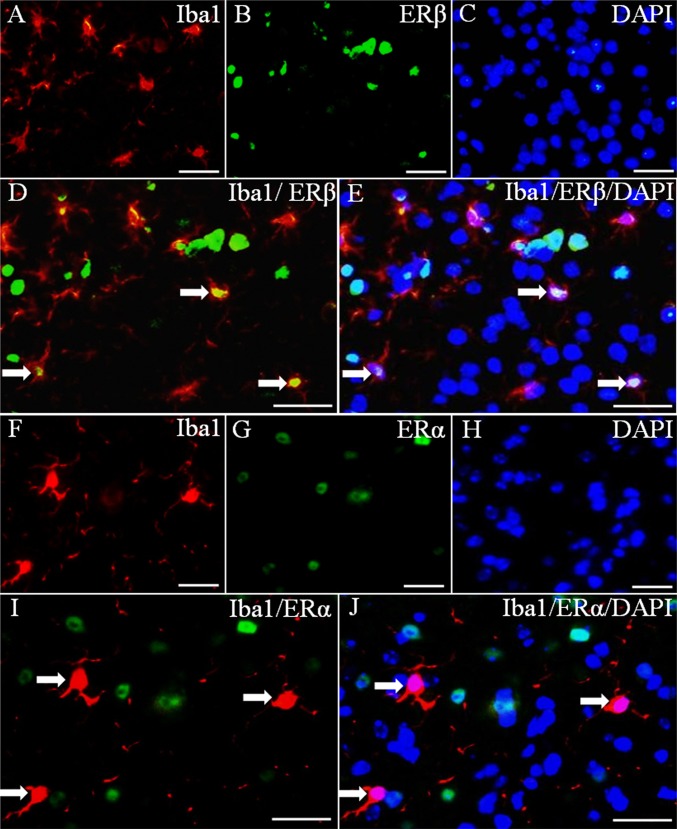
Two estrogen receptors, ERα and ERβ, mediate the actions of estrogen. To identify which ER is expressed in microglia, we used double-fluorescence staining with antibodies for ERβ, ERα, and Iba1 (a marker for microglia). Both ERβ and ERα were clearly expressed in nuclei of cells in the brain (Fig. 1 B and G); however, only ERβ was colocalized in cells with Iba1 (Fig. 1 D and E). There was no colocalization between Iba1 and ERα (Fig. 1 I and J). Thus, mouse microglia express ERβ, but not ERα.
Fig. 1.
Expression of ERα and ERβ in microglia. (A–E) Iba1 and ERβ double-fluorescence staining. (D) Colocalization of ERβ (green) and Iba1 (red) (arrows). (E) Colocalization of ERβ (green), Iba1 (red), and DAPI (blue) (arrows). (F–J) ERα and Iba1 double-fluorescence staining. (I and J) Lack of colocalization between ERα (green) and Iba1 (red). The arrows show ERα-negative microglia. Nuclei were counterstained with DAPI (blue). (Scale bars: 20 μm.)
LY3201 Inactivates Microglia in Spinal Cord of EAE Mice.
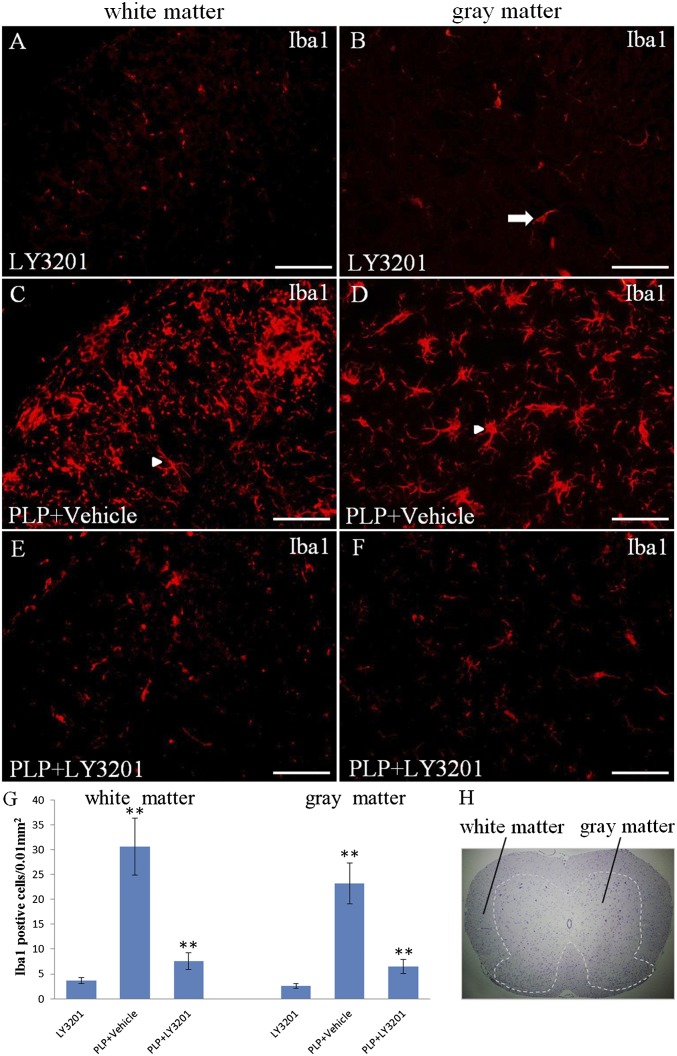
Microglia are innate immune cells residing in the CNS. Resting microglia are small cells with long, thin ramified processes. On activation of microglia, cell bodies become enlarged and processes become poorly ramified, short, and thick. To evaluate whether LY3201 can suppress activated microglia in EAE mice, we treated the mice with LY3201 before proteolipid protein (PLP). LY3201 treatment was applied for 3 d. Controls were not treated with PLP and are referred to as uninduced mice. The spinal cord of uninduced mice treated with LY3201 had few Iba1-positive cells with small cell bodies and long, thin ramified processes (Fig. 2 A and B). In PLP-treated mice, the resting microglia became activated, and the number of microglia was significantly increased in both white matter and gray matter of the spinal cord compared with uninduced mice (Fig. 2 C, D, and G). When mice were treated with LY3201 before PLP, there were few Iba1-positive cells in the spinal cord, and these Iba1-positive cells had small cell bodies with long, thin ramified processes (Fig. 2 E, F, and G).
Fig. 2.
Microglial cells were inactivated in the spinal cord of EAE mice treated with LY3201. (A and B) In noninduced mice treated with LY3201, there were very few Iba1-positive resting microglial cells (arrow) in the spinal cord. (C, D, and G) EAE mice treated with vehicle had more Iba1-positive cells (**P < 0.01) than noninduced mice treated with LY3201 both in white matter and in gray matter of the spinal cord. These activated cells had larger cell bodies and ramified morphology (arrowheads). (E, F, and G) EAE mice treated with LY3201 had fewer Iba1-positive cells in the spinal cord (**P < 0.010) compared with vehicle-treated EAE mice, and these Iba1-positive cells had small cell bodies and long, thin processes. (H) Hematoxylin staining shows the white matter and the gray matter of the spinal cord. (Scale bars: 50 μm.)
NF-κB Activation Is Down-Regulated in Spinal Cord of EAE Mice Treated with LY3201.
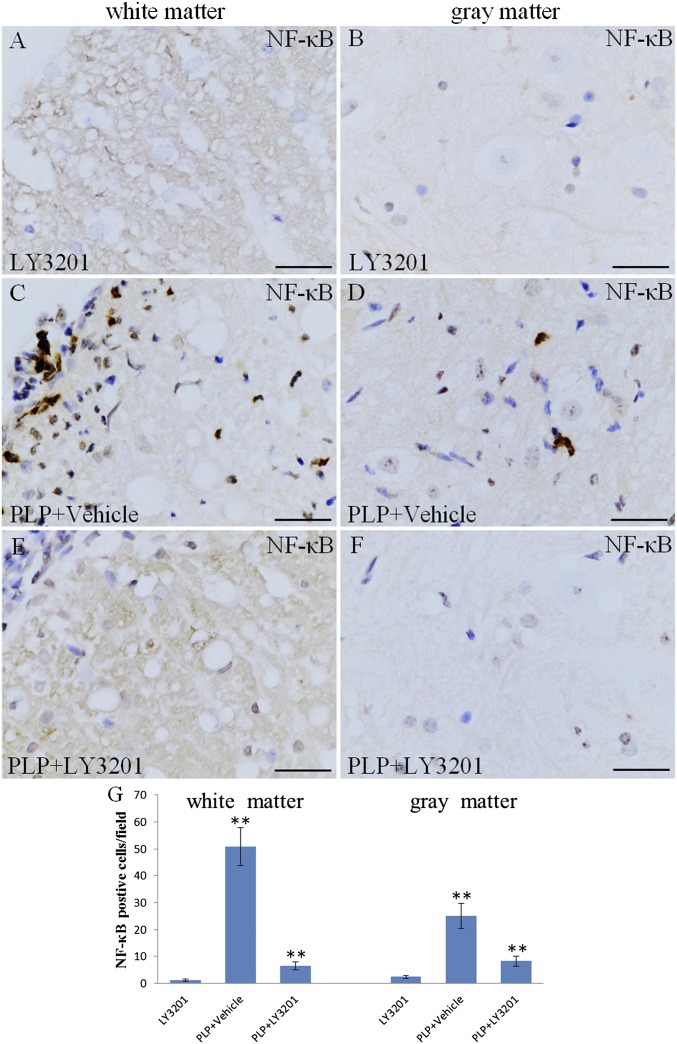
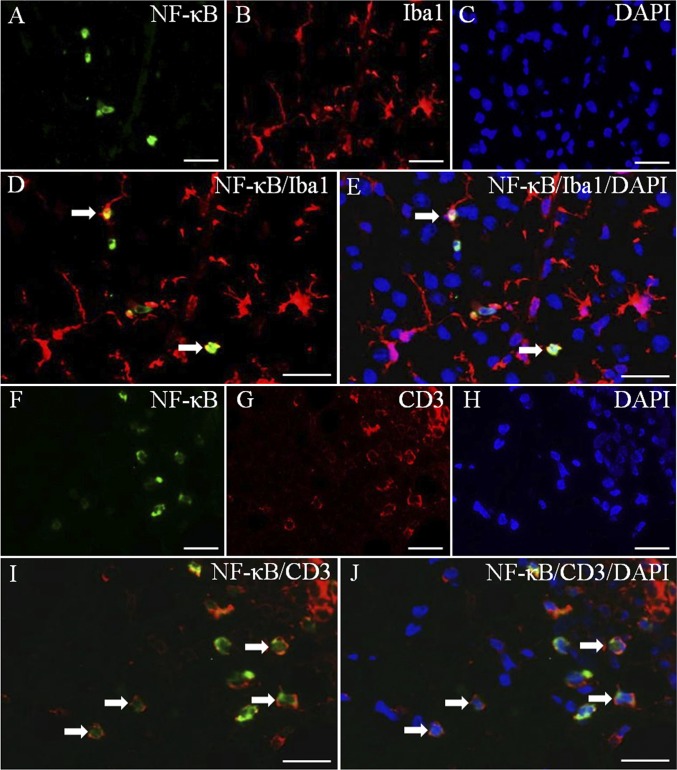
MS is an inflammatory disease of the CNS, and NF-κB plays an important role in controlling gene expression in inflammation. Activation of NF-κB results in the production of proinflammatory cytokines and potentially neurotoxic mediators in EAE. To evaluate whether NF-κB activation is down-regulated by LY3201, we performed NF- κB p65 immunohistochemistry in the spinal cord. In uninduced mice treated with LY3201, only a few NF-κB–positive nuclei were detected in the spinal cord (Fig. 3 A and B). Compared with uninduced mice, PLP mice exhibited a significant increase in the number of NF-κB–positive nuclei in both white matter and gray matter of the spinal cord (Fig. 3 C, D, and G). Mice treated with LY3201 before PLP had fewer NF-κB nuclei in the spinal cord (Fig. 3 E and F) compared with vehicle-treated PLP mice (Fig. 3 G). We used double-fluorescence staining to determine whether the NF-κB–expressing cells were microglia (Iba1) (Fig. 4 A–E) or T cells (CD3) (Fig. 4 F–J). The results demonstrate that NF-κB was expressed in both microglia (Fig. 4 D and E) and T cells (Fig. 4 I and J).
Fig. 3.
Down-regulation of activated NF-κB in the spinal cord of EAE mice treated with LY3201. (A and B) There were few NF-κB–positive cells in the spinal cord of noninduced mice treated with LY3201. (C, D, and G) There were more NF-κB–positive cells in vehicle-treated EAE mice (**P < 0.01) than in noninduced mice treated with LY3201. (E, F, and G) There were fewer NF-κB–positive cells in the spinal cords of EAE mice treated with LY3201 (**P < 0.01) than in vehicle-treated EAE mice. (Scale bars: 50 μm.)
Fig. 4.
Expression of NF-κB in microglia and T cells. NF-κB/Iba1 (A–E) and NF-κB/CD3 (F–J) double-fluorescence staining in gray matter of spinal cord of vehicle-treated EAE mice. (D) Colocalization of NF-κB (green) and Iba1 (red) (arrows). (E) Colocalization of NF-κB (green), Iba1 (red), and DAPI (blue) (arrows). (I) Colocalization of NF-κB (green) and CD3 (red) (arrows). (J) Colocalization of NF-κB (green), CD3 (red), and DAPI (blue) (arrows). (Scale bars: 20 μm.)
iNOS Expression Is Decreased in Spinal Cord of EAE Mice Treated with LY3201.
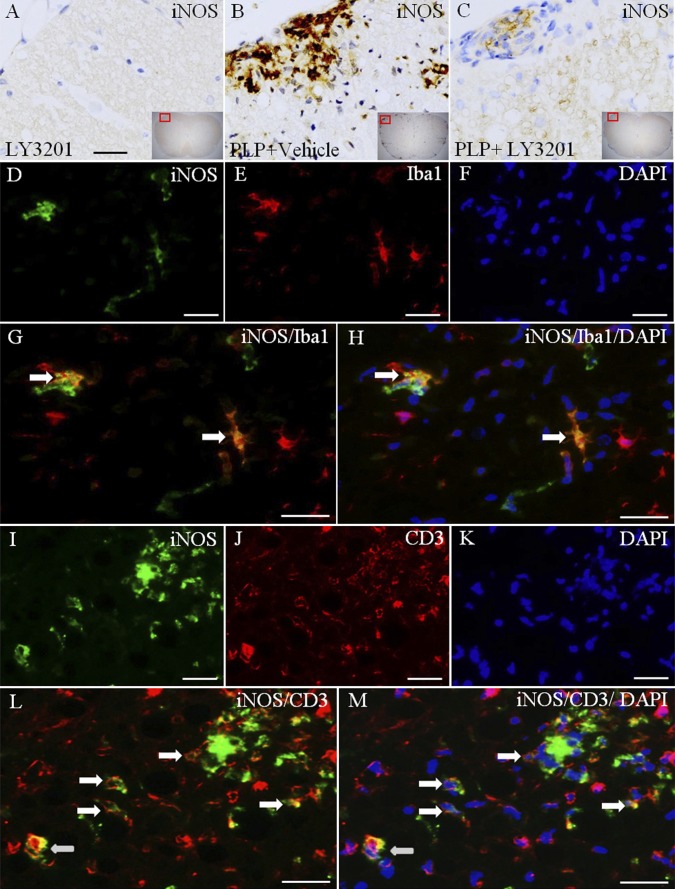
NO, synthesized by iNOS, can damage tissue. We examined iNOS expression to identify alterations of NO in the spinal cord. Our data show that iNOS-positive cells in the spinal cord of uninduced mice treated with LY3201 were very rare (Fig. 5 A). In EAE mice treated with vehicle, iNOS expression was readily detected, particularly in white matter of the spinal cord (Fig. 5 B). iNOS expression was lower in EAE mice treated with LY3201 compared with EAE vehicle-treated mice (Fig. 5 C). To identify the iNOS-expressing cells, we used double-fluorescence staining to colocalize iNOS/Iba1 (Fig. 5 D–H) and iNOS/CD3 (Fig. 5 I–M). Our results show that some iNOS is expressed in microglia (Fig. 5 G and H), but most is expressed in T cells (Fig. 5 L and M).
Fig. 5.
Reduced expression of iNOS in the spinal cord of LY3201-treated EAE mice. (A) There were very few iNOS-positive cells in the spinal cord of noninduced mice treated with LY3201. (B) In vehicle-treated EAE mice there was a markedly increased iNOS expression, especially in white matter. (C) The expression of iNOS was lower in LY3201-treated EAE mice than in vehicle-treated EAE mice. (D–H) iNOS and Iba1 double-fluorescence staining in white matter of the spinal cord of vehicle-treated EAE mice. (G) Colocalization of iNOS (green) and Iba1 (red) (arrows). (H) Colocalization of iNOS (green), Iba1 (red), and DAPI (blue) (arrows). (I–M) iNOS and CD3 double-fluorescence staining in white matter of spinal cord of vehicle-treated EAE mice. (L) Colocalization of iNOS (green) and CD3 (red) (arrows). (M) Colocalization of iNOS (green), CD3 (red), and DAPI (blue) (arrows). (Scale bars: 50 μm in A–C; 20 μm in D–M.)
Indoleamine-2,3-Dioxygenase–Positive Cells Are Sharply Decreased in Spinal Cord of EAE Mice Treated with LY3201.
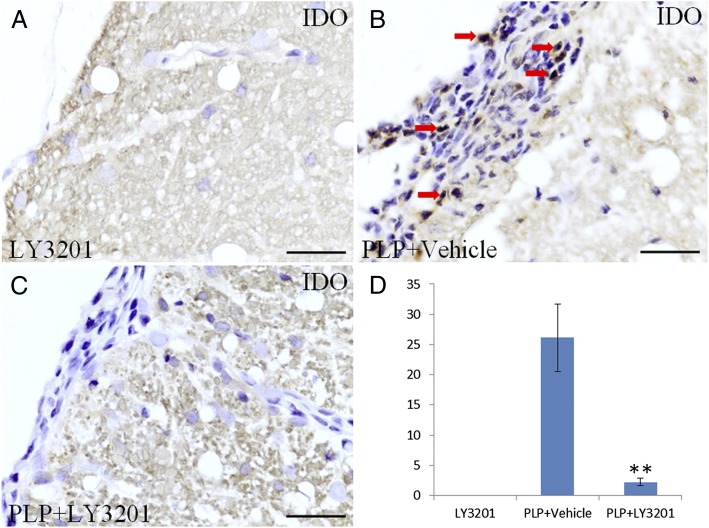
The enzyme indoleamine-2,3-dioxygenase (IDO), expressed in dendritic cells, catabolizes tryptophan and causes cell death by depleting tryptophan and generating quinolinic acid, a cytotoxic metabolite of tryptophan. IDO immunohistochemistry analysis in the spinal cord revealed many IDO-positive cells in vehicle-treated EAE mice, but none in uninduced mice (Fig. 6 A and B). There was a marked reduction in IDO-positive cells in EAE mice treated with LY3201 (Fig. 6 C and D).
Fig. 6.
Fewer IDO-positive cells in the spinal cord of EAE mice treated with LY3201. (A) There were no IDO-positive cells in the spinal cord of noninduced mice treated with LY3201. (B) IDO-positive cells (red arrow) were markedly increased in vehicle-treated EAE mice. (C) There were fewer IDO-positive cells in the spinal cord of EAE mice treated with LY3201. (D) The number of IDO-positive cells differed significantly in LY3201-treated and vehicle-treated EAE mice. **P < 0.01. (Scale bars: 50 μm.)
Three-Day Pretreatment with LY3201 Promotes Recovery and Decreases Mortality in EAE Mice.
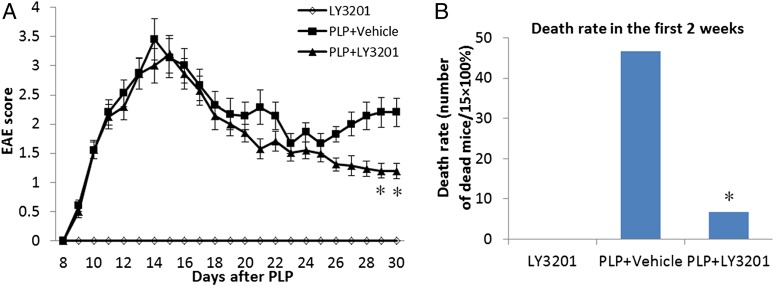
Mice were assessed daily for the development of clinical signs according to the following EAE scoring pattern: 0, no signs; 1, limp tail or hind limb weakness but not both; 2, limp tail and hind limb weakness; 3, partial hind limb paralysis; 4, complete hind limb paralysis. Treatment with LY3201 reduced the severity of EAE symptoms in mice surviving after 4 wk (Fig. 7A), and decreased the mortality rate in the first 2 wk (Fig. 7B).
Fig. 7.
LY3201 promoted recovery and decreased mortality in EAE mice. EAE score was determined daily for 30 d after PLP immunization. (A) Compared with vehicle-treated mice, LY3201-treated mice had less severe symptoms at 4 wk after PLP induction (*P < 0.05). (B) Treatment with LY3201 reduced the mortality of EAE mice in the first 2 wk. *P < 0.05.
Discussion
In the present work, we show that in EAE mice, the ERβ-selective agonist LY3201 can inactivate microglia and invading T cells by down-regulating two important inflammatory pathways, NF-κB and iNOS. There is much evidence that inhibition of proinflammatory mediators in microglia can attenuate the severity of MS, Parkinson disease, and cerebral ischemia (29–31). Thus, inhibition of microglial activation is considered a key strategy for preventing neurodegenerative diseases (32).
In the present study, immunohistochemistry and immunofluorescence analysis demonstrated expression of ERβ, but not ERα, in mouse microglia. The ERβ-selective agonist LY3201, administered before PLP, reduced mortality in EAE mice during the initiation phase of the disease, modulated the activation of microglia, and down-regulated expression of NF-κB and iNOS in both microglia and T cells.
Activated NF-κB regulates the expression of several inflammation-related genes, including iNOS, TNFβ, IL-6, and IL-1β. Gene expression profiling in MS patients and EAE models has shown increased expression of genes controlled by NF-κB, and drugs that decrease NF-κB activation can provide protection against EAE (24). These data indicate that the NF-κB pathway plays a critical role in the pathogenesis of MS and has potential as a therapeutic target in MS. Previous studies have demonstrated that both ERα and ERβ can inhibit NF-κB transcriptional activity (33, 34). Our present data show that NF-κB was activated in microglia and T cells of EAE mice, and that this NF-κB activation was down-regulated in the spinal cord of EAE mice treated with LY3201 before PLP.
In the CNS, NO, a damaging free radical (20), is an important weapon for attacking invading microbes. However, excessive iNOS activity may damage normal tissue, a factor involved in the pathophysiology of MS (35). NO metabolites detected in the cerebrospinal fluid of MS patients are associated with disease progression, and reduced NO synthesis can prevent the development of EAE (28). These results indicate an important role of NO in the onset of MS.
The target for inhibition of iNOS expression is NF-κB (36, 37). LPS, IL-1β, TNF-α, and oxidative stress induce iNOS expression by activating NF-κB, whereas glucocorticoid-induced inhibition of iNOS results from inhibition of NF-κB activation. Our data indicate that in EAE mice, iNOS expression was increased by PLP, particularly in white matter of the spinal cord. With double fluorescence, we identified iNOS expression in both microglia and invading T cells. iNOS expression was markedly decreased in mice treated with LY3201. This result is in accordance with findings of a previous in vitro study by Baker et al. (19), which demonstrated that activation of ERβ decreases iNOS expression and NO production in response to LPS stimulation of BV-2 microglia.
IDO is a key player in immunosuppression (38). Microarray studies comparing WT and ERβ−/− mice identified IDO as an ERβ-induced gene. IDO causes T-cell death through depletion of tryptophan. In the present study, IDO-positive cells were abundant in the spinal cord of vehicle-treated EAE mice. These cells resemble plasmacytoid T cells [dendritic cells (DCs)], which are needed for T-cell activation. No such cells were detectable in the LY3201-treated mice. We hypothesize that the ERβ agonist induced IDO in these cells and caused their death through depletion of tryptophan. ERβ is expressed in DCs (39), providing the possibility that ERβ-selective agonist modulates DC functions directly.
Concomitant with the modulation of microglial activity by LY3201 was amelioration of the clinical signs of EAE. LY3201 reduced the mortality of EAE mice in the first 2 wk and promoted the recovery of EAE mice surviving for more than 4 wk.
In summary, the present study demonstrates that in mice, ERβ, not ERα, is the receptor that modulates microglial activity. The ERβ-selective agonist LY3201 can suppress activated microglia and down-regulate PLP-induced NF-κB activation and iNOS expression in both microglia and T cells invading the spinal cord. The action on both microglia and T cells suggests that LY3201 may have a role in therapeutic intervention in MS and perhaps other neurodegenerative diseases in which both cell types play crucial roles in pathogenesis. All of this can be achieved without negative effects on the pituitary, mammary gland, and uterus, which are the organs adversely affected with the use of estradiol.
Materials and Methods
Materials, Animals, and Tissue Preparation.
The ERβ agonist LY3201, (3aS, 4R, 9bR)-2, 2-difluoro-4-(4-hydroxyphenyl)-3, 3a, 4, 9b-tetrahydro-1H-cyclopenta[c] chromen-8-ol (CAS 787621–78-7), was prepared as described previously (40). All animals were 8-wk-old SJL/J females, purchased from Jackson Laboratory. Thirty-five mice were divided randomly into three groups: (i) no induction, treated with LY3201 (n = 5); (ii) PLP treated with vehicle (n = 15); and (iii) PLP treated with LY3201 (n = 15). Mice from the second and third groups were immunized with 50-µL s.c. injections of a 1 mg/mL solution of PLP in equal volumes of PBS and complete Freund’s adjuvant containing Mycobacterium tuberculosis strain H37RA into the shoulders and flanks. Then 200 ng of pertussis toxin was injected i.p. on the day of PLP injection and again 2 d later. LY3201 was used in pellet form (0.04 mg/d), as produced by Innovative Research of America and implanted on the lateral side of the neck between the ear and the shoulder. The pellet is composed of a matrix fused with an active product; ingredients include cholesterol, cellulose, lactose, phosphates, and stearates. The mice were treated by insertion of pellets (vehicle or LY3201) 3 d before PLP. Mice were housed in a room at standard temperature (22 ± 1 °C) with a regular 12-h light/12-h dark cycle and given free access to water and standard rodent chow. EAE score was measured daily for 30 d after PLP. Mice were killed on day 36 with 60 mg/kg pentobarbital (Mebunat; Orion Pharma) and transcardially perfused with heparinized (2.5 IU/mL) saline followed by 4% paraformaldehyde in 0.1 M PBS (pH 7.4). These experiments were performed by The Jackson Laboratory, West 4910 Raley Blvd., Sacramento, CA 95838. All brains were dissected and postfixed in the same fixative overnight at 4 °C. After fixation, lumbar spinal cords were processed for paraffin sections (5 μm).
Immunohistochemistry.
Paraffin sections were deparaffinized in xylene, rehydrated through graded alcohol, and processed for antigen retrieval by boiling in 10 mM citrate buffer (pH 6.0) for 12–15 min in Pre-Treatment module. Sections were incubated in 0.3% H2O2 in 50% methanol for 30 min at room temperature to quench endogenous peroxidase. Sections were then incubated in 3% BSA for 30 min to block nonspecific binding, after which a biotin-blocking system (Dako) was used to block endogenous biotin. Sections were then incubated with anti–NF-κB (1:200; Abcam) and anti-iNOS (1:100; Abcam) at 4 °C after blocking of nonspecific binding in 3% BSA. BSA replaced primary antibodies in negative controls. After washing, sections were incubated with an HRP polymer kit (GHP516; Biocare Medical) for 30 min at room temperature, followed by 3,3′-diaminobenzidine tetrahydrochloride as the chromogen (41, 42). For immunofluorescence, quenching of endogenous peroxidase and blocking of endogenous biotin were omitted.
Sections were incubated overnight with anti-Iba1 (1:400; Abcam), anti-ERα (1:100; Abcam), anti-ERβ (1:100; made in our laboratory), anti–NF-κB (1:200; Abcam), anti-iNOS (1:100; Abcam), anti-CD3 (1:100; Santa Cruz Biotechnology), and anti-IDO (1:100; Santa Cruz Biotechnology) at 4 °C after blocking of nonspecific binding in 3% BSA. Primary antibodies were detected with donkey anti-goat Cy3 (1:400; Jackson ImmunoResearch), donkey anti rabbit FITC (1:400; Santa Cruz Biotechnology), and donkey anti-chicken FITC (1:400; Jackson ImmunoResearch). Sections were later counterstained with Vectashield mounting medium containing DAPI (Vector Laboratories) to label nuclei (43). The number of microglia and NF-κB–positive cells in the spinal cord of each mouse were counted under light microscopy as described previously. The ERβ antibody was homemade, raised in hens immunized with ERβ protein and purified from egg yolks. It has been used extensively in mouse brain and spinal cords (44–47), as well as in mouse and human prostates and mammary glands (48, 49). For cell counting, we took every fifth slice from 25 consecutive slices, that is, five slices from each mouse. One 400× magnification field was counted in each slice, for a total of five fields per mouse.
Data Analysis.
Data are expressed as mean ± SD. Statistical comparisons were done using one-way ANOVA followed by the Newman–Keuls post hoc test. Mortality was assessed using the χ2 (×2) test. P < 0.05 was considered to indicate statistical significance.
Acknowledgments
This work was supported by grants from the Robert A. Welch Foundation (E-0004), Eli Lilly, The Swedish Cancer Fund, and the Texas Emerging Technology Fund (Agreement 300-9-1958).
Footnotes
The authors declare no conflict of interest.
References
- 1.Noseworthy JH, Lucchinetti C, Rodriguez M, Weinshenker BG. Multiple sclerosis. N Engl J Med. 2000;343(13):938–952. doi: 10.1056/NEJM200009283431307. [DOI] [PubMed] [Google Scholar]
- 2.Dyment DA, Ebers GC, Sadovnick AD. Genetics of multiple sclerosis. Lancet Neurol. 2004;3(2):104–110. doi: 10.1016/s1474-4422(03)00663-x. [DOI] [PubMed] [Google Scholar]
- 3.Vukusic S, Confavreux C. Primary and secondary progressive multiple sclerosis. J Neurol Sci. 2003;206(2):153–155. doi: 10.1016/s0022-510x(02)00427-6. [DOI] [PubMed] [Google Scholar]
- 4.Wingerchuk DM. Current evidence and therapeutic strategies for multiple sclerosis. Semin Neurol. 2008;28(1):56–68. doi: 10.1055/s-2007-1019128. [DOI] [PubMed] [Google Scholar]
- 5.Wolinsky JS, et al. PROMiSe Trial Study Group Glatiramer acetate in primary progressive multiple sclerosis: Results of a multinational, multicenter, double-blind, placebo-controlled trial. Ann Neurol. 2007;61(1):14–24. doi: 10.1002/ana.21079. [DOI] [PubMed] [Google Scholar]
- 6.Hartung HP, et al. Mitoxantrone in Multiple Sclerosis Study Group (MIMS) Mitoxantrone in progressive multiple sclerosis: A placebo-controlled, double-blind, randomised, multicentre trial. Lancet. 2002;360(9350):2018–2025. doi: 10.1016/S0140-6736(02)12023-X. [DOI] [PubMed] [Google Scholar]
- 7.Polman CH, et al. AFFIRM Investigators A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med. 2006;354(9):899–910. doi: 10.1056/NEJMoa044397. [DOI] [PubMed] [Google Scholar]
- 8.Rice GP, Hartung HP, Calabresi PA. Anti-alpha4 integrin therapy for multiple sclerosis: Mechanisms and rationale. Neurology. 2005;64(8):1336–1342. doi: 10.1212/01.WNL.0000158329.30470.D0. [DOI] [PubMed] [Google Scholar]
- 9.Glass CK, Saijo K, Winner B, Marchetto MC, Gage FH. Mechanisms underlying inflammation in neurodegeneration. Cell. 2010;140(6):918–934. doi: 10.1016/j.cell.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ransohoff RM, Perry VH. Microglial physiology: Unique stimuli, specialized responses. Annu Rev Immunol. 2009;27:119–145. doi: 10.1146/annurev.immunol.021908.132528. [DOI] [PubMed] [Google Scholar]
- 11.Streit WJ. Microglia as neuroprotective, immunocompetent cells of the CNS. Glia. 2002;40(2):133–139. doi: 10.1002/glia.10154. [DOI] [PubMed] [Google Scholar]
- 12.Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308(5726):1314–1318. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- 13.Davalos D, et al. ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci. 2005;8(6):752–758. doi: 10.1038/nn1472. [DOI] [PubMed] [Google Scholar]
- 14.Benveniste EN. Role of macrophages/microglia in multiple sclerosis and experimental allergic encephalomyelitis. J Mol Med (Berl) 1997;75(3):165–173. doi: 10.1007/s001090050101. [DOI] [PubMed] [Google Scholar]
- 15.Ratchford JN, et al. Decreased microglial activation in MS patients treated with glatiramer acetate. J Neurol. 2012;259(6):1199–1205. doi: 10.1007/s00415-011-6337-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gold SM, et al. Estrogen treatment decreases matrix metalloproteinase (MMP)-9 in autoimmune demyelinating disease through estrogen receptor alpha (ERalpha) Lab Invest. 2009;89(10):1076–1083. doi: 10.1038/labinvest.2009.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saijo K, Collier JG, Li AC, Katzenellenbogen JA, Glass CK. An ADIOL-ERβ-CtBP transrepression pathway negatively regulates microglia-mediated inflammation. Cell. 2011;145(4):584–595. doi: 10.1016/j.cell.2011.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chadwick CC, et al. Identification of pathway-selective estrogen receptor ligands that inhibit NF-kappaB transcriptional activity. Proc Natl Acad Sci USA. 2005;102(7):2543–2548. doi: 10.1073/pnas.0405841102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baker AE, Brautigam VM, Watters JJ. Estrogen modulates microglial inflammatory mediator production via interactions with estrogen receptor beta. Endocrinology. 2004;145(11):5021–5032. doi: 10.1210/en.2004-0619. [DOI] [PubMed] [Google Scholar]
- 20.Vegeto E, et al. The endogenous estrogen status regulates microglia reactivity in animal models of neuroinflammation. Endocrinology. 2006;147(5):2263–2272. doi: 10.1210/en.2005-1330. [DOI] [PubMed] [Google Scholar]
- 21.Li Q, Verma IM. NF-kappaB regulation in the immune system. Nat Rev Immunol. 2002;2(10):725–734. doi: 10.1038/nri910. [DOI] [PubMed] [Google Scholar]
- 22.Karin M, Greten FR. NF-kappaB: Linking inflammation and immunity to cancer development and progression. Nat Rev Immunol. 2005;5(10):749–759. doi: 10.1038/nri1703. [DOI] [PubMed] [Google Scholar]
- 23.Baeuerle PA, Baltimore D. NF-kappa B: Ten years after. Cell. 1996;87(1):13–20. doi: 10.1016/s0092-8674(00)81318-5. [DOI] [PubMed] [Google Scholar]
- 24.van Loo G, et al. Inhibition of transcription factor NF-kappaB in the central nervous system ameliorates autoimmune encephalomyelitis in mice. Nat Immunol. 2006;7(9):954–961. doi: 10.1038/ni1372. [DOI] [PubMed] [Google Scholar]
- 25.Ousman SS, et al. Protective and therapeutic role for alphaB-crystallin in autoimmune demyelination. Nature. 2007;448(7152):474–479. doi: 10.1038/nature05935. [DOI] [PubMed] [Google Scholar]
- 26.Willenborg DO, Staykova M, Fordham S, O’Brien N, Linares D. The contribution of nitric oxide and interferon gamma to the regulation of the neuro-inflammation in experimental autoimmune encephalomyelitis. J Neuroimmunol. 2007;191(1-2):16–25. doi: 10.1016/j.jneuroim.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 27.Pautz A, et al. Regulation of the expression of inducible nitric oxide synthase. Nitric Oxide. 2010;23(2):75–93. doi: 10.1016/j.niox.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 28.Hooper DC, et al. Prevention of experimental allergic encephalomyelitis by targeting nitric oxide and peroxynitrite: Implications for the treatment of multiple sclerosis. Proc Natl Acad Sci USA. 1997;94(6):2528–2533. doi: 10.1073/pnas.94.6.2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Graeber MB, Streit WJ. Microglia: Biology and pathology. Acta Neuropathol. 2010;119(1):89–105. doi: 10.1007/s00401-009-0622-0. [DOI] [PubMed] [Google Scholar]
- 30.Koning N, Bö L, Hoek RM, Huitinga I. Downregulation of macrophage inhibitory molecules in multiple sclerosis lesions. Ann Neurol. 2007;62(5):504–514. doi: 10.1002/ana.21220. [DOI] [PubMed] [Google Scholar]
- 31.Qian L, Flood PM, Hong JS. Neuroinflammation is a key player in Parkinson’s disease and a prime target for therapy. J Neural Transm. 2010;117(8):971–979. doi: 10.1007/s00702-010-0428-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schieven GL. The biology of p38 kinase: A central role in inflammation. Curr Top Med Chem. 2005;5(10):921–928. doi: 10.2174/1568026054985902. [DOI] [PubMed] [Google Scholar]
- 33.Tyree CM, Zou A, Allegretto EA. 17beta-Estradiol inhibits cytokine induction of the human E-selectin promoter. J Steroid Biochem Mol Biol. 2002;80(3):291–297. doi: 10.1016/s0960-0760(02)00022-5. [DOI] [PubMed] [Google Scholar]
- 34.Valentine JE, Kalkhoven E, White R, Hoare S, Parker MG. Mutations in the estrogen receptor ligand binding domain discriminate between hormone-dependent transactivation and transrepression. J Biol Chem. 2000;275(33):25322–25329. doi: 10.1074/jbc.M002497200. [DOI] [PubMed] [Google Scholar]
- 35.Kröncke KD, Fehsel K, Kolb-Bachofen V. Inducible nitric oxide synthase in human diseases. Clin Exp Immunol. 1998;113(2):147–156. doi: 10.1046/j.1365-2249.1998.00648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mankan AK, Lawless MW, Gray SG, Kelleher D, McManus R. NF-kappaB regulation: The nuclear response. J Cell Mol Med. 2009;13(4):631–643. doi: 10.1111/j.1582-4934.2009.00632.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Basak S, Hoffmann A. Crosstalk via the NF-kappaB signaling system. Cytokine Growth Factor Rev. 2008;19(3-4):187–197. doi: 10.1016/j.cytogfr.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Munn DH, Mellor AL. Indoleamine 2,3 dioxygenase and metabolic control of immune responses. Trends Immunol. 2012 doi: 10.1016/j.it.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Komi J, Lassila O. Nonsteroidal anti-estrogens inhibit the functional differentiation of human monocyte-derived dendritic cells. Blood. 2000;95(9):2875–2882. [PubMed] [Google Scholar]
- 40.Richardson TI, et al. Benzopyrans as selective estrogen receptor beta agonists (SERBAs), part 3: Synthesis of cyclopentanone and cyclohexanone intermediates for C-ring modification. Bioorg Med Chem Lett. 2007;17(17):4824–4828. doi: 10.1016/j.bmcl.2007.06.052. [DOI] [PubMed] [Google Scholar]
- 41.Tan XJ, Dai YB, Wu WF, Warner M, Gustafsson JÅ. Anxiety in liver X receptor β knockout female mice with loss of glutamic acid decarboxylase in ventromedial prefrontal cortex. Proc Natl Acad Sci USA. 2012;109(19):7493–7498. doi: 10.1073/pnas.1205189109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dai YB, Tan XJ, Wu WF, Warner M, Gustafsson JA. Liver X receptor β protects dopaminergic neurons in a mouse model of Parkinson disease. Proc Natl Acad Sci USA. 2012;109:13112–13117. doi: 10.1073/pnas.1210833109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tan XJ, et al. Reduction of dendritic spines and elevation of GABAergic signaling in the brains of mice treated with an estrogen receptor β ligand. Proc Natl Acad Sci USA. 2012;109(5):1708–1712. doi: 10.1073/pnas.1121162109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Suzuki H, et al. Involvement of estrogen receptor β in maintenance of serotonergic neurons of the dorsal raphe. Mol Psychiatry. 2012 doi: 10.1038/mp.2012.62. [DOI] [PubMed] [Google Scholar]
- 45.Sugiyama N, et al. Spatiotemporal dynamics of the expression of estrogen receptors in the postnatal mouse brain. Mol Psychiatry. 2009;14(2):223–232, 117. doi: 10.1038/mp.2008.118. [DOI] [PubMed] [Google Scholar]
- 46.Fan X, Warner M, Gustafsson JA. Estrogen receptor beta expression in the embryonic brain regulates development of calretinin-immunoreactive GABAergic interneurons. Proc Natl Acad Sci USA. 2006;103(51):19338–19343. doi: 10.1073/pnas.0609663103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fan X, Kim HJ, Warner M, Gustafsson JA. Estrogen receptor beta is essential for sprouting of nociceptive primary afferents and for morphogenesis and maintenance of the dorsal horn interneurons. Proc Natl Acad Sci USA. 2007;104(34):13696–13701. doi: 10.1073/pnas.0705936104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Esslimani-Sahla M, et al. Increased estrogen receptor betacx expression during mammary carcinogenesis. Clin Cancer Res. 2005;11(9):3170–3174. doi: 10.1158/1078-0432.CCR-04-2298. [DOI] [PubMed] [Google Scholar]
- 49.Muthusamy S, et al. Estrogen receptor β and 17β-hydroxysteroid dehydrogenase type 6, a growth regulatory pathway that is lost in prostate cancer. Proc Natl Acad Sci USA. 2011;108(50):20090–20094. doi: 10.1073/pnas.1117772108. [DOI] [PMC free article] [PubMed] [Google Scholar]