Abstract
The continuous need for ion gradient restoration across the cell membrane, a prerequisite for synaptic transmission and conduction, is believed to be a major factor for brain’s high oxidative demand. However, do energy requirements of signaling and nonsignaling components of cortical neurons and astrocytes vary with activity levels and across species? We derived oxidative ATP demand associated with signaling (Ps) and nonsignaling (Pns) components in the cerebral cortex using species-specific physiologic and anatomic data. In rat, we calculated glucose oxidation rates from layer-specific neuronal activity measured across different states, spanning from isoelectricity to awake and sensory stimulation. We then compared these calculated glucose oxidation rates with measured glucose metabolic data for the same states as reported by 2-deoxy-glucose autoradiography. Fixed values for Ps and Pns were able to predict the entire range of states in the rat. We then calculated glucose oxidation rates from human EEG data acquired under various conditions using fixed Ps and Pns values derived for the rat. These calculated metabolic data in human cerebral cortex compared well with glucose metabolism measured by PET. Independent of species, linear relationship was established between neuronal activity and neuronal oxidative demand beyond isoelectricity. Cortical signaling requirements dominated energy demand in the awake state, whereas nonsignaling requirements were ∼20% of awake value. These predictions are supported by 13C magnetic resonance spectroscopy results. We conclude that mitochondrial energy support for signaling and nonsignaling components in cerebral cortex are conserved across activity levels in mammalian species.
Keywords: spike rate, field potentials, glutamate, functional MRI, bispectral index
The brain is one of the most energy demanding tissues in the body (1). 13C magnetic resonance spectroscopy (MRS) in the rat has shown that, in the resting awake state, ∼80% of cortical energy consumption is used to support signaling as reflected by the rate of glutamate neurotransmitter release and astroglial uptake (2, 3). Cerebral energy demand is also positively correlated with the rate of pyramidal neuron firing in rat cortex (4, 5). 13C MRS findings in the human cortex have been generally consistent with the rat results (6). However, there remain questions as to how well the energy costs of specific subcellular processes needed to support synaptic transmission and conduction are conserved over different activity levels and/or across species.
Recent bottom-up energy budgets for gray matter in the mammalian brain have attempted to understand the energetic costs of neuronal and glial electrical and neurotransmission events occurring in the neuropil (7, 8) by calculating the ATP used per neuron for signaling (Ps) and nonsignaling (Pns) events. In the awake cortex, the total ATP used per unit cortical volume per unit time (Etot; in units of ATP/s per centimeter3) was determined by multiplying the Ps (in units of ATP/neuron per spike) and Pns (in units of ATP/neuron per second) parameters with cellular densities (η) and average cortical firing rates (<f>) to give signaling (Es) and nonsignaling (Ens) components,
where the summation spans for neurons and astrocytes. These studies agreed with in vivo measurements in that the majority of brain energy consumption was used to support signaling and also concluded that both signaling and nonsignaling events in human were about two to three times more costly on a per neuron basis than in the rat. However, these budgets have limitations (7, 8); most notably, the histological and biophysical parameters used disagree with recent values, heterogeneity across cortical lamina was disregarded (9–11), and the comparison was made with only one the resting awake state.
Here, we use a top-down as opposed to a bottom-up approach to assess the fundamental links between electrical and chemical events at the neuropil. We tested the hypotheses that the energy requirements per cell for signaling (Ps) and nonsignaling (Pns) are independent of the state of neuronal activity (e.g., sensory activation, awake, asleep, or anesthetized) and are conserved across species (i.e., rat and human). We used layer-specific morphologic, neurophysiologic, and metabolic data in rat brain to calculate Ps and Pns, which were then tested on human data. In contrast to views highlighting differences between rat and human brain neurometabolic couplings (7, 8), our results suggest that, on a per cellular basis, the mitochondrial energy support for mammalian cortical functions during signaling and nonsignaling are conserved.
Calculations
Energetics of Signaling and Nonsignaling Components.
We did not sum bottom-up contributions to total oxidative ATP demand as previously done in other budgets (7, 8) but rather, tested whether unchanging values of Ps and Pns can account for measured in vivo results over a range of cortical activity levels (SI Text, section 1 and Tables S1 and S2). Although the Es term in Eq. 1 encompasses function of both neurons and astrocytes, we base our budget to neuronal activity data due to limited ability to quantify astrocytic signaling. Given that neuronal firing is statistically representative of neuropil activity (12), we used spike rate as a quantitative measure of cortical function as has been done before (7, 8). In Eq. 1, <f> is given in units of spike.neuron/s per centimeter3 and ηN and ηA are given in units of cells/cm3. Tables S1 and S2 list values of neuronal activity and glucose consumption in rat and human, respectively (SI Text, section 1). Recordings of cortical signaling were reflected by layer-specific microelectrodes in rats and EEG in humans, whereas metabolic measurements were made by 2-deoxyglucose (2DG) autoradiography in rats and fluoro-deoxyglucose PET in humans.
Energetics of Signaling from Layer-Specific Neuronal Recordings in the Rat Somatosensory Cortex.
Cortical signaling involves events like dendritic depolarization, axonal propagation, vesicular endocytosis and exocytosis, neurotransmitter cycling, ionotropic and metabotropic receptor activity, etc. To determine Es in Eq. 1, we first multiplied the spike rate of the ith cortical layer (fi in units of spike/s) by the cortical density in the same layer (ηN,i in units of neurons/cm3) to obtain the number of firing events per unit volume (fi ηN,i in units of spike.neuron/s per centimeter3). Next, we estimated the fraction of the ith cortical layer in relation to the entire cortical thickness (δi/Σδj). On multiplying, these two terms and summing across all layers gives <f> in Eq. 1 (in units of spike.neuron/s per centimeter3):
Using values of δ, η, and f from recent studies, we then determined if Eq. 1 was able to fit results from experimentally measured regional metabolism across different activity levels.
Energetics of Nonsignaling Events from Isoelectric Condition in the Rat Somatosensory Cortex.
We assigned the nonsignaling energy primarily to ion movement associated with maintaining neuronal and glial resting potentials described by Eq. 1. To separately calculate Pns,A and Pns,N, we needed input resistances of neurons (Rin,N) and astrocytes (Rin,A), which describe the energy demand of leaky cell membranes at rest (SI Text, section 2, and Fig. S1) and the average neuronal (ηN) and astrocytic (ηA) densities in the cerebral cortex (Table 1). Because recent Rin,x and ηx measurements in the rat show that values for neurons and astrocytes are quite similar (13–16), the calculations were slightly simplified by assuming that Rin,N ∼ Rin,A and ηN ∼ ηA (Table 1 and SI Text, section 2). To determine Pns, we needed the metabolic demand for nonsignaling, which was possible for the rat, because state of deep pentobarbital anesthesia in Table S1 induces an isoelectric condition and thus, just contains the Ens term. Because neuronal recordings do not show any significant cortical activity in the pentobarbital state, the nonsignaling energy described was uniform across all layers. By multiplying Pns (in units of ATP/cell per second) with ηx (in units of cells/cm3), we get Ens in Eq. 1 (in units of ATP/s per centimeter3). This empirically derived Ens was held constant for all other states.
Table 1.
Energy budget parameters in rat and human brains
| Source | ηN | ηA | Rin,N | Rin,A | Pns,N | Pns,A | Ps |
| This study (rat) | 4.75* | 4.75* | 74† | 74† | 9.20‡ | 6.85‡ | 4.81§ |
| This study (human) | 1.83* | 1.83* | 74† | 74† | 9.20‡ | 6.85‡ | 4.81§ |
| Ref. 7 (rat) | 9.2 | 9.2 | 200 | 500 | 3.42 | 1.02 | 0.71 |
| Ref. 8 (human) | 4.0 | 3.8 | 79 | 163 | 8.6 | 3.1 | 2.4 |
Average values of cortical density of neurons (ηN; ×107 neuron/cm3) and astrocytes (ηA; ×107 astrocyte/cm3) densities, input resistances of neurons (Rin,N; MΩ) and astrocytes (Rin,A; MΩ), rate of ATP use for nonsignaling per neuron (Pns,N; ×108 ATP/neuron per second) and astrocyte (Pns,A; ×108 ATP/astrocytes per second), and ATP use per signaling event per neuron (Ps; ×109 ATP/spike per neuron).
*Table 2 in ref. 15 shows average cortical neuronal density in rat brain, figure 2 in ref. 16 shows neuron vs. astrocyte densities are similar, and figure 2 in ref. 17 shows rat and human neuronal densities differ by ∼2.6.
†Estimated from Fig. S1A.
Converting Calculated Total ATP Production Rate to Cerebral Glucose Oxidation Rate.
We used the following formula to convert Etot in Eqs. 1 to rate of glucose oxidation [calcCMRglc(ox)],
where k depends on the oxygen-to-glucose index (OGI), which is given by the ratio of cerebral metabolic rates of oxygen (CMRO2) and glucose (CMRglc) consumption, and k itself is given by 107/(Avo ρ OGI), where ρ is the tissue density (1.05 g/mL) and Avo is the Avogadro constant (6.023 × 1023/mol). Similarly, the measured glucose oxidation [measCMRglc(ox)] was given by
where measCMRglc was obtained from 2DG autoradiography in rats and fluoro-deoxyglucose PET in humans (Tables S1 and S2); it is given by sum of oxidative [CMRglc(ox)] and nonoxidative [CMRglc(nonox)] terms, whereas CMRglc(ox) itself has neuronal [CMRglc(ox),N] and astrocytic [CMRglc(ox),A] components. Thus, it is possible to obtain calculated forms of CMRglc(nonox), CMRglc(ox),N, and CMRglc(ox),A across all activity states. Finally, we compared calcCMRglc(ox) with measCMRglc(ox) by least-square fitting to determine Ps and Pns:
Calculating Signaling and Nonsignaling Energetics in the Human Visual Cortex.
To test whether Pns,N, Pns,A, and Ps derived from the rat (Table 1) were representative of those values in human cerebral cortex, we calculated Ens in the human by simply multiplying the Pns,N and Pns,A terms with ηN and ηA, respectively [i.e., cellular density is about 2.6 times lower in human vs. rat (17)]. Neuronal activity for each state in the human was represented by EEG-measured bispectral index (BIS) values ranging from 0 to 100 (Table S2) as used intraoperatively (18). We calculated the Es term in the human similarly as in the rat, but because the neuronal activity data in the human originated from EEG recordings (SI Text, section 3), additional steps were needed to represent Eq. 2 to convert the BIS into units similar to the rat data (i.e., in units of spike.neuron/s per centimeter3):
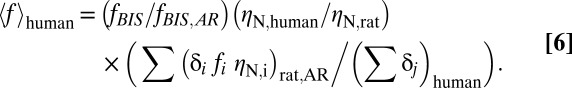 |
fBIS and fBIS,AR are BIS values for a given state and the awake condition (Table S2), ηN,human and ηN,rat are the average neuronal densities in the human and rat cortices (Table 1), Σ(δi fi ηN,i)rat,AR is the numerator of Eq. 2 for the awake condition in the rat (Table 2), and (Σδj)human is the cortical thickness in the human (Table 3). This conversion, from fBIS scale in humans to <f> scale in rats, accounts for differences in average neuronal density and cortical thickness between rat somatosensory and human visual cortices (15–17, 19).
Table 2.
Calculated CMRglc(ox) derived from neuronal activity in rat somatosensory cortex for OGI of 5.6
| Behavioral state* | PR | UR1† | US1† | AR† | AS† | UR2‡ | US2‡ | CR‡ | CS‡ | HR‡ | HS‡ |
| Σ(δi fi ηN,i) (×103 spike.neuron.cm/s per centimeter3) | 0.00 | 7,051 | 9,393 | 8,858 | 9,090 | 6,024 | 8,629 | 4,233 | 6,677 | 5,213 | 5,533 |
| Σ(δi fi ηN,i)/Σδj (×107 spike.neuron/s per centimeter3) | 0.00 | 3.71 | 4.95 | 4.67 | 4.79 | 13.36 | 19.13 | 9.39 | 14.80 | 11.56 | 12.27 |
| Ens = Σ Pns,x × ηN,x (×1017 ATP/s per centimeter3) | 0.76 | 0.76 | 0.76 | 0.76 | 0.76 | 0.76 | 0.76 | 0.76 | 0.76 | 0.76 | 0.76 |
| Es = Ps × Σ(δi fi ηN,i)/Σδj (×1017 ATP/s per centimeter3) | 0.00 | 1.79 | 2.38 | 2.24 | 2.30 | 1.53 | 2.19 | 1.07 | 1.69 | 1.32 | 1.40 |
| Etot = Ens + Es (×1017 ATP/s per centimeter3) | 0.76 | 2.55 | 3.14 | 3.01 | 3.07 | 2.29 | 2.95 | 1.84 | 2.45 | 2.08 | 2.16 |
| calcCMRglc(ox) (μmol/g per minute) | 0.22 | 0.72 | 0.89 | 0.85 | 0.87 | 0.65 | 0.83 | 0.52 | 0.69 | 0.59 | 0.61 |
| measCMRglc(ox) (μmol/g per minute) | 0.20 | 0.63 | 0.90 | 0.82 | 0.91 | 0.63 | 0.90 | 0.49 | 0.68 | 0.61 | 0.63 |
Details in Calculations. The calculated CMRglc(ox) [calcCMRglc(ox)] was determined from Eq. 3. The measured CMRglc(ox) [measCMRglc(ox)] was determined from Eq. 4 assuming OGI of 5.6. AR, awake rest; AS, awake stimulation; CR, α-chloralose rest; CS, α-chloralose stimulation; HR, halothane rest; HS, halothane stimulation; PR, pentobarbital; UR1, urethane rest; UR2, urethane rest; US1, urethane stimulation; US2, urethane stimulation.
*Details in SI Text, section 1 and see Fig. 1A for comparison between calcCMRglc(ox) and measCMRglc(ox).
†Σδj was 1.90 mm (with Ps = 4.81 × 109 ATP/spike per neuron; see red circles in Fig. 1A).
‡Σδj was 0.45 mm [with Ps′ = (0.45/1.90) × Ps; see orange circles in Fig. 1A].
Table 3.
Calculated CMRglc(ox) derived from neuronal activity in human visual cortex for OGI of 5.6
| Behavioral state* | VGP | VGA | PRO | SEV | HAL | SLP | AWK |
| Σ(δi fi ηN,i) (×103 spike.neuron.cm/s per centimeter3) | 282 | 1,192 | 1,317 | 1,306 | 1,959 | 2,674 | 3,407 |
| Σ(δi fi ηN,i)/Σδj (×107 spike.neuron/s per centimeter3) | 0.12 | 0.51 | 0.56 | 0.56 | 0.83 | 1.14 | 1.45 |
| Ens = Σ Pns,x × ηN,x (×1017 ATP/s per centimeter3) | 0.29 | 0.29 | 0.29 | 0.29 | 0.29 | 0.29 | 0.29 |
| Es = Ps × Σ(δi fi ηN,i)/Σδj (×1017 ATP/s per centimeter3) | 0.06 | 0.24 | 0.27 | 0.27 | 0.40 | 0.55 | 0.70 |
| Etot = Ens + Es (×1017 ATP/s per centimeter3) | 0.35 | 0.54 | 0.56 | 0.56 | 0.69 | 0.84 | 0.99 |
| calcCMRglc(ox) (μmol/g per minute) | 0.10 | 0.15 | 0.16 | 0.16 | 0.20 | 0.24 | 0.28 |
| measCMRglc(ox) (μmol/g per minute) | 0.08 | 0.14 | 0.15 | 0.14 | 0.18 | 0.24 | 0.31 |
Details in Calculations. To convert the EEG data in Table S2 into units of Σ(δi fi ηN,i)/Σδj, simple conversions were needed with Eq. 6. The calculated CMRglc(ox) [calcCMRglc(ox)] was determined from Eq. 3. The measured CMRglc(ox) [measCMRglc(ox)] was determined from Eq. 4 assuming OGI of 5.6. AWK, awake; HAL, halothane; PRO, propofol; SEV, sevoflurane; SLP, non-REM sleep; VGA, acute vegetative; VGP, persistent vegetative.
*Details in SI Text, section 1 and see Fig. 1A for comparison between calcCMRglc(ox) and measCMRglc(ox).
Results
Ps in the Rat Somatosensory Cortex.
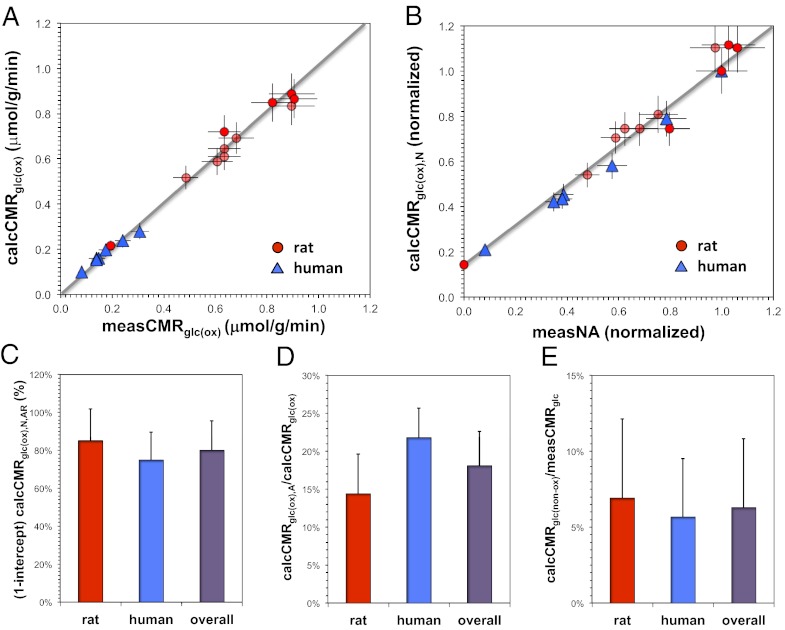
The averaged representation of normalized activity of neurons across all states showed dominant activity in the bottom two-thirds of cortical layers (Fig. S2 and Table S1), which is in good agreement with prior studies that investigated layer-specific representation of neuronal activity, 2DG autoradiography, and functional MRI (fMRI) data (9–11). The laminar activity was then used with Eqs. 1–6 to derive calcCMRglc(ox) and compared with measCMRglc(ox) by least-square fitting to determine Ps and Pns. An average Ps value of 4.81 × 109 ATP/spike per neuron was calculated by fitting Eq. 5 for all activity states in the rat, which is shown in Table 1. Because the neuronal activity data for some states in Table S1 were not available for all layers, Ps was estimated for those specific layers only [i.e., (0.45/1.90) × 4.81 × 109 ATP/spike per neuron) (Table 2). Although the current Ps value is derived for the entire cortex, layer-specific Ps values are unlikely to vary by more than 50% of the average value. Fig. 1A shows the goodness of fit for Eq. 5 to the rat data (red circles) for an OGI of 5.6 with an R2 value of 0.96 (gray line with σ2 = 0.0182 for 11 states), indicating that the assumption of a constant value of Ps over the full activity range is well-supported.
Fig. 1.
Relationship between glucose oxidation [CMRglc(ox)] and neuronal activity as a function of OGI. (A) Comparison between calculated CMRglc(ox) [calcCMRglc(ox)] and measured CMRglc(ox) [measCMRglc(ox)] values. Values of measCMRglc(ox) were derived from 2DG autoradiography in rat brain and PET in human brain, whereas values of calcCMRglc(ox) were derived for an OGI of 5.6. The goodness of fit between measCMRglc(ox) and calcCMRglc(ox) for the rat data (red circles) is indicated by the gray line with an R2 value of 0.96 (σ2 = 0.0182 for 11 states). The human data (blue triangles) also showed a strong correlation (R2 = 0.91 and σ2 = 0.0023 for seven states). (B) Comparison between measured NA (measNA) and calculated glucose oxidation in neurons [calcCMRglc(ox),N] in rat (red circles) and human (blue triangles) brains shows good correlation [R2 = 0.98, where calcCMRglc(ox),N = 0.90measNA + 0.12]. Because the data were normalized to the awake resting state values, the intercept on the vertical axis is about ∼20% of calcCMRglc(ox),N in the awake state for both species. (C–E) Comparison of calculated total glucose oxidation [calcCMRglc(ox)], calculated glucose oxidation in neurons [calcCMRglc(ox),N], calculated glucose oxidation in astrocytes [calcCMRglc(ox),A], calculated nonoxidative glucose consumption [calcCMRglc(nonox)], and measured total glucose consumption (measCMRglc), where calcCMRglc(ox) = calcCMRglc(ox),N + calcCMRglc(ox),A and calcCMRglc(nonox) = measCMRglc − calcCMRglc(ox). Ratios in rat brain (red), human brain (blue), and overall (purple) for (C) the value of the calcCMRglc(ox),N in B for the nonanesthetized awake resting state [calcCMRglc(ox),N,AR] minus the value of the intercept [i.e., (1-intercept)calcCMRglc(ox),N,AR] and the values of (D) calcCMRglc(ox),A/calcCMRglc(ox) and (E) calcCMRglc(nonox)/measCMRglc measured across all activity levels. All error bars indicate SDs from the mean. All calculations were obtained with Ps = 4.81 × 109 ATP/spike per neuron, Pns,N = 9.20 × 108 ATP/neuron per second, and Pns,A = 6.85 × 108 ATP/astrocytes per second). Details are in Tables 1, 2, and 3.
Pns in the Rat Somatosensory Cortex.
The measured CMRglc(ox) for the pentobarbital state in the rat (Table S1) was fitted to the Ens term (Table 2). The constant Ens term for all activity states was 0.76 × 1017 ATP/s per centimeter3, leading to average Pns,N and Pns,A values of 9.20 × 108 ATP/neuron per second and 6.85 × 108 ATP/astrocytes per second, respectively, for an OGI range of 5.1–6.0 (20). These values of Pns corresponded to an average Rin value of 74 MΩ (SI Text, section 2), which is well within the in vivo range measured for neurons and astrocytes (13, 14). As shown in Table 1, despite identical cortical densities of neurons and astrocytes, Pns,N and Pns,A were dissimilar, because slightly different Nernst potentials for Na+ and K+ and resting membrane potentials were used for neurons and astrocytes (SI Text, section 2), which has been done before (7, 8). However, similarity of contributions of astrocytes and neurons to nonsignaling energy consumption is in good agreement with measurements by 13C MRS made under isoelectric pentobarbital anesthesia, supporting the accuracy of the derived values (2, 21).
Es and Ens in the Human Visual Cortex.
At present, there are insufficient studies in which electrical signaling in the human cerebral cortex (e.g., by single-unit recording) has been measured over a range of activity states to empirically derive Ps and Pns, which was done for the rat cerebral cortex. To assess how different these values might be in the human, we used Eq. 1 with values for neuronal and glial densities determined for human and Ps and Pns derived from the rat data, which required normalizing the awake BIS scale in humans to rat neuronal activity scale in the awake state (Eq. 6). Laminar firing rates in the human are not available, but according to the difference of <f> between rats and humans (Tables 2 and 3), we expect the cortical neuronal firing rate in humans to be lower than in rats. Although instantaneous neuronal firing rates in the awake human cerebral cortex can be quite variable from moment to moment (22), average rates estimated from steady-state measurements (23) are about 30–40% of the highest firing rates typically observed in the awake rat (Table S1). Firing rate measurements in the awake primate cortex are slightly higher than values found in human brain (24). Given that CMRglc in awake primate cortex is about 0.5 μmol/g per minute (25), which is between awake CMRglc in rat and human (i.e., 0.8 vs. 0.3 μmol/g per minute, respectively), we expect that similar budget calculations can be made for primate cortex with the same Ps and Pns values based on cellular density variations across the species (17). Although neuronal recordings in primate brain do not usually report quantitative firing rates (26), basic features of action potentials in the human cerebral cortex are quite similar to those features typically found in other animals, including rats and primates (27–29). Fig. 1A shows the goodness of fit for Eq. 5 to the human data (blue triangles) for an OGI of 5.6 with an R2 value of 0.91 (gray line with σ2 = 0.0023 for seven states), indicating that the assumption of a constant value of Ps derived from the rat is well-supported over the full activity range in the human.
Relative Contributions of Es and Ens in the Resting Awake State.
To represent the magnitude of Ens vs. Es in the resting awake state, we plotted the calcCMRglc(ox),N vs. the measured neuronal activity in a normalized scale (Fig. 1B). Because the intercepts on the calcCMRglc(ox),N vertical axis—which indicates the isoelectric condition—were ∼0.1 and ∼0.05 μmol/g per minute for the rat and human, respectively (Fig. S3), or 15–20% of the calcCMRglc(ox),N in the awake state (Fig. 1B), the results suggest that a significant fraction of neuronal glucose oxidation in the awake state is dedicated for maintaining resting membrane potentials (Fig. 1C). Similarly, glial glucose oxidation is about ∼20% of total glucose oxidation in the awake state (Fig. 1D). Based on reported OGI values (20), nonoxidative glucose consumption is about ∼6% of total glucose consumption (Fig. 1E).
Comparison with 13C MRS Studies.
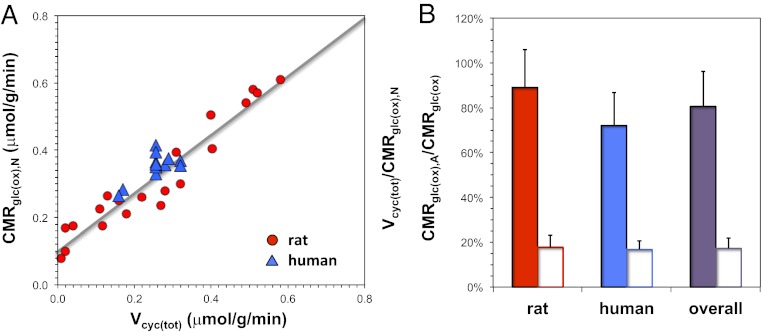
A similar linear relationship between neuronal activity and energy metabolism has been shown by results from 13C MRS—a method that simultaneously measures rates of total neurotransmitter cycling [Vcyc(tot)] and neuronal [CMRglc(ox),N] as well as astrocytic [CMRglc(ox),A] oxidative demand (2, 3). Fig. 2A shows the most up-to-date results from in vivo 13C MRS studies in rats and humans (Tables S3 and S4), which illustrate nearly a 1:1 relationship between ΔVcyc(tot) and ΔCMRglc(ox),N just beyond the isoelectric point when Vcyc(tot) approaches zero [i.e., gray line indicates an R2 value of 0.92, CMRglc(ox),N = 0.87 Vcyc(tot) + 0.10]. Moreover, the in vivo 13C MRS results of Vcyc(tot)/CMRglc(ox),N and CMRglc(ox),A/CMRglc(ox) ratios in the awake state for both species, shown in Fig. 2B, show that neurons and astrocytes in the awake state demand about ∼20% of oxidative ATP for nonsignaling factors. These results are similar to our budget calculations in Fig. 1.
Fig. 2.
In vivo 13C MRS experiments reporting rates of neurotransmitter cycling [Vcyc(tot)] and glucose oxidation in neurons [CMRglc(ox),N] and astrocytes [CMRglc(ox),A]. (A) Values of Vcyc(tot) and CMRglc(ox),N for rat (red circles) and human (blue triangles) cerebral cortex. The rat data represented many activity levels in the somatosensory cortex, whereas the human data were from the awake resting state in the visual cortex with varying degrees of gray vs. white matter partial volume (Table S3). Linear trends between changes in Vcyc(tot) and CMRglc(ox),N suggest strong coupling between neurotransmitter activity and energy metabolism [R2 = 0.92, where CMRglc(ox),N = 0.87 Vcyc(tot) + 0.10], where the finite intercept ranging between 0.05 and 0.15 μmol/g per minute indicates energy consumption for nonsignaling conditions. (B) Ratios of Vcyc(tot)/CMRglc(ox),N in the nonanesthetized awake resting state (filled bars) (Table S3) and CMRglc(ox),A/CMRglc(ox) for all conditions above isoelectricity (open bars) (Table S4) in rat (red), human (blue), and overall (purple). Similarity between the Vcyc(tot)/CMRglc(ox),N ratios (filled bars) in rat and human suggests that the relationship between Vcyc(tot) and CMRglc(ox),N in both species may be conserved. Likewise, correspondence between the CMRglc(ox),A/CMRglc(ox) ratios (open bars) suggests that the relationship between CMRglc(ox),A and CMRglc(ox) across species could be similar.
Discussion
Comparison of Derived Pns and Ps with Prior Calculations.
There are large differences between Pns,N and Pns,A estimates for the rat by Attwell and Laughlin (7) and our empirically derived values (Table 1). Values of Pns,N and Pns,A are susceptible to starting assumptions (SI Text, section 2 and Fig. S1). This difference principally occurs, because Attwell and Laughlin (7) assumed Rin,N and Rin,A to be around 200 and 500 MΩ, respectively, based on in vitro recordings (30, 31). However, our estimates of Rin,N and Rin,A values of 74 MΩ are in close agreement with in vivo measurements (13, 14). Because Pns depends on the reciprocal of Rin (SI Text, section 2), the higher Rin,N and Rin,A values by Attwell and Laughlin (7) are the main basis for their lower Pns,N and Pns,A estimates. Additionally, however, in the case of Pns,N, there could also be differences in average neuronal density, which in the study by Attwell and Laughlin (7), were based on the mouse cortex (32), whereas our values were obtained from the rat cortex (15). The Pns,N and Pns,A values estimated by Lennie (8) for the human are approximately similar to our values, despite large differences in cell densities across species (Table 1). There are similar differences between Ps values for the rat by Attwell and Laughlin (7) and the human by Lennie (8) as discussed in detail below. However, in a recent 31P MRS study of the human brain, Zhu et al. (33) estimated a Ps value of 4.7 × 109 ATP/neuron per second from measured cerebral metabolic rates of high-energy phosphate reactions catalyzed by ATPase. Assuming that, within 1 s, there is one spike in the awake brain, this value agrees well with Ps estimated in the current budget (Table 1).
Glial Energy Demand and Excitatory Vs. Inhibitory Neuronal Energy Requirements.
The calculated astrocytic energy demand (Fig. 1D) is consistent with 13C MRS results (Fig. 2B) but significantly higher than estimates from prior budgets (7, 8). The higher Ens,A values are because of using more recent values of astrocyte input resistances (14). The in vivo results were fit well with the assumption of the major energetic changes with activity being in the neurons. Relatively constant astrocytic energetics (compared with neurons) as a function of overall cortical activity may reflect the energetics associated with maintaining the high astrocyte K+ conductance dominating over additional functional demands (e.g., transporting glutamate and/or dealing with Ca2+ waves) as suggested by recent studies (34–37). 13C MRS results, in support of these observations, show that energy demand of astrocytes changes considerably less than neuronal energy demand over wide activity levels (Table S4). However, the glial data are relatively limited compared with neuronal data, and future studies are needed to better understand glial functional energy requirements.
As in previous bottom-up calculations (7, 8), to derive Ps and Pns, we could not separately include energy demand of inhibitory neurons, because the electrophysiological studies only included measurements of pyramidal neurons. Because CMRglc(ox),N contains the oxidative energy requirements of both excitatory and inhibitory neurons (15), the value of Ps likely reflects glutamatergic neurons working in conjunction with an ensemble of GABAergic neurons. Electrophysiological and 13C MRS studies have found that, over the range of activity that we examined, both function and energy demand of GABAergic neurons is proportional to activity/demand of glutamatergic neurons (38, 39), consistent with the fractional contribution of excitatory and inhibitory neurons to Ps being constant throughout the activity range and similar to glutamatergic and GABAergic neuronal fractions measured morphologically (15).
Constancy of Ps and Pns Across Activity Levels and Species.
Previously, Karbowski (40) suggested that basal metabolic cortical differences across species could be described on allometric rationale. However, Herculano-Houzel (17) pointed out that absolute metabolic difference across species could be explained by neuronal number variations. Both Karbowski (40) and Herculano-Houzel (17) dealt with only the awake state values and the total metabolic rate (i.e., only Etot in Eq. 1). In the current study, we partitioned Etot for each activity level examined in terms of signaling (Es) and nonsignaling (Ens) components to test if Ps and Pns are constant across species). Thus, neither Es nor Ens was assumed for any given state. Es was derived based on the neuronal firing for a given state in each cortical layer for the rat and across the cortex in the human, whereas Ens was based on leakiness of the cell membrane. To derive Ps, we fitted Etot to measured CMRglc(ox) data for each state, whereas to derive Pns, we fitted Ens to measured CMRglc(ox) data for pentobarbital. Because barbiturates may also inhibit mitochondrial respiration (41) and if so, could lead to an overestimate of Pns, we fitted the data in Fig. 1B with and without the isoelectric pentobarbital data and found negligible differences in the slope, intercept, and goodness of fit [rat data: CMRglc(ox) = 0.92 neuronal activity (NA) + 0.13, R2 = 0.97 with pentobarbital and CMRglc(ox) = 0.94 NA + 0.11, R2 = 0.93 without pentobarbital]. A fixed Pns does not necessitate a constant Ps. As shown in Fig. 1 A and B, a constant value of Ps gave an excellent fit to the data. A nonconstant Ps, most likely a decrease at higher firing rates, would have shown itself as a deviation from the best linear fit. It should be recalled that bottom-up energy budgets predict that Ps and Pns differ significantly across species (Table 1), and moreover, constancy of Ps and Pns beyond the awake state had not been tested (7, 8).
The finding of a constant Ps across activity levels suggests that energy-consuming subcellular processes of the neuropil (e.g., ion fluxes associated with action potentials, pre- and postsynaptic potentials, vesicular exocytosis/endocytosis, neurotransmitter release/uptake, etc.) are all tightly coupled, ensuring that the fidelity of impulse trafficking across a synapse is maintained independent of signaling frequency. Although the mechanisms upholding this neurometabolic linearity are not understood, the rapid rate with which surrounding astrocytic end feet clear synaptic glutamate is probably a critical component (3). The similarity of energetic efficiency with regard to electrical (Fig. 1) and chemical (Fig. 2) events across different activity levels suggests that there are physiological factors that limit the energy associated with cortical signaling (42) and that, within the normal physiological range of neuronal signaling, the electrical and chemical events are complimentary (3). Assuming a constant energetic cost of Na+ and K+ pumping, there is a direct relationship between synaptic strength (i.e., average current induced by a signaling event at a synapse, which can be increased through either induced conductivity or higher probability of presynaptic glutamate release) and energy consumption at the synapse.
The stability of Ps and Pns across species suggests that, early in evolution, the mammalian brain reached an optimum tradeoff between energy consumption and computational power at a cellular level (43). This constancy differs from most other tissues, where there is a decrease in cellular metabolic rate by a power of relative animal size (1). These findings imply that relative energy costs of fundamental features of cortical communication (e.g., action potentials, pre- and postsynaptic potentials, glial neurotransmitter and K+ clearance, etc.) as well as physiological mechanisms for supplying mitochondrial energy for neuropil operations (e.g., hemoglobin, voltage-gated ion channels, glutamate and GABA receptors, and glucose and oxygen transport) are well-preserved through evolution (17, 28, 29, 44–49). A constant interspecies ATP demand of cortical activity emphasizes the relevance of animal testing in experimental neuroscience studies. However, the approximately twofold difference in total cortical energy consumption between rats and humans may reflect limitations in blood flow in the human to supply oxygen and glucose and remove waste products and heat in relation to the number of cells that comprise each brain (50, 51).
Implications for fMRI and Resting Awake State Neuronal Activity.
fMRI has become a major tool for mapping neuronal activity in humans and animals. An important question in fMRI studies is whether the change in signal during tasks, which is produced by changes in blood flow, blood volume, and oxygen consumption, directly reflects changes in neuronal activity (52). Our finding that a constant Ps fits experimental data both within and across species supports that measurements of the oxygen consumption component of fMRI can provide a quantitative measure of changes in neuronal activity. This conclusion is consistent with experimental studies using calibrated fMRI and multiunit recordings that have found a linear relationship between the average pyramidal cell signaling and regional oxygen consumption (4, 5, 36, 37). Our findings also show that the high level of neuronal activity in the resting awake state by 13C MRS is consistent with metabolic measurements of 2DG and PET as well as electrical activity measurements (spanning from EEG to extracellular recordings) for the rat and human, supporting proposals that resting state activity be incorporated into fMRI models of brain function (53). Because these results are relating macroscopic energy measurements to microscopic electrical events, we expect that the empirically derived values of Ps and Pns can better guide bottom-up budgets of subcellular processes representing mammalian cortical function.
Materials and Methods
Tables S1 and S2 list values of neuronal activity and glucose consumption in rat and human for 11 and 7 different states, respectively, over a range of excitability (SI Text, section 1). In summary, the measured neuronal activity data were used to calculate CMRglc(ox) and then compared with measured CMRglc(ox) to determine values of Ps and Pns (Tables 1, 2, and 3). The budget is described in Calculations (SI Text, section 2).
Supplementary Material
Acknowledgments
We thank colleagues at Yale University and University of Sydney for insightful comments. This work was supported by National Institutes of Health Grants R01 MH-067528 (to F.H.), P30 NS-052519 (to F.H.), and R01 AG-034953 (to D.L.R.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1214912110/-/DCSupplemental.
See Commentary on page 3216.
References
- 1.Aiello LC, Wheeler P. The expensive-tissue hypothesis—the brain and the digestive-system in human and primate evolution. Curr Anthropol. 1995;36(2):199–221. [Google Scholar]
- 2.Sibson NR, et al. Stoichiometric coupling of brain glucose metabolism and glutamatergic neuronal activity. Proc Natl Acad Sci USA. 1998;95(1):316–321. doi: 10.1073/pnas.95.1.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hyder F, et al. Neuronal-glial glucose oxidation and glutamatergic-GABAergic function. J Cereb Blood Flow Metab. 2006;26(7):865–877. doi: 10.1038/sj.jcbfm.9600263. [DOI] [PubMed] [Google Scholar]
- 4.Smith AJ, et al. Cerebral energetics and spiking frequency: The neurophysiological basis of fMRI. Proc Natl Acad Sci USA. 2002;99(16):10765–10770. doi: 10.1073/pnas.132272199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maandag NJ, et al. Energetics of neuronal signaling and fMRI activity. Proc Natl Acad Sci USA. 2007;104(51):20546–20551. doi: 10.1073/pnas.0709515104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shulman RG, Rothman DL, Behar KL, Hyder F. Energetic basis of brain activity: Implications for neuroimaging. Trends Neurosci. 2004;27(8):489–495. doi: 10.1016/j.tins.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 7.Attwell D, Laughlin SB. An energy budget for signaling in the grey matter of the brain. J Cereb Blood Flow Metab. 2001;21(10):1133–1145. doi: 10.1097/00004647-200110000-00001. [DOI] [PubMed] [Google Scholar]
- 8.Lennie P. The cost of cortical computation. Curr Biol. 2003;13(6):493–497. doi: 10.1016/s0960-9822(03)00135-0. [DOI] [PubMed] [Google Scholar]
- 9.Armstrong-James M, Fox K. Spatiotemporal convergence and divergence in the rat S1 “barrel” cortex. J Comp Neurol. 1987;263(2):265–281. doi: 10.1002/cne.902630209. [DOI] [PubMed] [Google Scholar]
- 10.Kossut M, Hand PJ, Greenberg J, Hand CL. Single vibrissal cortical column in SI cortex of rat and its alterations in neonatal and adult vibrissa-deafferented animals: A quantitative 2DG study. J Neurophysiol. 1988;60(2):829–852. doi: 10.1152/jn.1988.60.2.829. [DOI] [PubMed] [Google Scholar]
- 11.Yang X, Hyder F, Shulman RG. Functional MRI BOLD signal coincides with electrical activity in the rat whisker barrels. Magn Reson Med. 1997;38(6):874–877. doi: 10.1002/mrm.1910380604. [DOI] [PubMed] [Google Scholar]
- 12.Buzsaki G. Rhythms of the Brain. New York: Oxford Univ Press; 2006. [Google Scholar]
- 13.Dégenètais E, Thierry AM, Glowinski J, Gioanni Y. Electrophysiological properties of pyramidal neurons in the rat prefrontal cortex: An in vivo intracellular recording study. Cereb Cortex. 2002;12(1):1–16. doi: 10.1093/cercor/12.1.1. [DOI] [PubMed] [Google Scholar]
- 14.Mishima T, Hirase H. In vivo intracellular recording suggests that gray matter astrocytes in mature cerebral cortex and hippocampus are electrophysiologically homogeneous. J Neurosci. 2010;30(8):3093–3100. doi: 10.1523/JNEUROSCI.5065-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beaulieu C. Numerical data on neocortical neurons in adult rat, with special reference to the GABA population. Brain Res. 1993;609(1–2):284–292. doi: 10.1016/0006-8993(93)90884-p. [DOI] [PubMed] [Google Scholar]
- 16.Herculano-Houzel S, Mota B, Lent R. Cellular scaling rules for rodent brains. Proc Natl Acad Sci USA. 2006;103(32):12138–12143. doi: 10.1073/pnas.0604911103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Herculano-Houzel S. Scaling of brain metabolism with a fixed energy budget per neuron: Implications for neuronal activity, plasticity and evolution. PLoS One. 2011;6(3):e17514. doi: 10.1371/journal.pone.0017514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kent CD, Domino KB. Depth of anesthesia. Curr Opin Anaesthesiol. 2009;22(6):782–787. doi: 10.1097/ACO.0b013e3283326986. [DOI] [PubMed] [Google Scholar]
- 19.la Fougère C, et al. Where in-vivo imaging meets cytoarchitectonics: The relationship between cortical thickness and neuronal density measured with high-resolution [18F]flumazenil-PET. Neuroimage. 2011;56(3):951–960. doi: 10.1016/j.neuroimage.2010.11.015. [DOI] [PubMed] [Google Scholar]
- 20.Shulman RG, Hyder F, Rothman DL. Lactate efflux and the neuroenergetic basis of brain function. NMR Biomed. 2001;14(7-8):389–396. doi: 10.1002/nbm.741. [DOI] [PubMed] [Google Scholar]
- 21.Choi IY, Lei H, Gruetter R. Effect of deep pentobarbital anesthesia on neurotransmitter metabolism in vivo: On the correlation of total glucose consumption with glutamatergic action. J Cereb Blood Flow Metab. 2002;22(11):1343–1351. doi: 10.1097/01.WCB.0000040945.89393.46. [DOI] [PubMed] [Google Scholar]
- 22.Bitterman Y, Mukamel R, Malach R, Fried I, Nelken I. Ultra-fine frequency tuning revealed in single neurons of human auditory cortex. Nature. 2008;451(7175):197–201. doi: 10.1038/nature06476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mukamel R, et al. Coupling between neuronal firing, field potentials, and FMRI in human auditory cortex. Science. 2005;309(5736):951–954. doi: 10.1126/science.1110913. [DOI] [PubMed] [Google Scholar]
- 24.Matsumoto K, Tanaka K. The role of the medial prefrontal cortex in achieving goals. Curr Opin Neurobiol. 2004;14(2):178–185. doi: 10.1016/j.conb.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 25.Shapiro HM, Greenberg JH, Reivich M, Ashmead G, Sokoloff L. Local cerebral glucose uptake in awake and halothane-anesthetized primates. Anesthesiology. 1978;48(2):97–103. doi: 10.1097/00000542-197802000-00004. [DOI] [PubMed] [Google Scholar]
- 26.Snodderly DM, Gur M. Organization of striate cortex of alert, trained monkeys (Macaca fascicularis): Ongoing activity, stimulus selectivity, and widths of receptive field activating regions. J Neurophysiol. 1995;74(5):2100–2125. doi: 10.1152/jn.1995.74.5.2100. [DOI] [PubMed] [Google Scholar]
- 27.Goldstein SR, Bak MJ, Oakley JC, Schmidt EM, Van Buren JM. An instrument for stable single cell recording from pulsating human cerebral cortex. Electroencephalogr Clin Neurophysiol. 1975;39(6):667–670. doi: 10.1016/0013-4694(75)90081-4. [DOI] [PubMed] [Google Scholar]
- 28.Williamson A, Spencer DD, Shepherd GM. Comparison between the membrane and synaptic properties of human and rodent dentate granule cells. Brain Res. 1993;622(1–2):194–202. doi: 10.1016/0006-8993(93)90819-9. [DOI] [PubMed] [Google Scholar]
- 29.Yu Y, Hill AP, McCormick DA. Warm body temperature facilitates energy efficient cortical action potentials. PLoS Comput Biol. 2012;8(4):e1002456. doi: 10.1371/journal.pcbi.1002456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Clark BA, Mobbs P. Voltage-gated currents in rabbit retinal astrocytes. Eur J Neurosci. 1994;6(9):1406–1414. doi: 10.1111/j.1460-9568.1994.tb01002.x. [DOI] [PubMed] [Google Scholar]
- 31.Hutcheon B, Miura RM, Puil E. Models of subthreshold membrane resonance in neocortical neurons. J Neurophysiol. 1996;76(2):698–714. doi: 10.1152/jn.1996.76.2.698. [DOI] [PubMed] [Google Scholar]
- 32.Braitenberg V, Schüz A. Cortex: Statistics and Geometry of Neuronal Connectivity. 2nd Ed. Berlin: Springer; 1998. [Google Scholar]
- 33.Zhu XH, et al. Quantitative imaging of energy expenditure in human brain. Neuroimage. 2012;60(4):2107–2117. doi: 10.1016/j.neuroimage.2012.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hirase H, Qian L, Barthó P, Buzsáki G. Calcium dynamics of cortical astrocytic networks in vivo. PLoS Biol. 2004;2(4):E96. doi: 10.1371/journal.pbio.0020096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dombeck DA, Khabbaz AN, Collman F, Adelman TL, Tank DW. Imaging large-scale neural activity with cellular resolution in awake, mobile mice. Neuron. 2007;56(1):43–57. doi: 10.1016/j.neuron.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sanganahalli BG, Herman P, Blumenfeld H, Hyder F. Oxidative neuroenergetics in event-related paradigms. J Neurosci. 2009;29(6):1707–1718. doi: 10.1523/JNEUROSCI.5549-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Herman P, Sanganahalli BG, Blumenfeld H, Hyder F. Cerebral oxygen demand for short-lived and steady-state events. J Neurochem. 2009;109(Suppl 1):73–79. doi: 10.1111/j.1471-4159.2009.05844.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shu Y, Hasenstaub A, Duque A, Yu Y, McCormick DA. Modulation of intracortical synaptic potentials by presynaptic somatic membrane potential. Nature. 2006;441(7094):761–765. doi: 10.1038/nature04720. [DOI] [PubMed] [Google Scholar]
- 39.Patel AB, et al. The contribution of GABA to glutamate/glutamine cycling and energy metabolism in the rat cortex in vivo. Proc Natl Acad Sci USA. 2005;102(15):5588–5593. doi: 10.1073/pnas.0501703102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Karbowski J. Global and regional brain metabolic scaling and its functional consequences. BMC Biol. 2007;5:18. doi: 10.1186/1741-7007-5-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Anderson CM, et al. Barbiturates induce mitochondrial depolarization and potentiate excitotoxic neuronal death. J Neurosci. 2002;22(21):9203–9209. doi: 10.1523/JNEUROSCI.22-21-09203.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Niven JE, Laughlin SB. Energy limitation as a selective pressure on the evolution of sensory systems. J Exp Biol. 2008;211(Pt 11):1792–1804. doi: 10.1242/jeb.017574. [DOI] [PubMed] [Google Scholar]
- 43.Laughlin SB, Sejnowski TJ. Communication in neuronal networks. Science. 2003;301(5641):1870–1874. doi: 10.1126/science.1089662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Storz JF. Hemoglobin function and physiological adaptation to hypoxia in high-altitude mammals. J Mammal. 2007;88(1):24–31. [Google Scholar]
- 45.Vacher H, Mohapatra DP, Trimmer JS. Localization and targeting of voltage-dependent ion channels in mammalian central neurons. Physiol Rev. 2008;88(4):1407–1447. doi: 10.1152/physrev.00002.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dingledine R, Borges K, Bowie D, Traynelis SF. The glutamate receptor ion channels. Pharmacol Rev. 1999;51(1):7–61. [PubMed] [Google Scholar]
- 47.Young AB, Chu D. Distribution of Gaba-A and Gaba-B receptors in mammalian brain—potential targets for drug development. Drug Dev Res. 1990;21(3):161–167. [Google Scholar]
- 48.Pessin JE, Bell GI. Mammalian facilitative glucose transporter family: Structure and molecular regulation. Annu Rev Physiol. 1992;54:911–930. doi: 10.1146/annurev.ph.54.030192.004403. [DOI] [PubMed] [Google Scholar]
- 49.Hyder F, Shulman RG, Rothman DL. A model for the regulation of cerebral oxygen delivery. J Appl Physiol. 1998;85(2):554–564. doi: 10.1152/jappl.1998.85.2.554. [DOI] [PubMed] [Google Scholar]
- 50.Trübel HK, Sacolick LI, Hyder F. Regional temperature changes in the brain during somatosensory stimulation. J Cereb Blood Flow Metab. 2006;26(1):68–78. doi: 10.1038/sj.jcbfm.9600164. [DOI] [PubMed] [Google Scholar]
- 51.Rango M, Arighi A, Bresolin N. Brain temperature: What do we know? Neuroreport. 2012;23(8):483–487. doi: 10.1097/WNR.0b013e3283534a60. [DOI] [PubMed] [Google Scholar]
- 52.Hyder F. Neuroimaging with calibrated FMRI. Stroke. 2004;35(11 Suppl 1):2635–2641. doi: 10.1161/01.STR.0000143324.31408.db. [DOI] [PubMed] [Google Scholar]
- 53.Shulman RG, Hyder F, Rothman DL. Baseline brain energy supports the state of consciousness. Proc Natl Acad Sci USA. 2009;106(27):11096–11101. doi: 10.1073/pnas.0903941106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.