Abstract
The refractoriness of acute promyelocytic leukemia (APL) with t(11;17)(q23;q21) to all-trans retinoic acid (ATRA)-based therapy concerns clinicians and intrigues basic researchers. By using a murine leukemic model carrying both promyelocytic leukemia zinc finger/retinoic acid receptor-α (PLZF/RARα) and RARα/PLZF fusion genes, we discovered that 8-chlorophenylthio adenosine-3′, 5′-cyclic monophosphate (8-CPT-cAMP) enhances cellular differentiation and improves gene trans-activation by ATRA in leukemic blasts. Mechanistically, in combination with ATRA, 8-CPT-cAMP activates PKA, causing phosphorylation of PLZF/RARα at Ser765 and resulting in increased dissociation of the silencing mediator for retinoic acid and thyroid hormone receptors/nuclear receptor corepressor from PLZF/RARα. This process results in changes of local chromatin and transcriptional reactivation of the retinoic acid pathway in leukemic cells. Meanwhile, 8-CPT-cAMP also potentiated ATRA-induced degradation of PLZF/RARα through its Ser765 phosphorylation. In vivo treatment of the t(11;17) APL mouse model demonstrated that 8-CPT-cAMP could significantly improve the therapeutic effect of ATRA by targeting a leukemia-initiating cell activity. This combined therapy, which induces enhanced differentiation and oncoprotein degradation, may benefit t(11;17) APL patients.
Keywords: leukemogenesis, PKA pathway, PML/RARα, SMRT, NcoR1
Acute promyelocytic leukemia (APL) comprises up to 10–15% of acute myeloid leukemia (AML), and its unique morphological, cytogenetic and clinical features make it a distinct AML subtype (1). The majority of these patients carry the hallmark t(15;17)(q22;q21) chromosomal translocation, which gives rise to the promyelocytic leukemia/retinoic acid receptor-α (PML/RARα) fusion gene. These leukemic cells undergo induced terminal differentiation both in vitro and in vivo upon treatment of all-trans retinoic acid (ATRA), a natural pan-retinoic acid-receptor agonist. Modern therapeutic modalities integrating ATRA in both remission-induction and postremission stages have greatly improved the 5-y survival of these patients up to 90% or even higher (2). In contrast, the 1–2% of APL patients, with t(11;17)(q23;q21) translocation that fuses the promyelocytic leukemia zinc finger (PLZF) to the RARα gene, respond poorly to ATRA treatment. In addition, these APL patients are refractory to concurrent cytotoxic chemotherapy and generally have poor outcomes (3). These features make t(11;17) APL a distinct variant from typical APL. Effective therapeutic regimens for this subtype of APL are imperatively needed.
There have been sporadic case-reports documenting successful differentiation of t(11;17) APL using ATRA in combination with other differentiation inducers, highlighting the possibility and significance of reversing retinoic acid (RA) resistance in the treatment of this peculiar leukemia (4, 5). However, combinatorial differentiation inducers are usually chosen on an empirical basis, and their therapeutic mechanisms are still mostly undefined. This choice can be largely attributed to the scarcity of clinical samples and lack of a suitable disease model.
To facilitate pathophysiological mechanistic studies and the therapeutic exploration of APL with the PLZF/RARα fusion gene, we established a murine leukemic model with both PLZF/RARα and RARα/PLZF fusions expressed in myeloid cells by transgene technology (6). This t(11;17) APL model largely recapitulated the phenotype of human disease. We and others have previously shown that cAMP and the PKA pathway plays important roles in drug-induced APL cell differentiation (7–9). We found that 8-chlorophenylthio adenosine-3′, 5′-cyclic monophosphate (8-CPT-cAMP) could enhance the therapeutic efficacy of ATRA through both induction of differentiation and enhanced PLZF/RARα protein degradation. These activities significantly extended the survival of leukemic mice, raising hopes that they could be used in patients.
Results
Combined Treatment with 8-CPT-cAMP and ATRA Induces PLZF/RARα APL Cell Differentiation.
We and others have shown that transgenic mice expressing PLZF/RARα under the control of a minigene cassette derived from the human Cathepsin G gene promoter develop chronic myeloid leukemia-like phenotypes (10, 11). We subsequently generated transgenic mice expressing both PLZF/RARα and RARα/PLZF fusion genes. The double-transgenic (DT) mice developed myeloid leukemia resembling the human t(11;17) APL as regards accumulation of immature myeloblastic cells in hematopoietic tissues, impairment of normal hematopoiesis, and infiltration of nonhematopoietic organs, including liver, lung, gastrointestinal tract, and kidney (6). These APL can be transplanted to syngeneic recipient mice by transferring leukemic blasts from DT mice bone marrow (BM) or spleen (Fig. S1 A–D). As few as 104 unselected nucleated BM cells could invariably induce overt disease in sublethally irradiated recipient mice (Fig. S1E). Leukemic blasts could be morphologically traced in BM and spleen of recipient mice 10 d posttransplantation (Fig. S1F). A cDNA microarray analysis of CD34+ BM cells from both PLZF/RARα (three PLZF-RARα and two PLZF/RARα–RARα/PLZF animals) as well as PML/RARα (four animals) transgenic mice with leukemia revealed distinct gene-expression patterns (Fig. S2), consistent with the distinct nature of the driving fusions.
To identify compounds that might synergize with ATRA to induce APL cell differentiation, we screened several potential differentiation inducers with ATRA, using a nitroblue tetrazolium (NBT) reduction assay of primary BM blasts as an endpoint. We observed a remarkable synergy between 8-CPT-cAMP and ATRA for cellular differentiation (Fig. S3A) (P = 0.0003). Morphological analysis of the leukemic cells confirmed enhanced cellular differentiation by these two drugs (Fig. S3B).
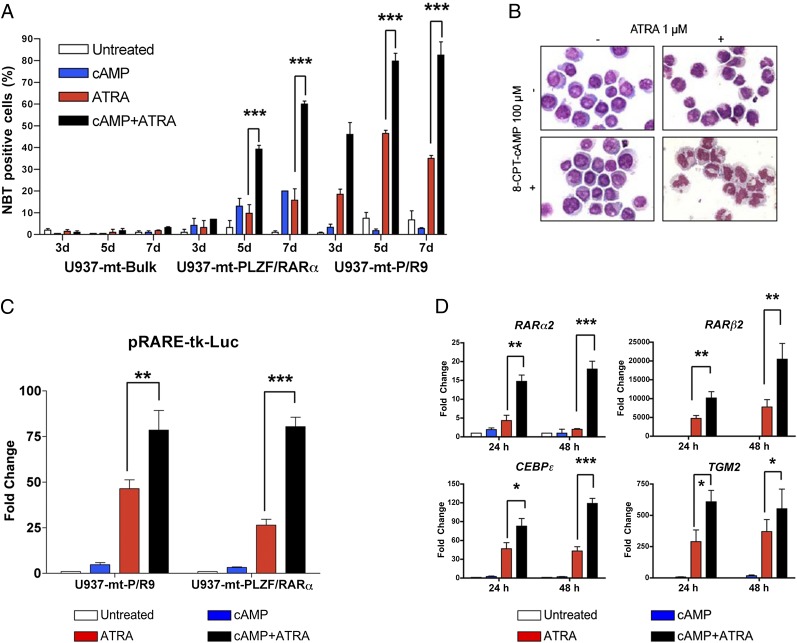
To validate this cross-talk in a human leukemic system, we used U937 cell sublines harboring stably transfected, conditionally inducible fusion genes (U937-mt-PLZF/RARα and U937-mt-P/R9) and their parental control (U937-mt-Bulk) (12) (Fig. S3C). Using the NBT reduction assay, we confirmed that U937-mt-PLZF/RARα cells are quite resistant to ATRA-triggered differentiation, but efficiently mature when 8-CPT-cAMP is added. The quantitative extent of leukemic cell maturation represented by the percentage of NBT reduction-positive cells (Fig. 1A) (39.3 ± 1.8% and 60.0 ± 1.4% at 5 and 7 d, P = 0.0003 and P = 0.0002, respectively) was equivalent to that of PML/RARα-expressing U937-mt-P/R9 cells upon ATRA treatment. Moreover, morphological analysis of U937-mt-PLZF/RARα cells further validated enhanced differentiation induction effect of combined treatment (Fig. 1B), as did FACS analysis of CD11b and CD11c expression (Fig. S3 E and F).
Fig. 1.
The 8-CPT-cAMP/ATRA combined treatment induces APL cells differentiation. (A) NBT reduction assays of three U937 cell strains posttreatment of 8-CPT-cAMP (100 μM) and/or ATRA (1 μM) for 3, 5, and 7 d, respectively. (B) Morphological analysis of U937-mt-PLZF/RARα cells after 8-CPT-cAMP (100 μM) and/or ATRA (1 μM) treatment for 7 d (magnification: 1,000×). (C) Activities of pRARE-tk-Luc reporter transfected in both U937-mt-P/R9 and U937-mt-PLZF/RARα cells were detected after 8-CPT-cAMP (100 μM) and/or ATRA (1 μM) treatment for 24 h. (D) Real-time RT-PCR quantified the mRNA expression of RARα2, RARβ2, CEBPɛ, and TGM2 in U937-mt-PLZF/RARα cells after 8-CPT-cAMP (100 μM) and/or ATRA (1 μM) treatment for 24 and 48 h, respectively. Results were presented as mean ± SD of three independent experiments. Briefly, *P < 0.05, **P < 0.01, and ***P < 0.001 in comparison.
Next, by using a retinoid-responsive luciferase reporter (pRARE-tk-luc), we found that 1 μM ATRA modestly activated RA-response in U937-mt-PLZF/RARα cells, but the combination of 8-CPT-cAMP and ATRA dramatically increased reporter activation (Fig. 1C) (P = 0.0001). U937-mt-P/R9 cells were significantly more responsive to ATRA treatment (P = 0.009) and also superactivated by coexposure to 8-CPT-cAMP. Collectively, transcriptional response to 8-CPT-cAMP and ATRA in PLZF/RARα- or PML/RARα-expressing leukemic cells mirrors differentiation (Fig. 1A). We then investigated in U937-mt-PLZF/RARα cells the effect of combined treatment in well-known RA target genes, RARα2, RARβ2, CEBPɛ, and TGM2. Real-time PCR and semiquantitative RT-PCR again demonstrated that 8-CPT-cAMP significantly enhanced the transactivating effect of ATRA (Fig. 1D and Fig. S3D), in line with the results with artificial reporters.
Silencing Mediator for RA and Thyroid Hormone Receptor Dissociation from PLZF/RARα Through Ser765 Phosphorylation by PKA Is Facilitated by 8-CPT-cAMP.
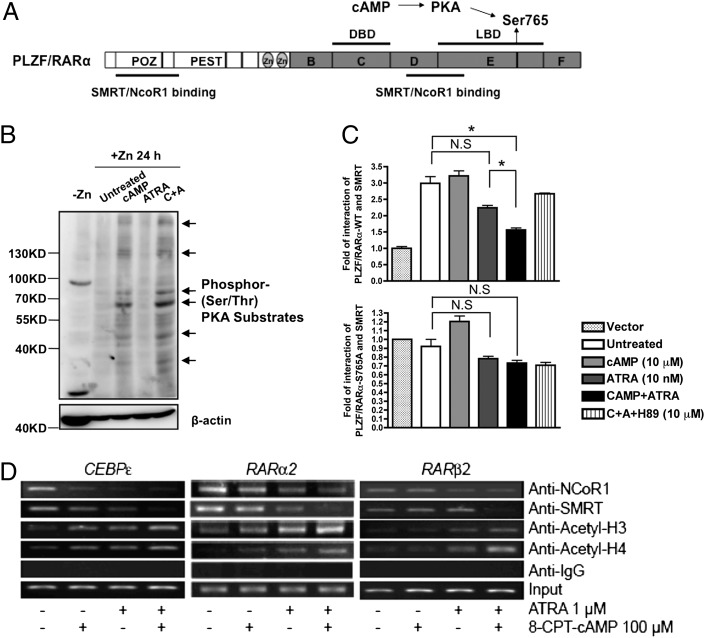
8-CPT-cAMP activates the PKA pathway and the latter targets RARα (Fig. 2A), prompting us to examine the role of PKA pathway activation in ATRA and 8-CPT-cAMP synergy. Serine/threonine-specific phosphorylation was enhanced in U937-mt-PLZF/RARα cells treated with 8-CPT-cAMP (Fig. 2B) and cAMP-response element binding protein (13) became phosphorylated on Ser133 upon 8-CPT-cAMP treatment; both were further enhanced by ATRA (Fig. S4A).
Fig. 2.
8-CPT-cAMP facilitates SMRT disassociation from PLZF/RARα through its Ser765 phosphorylation by activating the PKA pathway. (A) Schematic illustration of PLZF/RARα protein structure. Several potential sites were denoted in the corresponding domains of the fusion protein, and possible upstream regulators were also annotated aside. (B) U937-mt-PLZF/RARα cells were exposed to 100 μM ZnSO4 for 12 h before treatment with 8-CPT-cAMP (100 μM) and/or ATRA (1 μM) for additional 24 h. Serine/threonine-specific phosphorylation level of PKA downstream substrates was assessed by Western blot (Upper). Arrows indicate the bands specific to the antibodies. Equal loading of protein was assessed with β-actin (Lower). (C) Mammalian two-hybrid analysis of PLZF/RARα-WT (Upper) or S765A mutant (Lower) with SMRT was done when exposed to different drug treatments. COS-7 cells were transiently transfected with plasmids, as indicated in SI Materials and Methods for 18 hours, and then 8-CPT-cAMP and/or ATRA were added for additional 6 hours. (D) ChIP assays in U937-mt-PLZF/RARα cells treated with indicated agents for 24 h. Briefly, *P < 0.05; N.S. refers to “not significant” in comparison by Student t test.
The cAMP/PKA pathway phosphorylates RARα at Ser369 (14). To test the potential impact of this phosphorylation on PLZF/RARα-mediated transcriptional regulation, we constructed a mutant S765A in its RARα moiety (equivalent to Ser369 of RARα). Then, we examined their affinity to silencing mediator for RA and thyroid hormone receptor/nuclear receptor corepressor (SMRT/NcoR1) in mammalian two-hybrid experiments in COS-7 cells. As shown in Fig. 2C, Upper and Fig. S4B, coexpression of PLZF/RARα-WT and SMRT or NCoR1 could remarkably increase the transcriptional activities of pGal4-(RE)5-tk-Luc reporter in COS-7 cells, indicating a direct association between PLZF/RARα and SMRT or NCoR1 proteins. ATRA treatment (10 nM) had minor effects on the association of PLZF/RARα and SMRT, whereas the addition of 8-CPT-cAMP with ATRA significantly loosened SMRT binding to PLZF/RARα (P = 0.019) (Fig. 2C, Upper). Notably, this was reverted by 10 μM H89, a potent selective PKA inhibitor. Of note, the PLZF/RARα-S765A mutant itself failed to bind SMRT (Fig. 2C, Lower), suggesting that Ser765 is essential for both corepressor interaction and 8-CPT-cAMP response. Similar results were found when analyzing the binding ability of PLZF/RARα to NcoR1 regarding the 8-CPT-cAMP response, although the interaction pattern between PLZF/RARα and NcoR1 was different from that between PLZF/RARα and SMRT (Fig. S4B).
Using ChIP assays, we tested if this combined treatment could affect the amount of corepressors recruited to regulatory regions of RA responsive genes, presumably by PLZF/RARα oncoprotein. As shown in Fig. 2D, ATRA treatment mildly affected the association of SMRT protein with retinoic acid response element (RARE)-containing promoter regions of RARα2, RARβ2, and CEBPɛ in U937-mt-PLZF/RARα cells. Nevertheless, the combined treatment greatly enhanced the dissociation of SMRT from the promoter regions of these genes. For NCoR1, treatment with ATRA decreased its promoter binding, but addition of 8-CPT-cAMP did not increase corepressor release. The acetylation levels of histone 3/4 were significantly increased under drug combination compared with single-drug treatment (Fig. 2D), consistent with enhanced transcriptional activations.
To investigate the mechanism of enhanced cell differentiation induced by 8-CPT-cAMP and ATRA in PLZF/RARα cells, we compared the dynamic interaction status of the same gene locus, a RARE region of RARβ2, between U937-mt-PLZF/RARα cells and U937-mt-P/R9 cells (Fig. S4C). Consistent with previous observations, ATRA alone was enough to activate RARβ2 gene by elevating acetylated histone 3/4, increasing trimethylated H3K4Me3, and decreasing trimethylated H3K27Me3 in U937-mt-P/R9 cells; 8-CPT-cAMP could not further enhance these effects. In contrast, in U937-mt-PLZF/RARα cells, 8-CPT-cAMP potentiated the ATRA-induced increment in acetylated histone 3 and trimethylated H3K4Me3, and contrary to its effect in PML/RARα-expressing cells, increased trimethylated H3K27Me3.
ATRA-Induced Degradation of PLZF/RARα Protein Through Ser765 Phosphorylation Is Enhanced by 8-CPT-cAMP.
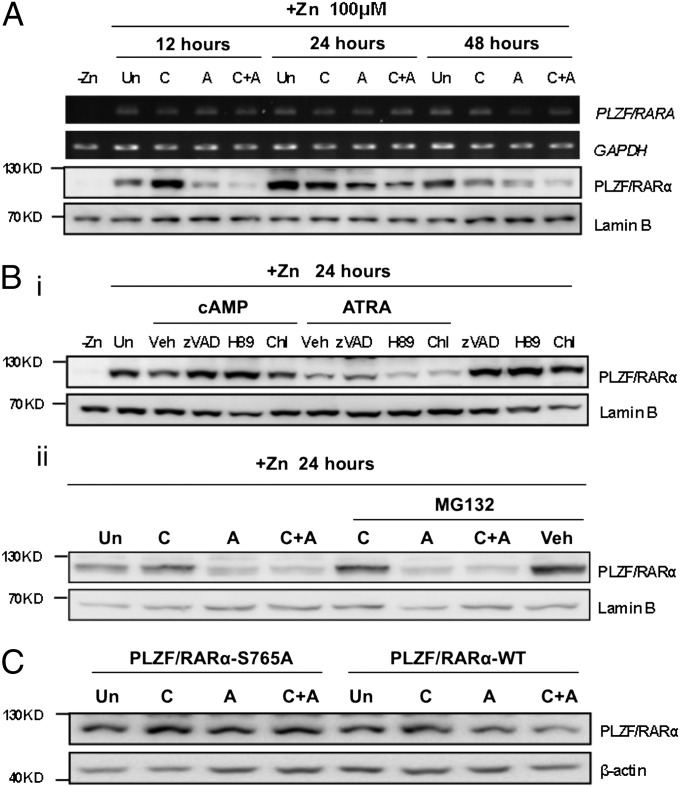
Phosphodiesterase inhibitors elevate endogenous cAMP and facilitate ATRA-induced PML/RARα degradation through Ser873 phosphorylation (15). PLZF/RARα levels were decreased by treatment with 8-CPT-cAMP, ATRA, as well as both at most of the time points analyzed (Fig. 3A). PLZF/RARα mRNA expression was unchanged in these conditions. We then used inhibitors of degradation pathways, including caspases (z-VAD-fmk), lysosome (chloroquine), proteasome (MG132), and PKA (H89). A pan-caspase inhibitor, z-VAD-fmk, but not the MG132 proteasome inhibitor, reverted PLZF/RARα degradation by both 8-CPT-cAMP and ATRA, although H89 selectively inhibited 8-CPT-cAMP–induced PLZF/RARα degradation (Fig. 3B), suggesting that PKA activation favors the turnover of PLZF/RARα. To confirm this hypothesis, we overexpressed either wild-type or mutant PLZF/RARα by transient transfection in 293T cells before 24-h treatment with 8-CPT-cAMP and/or ATRA (Fig. 3C). As expected, absence of S765 phosphorylation impeded ATRA-induced PLZF/RARα protein degradation. Taken together, these data show that 8-CPT-cAMP enhances ATRA-induced degradation of PLZF/RARα proteins through S765 phosphorylation.
Fig. 3.
8-CPT-cAMP enhances ATRA-induced degradation of PLZF/RARα proteins through its Ser765 phosphorylation by PKA pathway activation. (A) A time course of PLZF/RARα expression in U937-mt-PLZF/RARα cells was determined at both mRNA and protein level after 8-CPT-cAMP and/or ATRA treatment. The cells were initially induced by 100 μM ZnSO4 before treatment with 8-CPT-cAMP (100 μM) and/or ATRA (1 μM) for 12, 24, and 48 h, respectively. Nuclear proteins were extracted and detected by anti-RARα antibody. (B) Zn2+ induced-U937-mt-PLZF/RARα cells were preincubated with pan-caspases inhibitor z-VAD-fmk (40 μM), PKA pathway inhibitor H89 (10 μM), lysosome inhibitor chloroquine (100 μM), proteasome inhibitor MG132 (1 μM) for 2 h, and then treated with 8-CPT-cAMP (100 μM) and/or ATRA (1 μM) for an additional 24 h. PLZF/RARα and Lamin B proteins were examined by Western blot. (C) 293T cells transiently expressing pVP16-PLZF/RARα-WT and S765A proteins were treated by 8-CPT-cAMP (100 μM) and/or ATRA (1 μM) for 24 h. Both wild-type and mutant PLZF/RARα were immunoblotted by anti-RARα antibody. Equal loading was assessed by Lamin B or β-actin expression. Abbreviations: +/− Zn, with or without ZnSO4; Un, untreated; C, 8-CPT-cAMP; A, ATRA; zVAD, z-VAD-fmk; Chl, chloroquine; Veh, vehicle.
In Vivo Combination Treatment with 8-CPT-cAMP and ATRA Improves the Survival of t(11;17) APL Mice.
To assess the in vivo relevance of this 8-CPT-cAMP/ATRA synergy, we treated t(11;17) APL transplantable mouse model with 8-CPT-cAMP and ATRA. First, we conducted a preliminary short-term experiment (Fig. S5A). The mice treated with the combination showed significantly decreased spleen index (Fig. S5B). Moreover, PLZF/RARα expression was sharply decreased in BM cells (Fig. S5C), likely reflecting both clearance of APL blasts and the fact that these two drugs induced degradation of PLZF/RARα in ex vivo treatment (Fig. S5D). FACS analysis of BM cells further confirmed that normal hematopoiesis was partially restored upon combined treatment (Fig. S5E). Pathological analysis, moreover, demonstrated greatly reduced leukemic cell infiltration in both the liver and spleen in mice that received combined treatment (Fig. S5F), suggestive of a differentiation-enhancing and antiproliferative potency of this combined therapy.
Next, we attempted to evaluate the long-term effect on t(11;17) APL leukemia-initiating cells (LIC) in the transplantable model. We first defined the LICs by isolating the leukemic BM cell populations with an appropriate combination of cell surface markers. Using a stepwise sorting strategy and limited-dilution assay, we characterized cell populations with the strongest leukemia-initiating ability in vivo (for details, see Fig. S6 and Table S1). Myeloid-lineage committed cells (Mac-1+/Gr-1+/c-Kit+) had more primitive features, such as round-oval nucleus with a high nuclear-cytoplasmic ratio, expressed higher levels of PLZF/RARα, and were highly clonogenic (Fig. S6F). In contrast, cells from the Mac-1+/Gr-1+/c-Kit− fraction had a mature myeloid appearance with lower PLZF/RARα expression (Fig. S6 B–E) and were poorly clonogenic (Fig. S6F).
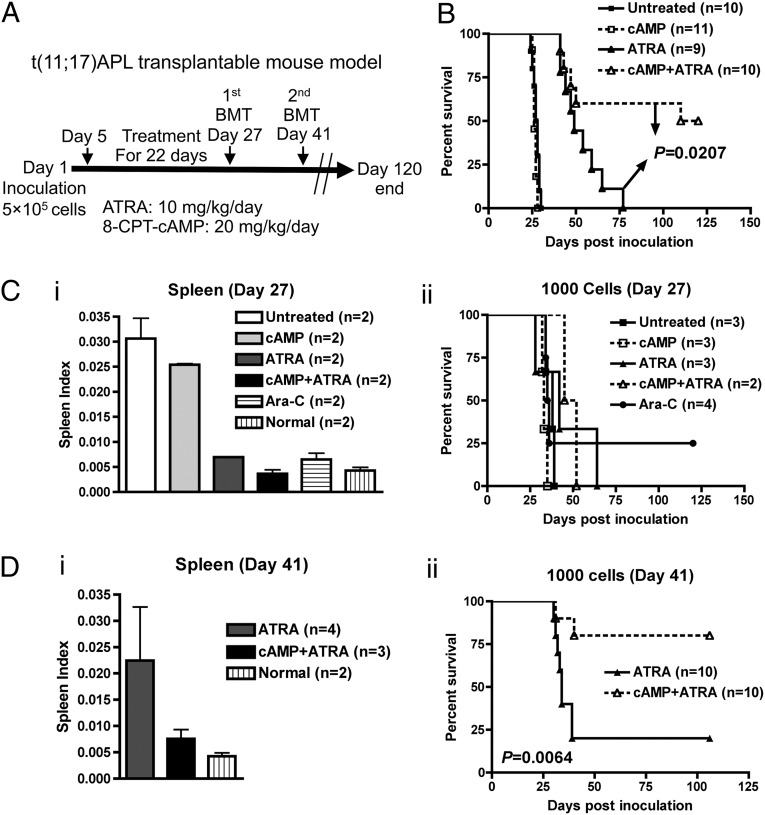
Next, sublethally irradiated animals inoculated with 5 × 105 transplantable t(11;17) APL cells were treated with 8-CPT-cAMP (20 mg⋅kg⋅d) and ATRA (10 mg⋅kg⋅d) for 22 d beginning on day 5 postinoculation (Fig. 4A). As shown in Fig. 4B, within a 120-d observation, those treated with the 8-CPT-cAMP/ATRA combination showed a significantly prolonged survival time compared with those treated with ATRA-alone (median 115 d vs. 52 d, P = 0.0207), demonstrating that the 8-CPT-cAMP/ATRA synergy for differentiation and PLZF/RARα degradation could be translated into a sharp survival benefit.
Fig. 4.
In vivo 8-CPT-cAMP/ATRA combined treatment improves the survival of t(11;17) APL mice by targeting of LICs. (A) Scheme of the long-term drug treatment in vivo and secondary BM transplantations (BMT). (B) Kaplan–Meier survival curve of primary mice under different drug treatments. (C) Spleen index of primary mice on day 27 (i) and the survival curve of their secondary recipients inoculated with 1,000 LICs (Mac-1+/Gr-1+/c-Kit+) each (ii). (D) Spleen index of primary mice on day 41 (i) and the survival curve of their secondary recipients inoculated with 1,000 LICs (Mac-1+/Gr-1+/c-Kit+) each (ii).
As shown in the treatment scheme (Fig. 4A), sorted BM cells (Mac-1+/Gr-1+/c-Kit+) were transplanted to secondary recipients either immediately after the termination of treatment (day 27) or 2 wk after the termination of treatment (day 41). In the day 27 transplant recipients, no significant differences were found in survival between the ATRA-alone and combined therapy groups (Fig. 4 C, ii). On day 41, only two groups of primary mice survived (ATRA alone and in combination). Although there was no statistical difference in spleen index between these two groups (Fig. 4 D, i), both the frequency and absolute numbers of the LIC-containing subpopulation were significantly reduced by combined treatment (Table S2). During nearly 100-d follow-up, we found that mice receiving combined therapy showed a significantly prolonged survival time in contrast to ATRA alone, especially at 1,000-cell dose (Fig. 4 D, ii) (P = 0.006). Of note, two mice were additionally treated with a standard dose of Ara-C (250 mg⋅kg⋅d) for 5 d, which produced a good response (Fig. 4 C, i) but was not as efficient as combined 8-CPT-cAMP/ATRA therapy in eliminating LICs (Table S2).
In an independently derived DT model previously described (15), a shorter (10 d) treatment initiated 7 d after APL inoculation, sharply prolonged survival and exhibited a clear-cut synergy between 8-CPT-cAMP and ATRA, which normalized spleen weight but was not sufficient to eradicate the disease (Fig. S7 A, i and ii). In fact, BM transplantation experiments in secondary recipients to assess the effect of treatment on LIC activity demonstrated a clear loss of clonogenic activity after 8 d of combined treatment, particularly with the highest dose of ATRA (Fig. S7 A, iii). In contrast, 5-Aza-2-Deoxycytidine (DAC), an inhibitor of DNA methylation that had little effect on its own, was dramatically synergistic with ATRA to clear the disease, as assessed by spleen weight and the DAC/ATRA combination could actually even eradicate leukemia-initiating activity in secondary recipients (Fig. S7B).
Collectively, these data suggest that the ATRA/8-CPT-cAMP combination favors survival by targeting t(11;17) APL LICs in vivo.
Discussion
Here we used a unique model of APL with PLZF/RARα and RARα/PLZF fusion genes (6). We demonstrate that ATRA triggers both differentiation and PLZF/RARα degradation, both of which are sharply enhanced by PKA activation. It was previously reported that the c-Myc gene is a direct target up-regulated by PLZF/RARα oncoprotein by dominant-negative inhibition of wild-type PLZF (16). We indeed found clues of c-Myc deregulation in our microarray of CD34+ leukemia cells from t(11;17) APL mice (Fig. S2). Full-length PLZF can transcriptionally repress c-Kit expression in CD34+ cells, similar to Myc (17). Reversal of c-Kit repression by PLZF/RARα or RARα/PLZF could account for elevated KIT expression in PLZF/RARα leukemia cells, possibly contributing to their immature features. Enrichment of LICs within the Mac-1+/Gr-1+/c-Kit+ population could illustrate a hierarchical structure within the leukemia. Such disordered immunophenotype of the leukemia blasts is distinct from that of hematopoietic stem cell-like LIC in BCR/ABL+ chronic myeloid leukemia (18). Nevertheless, our results are consistent with PML/RARα-positive APL, where a population of committed myeloid cells (Gr-1int/c-Kit+/FcγRIII/II+/CD34+) was identified as the LIC compartment in different t(15;17) APL models (19, 20). These LICs morphologically resemble our LIC-enriched cell, reflecting possible common origin and oncogenic mechanism.
Using this model of t(11;17) APL, we found that 8-CPT-cAMP could significantly enhance both the transcriptional activity and cellular differentiation effects of ATRA both ex vivo and in vivo. In APL, ATRA target genes are repressed by PML/RARα and PLZF/RARα through recruitment of a transcriptional corepressor complex containing histone deacetylases (HDACs) (21). The PLZF moiety of PLZF/RARα can also independently recruit SMRT/NCoR1 through its N-terminal POZ/BTB domain (22, 23). Although the effects of various HDAC inhibitors have been tested in APL with t(11;17) and other AMLs, such as those with t(8;21), these agents induce apoptosis rather than differentiation of leukemic blasts (24). In our experiments, addition of 8-CPT-cAMP to ATRA specifically increased acetylation levels of histone 3/4 in ATRA-regulated genes and also enhanced dissociation of SMRT from PLZF/RARα fusion protein. Thus, 8-CPT-cAMP–triggered PKA signaling (25) may cross-talk with the RA pathway and modulate the differentiation of APL cells. PKA-enhanced leukemia differentiation may reflect the “desubordination” of RXRα over RARα by inducing corepressor release from the RARα component of RXR-RARα heterodimers (26). It was also reported that RARα Ser369 phosphorylated by PKA facilitates Ser77 phosphorylation by CDK7/CyclinH complex, which then further potentiates RARα’s transcription ability (14). Indeed, we provide evidence that in the presence of ATRA, PKA-induced Ser765 phosphorylation in PLZF/RARα decreases SMRT/NcoR1 affinity to the fusion protein, thereby facilitating ATRA-induced transactivation.
Besides the effect on transcriptional regulation, substrate phosphorylation by PKA can also affect RARα metabolism. In t(15;17) APL, phosphorylation of PML/RARα Ser873 by PKA led to PML/RARα degradation and, eventually, to eradication of LICs in vivo (15, 27). Similarly, our results demonstrate that 8-CPT-cAMP and ATRA sharply reduce PLZF/RARα levels, contributing to clearance of t(11;17) APL. The primary mechanisms proposed to be implicated in APL response to ATRA are cell differentiation and oncoprotein degradation (28, 29). Enforced PML/RARα or PLZF/RARα through ATRA binding facilitates granulocyte maturation of myeloid cell lines (30, 31). Accordingly, although standard-dose ATRA can induce complete differentiation of APL cells with partial degradation of PML/RARα protein, it fails to eradicate LICs (2, 15). By elevating the intracellular concentration of ATRA, the application of high-dose ATRA or liposomal ATRA results in full PML/RARα degradation and LIC clearance (9, 32, 33). Similarly, ATO, cAMP, or phosphodiesterase inhibitors can also clear t(15;17) APL LICs via complete degradation of the PML/RARα protein (15). Here, we provide evidence that combined therapy of 8-CPT-cAMP and ATRA can induce enhanced differentiation and oncoprotein degradation in this model, and raise hopes for better clinical management of t(11;17) APL.
Materials and Methods
Mouse Model and in Vivo Drug Treatment.
Six- to eight-wk-old female C57BL/6J mice were preconditioned with high-energy γ-ray irradiation at a sublethal dose of 4.0 Gy at the day before leukemic cells inoculation. Nucleated cells collected from spleens of mice with full-blown leukemia were injected into each recipient mouse via tail vein to establish a transplantable leukemia mouse model. For in vivo drug treatment, ATRA was administrated through oral gavage at the dosage of 10 or 20 mg⋅kg⋅d. 8-CPT-cAMP was injected intravenously at the dosage of 20 or 40 mg⋅kg⋅d. Mice of the untreated group received mock treatment of placebo. For the Ara-C treatment cohort, 250 mg⋅kg⋅d Ara-C was injected intravenously for 5 d. All the animal experiments were approved by The Animal Care & Welfare Committee of Shanghai Jiao Tong University School of Medicine.
Identification of t(11;17) APL LIC.
For t(11;17) APL LICs sorting, 2 × 106 APL BM blasts were stained with anti-mouse Mac-1-FITC, Gr-1-PerCP-cy5.5, and c-Kit-APC antibodies together and sorted by a MoFlo high-speed cell sorter (Dako-Cytomation). Then, each population was diluted to three different cell doses: 1,000 cells, 200 cells, and 50 cells supplemented with congenic normal spleen cells. After that procedure, sorted cells were intravenously injected into sublethally irradiated secondary recipients. A long-term follow-up study was performed to record disease incidence of secondary transplants.
Microarray Analysis, Immunophenotyping, Cytochemistry, and Histology.
Details on analysis of microarray, immunophenotype, cytochemistry and histology can be found in SI Materials and Methods.
Luciferase Reporter Analysis and Mammalian Two-Hybrid Analysis.
Luciferase reporter analysis and mammalian two-hybrid analysis were performed as in SI Materials and Methods.
ChIP, RT-PCR, and Western Blot.
ChIP, RT-PCR, and Western Blot assays were done as described in SI Materials and Methods.
Statistical Analysis.
Graphpad Prism 4 software was used for statistical data analysis in this study. L-Calc software (v1.1, StemCell Technologies) was used to calculate and compare the LIC frequencies. Student t test was exploited for mean comparison, and the Kaplan–Meier method was used for survival analysis. Statistical significance threshold was set at 0.05. Briefly, *P < 0.05, **P < 0.01, and ***P < 0.001 in comparison; N.S. refers to “not significant.”
Supplementary Material
Acknowledgments
This work was supported by a Chinese National Key Basic Research Project (973, 2013CB966800, 2010CB529200), Mega-Projects of Science Research for the 12th Five-Year Plan (2013ZX09303302), Special Research Fund of Ministry of Health (201202003), National High Tech Program for Biotechnology Grant 863 (2012AA02A505), National Natural Science Foundation of China (30830119, 81170506, 81270624), and the Shanghai Rising Star Program (11QA1404300).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1222863110/-/DCSupplemental.
References
- 1.Zelent A, Guidez F, Melnick A, Waxman S, Licht JD. Translocations of the RARalpha gene in acute promyelocytic leukemia. Oncogene. 2001;20(49):7186–7203. doi: 10.1038/sj.onc.1204766. [DOI] [PubMed] [Google Scholar]
- 2.Wang ZY, Chen Z. Acute promyelocytic leukemia: From highly fatal to highly curable. Blood. 2008;111(5):2505–2515. doi: 10.1182/blood-2007-07-102798. [DOI] [PubMed] [Google Scholar]
- 3.Licht JD, et al. Clinical and molecular characterization of a rare syndrome of acute promyelocytic leukemia associated with translocation (11;17) Blood. 1995;85(4):1083–1094. [PubMed] [Google Scholar]
- 4.Jansen JH, et al. Complete remission of t(11;17) positive acute promyelocytic leukemia induced by all-trans retinoic acid and granulocyte colony-stimulating factor. Blood. 1999;94(1):39–45. [PubMed] [Google Scholar]
- 5.Petti MC, et al. Complete remission through blast cell differentiation in PLZF/RARalpha-positive acute promyelocytic leukemia: in vitro and in vivo studies. Blood. 2002;100(3):1065–1067. doi: 10.1182/blood-2001-12-0368. [DOI] [PubMed] [Google Scholar]
- 6.Chen L-J, et al. [hCG-PLZF-RARalpha/hCG-RARalpha-PLZF transgenic mice developing into leukemia] Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2005;13(6):924–931. [PubMed] [Google Scholar]
- 7.Zhao Q, et al. Rapid induction of cAMP/PKA pathway during retinoic acid-induced acute promyelocytic leukemia cell differentiation. Leukemia. 2004;18(2):285–292. doi: 10.1038/sj.leu.2403226. [DOI] [PubMed] [Google Scholar]
- 8.Zhu Q, et al. Synergic effects of arsenic trioxide and cAMP during acute promyelocytic leukemia cell maturation subtends a novel signaling cross-talk. Blood. 2002;99(3):1014–1022. [PubMed] [Google Scholar]
- 9.Guillemin MC, et al. In vivo activation of cAMP signaling induces growth arrest and differentiation in acute promyelocytic leukemia. J Exp Med. 2002;196(10):1373–1380. doi: 10.1084/jem.20021129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheng GX, et al. Distinct leukemia phenotypes in transgenic mice and different corepressor interactions generated by promyelocytic leukemia variant fusion genes PLZF-RARalpha and NPM-RARalpha. Proc Natl Acad Sci USA. 1999;96(11):6318–6323. doi: 10.1073/pnas.96.11.6318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.He LZ, et al. Two critical hits for promyelocytic leukemia. Mol Cell. 2000;6(5):1131–1141. doi: 10.1016/s1097-2765(00)00111-8. [DOI] [PubMed] [Google Scholar]
- 12.Ruthardt M, et al. Opposite effects of the acute promyelocytic leukemia PML-retinoic acid receptor alpha (RAR alpha) and PLZF-RAR alpha fusion proteins on retinoic acid signalling. Mol Cell Biol. 1997;17(8):4859–4869. doi: 10.1128/mcb.17.8.4859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Delghandi MP, Johannessen M, Moens U. The cAMP signalling pathway activates CREB through PKA, p38 and MSK1 in NIH 3T3 cells. Cell Signal. 2005;17(11):1343–1351. doi: 10.1016/j.cellsig.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 14.Gaillard E, et al. Phosphorylation by PKA potentiates retinoic acid receptor alpha activity by means of increasing interaction with and phosphorylation by cyclin H/cdk7. Proc Natl Acad Sci USA. 2006;103(25):9548–9553. doi: 10.1073/pnas.0509717103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nasr R, et al. Eradication of acute promyelocytic leukemia-initiating cells through PML-RARA degradation. Nat Med. 2008;14(12):1333–1342. doi: 10.1038/nm.1891. [DOI] [PubMed] [Google Scholar]
- 16.Rice KL, et al. Comprehensive genomic screens identify a role for PLZF-RARalpha as a positive regulator of cell proliferation via direct regulation of c-MYC. Blood. 2009;114(27):5499–5511. doi: 10.1182/blood-2009-03-206524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spinello I, et al. PLZF-mediated control on c-kit expression in CD34(+) cells and early erythropoiesis. Oncogene. 2009;28(23):2276–2288. doi: 10.1038/onc.2009.87. [DOI] [PubMed] [Google Scholar]
- 18.Sengupta A, Arnett J, Dunn S, Williams DA, Cancelas JA. Rac2 GTPase deficiency depletes BCR-ABL+ leukemic stem cells and progenitors in vivo. Blood. 2010;116(1):81–84. doi: 10.1182/blood-2009-10-247437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wojiski S, et al. PML-RARalpha initiates leukemia by conferring properties of self-renewal to committed promyelocytic progenitors. Leukemia. 2009;23(8):1462–1471. doi: 10.1038/leu.2009.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guibal FC, et al. Identification of a myeloid committed progenitor as the cancer-initiating cell in acute promyelocytic leukemia. Blood. 2009;114(27):5415–5425. doi: 10.1182/blood-2008-10-182071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grignani F, et al. Fusion proteins of the retinoic acid receptor-alpha recruit histone deacetylase in promyelocytic leukaemia. Nature. 1998;391(6669):815–818. doi: 10.1038/35901. [DOI] [PubMed] [Google Scholar]
- 22.Guidez F, et al. Reduced retinoic acid-sensitivities of nuclear receptor corepressor binding to PML- and PLZF-RARalpha underlie molecular pathogenesis and treatment of acute promyelocytic leukemia. Blood. 1998;91(8):2634–2642. [PubMed] [Google Scholar]
- 23.Mengeling BJ, Phan TQ, Goodson ML, Privalsky ML. Aberrant corepressor interactions implicated in PML-RAR(alpha) and PLZF-RAR(alpha) leukemogenesis reflect an altered recruitment and release of specific NCoR and SMRT splice variants. J Biol Chem. 2011;286(6):4236–4247. doi: 10.1074/jbc.M110.200964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Insinga A, et al. Inhibitors of histone deacetylases induce tumor-selective apoptosis through activation of the death receptor pathway. Nat Med. 2005;11(1):71–76. doi: 10.1038/nm1160. [DOI] [PubMed] [Google Scholar]
- 25.Lamb D, Steinberg RA. Anti-proliferative effects of 8-chloro-cAMP and other cAMP analogs are unrelated to their effects on protein kinase A regulatory subunit expression. J Cell Physiol. 2002;192(2):216–224. doi: 10.1002/jcp.10131. [DOI] [PubMed] [Google Scholar]
- 26.Altucci L, et al. Rexinoid-triggered differentiation and tumor-selective apoptosis of acute myeloid leukemia by protein kinase A-mediated desubordination of retinoid X receptor. Cancer Res. 2005;65(19):8754–8765. doi: 10.1158/0008-5472.CAN-04-3569. [DOI] [PubMed] [Google Scholar]
- 27.Nasr R, de Thé H. Eradication of acute promyelocytic leukemia-initiating cells by PML/RARA-targeting. Int J Hematol. 2010;91(5):742–747. doi: 10.1007/s12185-010-0582-0. [DOI] [PubMed] [Google Scholar]
- 28.Ablain J, de The H. Revisiting the differentiation paradigm in acute promyelocytic leukemia. Blood. 2011;117(22):5795–5802. doi: 10.1182/blood-2011-02-329367. [DOI] [PubMed] [Google Scholar]
- 29.Chen S-J, et al. From an old remedy to a magic bullet: Molecular mechanisms underlying the therapeutic effects of arsenic in fighting leukemia. Blood. 2011;117(24):6425–6437. doi: 10.1182/blood-2010-11-283598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Testa U, et al. PML/RAR alpha+ U937 mutant and NB4 cell lines: Retinoic acid restores the monocytic differentiation response to vitamin D3. Cancer Res. 1994;54(16):4508–4515. [PubMed] [Google Scholar]
- 31.Grignani F, et al. The acute promyelocytic leukemia-specific PML-RAR alpha fusion protein inhibits differentiation and promotes survival of myeloid precursor cells. Cell. 1993;74(3):423–431. doi: 10.1016/0092-8674(93)80044-f. [DOI] [PubMed] [Google Scholar]
- 32.Westervelt P, et al. Adaptive immunity cooperates with liposomal all-trans-retinoic acid (ATRA) to facilitate long-term molecular remissions in mice with acute promyelocytic leukemia. Proc Natl Acad Sci USA. 2002;99(14):9468–9473. doi: 10.1073/pnas.132657799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tsimberidou A-M, et al. Single-agent liposomal all-trans retinoic acid can cure some patients with untreated acute promyelocytic leukemia: An update of The University of Texas M. D. Anderson Cancer Center Series. Leuk Lymphoma. 2006;47(6):1062–1068. doi: 10.1080/10428190500463932. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.