Abstract
The Nuclear Envelope (NE) contains over 100 different proteins that associate with nuclear components such as chromatin, the lamina and the transcription machinery. Mutations in genes encoding NE proteins have been shown to result in tissue-specific defects and disease, suggesting cell-type specific differences in NE composition and function. Consistent with these observations, recent studies have revealed unexpected functions for numerous NE associated proteins during cell differentiation and development. Here we review the latest insights into the roles played by the NE in cell differentiation, development, disease and aging, focusing primarily on inner nuclear membrane (INM) proteins and nuclear pore components.
Introduction
One of the major transitions in the evolution of life on earth was the appearance of an endomembrane system that allowed for cellular compartmentalization. The most conspicuous manifestation of this event, which occurred between 2.1 and 1.8 billion years ago and gave rise to the entire domain Eukaryota, was the emergence of a physical membrane barrier separating nuclear and cytoplasmic components. It has only recently become evident, however, that beyond its classical barrier function, the NE together with its associated proteins is a major player in cellular organization, gene expression and the regulation of development [1–3].
The first experimental evidence for the presence of the NE came from micromanipulation studies of the nucleus almost 100 years ago [4]. With the emergence of the electron microscope in the 1950s, it was established that the NE consists of two concentric membranes, the inner nuclear membrane (INM) and the outer nuclear membrane (ONM), both perforated by nuclear pores [5,6]. While the ONM is contiguous with the endoplasmic reticulum (ER) and studded with ribosomes in the cytoplasmic side, the INM is in direct contact with nuclear contents such as chromatin and the nuclear lamina, a meshwork of intermediate filaments important for the maintenance of nuclear architecture [7–9]. Although the NE represents a continuous membrane system, protein composition differs dramatically between the ONM and the INM [10]. For example, several transmembrane proteins called Nesprins, characterized by a Klarsicht/ANC-1/Syne-1 homology (KASH) domain, are targeted to the ONM to specifically interact with structural components of the cytoplasm and SUN proteins [11,12]. The latter belong to a large group of integral membrane proteins that exclusively reside at the INM and bind to the nuclear lamina and chromatin, participating in the regulation of diverse nuclear functions [13,14].
Transport of macromolecules between the nucleus and the cytoplasm is regulated by massive multiprotein channels known as Nuclear Pore Complexes (NPCs). NPCs are composed of ~30 different nucleoporins (Nups) and can be generally subdivided into at least two classes. The first class represents the structural scaffold of the NPC, which is stably embedded into the NE, including the NUP107/160 complex and the NUP205/188/93 complex. The second class includes approximately 15 Nups that form peripheral components of the NPC. Most of these Nups contain phenylalanine-glycine (FG) repeat motifs, which bind directly to soluble transport receptors and facilitate transport through the nuclear pore [2]. Interestingly, many nucleoporins of this group are mobile and exhibit shuttling off and on the NPC [15]. Whether this dynamic behavior might relate to their role as transport mediators remains unclear.
In recent years there has been a growing appreciation that changes in NE composition contribute to both development and disease [1,2,16,17]. Genetic mutation of widely expressed NE protein genes commonly give rise to tissue-specific disorders; this implies the existence of functional differences between nuclear envelopes of different cell types, conceivably acting as specialized ‘nuclear outfits’ for diverse cell fate determination events. Well-known examples of tissue-specific diseases are the ones that result from mutations in lamins or lamin-interacting proteins. Furthermore, certain NPC components also exhibit tissue-specific disease phenotypes, and are essential during decisive steps of differentiation and development. These observations support a new vision of the NE as a hub at which fundamental signaling pathways converge to facilitate gene regulation during cell differentiation and development. Still, distinguishing precisely how these processes occur at the NE and how tissue-selective abnormalities arise from ubiquitously expressed proteins has been a long-standing conundrum. Here we will review emerging roles of NE-associated proteins during development and disease and discuss the fascinating biological implications of these findings.
Inner nuclear membrane proteins play a role in cell signaling and differentiation
The INM harbors a unique set of transmembrane proteins, most of which have not been fully characterized. Based on biochemical evidence obtained from isolated rat liver NEs, approximately 70 different proteins reside in the INM, many of which interact with the lamina and/or chromatin [18]. However, recent proteomic studies performed in different tissues identified novel INM proteins, suggesting that NE composition is tissue-specific [19,20]. Consistent with these findings, mutations and misregulation of genes encoding INM proteins result in tissue-specific defects and numerous diseases, ranging from muscular dystrophies and cardiomyopathies to bone disorders [21–25]. An emerging theme is that tissue-specific INM proteins are required for proper regulation of numerous cell signaling pathways, disclosing previously unanticipated roles of the NE during cell differentiation and development (Table 1).
Table 1.
Tissue-specific roles of nuclear envelope proteins
| NE component | Protein | Expression | Tissue-specific disorder or developmental role | References |
|---|---|---|---|---|
| Nuclear Lamina | Lamin A, Lamin C | Most differentiated cells | Muscular dystrophies, cardiomyopathies, lypodystrophies and peripheral neuropathies. Progeroid syndromes, affecting various tissues including skin, bone, adipose, cardiac and skeletal muscle. | [72,73,77] |
| Inner Nuclear Membrane | Emerin | Predominantly expressed in cardiac and skeletal muscle. | Cardiomyopathy and Emery-Dreifuss muscular dystrophy (EDMD). Antagonizes ERK and Wnt signaling. | [21,34,37–40, 83,78] [74] |
| LBR | Predominantly expressed in neutrophils. | Pelger-Huet anomaly (blood laminopathy affecting neutrophils); Greenberg skeletal dysplasia (mainly affecting bone tissue). Regulates growth and maturation of myeloid progenitors. | [24,35] | |
| Nesprin1 | Predominantly expressed in brain, pancreas, kidney, cardiac, and skeletal muscle. | EDMD-like phenotype, cardiomyopathy and cerebellar ataxia. | [75] | |
| LEM2 | Predominantly expressed in skeletal muscle. | Negatively regulates ERK signaling during muscle differentiation. | [36] | |
| MAN1 | Negatively regulates TGF-β signaling. Loss of function mutations lead to sclerosing bone dysplasias in humans. | [22,25–29] | ||
| NET37 | Involved in AKT signaling. Maturation and secretion of IGFII during myogenesis. | [42] | ||
| NET39 | Repression of mTOR signaling during muscle differentiation. | [41] | ||
| SUN1 | Predominantly expressed in brain, testis, heart and skeletal muscle. | Progeric and dystrophic laminopathies. Gametogenesis in mice. | [14,76••] | |
| Nuclear Pore Complex | NUP155 | Predominantly expressed in cardiac and skeletal muscle.a | Missense mutation causes atrial fibrillation in cardiac muscle in humans. Regulates hypertrophy-related genes by modulating HDAC4 function in rat cardiomyocytes. | [50•,55] |
| NUP133 | Differentially expressed during embryogenesis.a | Neuronal cell differentiation during mouse embryogenesis. | [51•] | |
| NUP210 | Only in certain cell types including epithelial cells, differentiated myotubes and neuroprogenitor cells. | Muscle and neuronal cell differentiation. | [60,61,62••] | |
| NUP50 | Highly expressed in developing neural tube and testis in mice. | Neural tube formation during embryogenesis in mouse. Gene regulatory function during development in D. melanogaster. | [69••,71] | |
| NUP98 | Ubiquituous. | Acute myeloid leukemia; hematopoietic stem-cell proliferation in humans. Activation of genes involved in developmental regulation in D. melanogaster. | [49,68••,69••] |
Differential expression of nucleoporins only determined by mRNA levels.
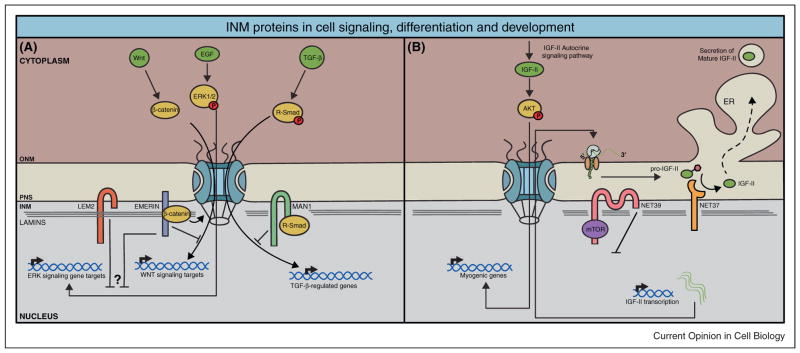
The most well documented example of NE-mediated signaling is MAN1 and its role in transforming growth factor beta (TGF-β) signaling. MAN1 belongs to the family of LEM-domain proteins, defined by a ~40 residue protein-protein interaction motif termed the LEM domain. In humans, heterozygous loss of function mutations of MAN1 lead to several sclerosing bone dysplasias, while knockout of the MAN1 gene in mice causes embryonic lethality [22,26,27]. Loss of MAN1 has been linked to enhanced TGF-β signaling, and is now recognized as a negative regulator of this pathway. MAN1 binds and inhibits R-SMADs, which are known mediators of TGF-β signaling [22,28–30]. Importantly, members of TGF-β superfamily of cytokines were originally isolated as cartilage and bone-inducing factors, which could explain why partial loss of MAN1 causes bone sclerosis and overgrowth of connective tissue [31–33]. The current model proposes that MAN1 sequesters R-SMADs at the NE, preventing transcription of R-SMAD target genes (Figure 1A).
Figure 1.
(A) LEM2 is a negative regulator of ERK signaling during muscle differentiation. Although the mechanism by which ERK1/2 is inhibited is unclear, one possibility could be through the sequestration of ERK1/2 to the nuclear periphery. Emerin has been shown to play a role in two different signaling cascades. First, it negatively regulates Wnt signaling by preventing accumulation of β-catenin in the nucleus, presumably by stimulating export of β-catenin. Second, emerin negatively regulates ERK signaling through unclear mechanisms. MAN1 negatively regulates TGF-β signaling by sequestering R-Smads away from gene targets. (B) During myogenesis, NET39 acts as a repressor of the mTOR-IGF-II signaling pathway. NET39 blocks IGF-II transcription by inhibiting mTOR activity at the NE. NET37, an INM protein with glycosidase activity promotes myogenesis by activating AKT signaling through the maturation and secretion of IGF-II in the PNS/ER.
Other LEM domain containing INM proteins such as emerin, Lamin B receptor (LBR), LEM2 and Lamina-associated polypeptide 2 beta (LAP2β) are also involved in different signaling cascades [24,34–36]. LEM2 negatively regulates Extracellular signal-regulated kinases (ERK) signaling during myoblast differentiation in C2C12 cells, as depletion of LEM2 blocks C2C12 differentiation and leads to the hyperactivation of ERK1/2 [36]. Likewise, cardiomyocytes from emerin-null mice as well as knockdown cells in culture show accumulation of activated ERK1/2 in the nucleus, suggesting that emerin attenuates ERK signaling [37,38]. Emerin also regulates the Wnt pathway by preventing the accumulation of β-catenin in the nucleus, presumably by stimulating β-catenin export [34]. Surprisingly, emerin can be found at adherens junctions of the intercalated disc (ID) in cardiomyocytes [39]. Loss of emerin at this junctions perturbs β-catenin distribution and ID architecture [40], suggesting that cell-type specific localization of INM proteins may also account for the emergence of NE tissue-specific diseases. Overall, these findings indicate that several INM proteins utilize a common mode of action in which they sequester transcriptional activators away from target genes (Figure 1A).
Signal transduction events at the NE are not restricted to LEM domain-containing INM proteins [41,42]. For instance, Nuclear Envelope Transmembrane protein 39 (NET39) antagonizes myoblast differentiation by directly binding to mammalian target of rapamycin (mTOR). NET39 overexpression leads to mTOR inactivation and the repression of mTOR-insulin-like growth factor 2 (IGFII) signaling, central for the initiation of myogenesis (Figure 1B) [41]. Another example is NET37, which promotes C2C12 differentiation via the maturation and secretion of IGFII, an autocrine factor critical for the activation of AKT signaling. NET37 functions by utilizing its glycosidase activity within the perinuclear space (PNS) where IGFII is processed into a mature form (Figure 1B) [42].
Apart from their roles in signaling, INM proteins also participate in nuclear architecture, chromatin configuration and cytoskeleton organization [11–14,19,20]. For example, emerin binds structural proteins (i.e. SUN1, lamins), chromatin regulators (i.e. barrier-to-autointegration factor (BAF), Lmo7) [43,44], and cell signaling factors (i.e. β-catenin) [34], but how these interactions are orchestrated to execute specific functions is unclear. These interactions may be partially regulated by post-translational modification, as new evidence suggests emerin is phosphorylated on as many as twelve residues [45]. Establishing the precise mechanism of post-translational modification of INM proteins and how these alterations modulate protein function remains an important area of future research that may render insight into tissue-specific roles of INM proteins.
Tissue-specific roles of nucleoporins in cell differentiation, development, and disease
The primary function of NPCs has traditionally been viewed to be nucleo-cytoplasmic transport. Yet recent studies indicate that NPCs might play additional roles in nuclear processes such as chromatin organization and gene regulation [46–48]. Moreover, accumulating evidence has established unambiguous connections between single nucleoporin mutations and tissue specific disorders [49,50•,51•] (Table 1). These observations suggest not only that NPCs may have cell type-specific composition, but also that tissue-specific nucleoporins might act as critical regulators of gene expression programs during differentiation and development.
In humans, NUP155 is predominantly enriched in heart and skeletal muscle [52]. Interestingly, a recessive Mendelian mutation in NUP155 cosegregates with atrial fibrillation (AF), a form of clinical arrhythmia that can lead to sudden cardiac death. Nup155−/− mice die early in embryogenesis but heterozygous animals show an AF phenotype, suggesting that reduction of NUP155 leads to a tissue-specific defect [50•]. Further analysis revealed that NUP155 is required for proper export of Hsp70 mRNA and import of HSP70 protein, a factor previously linked to this disease (Figure 2A) [53,54]. Supporting its importance in cardiac muscle, NUP155 also interacts with HDAC4 in rat cardiomyocytes, where it is required for the localization and regulation of hypertrophy-related genes. Disruption of this interaction by overexpression of a NUP155 C-terminal truncation mutant suppressed HDAC4-induced gene expression changes, indicating NUP155-mediated localization, presumably at the pore, is crucial for HDAC4 function (Figure 2A) [55]. Curiously, inherited mutation of NUP155 in AF patients leads to NUP155 mislocalization. Since HDAC4 regulation of hypertrophy-related genes is dependent on NUP155 localization in cardiomyocytes, and complication of hypertrophic cardiomyopathy can lead to AF, it is intriguing to speculate that malfunction of HDAC4 in hearts carrying this mutation may also contribute to AF.
Figure 2.
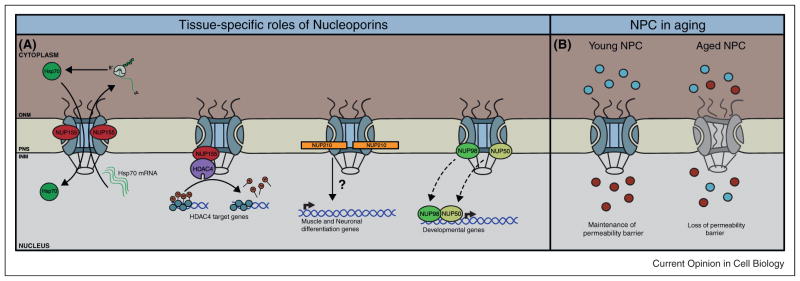
(A) NUP155 regulates the export of Hsp70 mRNA and import of Hsp70 protein in cardiac muscle, a function NUP155 loses in atrial fibrillation. In cardiomyocytes, NUP155 mediates localization and activity of HDAC4. Addition of NUP210 to NPCs induces activation of genes critical for myogenesis and the differentiation of ES cells into NP cells. In Drosophila, several dynamic NPC components shuttle off the pore to regulate the expression of developmental genes inside the nucleoplasm. (B) Lack of a replacement mechanism of NPCs in post-mitotic cells results in deterioration of NPCs over time and progressive loss of cell compartmentalization.
Both stable and dynamic NPC components play a role in particular aspects of development. NUP133, a component of the stable NUP107-160 subcomplex, is required for neuronal differentiation during mouse development. Nup133-null mice die during embryogenesis owing to impaired neuronal development, while in vitro, Nup133-null embryonic stem (ES) cells are unable to efficiently produce terminally differentiated neurons [51•]. Utilizing in situ hybridization, the authors determined NUP133 is expressed in a tissue-specific manner in the developing embryo, suggesting the existence of cell-type-specific nuclear pores. However, it is important to note that the lack of transcription of Nups does not always correlate with absence of protein at the NPC. For example, scaffold nucleoporin transcripts, such as those belonging to the NUP107–160 subcomplex, are down-regulated in developing C. elegans embryos and adult worms without affecting their protein levels at NPCs [56••]. This finding suggests scaffold Nups can be extremely long-lived proteins in post-mitotic cells even when their expression is strongly reduced [56••,57••]. In this regard, differential expression of Nups during differentiation and development should always be validated at the protein level, especially when studying stable NPC components.
Being the first transmembrane nucleoporin to be discovered [58], NUP210 was first proposed to be involved in anchoring the NPC to the NE membranes [59]. Nonetheless, several current findings challenge this perception, as NUP210 is absent in many tissue types. Also, NUP210 is differentially expressed in the kidney during mesenchymal to epithelial transition, raising the possibility of cell type-specific nuclear pores and a potential role for NUP210 in regulating cell differentiation [60,61]. In support of this idea, NUP210 has been recently identified to be upregulated and to play a pivotal role during myogenic and neuronal differentiation [62••]. Expression analysis showed NUP210 is absent in myoblasts and ES cells but becomes expressed and incorporated into NPCs in myotubes and neuroprogenitor (NP) cells, respectively. Depletion of NUP210 by RNAi blocks myogenesis and the differentiation of ES cells into NP cells without affecting global nuclear transport. Significantly, NUP210 was found to be required for the induction of genes essential for cell differentiation (Figure 2A) [62••]. Given that the NPC is considered a site coupled with transcription, one potential function of NUP210 might be to recruit transcription or mRNA export factors to the NPC to facilitate gene expression. Taking this into consideration, one could envision a scenario where NPC composition can be modulated in a tissue-specific manner to accommodate diverse regulatory functions within a multicellular organism. Intriguingly, some NPC components have alternative transcription variants, an unstudied feature in most nucleoporins [63,64]. It is interesting to speculate whether splicing variants contribute to diversity and functionality of NPCs across different tissues.
Multiple studies in S. cerevisiae indicate NPCs may serve as anchoring structures to help stabilize and promote gene transcription [65–67]. Yet, how NPCs influence gene regulation in multicellular organisms during development remains poorly understood. Three independent studies have recently reported participation of several Nups in transcriptional activation in Drosophila [68••,69••,70••]. NUP98, Sec13, NUP62, NUP50, and additional FG Nups dynamically associate with chromatin in a stage-specific manner during larvae development [68••,69••]. Strikingly, these interactions were commonly observed to occur inside the nucleoplasm (i.e. away from the NE), expanding the functional reach of NPCs in higher eukaryotes. Genome-wide association studies in Drosophila embryonic cells revealed NUP98 and NUP50 preferentially bind to highly transcribed genes involved in developmental regulation (Figure 2A) [68••,69••]. Particularly, target gene expression was reduced after knockdown of NUP98, whereas overexpression stimulated their expression [68••]. Together, these findings portray a much more complex picture of NPC components in gene regulation and uncover a novel role of nucleoplasmic Nups in stimulating gene activity and regulating developmental programs in higher eukaryotes. Interestingly, murine NUP50 exhibits tissue-specific protein expression and Nup50−/− mice die during embryogenesis owing to abnormal neural tube formation [71]. Whether NUP50, or other nucleoplasmic Nups, drive tissue morphogenesis in mammals via cell-type-specific NUP-chromatin interactions remains a fascinating possibility.
The lamins and associated INM proteins in disease
A wide array of human disorders is caused by mutations in genes encoding NE proteins [1,17,23], commonly referred to as nuclear envelopathies and laminopathies. Laminopathies originate from a variety of different mutations in LMNA, the gene encoding A-type lamins [1,23]. Although A-type lamins are expressed in almost all tissues, LMNA mutations frequently give rise to tissue-specific diseases, ranging from muscular dystrophies to progeria [13,72,73]. Intriguingly, mutations in certain INM proteins that are anchored to the NE by A-type lamins have been implicated in Emery-Dreifuss muscular dystrophy, a disorder that phenocopies diseases caused by certain LMNA mutations [74,75]. Specifically, a recent study demonstrates SUN1, another INM protein known to interact with A-type lamins [13], to be directly involved in progeric and dystrophic laminopathies, as knockout of Sun1 in progeric and dystrophic mice reverts tissue pathogenesis and averts premature death by reducing cell cytotoxicity from SUN1 mislocalization [76••]. The fact that many INM proteins are both functional interactors of A-type lamins and tissue-specific may explain why LMNA mutations can lead to distinct diseases in different tissues.
Loss of cell compartmentalization in laminopathies and cancer
As key structural components of the nucleus, nuclear lamins cause loss of nuclear integrity when mutated [77,78]. These lamin mutations often result in dramatic morphological deformations of the NE. Strikingly, two recent studies have shown that mutation or downregulation of nuclear lamins can also lead to a temporal disruption of the nuclear membrane, resulting in the mislocalization of nuclear and cytoplasmic components [79,80]. Isolated human fibroblasts from patients with different lamin A/C mutations exhibit non-lethal NE ruptures during interphase [79]. Moreover, knock down of lamins in human cancer cells, which already display a certain degree of NE rupturing events, increases the frequency of nuclear ruptures [80]. These findings suggest that loss of cell compartmentalization by transient NE rupturing may lead to cell dysfunction and pathological hallmarks of laminopathies as well as cancer.
The NPC in aging and neurodegenerative disease
Deterioration of NPCs in aged cells has been recently shown to cause loss of NE integrity [56••]. In dividing cells, NPCs are disassembled at the onset of mitosis and reassembled in the newly forming nuclei. However, in post-mitotic cells, scaffold nucleoporins remain stably associated at the NE for the entire lifespan of a cell [56••,57••]. The lack of a replacement mechanism eventually leads to the deterioration of NPCs and loss of the nuclear permeability barrier (Figure 2B) [56••]. Strikingly, cytoplasmic tubulin accumulates inside nuclei of aged rat neurons, a characteristic that has been previously described in various neurological disorders including Parkinson’s disease [81,82]. This implies that gradual deterioration of NPCs might contribute to pathological progression of neurodegenerative diseases in the brain.
Concluding remarks
The view of the NE has been greatly revolutionized since the first pathological mutation of the NE-associated protein, emerin, was unveiled in the mid 1990s [21,83]. Since then, multiple discoveries have continued to link human disease and tissue-selective defects to mutations in genes encoding NE proteins. Nevertheless, future research must attempt to elucidate the molecular biology hidden behind these phenotypes. Recent studies have demonstrated induced pluripotent stem cells (iPSC) can be generated from patients with laminopathies [84,85••]. These iPSCs are able to recapitulate the laminopathy phenotype when induced to differentiate in culture [84,85••]. These findings not only provide an in vitro model to study laminopathies but hold the potential to provide new insights into the pathology of nuclear envelopathies in the future. Furthermore, since many NE proteins have been found to be vital during embryogenesis [26,50•,51•,71], it is not surprising that many knockout mice are embryonic lethal. Thus, to examine the individual contribution of particular NE proteins in different cell-types and stages during development, it would be extremely useful to utilize tissue-specific conditional knock outs. Dissecting the pathways and identifying the molecular players responsible for these phenotypes will shed light on potential novel mechanisms involved in the regulation of cell differentiation and development.
Acknowledgments
We thank the members of the Hetzer laboratory and Jennifer M. Higginbotham for helpful suggestions and critical reading of the manuscript. J.S.G.C. is supported by UC-MEXUS CONACYT Fellowship, M.W.H. was supported by NIH GM098749.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Dauer WT, Worman HJ. The nuclear envelope as a signaling node in development and disease. Dev Cell. 2009;17:626–638. doi: 10.1016/j.devcel.2009.10.016. http://dx.doi.org/10.1016/j.devcel.2009.10.016. [DOI] [PubMed] [Google Scholar]
- 2.Capelson M, Hetzer MW. The role of nuclear pores in gene regulation, development and disease. EMBO Rep. 2009;10:697–705. doi: 10.1038/embor.2009.147. http://dx.doi.org/10.1038/embor.2009.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mekhail K, Moazed D. The nuclear envelope in genome organization, expression and stability. Nat Rev Mol Cell Biol. 2010;11:317–328. doi: 10.1038/nrm2894. http://dx.doi.org/10.1038/nrm2894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kite G. Studies on the physical properties of protoplasm. Am J Physiol. 1913;146 [Google Scholar]
- 5.Callan HG, Tomlin SG. Experimental studies on amphibian oocyte nuclei. I. Investigation of the structure of the nuclear membrane by means of the electron microscope. Proc R Soc Lond B Biol Sci. 1950;137:367–378. doi: 10.1098/rspb.1950.0047. [DOI] [PubMed] [Google Scholar]
- 6.Watson ML. Further observations on the nuclear envelope of the animal cell. J Biophys Biochem Cytol. 1959;6:147–156. doi: 10.1083/jcb.6.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Watson ML. The nuclear envelope; its structure and relation to cytoplasmic membranes. J Biophys Biochem Cytol. 1955;1:257–270. doi: 10.1083/jcb.1.3.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Franke WW, Scheer U, Krohne G, Jarasch ED. The nuclear envelope and the architecture of the nuclear periphery. J Cell Biol. 1981;91(3 Pt 2):39s–50s. doi: 10.1083/jcb.91.3.39s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.D’Angelo MA, Hetzer MW. The role of the nuclear envelope in cellular organization. Cell Mol Life Sci. 2006;63:316–332. doi: 10.1007/s00018-005-5361-3. http://dx.doi.org/10.1007/s00018-005-5361-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stewart CL, Roux KJ, Burke B. Blurring the boundary: the nuclear envelope extends its reach. Science (New York, NY) 2007;318:1408–1412. doi: 10.1126/science.1142034. http://dx.doi.org/10.1126/science.1142034. [DOI] [PubMed] [Google Scholar]
- 11.Zhang Q, Ragnauth C, Greener MJ, Shanahan CM, Roberts RG. The nesprins are giant actin-binding proteins, orthologous to drosophila melanogaster muscle protein MSP-300. Genomics. 2002;80:473–481. [PubMed] [Google Scholar]
- 12.Wilhelmsen K, Litjens SH, Kuikman I, Tshimbalanga N, Janssen H, van den Bout I, Sonnenberg A. Nesprin-3, a novel outer nuclear membrane protein, associates with the cytoskeletal linker protein plectin. J Cell Biol. 2005;171:799–810. doi: 10.1083/jcb.200506083. http://dx.doi.org/10.1083/jcb.200506083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haque F, Lloyd DJ, Smallwood DT, Dent CL, Shanahan CM, Fry AM, Shackleton S. SUN1 interacts with nuclear lamin A and cytoplasmic nesprins to provide a physical connection between the nuclear lamina and the cytoskeleton. Mol Cell Biol. 2006;26:3738–3751. doi: 10.1128/MCB.26.10.3738-3751.2006. http://dx.doi.org/10.1128/MCB.26.10.3738-3751.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ding X, Xu R, Yu J, Xu T, Zhuang Y, Han M. SUN1 is required for telomere attachment to nuclear envelope and gametogenesis in mice. Dev Cell. 2007;12:863–872. doi: 10.1016/j.devcel.2007.03.018. [DOI] [PubMed] [Google Scholar]
- 15.Rabut G, Doye V, Ellenberg J. Mapping the dynamic organization of the nuclear pore complex inside single living cells. Nat Cell Biol. 2004;6:1114–1121. doi: 10.1038/ncb1184. http://dx.doi.org/10.1038/ncb1184. [DOI] [PubMed] [Google Scholar]
- 16.Wilson KL, Berk JM. The nuclear envelope at a glance. J Cell Sci. 2010;123(Pt 12):1973–1978. doi: 10.1242/jcs.019042. http://dx.doi.org/10.1242/jcs.019042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mendez-Lopez I, Worman HJ. Inner nuclear membrane proteins: impact on human disease. Chromosoma. 2012;121:153–167. doi: 10.1007/s00412-012-0360-2. http://dx.doi.org/10.1007/s00412-012-0360-2; http://dx.doi.org/10.1007/s00412-012-0360-2. [DOI] [PubMed] [Google Scholar]
- 18.Schirmer EC, Florens L, Guan T, Yates JR, 3rd, Gerace L. Nuclear membrane proteins with potential disease links found by subtractive proteomics. Science (New York, NY) 2003;301:1380–1382. doi: 10.1126/science.1088176. http://dx.doi.org/10.1126/science.1088176. [DOI] [PubMed] [Google Scholar]
- 19.Korfali N, Wilkie GS, Swanson SK, Srsen V, Batrakou DG, Fairley EA, Schirmer EC. The leukocyte nuclear envelope proteome varies with cell activation and contains novel transmembrane proteins that affect genome architecture. Mol Cell Proteomics. 2010;9:2571–2585. doi: 10.1074/mcp.M110.002915. http://dx.doi.org/10.1074/mcp.M110.002915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wilkie GS, Korfali N, Swanson SK, Malik P, Srsen V, Batrakou DG, Schirmer EC. Several novel nuclear envelope transmembrane proteins identified in skeletal muscle have cytoskeletal associations. Mol Cell Proteomics. 2011;10:M110. doi: 10.1074/mcp.M110.003129. http://dx.doi.org/10.1074/mcp.M110.003129.003129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nagano A, Koga R, Ogawa M, Kurano Y, Kawada J, Okada R, Arahata K. Emerin deficiency at the nuclear membrane in patients with emery-dreifuss muscular dystrophy. Nat Genet. 1996;12:254–259. doi: 10.1038/ng0396-254. http://dx.doi.org/10.1038/ng0396-254. [DOI] [PubMed] [Google Scholar]
- 22.Hellemans J, Preobrazhenska O, Willaert A, Debeer P, Verdonk PC, Costa T, Mortier GR. Loss-of-function mutations in LEMD3 result in osteopoikilosis, buschke-ollendorff syndrome and melorheostosis. Nat Genet. 2004;36:1213–1218. doi: 10.1038/ng1453. http://dx.doi.org/10.1038/ng1453. [DOI] [PubMed] [Google Scholar]
- 23.Worman HJ, Ostlund C, Wang Y. Diseases of the nuclear envelope. Cold Spring Harb Perspect Biol. 2010;2:a000760. doi: 10.1101/cshperspect.a000760. http://dx.doi.org/10.1101/cshperspect.a000760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Subramanian G, Chaudhury P, Malu K, Fowler S, Manmode R, Gotur D, Zwerger M, Ryan D, Roberti R, Gaines P. Lamin B receptor regulates the growth and maturation of myeloid progenitors via its sterol reductase domain: implications for cholesterol biosynthesis in regulating myelopoiesis. J Immunol (Baltimore, MD: 1950) 2012;188:85–102. doi: 10.4049/jimmunol.1003804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shultz LD, Lyons BL, Burzenski LM, Gott B, Samuels R, Schweitzer PA, Hoffmann K. Mutations at the mouse ichthyosis locus are within the lamin B receptor gene: a single gene model for human pelger-huet anomaly. Hum Mol Genet. 2003;12:61–69. doi: 10.1093/hmg/ddg003. [DOI] [PubMed] [Google Scholar]
- 26.Ishimura A, Ng JK, Taira M, Young SG, Osada S. Man1, an inner nuclear membrane protein, regulates vascular remodeling by modulating transforming growth factor beta signaling. Development (Cambridge, England) 2006;133:3919–3928. doi: 10.1242/dev.02538. http://dx.doi.org/10.1242/dev.02538. [DOI] [PubMed] [Google Scholar]
- 27.Cohen TV, Kosti O, Stewart CL. The nuclear envelope protein MAN1 regulates TGFbeta signaling and vasculogenesis in the embryonic yolk sac. Development (Cambridge, England) 2007;134:1385–1395. doi: 10.1242/dev.02816. http://dx.doi.org/10.1242/dev.02816. [DOI] [PubMed] [Google Scholar]
- 28.Lin F, Morrison JM, Wu W, Worman HJ. MAN1, an integral protein of the inner nuclear membrane, binds Smad2 and Smad3 and antagonizes transforming growth factor-beta signaling. Hum Mol Genet. 2005;14:437–445. doi: 10.1093/hmg/ddi040. http://dx.doi.org/10.1093/hmg/ddi040. [DOI] [PubMed] [Google Scholar]
- 29.Pan D, Estevez-Salmeron LD, Stroschein SL, Zhu X, He J, Zhou S, Luo K. The integral inner nuclear membrane protein MAN1 physically interacts with the R-smad proteins to repress signaling by the transforming growth factor-{beta} superfamily of cytokines. J Biol Chem. 2005;280:15992–16001. doi: 10.1074/jbc.M411234200. http://dx.doi.org/10.1074/jbc.M411234200. [DOI] [PubMed] [Google Scholar]
- 30.Konde E, Bourgeois B, Tellier-Lebegue C, Wu W, Perez J, Caputo S, Zinn-Justin S. Structural analysis of the Smad2-MAN1 interaction that regulates transforming growth factor-beta signaling at the inner nuclear membrane. Biochemistry. 2010;49:8020–8032. doi: 10.1021/bi101153w. http://dx.doi.org/10.1021/bi101153w. [DOI] [PubMed] [Google Scholar]
- 31.Seyedin SM, Segarini PR, Rosen DM, Thompson AY, Bentz H, Graycar J. Cartilage-inducing factor-B is a unique protein structurally and functionally related to transforming growth factor-beta. J Biol Chem. 1987;262:1946–1949. [PubMed] [Google Scholar]
- 32.Urist MR. Bone: formation by autoinduction. Science (New York, NY) 1965;150:893–899. doi: 10.1126/science.150.3698.893. [DOI] [PubMed] [Google Scholar]
- 33.Luyten FP, Cunningham NS, Ma S, Muthukumaran N, Hammonds RG, Nevins WB, Reddi AH. Purification and partial amino acid sequence of osteogenin, a protein initiating bone differentiation. J Biol Chem. 1989;264:13377–13380. [PubMed] [Google Scholar]
- 34.Nili E, Cojocaru GS, Kalma Y, Ginsberg D, Copeland NG, Gilbert DJ, Rechavi G. Nuclear membrane protein LAP2beta mediates transcriptional repression alone and together with its binding partner GCL (germ-cell-less) J Cell Sci. 2001;114(Pt 18):3297–3307. doi: 10.1242/jcs.114.18.3297. [DOI] [PubMed] [Google Scholar]
- 35.Markiewicz E, Tilgner K, Barker N, van de Wetering M, Clevers H, Dorobek M, Hutchison CJ. The inner nuclear membrane protein emerin regulates beta-catenin activity by restricting its accumulation in the nucleus. EMBO J. 2006;25:3275–3285. doi: 10.1038/sj.emboj.7601230. http://dx.doi.org/10.1038/sj.emboj.7601230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huber MD, Guan T, Gerace L. Overlapping functions of nuclear envelope proteins NET25 (Lem2) and emerin in regulation of extracellular signal-regulated kinase signaling in myoblast differentiation. Mol Cell Biol. 2009;29:5718–5728. doi: 10.1128/MCB.00270-09. http://dx.doi.org/10.1128/MCB.00270-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Muchir A, Pavlidis P, Bonne G, Hayashi YK, Worman HJ. Activation of MAPK in hearts of EMD null mice: similarities between mouse models of X-linked and autosomal dominant emery dreifuss muscular dystrophy. Hum Mol Genet. 2007;16:1884–1895. doi: 10.1093/hmg/ddm137. http://dx.doi.org/10.1093/hmg/ddm137. [DOI] [PubMed] [Google Scholar]
- 38.Muchir A, Wu W, Worman HJ. Reduced expression of A-type lamins and emerin activates extracellular signal-regulated kinase in cultured cells. Biochim Biophys Acta. 2009;1792:75–81. doi: 10.1016/j.bbadis.2008.10.012. http://dx.doi.org/10.1016/j.bbadis.2008.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cartegni L, di Barletta MR, Barresi R, Squarzoni S, Sabatelli P, Maraldi N, Toniolo D. Heart-specific localization of emerin: new insights into emery-dreifuss muscular dystrophy. Hum Mol Genet. 1997;6:2257–2264. doi: 10.1093/hmg/6.13.2257. [DOI] [PubMed] [Google Scholar]
- 40.Wheeler MA, Warley A, Roberts RG, Ehler E, Ellis JA. Identification of an emerin-beta-catenin complex in the heart important for intercalated disc architecture and beta-catenin localisation. Cell Mol Life Sci. 2010;67:781–796. doi: 10.1007/s00018-009-0219-8. http://dx.doi.org/10.1007/s00018-009-0219-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu GH, Guan T, Datta K, Coppinger J, Yates J, 3rd, Gerace L. Regulation of myoblast differentiation by the nuclear envelope protein NET39. Mol Cell Biol. 2009;29:5800–5812. doi: 10.1128/MCB.00684-09. http://dx.doi.org/10.1128/MCB.00684-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Datta K, Guan T, Gerace L. NET37, a nuclear envelope transmembrane protein with glycosidase homology, is involved in myoblast differentiation. J Biol Chem. 2009;284:29666–29676. doi: 10.1074/jbc.M109.034041. http://dx.doi.org/10.1074/jbc.M109.034041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Holaska JM, Rais-Bahrami S, Wilson KL. Lmo7 is an emerin-binding protein that regulates the transcription of emerin and many other muscle-relevant genes. Hum Mol Genet. 2006;15:3459–3472. doi: 10.1093/hmg/ddl423. http://dx.doi.org/10.1093/hmg/ddl423. [DOI] [PubMed] [Google Scholar]
- 44.Holaska JM, Wilson KL. An emerin “proteome”: purification of distinct emerin-containing complexes from HeLa cells suggests molecular basis for diverse roles including gene regulation, mRNA splicing, signaling, mechanosensing, and nuclear architecture. Biochemistry. 2007;46:8897–8908. doi: 10.1021/bi602636m. http://dx.doi.org/10.1021/bi602636m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Holaska JM. Emerin and the nuclear lamina in muscle and cardiac disease. Circ Res. 2008;103:16–23. doi: 10.1161/CIRCRESAHA.108.172197. http://dx.doi.org/10.1161/CIRCRESAHA.108.172197. [DOI] [PubMed] [Google Scholar]
- 46.Akhtar A, Gasser SM. The nuclear envelope and transcriptional control. Nat Rev Genet. 2007;8:507–517. doi: 10.1038/nrg2122. http://dx.doi.org/10.1038/nrg2122. [DOI] [PubMed] [Google Scholar]
- 47.Brown CR, Silver PA. Transcriptional regulation at the nuclear pore complex. Curr Opin Genet Dev. 2007;17:100–106. doi: 10.1016/j.gde.2007.02.005. http://dx.doi.org/10.1016/j.gde.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 48.Liang Y, Hetzer MW. Functional interactions between nucleoporins and chromatin. Curr Opin Cell Biol. 2011;23:65–70. doi: 10.1016/j.ceb.2010.09.008. http://dx.doi.org/10.1016/j.ceb.2010.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nakamura T, Largaespada DA, Lee MP, Johnson LA, Ohyashiki K, Toyama K, Shaughnessy JD., Jr Fusion of the nucleoporin gene NUP98 to HOXA9 by the chromosome translocation t(7;11)(p15;p15) in human myeloid leukaemia. Nat Genet. 1996;12:154–158. doi: 10.1038/ng0296-154. http://dx.doi.org/10.1038/ng0296-154. [DOI] [PubMed] [Google Scholar]
- 50.Zhang X, Chen S, Yoo S, Chakrabarti S, Zhang T, Ke T, Wang QK. Mutation in nuclear pore component NUP155 leads to atrial fibrillation and early sudden cardiac death. Cell. 2008;135:1017–1027. doi: 10.1016/j.cell.2008.10.022. http://dx.doi.org/10.1016/j.cell.2008.10.022. • Identification of an inherited missense mutation in the NUP155 gene that causes atrial fibrillation. NUP155 mutant fails to export HSP70 mRNA and import HSP70 protein. [DOI] [PubMed] [Google Scholar]
- 51.Lupu F, Alves A, Anderson K, Doye V, Lacy E. Nuclear pore composition regulates neural stem/progenitor cell differentiation in the mouse embryo. Dev Cell. 2008;14:831–842. doi: 10.1016/j.devcel.2008.03.011. http://dx.doi.org/10.1016/j.devcel.2008.03.011. • Genetic deletion of NUP133 impairs differentiation of the neural lineage. NUP133 mRNA expression is cell-type and developmental stage specific during embryogenesis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang X, Yang H, Corydon MJ, Zhang X, Pedersen S, Korenberg JR, Chen XN, Laporte J, Gregersen N, Niebuhr E, et al. Localization of a human nucleoporin 155 gene (NUP155) to the 5p13 region and cloning of its cDNA. Genomics. 1999;57:144–151. doi: 10.1006/geno.1999.5741. [DOI] [PubMed] [Google Scholar]
- 53.Kim YK, Suarez J, Hu Y, McDonough PM, Boer C, Dix DJ, Dillmann WH. Deletion of the inducible 70-kDa heat shock protein genes in mice impairs cardiac contractile function and calcium handling associated with hypertrophy. Circulation. 2006;113:2589–2597. doi: 10.1161/CIRCULATIONAHA.105.598409. http://dx.doi.org/10.1161/CIRCULATIONAHA.105.598409. [DOI] [PubMed] [Google Scholar]
- 54.Afzal AR, Mandal K, Nyamweya S, Foteinos G, Poloniecki J, Camm AJ, Xu Q. Association of Met439Thr substitution in heat shock protein 70 gene with postoperative atrial fibrillation and serum HSP70 protein levels. Cardiology. 2008;110:45–52. doi: 10.1159/000109406. http://dx.doi.org/10.1159/000109406. [DOI] [PubMed] [Google Scholar]
- 55.Kehat I, Accornero F, Aronow BJ, Molkentin JD. Modulation of chromatin position and gene expression by HDAC4 interaction with nucleoporins. J Cell Biol. 2011;193:21–29. doi: 10.1083/jcb.201101046. http://dx.doi.org/10.1083/jcb.201101046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.D’Angelo MA, Raices M, Panowski SH, Hetzer MW. Age-dependent deterioration of nuclear pore complexes causes a loss of nuclear integrity in postmitotic cells. Cell. 2009;136:284–295. doi: 10.1016/j.cell.2008.11.037. http://dx.doi.org/10.1016/j.cell.2008.11.037. •• This study implicates, for the first time, NPC structures in the aging process. Also it demonstrates stable NPC components are downregulated upon cell-cycle exit and can be long-lived proteins in post-mitotic cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Savas JN, Toyama BH, Xu T, Yates JR, 3rd, Hetzer MW. Extremely long-lived nuclear pore proteins in the rat brain. Science (New York, NY) 2012;335:942. doi: 10.1126/science.1217421. http://dx.doi.org/10.1126/science.1217421. •• Utilizing pulse-chase labeling of whole rats, this study shows stable nuclear pore components can be extremely long-lived proteins in rat brains. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gerace L, Ottaviano Y, Kondor-Koch C. Identification of a major polypeptide of the nuclear pore complex. J Cell Biol. 1982;95:826–837. doi: 10.1083/jcb.95.3.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wozniak RW, Bartnik E, Blobel G. Primary structure analysis of an integral membrane glycoprotein of the nuclear pore. J Cell Biol. 1989;108:2083–2092. doi: 10.1083/jcb.108.6.2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Olsson M, Ekblom M, Fecker L, Kurkinen M, Ekblom P. cDNA cloning and embryonic expression of mouse nuclear pore membrane glycoprotein 210 mRNA. Kidney Int. 1999;56:827–838. doi: 10.1046/j.1523-1755.1999.00618.x. http://dx.doi.org/10.1046/j.1523-1755.1999.00618.x. [DOI] [PubMed] [Google Scholar]
- 61.Olsson M, Scheele S, Ekblom P. Limited expression of nuclear pore membrane glycoprotein 210 in cell lines and tissues suggests cell-type specific nuclear pores in metazoans. Exp Cell Res. 2004;292:359–370. doi: 10.1016/j.yexcr.2003.09.014. [DOI] [PubMed] [Google Scholar]
- 62.D’Angelo MA, Gomez-Cavazos JS, Mei A, Lackner DH, Hetzer MW. A change in nuclear pore complex composition regulates cell differentiation. Dev Cell. 2012;22:446–458. doi: 10.1016/j.devcel.2011.11.021. http://dx.doi.org/10.1016/j.devcel.2011.11.021. •• This study provides evidence for the existence of cell-type specific nuclear pores. This study identifies NUP210 as a key regulator of cell differentiation of muscle and neurons. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang X, Yang H, Yu J, Chen C, Zhang G, Bao J, Bolund L. Genomic organization, transcript variants and comparative analysis of the human nucleoporin 155 (NUP155) gene. Gene. 2002;288:9–18. doi: 10.1016/s0378-1119(02)00470-5. [DOI] [PubMed] [Google Scholar]
- 64.Cai Y, Gao Y, Sheng Q, Miao S, Cui X, Wang L, Koide SS. Characterization and potential function of a novel testis-specific nucleoporin BS-63. Mol Reprod Dev. 2002;61:126–134. doi: 10.1002/mrd.1139. http://dx.doi.org/10.1002/mrd.1139. [DOI] [PubMed] [Google Scholar]
- 65.Casolari JM, Brown CR, Komili S, West J, Hieronymus H, Silver PA. Genome-wide localization of the nuclear transport machinery couples transcriptional status and nuclear organization. Cell. 2004;117:427–439. doi: 10.1016/s0092-8674(04)00448-9. [DOI] [PubMed] [Google Scholar]
- 66.Schmid M, Arib G, Laemmli C, Nishikawa J, Durussel T, Laemmli UK. Nup-PI: the nucleopore-promoter interaction of genes in yeast. Mol Cell. 2006;21:379–391. doi: 10.1016/j.molcel.2005.12.012. http://dx.doi.org/10.1016/j.molcel.2005.12.012. [DOI] [PubMed] [Google Scholar]
- 67.Taddei A, Van Houwe G, Hediger F, Kalck V, Cubizolles F, Schober H, Gasser SM. Nuclear pore association confers optimal expression levels for an inducible yeast gene. Nature. 2006;441:774–778. doi: 10.1038/nature04845. http://dx.doi.org/10.1038/nature04845. [DOI] [PubMed] [Google Scholar]
- 68.Capelson M, Liang Y, Schulte R, Mair W, Wagner U, Hetzer MW. Chromatin-bound nuclear pore components regulate gene expression in higher eukaryotes. Cell. 2010;140:372–383. doi: 10.1016/j.cell.2009.12.054. http://dx.doi.org/10.1016/j.cell.2009.12.054. ••. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kalverda B, Pickersgill H, Shloma VV, Fornerod M. Nucleoporins directly stimulate expression of developmental and cell-cycle genes inside the nucleoplasm. Cell. 2010;140:360–371. doi: 10.1016/j.cell.2010.01.011. http://dx.doi.org/10.1016/j.cell.2010.01.011. ••. [DOI] [PubMed] [Google Scholar]
- 70.Vaquerizas JM, Suyama R, Kind J, Miura K, Luscombe NM, Akhtar A. Nuclear pore proteins nup153 and megator define transcriptionally active regions in the drosophila genome. PLoS Genet. 2010;6:e1000846. doi: 10.1371/journal.pgen.1000846. http://dx.doi.org/10.1371/journal.pgen.1000846. •• These three papers [68••,69••,70••] show for the first time that NPC components are required for gene expression in higher eukaryotes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Smitherman M, Lee K, Swanger J, Kapur R, Clurman BE. Characterization and targeted disruption of murine Nup50, a p27(Kip1)-interacting component of the nuclear pore complex. Mol Cell Biol. 2000;20:5631–5642. doi: 10.1128/mcb.20.15.5631-5642.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Krimm I, Ostlund C, Gilquin B, Couprie J, Hossenlopp P, Mornon JP, Zinn-Justin S. The ig-like structure of the C-terminal domain of lamin A/C, mutated in muscular dystrophies, cardiomyopathy, and partial lipodystrophy. Structure (London, England: 1993) 2002;10:811–823. doi: 10.1016/s0969-2126(02)00777-3. [DOI] [PubMed] [Google Scholar]
- 73.Eriksson M, Brown WT, Gordon LB, Glynn MW, Singer J, Scott L, Collins FS. Recurrent de novo point mutations in lamin A cause Hutchinson–Gilford progeria syndrome. Nature. 2003;423:293–298. doi: 10.1038/nature01629. http://dx.doi.org/10.1038/nature01629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fairley EA, Kendrick-Jones J, Ellis JA. The emery-dreifuss muscular dystrophy phenotype arises from aberrant targeting and binding of emerin at the inner nuclear membrane. J Cell Sci. 1999;112(Pt 15):2571–2582. doi: 10.1242/jcs.112.15.2571. [DOI] [PubMed] [Google Scholar]
- 75.Puckelwartz MJ, Kessler E, Zhang Y, Hodzic D, Randles KN, Morris G, McNally EM. Disruption of nesprin-1 produces an emery dreifuss muscular dystrophy-like phenotype in mice. Hum Mol Genet. 2009;18:607–620. doi: 10.1093/hmg/ddn386. http://dx.doi.org/10.1093/hmg/ddn386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chen CY, Chi YH, Mutalif RA, Starost MF, Myers TG, Anderson SA, Jeang KT. Accumulation of the inner nuclear envelope protein Sun1 is pathogenic in progeric and dystrophic laminopathies. Cell. 2012;149:565–577. doi: 10.1016/j.cell.2012.01.059. http://dx.doi.org/10.1016/j.cell.2012.01.059. •• This study demonstrates that Sun1 overexpression and mislocalization drive pathology of multiple laminopathies. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sullivan T, Escalante-Alcalde D, Bhatt H, Anver M, Bhat N, Nagashima K, Burke B. Loss of A-type lamin expression compromises nuclear envelope integrity leading to muscular dystrophy. J Cell Biol. 1999;147:913–920. doi: 10.1083/jcb.147.5.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Vohanka S, Vytopil M, Bednarik J, Lukas Z, Kadanka Z, Schildberger J, Toniolo D. A mutation in the X-linked emery-dreifuss muscular dystrophy gene in a patient affected with conduction cardiomyopathy. Neuromuscul Disord. 2001;11:411–413. doi: 10.1016/s0960-8966(00)00206-6. [DOI] [PubMed] [Google Scholar]
- 79.De Vos WH, Houben F, Kamps M, Malhas A, Verheyen F, Cox J, Broers JL. Repetitive disruptions of the nuclear envelope invoke temporary loss of cellular compartmentalization in laminopathies. Hum Mol Genet. 2011;20:4175–4186. doi: 10.1093/hmg/ddr344. http://dx.doi.org/10.1093/hmg/ddr344. [DOI] [PubMed] [Google Scholar]
- 80.Vargas JD, Hatch EM, Anderson DJ, Hetzer MW. Transient nuclear envelope rupturing during interphase in human cancer cells. Nucleus (Austin, Tex) 2012;3:88–100. doi: 10.4161/nucl.18954. http://dx.doi.org/10.4161/nucl.18954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Woulfe J, Gray D, Prichett-Pejic W, Munoz DG, Chretien M. Intranuclear rodlets in the substantia nigra: interactions with marinesco bodies, ubiquitin, and promyelocytic leukemia protein. J Neuropathol Exp Neurol. 2004;63:1200–1207. doi: 10.1093/jnen/63.11.1200. [DOI] [PubMed] [Google Scholar]
- 82.Woulfe JM. Abnormalities of the nucleus and nuclear inclusions in neurodegenerative disease: a work in progress. Neuropathol Appl Neurobiol. 2007;33:2–42. doi: 10.1111/j.1365-2990.2006.00819.x. http://dx.doi.org/10.1111/j.1365-2990.2006.00819.x. [DOI] [PubMed] [Google Scholar]
- 83.Bione S, Maestrini E, Rivella S, Mancini M, Regis S, Romeo G, Toniolo D. Identification of a novel X-linked gene responsible for emery-dreifuss muscular dystrophy. Nat Genet. 1994;8:323–327. doi: 10.1038/ng1294-323. http://dx.doi.org/10.1038/ng1294-323. [DOI] [PubMed] [Google Scholar]
- 84.Ho JC, Zhou T, Lai WH, Huang Y, Chan YC, Li X, Esteban MA. Generation of induced pluripotent stem cell lines from 3 distinct laminopathies bearing heterogeneous mutations in lamin A/C. Aging (Milano) 2011;3:380–390. doi: 10.18632/aging.100277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Liu GH, Barkho BZ, Ruiz S, Diep D, Qu J, Yang SL, Izpisua Belmonte JC. Recapitulation of premature ageing with iPSCs from Hutchinson–Gilford progeria syndrome. Nature. 2011;472:221–225. doi: 10.1038/nature09879. http://dx.doi.org/10.1038/nature09879. •• This work achieves successful reprogramming of Hutchinson–Gilford progeria syndrome into IPSCs and the recapitulation of the premature aging phenotype in vitro upon their differentiation. [DOI] [PMC free article] [PubMed] [Google Scholar]