Abstract
Objective
Procyanidins (PC) are widely available natural polyphenols. The present study is designed to investigate if PC can inhibit angiogenesis in lung adenocarcinoma xenografts through crosslinking vascular extracellular matrix (ECM) and preventing proteolysis by matrix metalloproteinases (MMPs).
Methods
Using the in vitro MMP-2 proteolysis and in vivo subcutaneous implantation models, we investigated if PC crosslinking inhibits MMP-mediated proteolysis. Using a cultured cell detachment assay, an in vitro angiogenesis assay, and a cell proliferation assay, we investigated if PC inhibits MMP-2-mediated endothelial cell detachment, angiogenesis, and cell proliferation, respectively. Using tumor xenografts, we evaluated if PC can inhibit growth of lung adenocarcinoma.
Results
PC crosslink vascular ECM proteins, protecting them against proteolysis by MMPs in vitro and in vivo, protecting cultured human umbilical vein endothelial cells from detachment by MMP-2, and inhibiting in vitro angiogenesis. However, PC (0.75-100 µg/ml) did not inhibit vascular and tumor cells proliferation. PC injections (30 mg PC/kg bodyweight) in situ had anticancer effects on xenografts of lung adenocarcinoma, most likely by inhibiting angiogenesis during ECM proteolysis by MMPs.
Conclusion
The results suggest that PC may be important MMP inhibitors that can be used as therapeutic anticancer agents.
Key words: Procyanidins, Crosslinking, Extracellular matrix, Matrix metalloproteinases, Angiogenesis
INTRODUCTION
Angiogenesis accelerates the development of tumors by providing oxygen and nutrients to neighboring cancer tissue. One of the earliest angiogenic events is the proteolytic degradation of extracellular matrix (ECM) of existing vessels, which results in endothelial cell migration, proliferation and formation of new blood vessels [1]. Matrix metalloproteinases (MMPs), a family of structurally related zinc endopeptidases, contribute to the degradation of ECM[1-3] and angiogenic events in tumorigenesis[4]. MMP-2 (collagenase 4), an important member of the MMP family, remains important target for cancer therapy [5] in that it contributes to the hydrolysis of gelatin and type IV collagen[6], which are the major structural component of basement membrane. Previous clinical studies have attempted to treat various types of cancers with MMPs inhibitor monotherapy or in combination with other medicines, but almost of all these treatments were not effective[7].
Procyanidins (PC) are naturally occurring polyphenols and are widely available in various vegetables, nuts, seeds, flowers and bark[8,9]. They are widely used as natural antioxidants, free-radical scavengers and cardiovascular protectors[8,9]. Among them, grape seed PC are composed mainly of dimers, trimers, tetramers and oligomers of catechin/ epicatechin[9]. Although only monomers and dimers are bioavailable and could be metabolized in vivo [10,11], several reports have revealed that PC from different sources with different composition of the aforementioned components can be used as chemopreventive or chemotherapeutic agents for cancers because of their antioxidant and anti-inflammatory properties[12,13]. PC targets NF-kB, mitogen-activated protein kinases, PI3K/Akt, caspases, cytokines, angiogenic, metastatic and cell cycle regulatory proteins and other check points in several cancer cell lines without significant toxicity to non-cancer cells[5,14]. PC from various sources can reduce the expression and secretion of MMP-2 and inhibit its activation and activity[4,5,15-17]. In human umbilical vein endothelial cells (HUVECs), grape seed extract could inhibit capillary tube formation on Matrigel and MMP-2 secretion[18]. However, evidence for in vivo antitumor activity is limited[19,20]. Further research is needed to apply these in vitro anticancer effects, in particular the inhibitory effect on MMPs, to in vivo antitumor growth.
Previously, Han et al[21,22] reported that PC could crosslink collagen by forming hydrogen bonds with proline-rich proteins. Recently, we found that PC could crosslink ECM in porcine heart valves, which are a part of the aorta, and this crosslinking effect is resistant to MMP-8 (collagenase 2) proteolysis[23]. Based on these findings, we speculate that the PC crosslinking may prevent the vascular ECM from proteolysis by MMPs and therefore inhibit tumor angiogenesis. Here, we show that PC-crosslinking could enable vascular ECM resistant to proteolysis by MMPs and protect cells from MMP-2- caused detachment. We also demonstrated significant anticancer effects of PC using a lung cancer xenograft model.
MATERIALS AND METHODS
Chemical Reagents
Grape seed procyanidins (JianfnolR, purity > 98.9%), including dimers (1.8%) and oligomers (60%), were purchased from Tianjin Jianfeng Natural Product Co. Ltd. Irinotecan was purchased from Knowshine Pharmachemicals (Shanghai, China). MMP-2 (Cat. No. 17104-019; 265.00 units/mg) and 3-(4,5-dimethylthiazol-2 -yl)-2,5-di-phenyl tetrazolium bromide (MTT) were bought from Invitrogen. Glutaraldehyde, triton χ-100, sodium deoxycholate, ethylenediaminetetra-acetic acid (EDTA), ribonuclease A and deoxyribonuclease were purchased from Sigma Aldrich. The In Vitro Angiogenesis Assay Kit was obtained from Millipore. Rat anti-CD31 and FITC-conjugated secondary antibodies were purchased from BD Pharmingen and Molecular Probes, respectively.
ECM Preparation and Denaturation Temperature Determination
Heart valve ECM, obtained by treating porcine aortic valves according to a previously described method[23], was used as substrate to determine PC crosslinking. The heart valve, which can be completely decellularized and easily treated in in vivo experiments, is actually a part of the aorta and shares similar structural components such as collagens and elastin. Briefly, ECM was treated with procyanidins (0.01, 0.05, 0.1, 1 and 5 mg/ml) at 37°C under continuous shaking for 4 h. Glutaraldehyde (6.25 mg/ml) was used as a positive control. The thermal denaturation temperature (Td) of crosslinked protein will be increase at some extent according to the crosslinking extent. The Td was determined using differential scanning calorimetry (Model DSC 7, Perkin-Elmer, Boston, MA, USA) as previously reported with slight modification[16–18]. Briefly, weighed samples (n=3) of crosslinked heart valve samples were heated at a rate of 2 oC/min from 28 to 110°C in hermetically sealed aluminum pans. The temperature at the endothermic peak was taken as Td.
In vitro Proteolysis Assay
To evaluate the resistance of PC-crosslinked ECM to hydrolysis by MMPs, the crosslinked ECM was washed with PBS, air-dried and weighed[23]. Dried specimens were immersed in a PBS solution (pH 7.4) containing 1.5 mg/ml MMP-2 and incubated at 37°C for 4 h under continuous shaking. The proteolysis was stopped by adding 50 μl EDTA (10 mmol/L). The residual specimens were dried and weighed again. The degradation rate (ΔW%) was calculated according to the formula: ΔW% = (W0 – Wt)/ W0 × 100, where W0 represents the original weight of each sample and Wt represents the weight of the corresponding sample after proteolysis.
In vivo Proteolysis Assay
Various MMPs can be secreted continuously in the inflammatory process in vivo[1]. To determine whether PC-crosslinking could resist to proteolysis by various MMPs, we further evaluated the degradation of PC-crosslinked ECM using a Sprague Dawley (SD) rat subcutaneous implantation model[24]. SD rats (SLAC Experimental Animal Co., Shanghai, China). with a body weight of 200 ± 2 g, were housed under a standard facility (filtrated air, 20–25°C temperature, relative humidity at 50–60%, 12 h light:dark cycle), and had unlimited access to standard diet and water throughout the experimental period.. Crosslinked ECMs were implanted subcutaneously on the back of the animal (n=4). Three weeks later, the implants were removed and fixed with 2% paraformaldehyde. For histological analysis, the specimens were embedded in paraffin, sectioned and stained with hematoxylin and eosin (HE). All animal experiments were approved by Shanghai Jiao Tong University School of Medicine.
Cells Detachment Assay
HUVECs (Invitrogen) were cultured in M199 medium (Gibco) supplemented with 2 mmol/L glutamine, 100 U/ml penicillin, 100 mg/ml streptomycin and 10% fetal bovine serum (FBS, Sijiqing, Hangzhou, China) in a humidified atmosphere (5% CO2 and 95% air) at 37°C. At 24 h after seeding in 6-well plates at 105 cells/well, the cells were treated with PC for 5 min. After stringent washes that removed procyanidins, 0.5 ml solution containing 0.5 mg/ml MMP-2 was added to the cells. Cells were incubated at 37°C. The number of cells detached from the culture well was measured at 5, 10 and 20 min by a hemacytometer under light microscopy.
In vitro Angiogenesis Assay
The angiogenesis in vitro assay was conducted in 96-well plates coated with ECMatrixTM (Milipore, Cat. No. ECM625), Culture plates (96-well) were coated with ECMatrixTM according to the manufacturer’s instructions. HUVECs (3×104 cells/well) were treated with PC solutions (0.1, 0.5, 1.0, 1.5 and 100 µg/ml) in M199 with 1% FBS. After the cultures were grown at 37ºC for 16 h, the in vitro angiogenesis at the core of microplate wells (n=5) was photographed using an inverted light microscope (Olympus, Japan)[25].
Cell Proliferation Assay
HUVECs were cultured under conditions described above. Lung adenocarcinoma A549 cells (ATCC, USA) were cultured in F-12K medium supplemented 2 mmol/L glutamine, 100 U/ml penicillin, 100 mg/ml streptomycin, and 10% FBS under the same conditions described above for the HUVECs. For the cell proliferation assay, HUVEC and A549 cells were seeded in 96-well plates at a density of 103 cells/well. After 24 h, the culture medium was replaced with fresh medium supplemented with 0, 0.75, 1.5, 3.1, 6.3, 12.5, 25, 50, and 100 μg/ml PC or 1.5 μg/ml irinotecan as a positive control. After 4 d, cell proliferation was determined using the MTT assay[23]. PC solutions were prepared in M199 or F-12K without FBS immediately before use. The irinotecan solution was prepared by dissolving irinotecan powder in distilled water and diluting to the desired concentration.
In vivo Antitumor Assay
Nude mice (SLAC) were obtained with a body weight of 20 ±0.4 g. Mice were housed, fed, and handled under the same conditions as the SD rats. All mice were inoculated with tumor xenografts of lung adeno- carcinoma A549 cells. After one week, conscious mice were randomly divided into three groups. Control mice (n=9) received 0.9% saline in situ injections (100 μl) twice a week for three weeks. Mice in the low dose PC group (n=9, PC10 group) and high dose PC group (n=9, PC30 group) received in situ injections of 10 and 30 mg PC/kg bodyweight in 0.9% saline, respectively. All PC solutions were prepared immediately before injection. Beginning 7 d after the first injection, the tumors were measured every four days with a caliper. Tumor volume (V) was calculated by V=π × [d2 × D]/6, where d and D are the minor and major tumor axis, respectively. After 21 d, the animals were killed and the tumors were weighed and photographed.
Histological and Immunological Fluorescent Staining
After the animals were killed, four tumors from each group were fixed with 2% paraformaldehyde overnight at 4ºC, dehydrated, and embedded in paraffin. The tumors were sectioned and stained with HE for histological analysis. Blood vessels were identified by erythrocytes within the blood vessel lumen[26]. For immunological fluorescent staining of the vascular endothelium, tumor samples were immediately frozen in OCT compound, and serial sections were cut (40 μm) using a cryostat (Leica CM3050S XP, Germany). The sections were probed with the primary antibody rat anti-CD31 and the secondary FITC-conjugated antibody[27]. The images were visualized using a fluorescence microscope (Iχ 51, Olympus, Japan) and quantified using Image-Pro Plus 5.1 (Media Cybernetics, Inc. Bethesda, MD, USA). To determine the number of CD31+ cells, three images were taken of each slice from all four tumors from each group. The fluorescence intensity of each pixel was normalized to the intensity of the control.
Proteolysis of Excised Tumor Using MMP-2
For proteolysis of the excised tumors, five tumors from each group were digested with 1.5 mg/ml MMP-2 at 37ºC under constant rotation (120 rpm). After 48 h, degraded products were filtered through pre-weighed filter paper and air dried to measure the debris weight. The extent of digestion (ΔW%) was calculated by ΔW% = (W0 - Wt)/ W0 × 100, where W0 is the original weight of the tumor and Wt is the weight of the tumor after proteolysis.
Statistical Analysis
All data are presented as mean ± standard deviation. At a minimum, each data point represents five samples. Statistical significance between groups was calculated using two-tailed analysis of variance, performed with a computer statistical program. P<0.05 is considered statistically significant.
RESULTS
Td of PC-crosslinked ECM
The Td of five groups (non-, GA- and PC-crosslinked ECMs) are shown in Table 1. The Td values of PC- crosslinked ECMs increased with the PC concentration increase. The Td of the ECM treated with two lower concentrations of PC was approximately equal to that of the non-crosslinked matrix, and only the highest concentration of PC resulted in a Td (81.3 oC) close to that of GA-crosslinked ECM (91.2 oC), indicating an effective crosslinking of heart valve ECM with higher concentrations of PC.
Table 1. The thermal denaturation temperature (Td) of PC-crosslinked ECM.
| ECM | NONa | GAb | PC0.01c | PC0.1d | PC5e |
|---|---|---|---|---|---|
| Td(oC) | 59.1±0.3 | 89.9±0.4 | 59.1±0.2 | 59.7±0.2* | 81.3±0.4* |
a: NON: ECM without crosslinking; b: GA: glutaraldehyde- crosslinked ECM; c: PC0.01: 0.01 mg /ml PC-crosslinked ECM; d: PC0.1: 0.1 mg/ml PC-crosslinked ECM; e: PC5: 5 mg/ml PC- crosslinked ECM; *P< 0.05 when compared with NON group
PC-crosslinked Vascular ECM Prevents against Hydrolysis by MMP-2 in vitro
After 24 h of crosslinking, the color of non-, GA- and PC-crosslinked valvular ECMs gradually changed from white to red-brown with the PC concentrations increase (Figure 1). After in vitro proteolysis using MMP-2 for 4 h, the degradation rate of all crosslinked samples was significantly lower than that of non-crosslinked samples as shown in Figure 1. The untreated ECM was almost completely hydrolyzed after 4 h proteolysis. By contrast, the GA-crosslinked ECM degraded only 9.44%. PC-crosslinked ECM degraded 44.26% at 0.01 mg/ml and 12.37% at 0.1 mg/ml.
Figure 1.
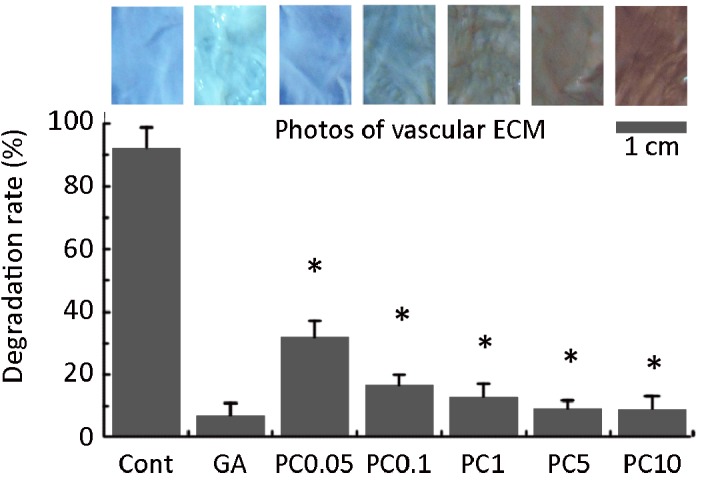
The appearance (top) of PC-crosslinked ECM against blue background and the degradation resistance of crosslinked ECM to proteolysis by MMP-2 in vitro (Bottom). Cont: control; GA: glutaraldehyde; PC0.01 to PC5 were 0.01, 0.05, 0.1, 1 and 5 mg/mL PC crosslinked ECM, respectively. * represents p < 0.01 compared to control. Scale bar is 1 cm.
Resistant Effect to Proteolysis by MMPs in vivo
In vivo experiments showed that, 3 weeks after implantation, most non-crosslinked ECMs had degraded. These non-crosslinked ECMs displayed common inflammatory responses, such as a large number of inflammatory cells and the penetration of native fibroblasts into the ECMs (Figure 2A). Similarly, GA- crosslinked ECMs were also invaded by inflammatory cells and native fibroblasts. By contrast, the ECM crosslinked with 0.1 mg/ml PC maintained its integrity with almost no inflammatory cells (Figure 2C,D) which stayed only close to the ECM surface (Figure 2C). This result demonstrates that PC-crosslinked ECMs are resistant to proteolysis by all kinds of inflammatory MMPs.
Figure 2.
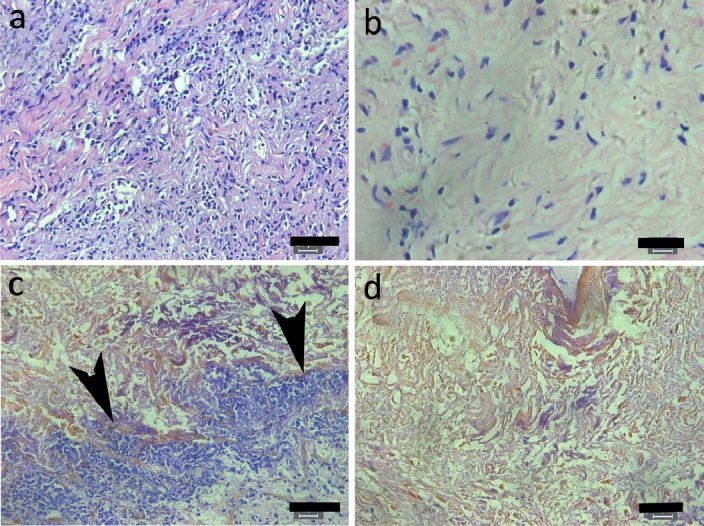
In vivo degradation of PC-crosslinked ECM three weeks after subcutaneous implantation in SD rats. Inflammatory cells and native fibroblasts penetrated in the non-crosslinked (A) and GA-crosslinked (B) ECMs. By contrast, they stayed close to surface (C) and not in the inner part of ECM (D) of 0.1 mg/ml PC- crosslinked ECMs. Arrows indicate inflammatory congregated at the ECM surface. Scale bars in A and C is 50 μm, in B and D 20 μm
Cell Detachment Response to Proteolysis by MMP-2
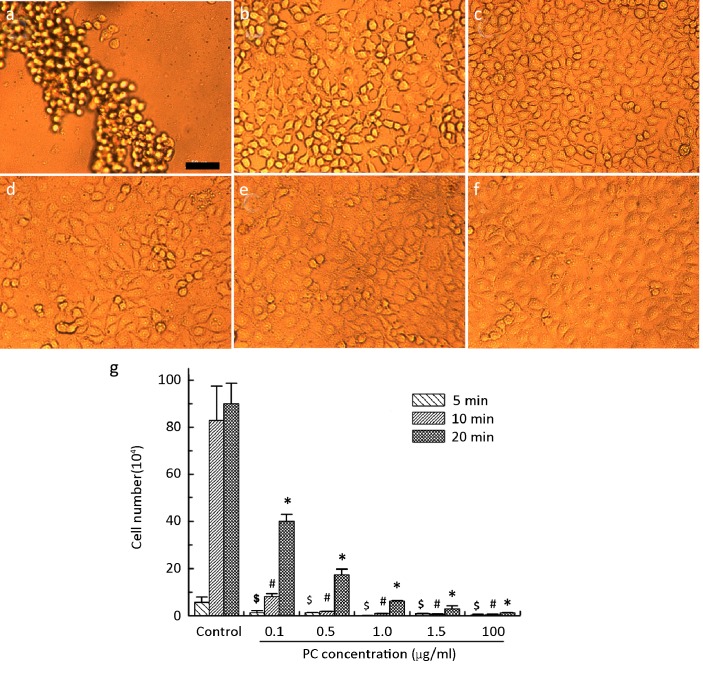
Confluent HUVECs pretreated with PC for 5 min were resistant to MMP-2 proteolysis (Figure 3). After an additional 5 min, few cells had detached from the culture surface in either the PC-treated or control wells. However, after 10 min, most of cells in the control wells had detached from the surface (Figure 3a) while PC-treated cells remained attached and maintained the normal cobblestone-like morphology. As the PC concentration decreased, the cells became gradually isolated from each other, but still displayed no detachment (Figure 3b-f). Over time, the number of detached cells increased in all PC-treated group. Cell detachment was dose dependent with low PC concentrations resulting in more detached cells (Figure 3g). On average, after all cells in the control group had detached, cells in the highest PC concentration group remained almost completely unaffected.
Figure 3.
Detachment of HUVEC cultures treated with 0 (A), 0.1 (B), 0.5 (C), 1 (D), 1.5 (E), and 10 (F) μg/ml PC before enzymatic hydrolysis with 0.5 mg/ml MMP-2 (0.5 ml). (g) The number of cells detached from the PC-pretreated HUVEC cultures for 5, 10, and 20 min after introduction of MMP-2. $, #, and * indicate significant difference (P<0.01) compared to control. Scale bar is 100 μm.
In vitro Angiogenesis Inhibition
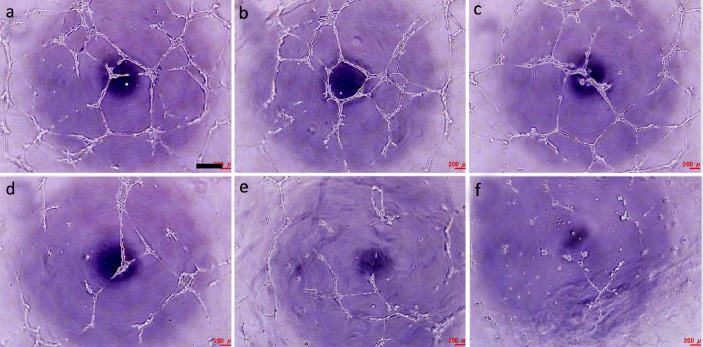
Angiogenesis in vitro assay showed that HUVECs on ECMatrixTM could form abundant mesh-like tubes just like capillary vessels (Figure 4a). When the culture medium supplemented with PC, the mesh gradually became sparsity as PC concentrations increase (Figure 4a -f). At 1.5 μg/ml, only few tubes were formed and at 100 μg/ml the angiogenesis was almost completely inhibited (Figure 4e-f).
Figure 4.
In vitro angiogenesis in the presence of PC at 0 (a), 0.1 (b), 0.5 (c), 1.0 (d), 1.5 (e), and 100 (f) μg/ml. Scale bar is 400 μm.
Cell proliferation in presence of PC
Both HUVECs and A549 cells grown with the positive control irinotecan showed inhibition of proliferation. By contrast, PC failed to inhibit the proliferation of HUVECs (Figure 5a) and A549 cells (Figure 5b) even at the highest concentration.
Figure 5.
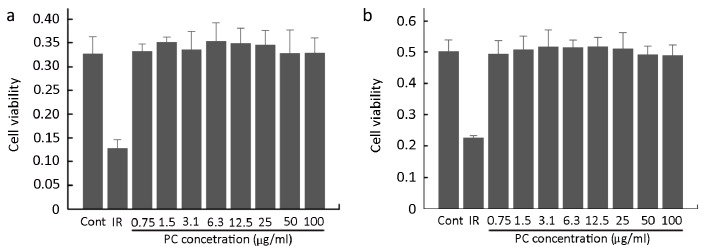
Proliferation of HUVECs (a) and A549 cells (b) after 4 d in culture with PC. IR is 1.5 μg/ml irinotecan.
In vivo Ant-tumor Effect
After mice with tumor xenografts of lung adenocarcinoma were treated with 10 or 30 mg PC/kg bodyweight for 7 d, tumor volumes were almost the same as in the control group. However, after the first week, tumor volume in PC-treated mice increased more slowly compared to control mice, exhibiting a significant antitumor effect at 15 day (22.5% for PC10 and 60.8% for PC30) (Figure 6a). By the end of the experiment, control tumors were larger and more irregularly shaped than tumors from PC-treated mice (Figure 6b). The tumor weights from PC-treated mice were 19.1% (10 mg PC/kg bodyweight, P>0.05) and 45.5% (30 mg PC/kg bodyweight, P<0.05) less than those of control mice (Figure 6c).
Figure 6.
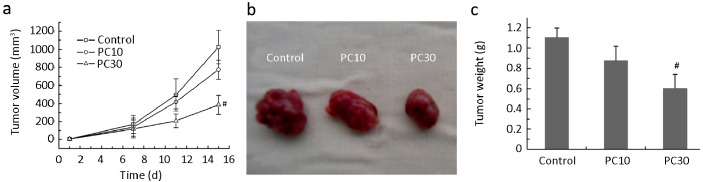
Antitumor effect of PC on tumor volume (a), tumor morphology (b), and tumor weight (c). PC10 and PC30 are 10 and 30 mg PC/kg bodyweight, respectively. # indicates P<0.05 compared to control.
Tumor Histological Characteristics and Angiogenesis
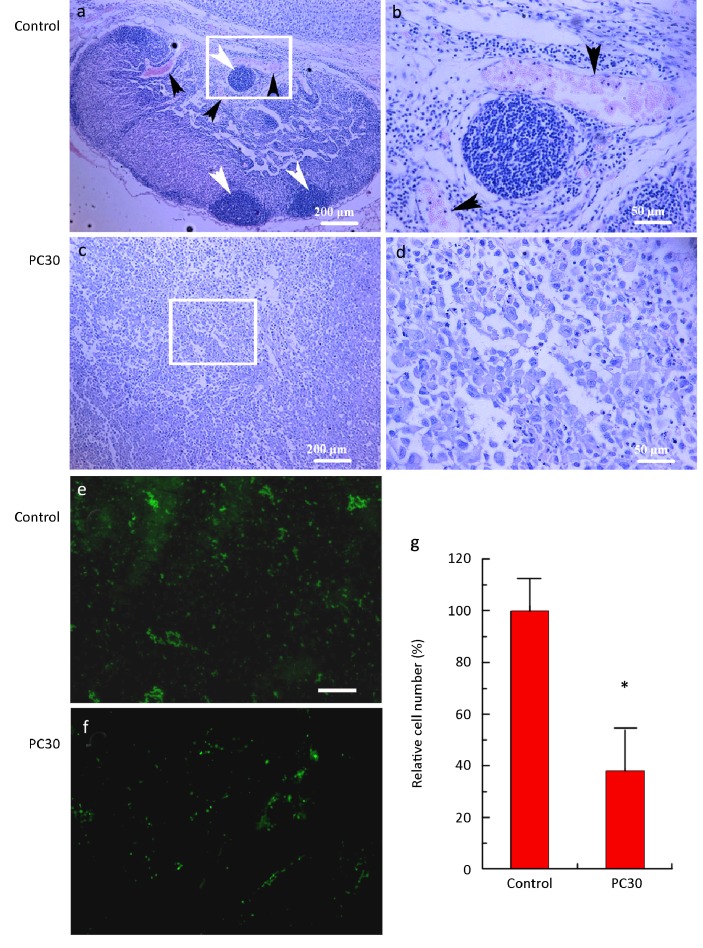
HE staining of control tumors revealed considerable amount of invasive tissue composed of many new invasive sites (Figure 7a). These sites were supplied by abundant vessels with red blood cells within the lumen (Figure 7a-b). By contrast, tumors from PC-treated mice (30 mg PC/kg bodyweight) had few invasive growth sites and almost no discernable vessels, resulting in a large necrotic area (Figure 7c-d). In tumors from these PC-treated mice, angiogenesis was inhibited (Figure 7e-f). Immunofluorescent staining showed that the fluorescence intensity in PC (30 mg/kg) group was only 38.2% ± 8.9% to that in control group, suggesting that the CD 31+ cells is much less in the PC (30 mg/kg) group than in control group (Figure 7e,f-g). Because endothelial cells are CD 31+ cells, this result demonstrated that the endothelial cells of capillary vessels in the tumor tissue were lower in PC group than in control, indicating that PC inhibits angiogenesis.
Figure 7.
HE staining of lung adenocarcinoma tissue in control (a) and PC30 (c) treatments. PC30 is 30 mg PC/kg bodyweight. Areas in the white rectangles are magnified 4× for control (b) and PC30 (d) treatments. For (a) and (c), the scale bar is 200 μm. For (b) and (d), the scale bar is 50 μm. White arrow head indicates invasive growth sites. Black arrow head indicates blood vessel. The images (e) and (f) (40×, bar = 200 μm) were the sections probed with anti-cd31+ cells (green). (g) The fluorescence intensity of cd31+ cells normalized to control (n=6). *indicates P<0.05 compared to control.
In vivo resistance to proteolysis by MMP-2
Following a 48-hour treatment with MMP-2, tumors in the control group were almost completely degraded. By contrast, tumors from mice treated with PC (30 mg PC/kg bodyweight) were significantly resistant to proteolysis and exhibited almost no degradation (Figure 8). This result suggests that PC crosslinking in the tumor tissue prevented hydrolysis by MMP-2.
Figure 8.
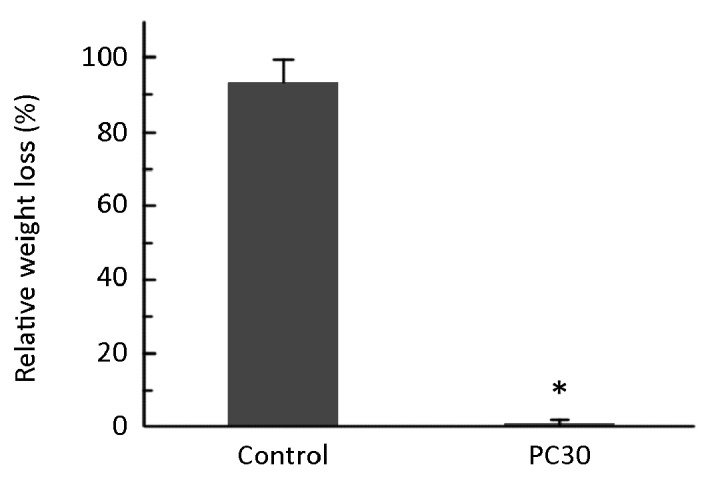
Relative weight loss of tumors after MMP-2 proteolysis for 48 h. PC30 is 30 mg PC/kg bodyweight. *indicates P<0.05 compared to control
DISCUSSION
Previous studies demonstrated an in vitro anticancer effect of PC through several pathways by inhibiting the activity of angiogenic factors, such as MMP-2 and MMP-9. Because PC are non-toxic and exist in everyday foods, these in vitro anticancer effects have garnered much attention. However, few of these anticancer effects have been confirmed in animal models or clinical trials[19,20]. Previous studies revealed that PC can easily bind to vascular endothelial tissues[9]. Therefore, intravenous injections may result in a large loss of PC, while they circulate through the blood. Because of poor digestion and absorption, oral administration of PC provides only a limited amount of monomeric and dimeric PC in the bloodstream[10,11]. These disadvantages might markedly decrease the PC concentration in the target tissue, and most of the in vitro anticancer effect may disappear in vivo. To overcome these disadvantages, we used in situ injection to deliver PC, and found that at a dose of 30 mg PC/kg bodyweight, the vascular ECM is crosslinked by PC, providing resistance to proteolysis by MMP and inhibiting angiogenesis-mediated tumor growth.
During inflammatory period in vivo, the MMPs are secreted from inflammatory cells to degrade ECM proteins[1]. The Td determination result evidently demonstrated the PC crosslinking effect on vascular ECM (Table 1). In present research, we showed that PC crosslinking of ECM (Table 1) limits both in vitro and in vivo proteolysis by MMPs (Figure 1, 2), suggesting that PC crosslinking inhibits the initial step of tumor angiogenesis[28]. This protection against proteolysis by MMPs was confirmed in the direct hydrolysis of tumors by MMP-2 (Figure 8). Additionally, ECM proteolysis by MMPs may free cells from existing blood vessels to move to neovascularization sites[29-31]. In this study, PC inhibited HUVECs detachment from the culture surface (Figure 3), suggesting that PC pretreatment could inhibit angiogenesis by preventing the movement of endothelial cells to the neovascular sites. Based on the detachment-resistant effect of PC-crosslinking, this effect must be also due to PC crosslinking on the living cellular ECM. Furthermore, PC may effectively inhibit endothelial cells forming new vessels in tumor tissue (Figure 4).
Recently, Levental, et al[32] reported that matrix covalent crosslinking remodels the ECM by stiffening and enhancing integrin signaling, which causes tumor progression. However, PC crosslinking is due to hydrogen bonds, not covalent bonds, resulting in crosslinking with hydroxyproline in ECM proteins and maintaining a soft and pliable tissue[9,21-23]. Proline is an efficient hydrogen bond acceptor, so PC has an extremely high affinity for proline-rich proteins[9,22,33-36]. ECM proteins collagen and elastin are proline-rich, which promote PC to form hydrogen bonds[22,23]. Therefore, PC crosslinking may obstruct MMPs from accessing their substrate ECM proteins, offering protection for ECM from proteolysis. The present data demonstrated that PC crosslinking directly reduced the proteolysis by MMPs on the ECM, which subsequently inhibited angiogenesis in tumor xenografts.
We also observed that lung cancer xenograft growth was significantly inhibited by PC injection in situ (Figure 6). Considering that PC did not inhibit HUVEC and A549 proliferation in vitro (Figure 5), this in vivo inhibition does not result from PC cytotoxicity. Instead, it is more likely that the anti-angiogenesis effect resulted from inhibiting proteolysis by MMPs. Accordingly, HE and immuno- fluorescent staining revealed that PC crosslinking reduced the tumor angiogenesis (Figure 7).
Recently, Punathil[37] and Akhtar[38] reported that non-small cell lung cancer cells, including A549 cells, were inhibited in vitro by grape seed PC at concentrations from 20 to 60 μg/mL. These concentrations are lower than those used here. This difference is likely due to a difference in the PC combination and relative ratio of monomers, dimers and oligomers. Here, a PC combination of 1.8% dimers and 60% oligomers, exerted an anticancer effect on A549 lung cancer cells by inhibiting MMP-mediated angiogenesis through crosslinking existing vascular ECM.
In conclusion, results of this study revealed that PC effectively inhibited tumor angiogenesis, at least in part by inhibiting proteolysis by MMPs, detachment of endothelial cells and the formation of capillary vessels. Therefore, PC can exert an anticancer effect on xenografts of lung adenocarcinoma. These results suggested that PC may be used as MMPs inhibitors in anticancer therapies.
REFERENCES
- 1.Kessenbrock K, Plaks V, Werb Z.Matrix Metalloproteinases: Regulators of the Tumor Microenvironment. Cell 2010; 141: 52-67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Visse R, Nagase H.Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ Res 2003; 92: 827-39 [DOI] [PubMed] [Google Scholar]
- 3.Galis ZS, Khatri JJ. Matrix metalloproteinases in vascular remodeling and atherogenesis: the good, the bad, and the ugly. Circ Res 2002; 90: 251-62 [PubMed] [Google Scholar]
- 4.Oak MH, EI Bedoui J, Schini-Kerth VB. Antiangiogenic properties of natural polyphenols from red wine and green tea. J Nutri Bioch 2005; 16:1-8 [DOI] [PubMed] [Google Scholar]
- 5.Nandakumar V, Singh T, Katiyar SK. Multi-targeted prevention and therapy of cancer by proanthocyanidins. Cancer Lett 2008; 269: 378-87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stamenkovic I.Matrix metalloproteinases in tumor invasion and metastasis. Semin Cancer Biol 2000; 10: 415-33 [DOI] [PubMed] [Google Scholar]
- 7.Coussens LM, Fingleton B, Matrisian LM. Matrix Metalloproteinase Inhibitors and Cancer: Trials and Tribulations. Science 2002; 295: 2387-92 [DOI] [PubMed] [Google Scholar]
- 8.Yamakoshi J, Saito M, Kataoka S, et al. Safety evaluation of proanthocyanidins-rich extract from grape seeds. Food Chem Toxicol 2002; 40: 599-607 [DOI] [PubMed] [Google Scholar]
- 9.Packer L, Rimbach G, Virgili F.Antioxidant activity and biologic properties of a procyanidin-rich extract from pine (Pinus Maritima) bark, pycnogenol. Free Radic Biol Med 1999; 27: 704-24 [DOI] [PubMed] [Google Scholar]
- 10.Gonther MP, Donovan JL, Texier O, et al. Metabolism of dietary procyanidins in rats. Free Radic Boil Med 2003; 35: 837-44 [DOI] [PubMed] [Google Scholar]
- 11.Urpi-Sarda M, Monagas M, Khan N, et al. Epicatechin, procyanidins, and phenolic microbial metabolisms after cocoa intake in humans and rats. Anal Bioanal Chem 2009; 394: 1545-56 [DOI] [PubMed] [Google Scholar]
- 12.Mantena SK, Baliga MS, Katiyar SK. Grape seed proanthocyanidins induce apoptosis and inhibit metastasis of highly metastatic breast carcinoma cells. Carcinogenesis 2006; 27: 1682-91 [DOI] [PubMed] [Google Scholar]
- 13.Singh RP, Tyagi AK, Dhanalakshmi S, et al. Grape seed extract inhibits advanced human prostate tumor growth and angiogenesis and up-regulates insulin-like growth factor binding protein-3. Int J Cancer 2004; 108: 733-40 [DOI] [PubMed] [Google Scholar]
- 14.Mittal A, Elmets CA, Katiyar SK. Dietary feeding of proanthocyanidins from grape seeds prevents photocarcino- genesis in SKH-1 hairless mice: relationship to decreased fat and lipid peroxidation. Carcinogenesis 2003; 24: 1379-88 [DOI] [PubMed] [Google Scholar]
- 15.Oak MH, EI Bedoui J, Anglard P, et al. Red wine polyphenolic compounds strongly inhibit pro-MMP-2 expression and its activation in response to thrombin via direct inhibition of MT1-MMP in vascular smooth muscle cells. Circulation 2004; 110:1861-7 [DOI] [PubMed] [Google Scholar]
- 16.Strek M, Gorlach S, Podsedek A, et al. Procyanidin oligomers from Japanese quince (Chaenomeles japonica) fruit inhibit activity of MMP-2 and MMP-9 metalloproteinases. J Agric Food Chem 2007; 55: 6447-52 [DOI] [PubMed] [Google Scholar]
- 17.Vayalil PK, Mittal A, Katiyar SK. Proanthocyanidins from grape seeds inhibit expression of matrix metalloproteinases in human prostate carcinoma cells which is associated with the inhibition of activation of MAPK and NFkB. Carcinogenesis 2004; 25: 987-95 [DOI] [PubMed] [Google Scholar]
- 18.Agarwal C, Singh RP, Dhanalakshmi S, et al. Anti-angiogenic efficacy of grape seed extract in endothelial cells. Oncol Rep 2004; 11: 681-5 [PubMed] [Google Scholar]
- 19.Meeran SM, Katiyar SK. Proanthocyanidins inhibit mitogenic and survival-signaling in vitro and tumor growth in vivo. Front Biosci 2008; 13: 887-97 [DOI] [PubMed] [Google Scholar]
- 20.Nassiri-Asl M, Hosseinzadeh H.Review of the pharmacological effects of vitis vinifera (Grape) and its bioactive compounds. Phytother Res 2009; 23:1197-204 [DOI] [PubMed] [Google Scholar]
- 21.Han B, Jaurequi J, Tang WB, Nimni ME. Proanthocyanidin: a natural crosslinking reagent for stabilizing collagen matrix. J Biomed Mater Res 2003; 65A:118-24 [DOI] [PubMed] [Google Scholar]
- 22.Hagerman AE, Butler LG. The specificity of proanthocyanidin- protein interactions. J Biol Chem 1981; 256: 4494-7 [PubMed] [Google Scholar]
- 23.Zhai W, Chang J, Lin K, et al. Crosslinking of decellularized porcine heart valve matrix by procyanidins. Biomaterials 2006; 27: 3684-90 [DOI] [PubMed] [Google Scholar]
- 24.Cheung DT, Tong D, Perelman N, et al. Mechanism of crosslinking of proteins by glutaraldehyde. IV: In vitro and in vivo stability of a crosslinked collagen matrix. Connect Tissue Res 1990; 25: 27-34 [DOI] [PubMed] [Google Scholar]
- 25.Heinke J, Wehofsits L, Zhou Q, et al. BMPER is an endothelial cell regulator and controls bone morphogenetic protein-4-dependent angiogenesis. Circ Res 2008; 103: 804-12 [DOI] [PubMed] [Google Scholar]
- 26.Day RM, Boccaccini AR, Shurey S, et al. Assessment of polyglycolic acid mesh and bioactive glass for soft-tissue engineering scaffolds. Biomaterials 2004; 25: 5857-66 [DOI] [PubMed] [Google Scholar]
- 27.Fischer C, Jonckx B, Mazzone M, et al. Anti-PlGF inhibits growth of VEGF(R)-inhibitor-resistant tumors without affecting healthy vessels. Cell 2007; 131: 463-75 [DOI] [PubMed] [Google Scholar]
- 28.Cook KM, Figg WD. Angiogenesis inhibitors: current strategies and future prospects. CA Cancer J Clin 2010; 60: 222-43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gingras D, Bousquet-Gagnon N, Langois S, et al. Activation of the extracellular signal regulated signal-regulated protein kinase (ERK) cascade by membrane type-1matrix metalloproteinase (MT1-MMP). FEBS Lett 2001; 507: 231-6 [DOI] [PubMed] [Google Scholar]
- 30.Kiran MS, Sameer-Kumar VB, Viji RI, et al. Temporal relationship between MMP production and angiogenic process in HUVECs. Cell Biol Int 2006; 30: 704-13 [DOI] [PubMed] [Google Scholar]
- 31.Yu Q, Stamenkovic I.Localization of matrix metalloproteinase 9 to the cell surface provides a mechanism for CD44-mediated tumor invasion. Genes Dev 1999; 13: 35-48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Levental KR, Yu H, Kass L, et al. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell 2009; 139: 891-906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shahat AA, Cos P, De Bruyne T, et al. Antiviral and antioxidation activity of flavonoids and proanthocyanidins from Crataegus sinaica. Planta Med 2002; 68: 539-41 [DOI] [PubMed] [Google Scholar]
- 34.Nakane H, Ono K.Differential inhibitory effects of some catechin derivatives on the activities of human immunodeficienct virus reverse transcriptase and cellular deoxyribonucleic and ribonucleic acid polymerase. Biochemistry 1990; 29: 2841-5 [DOI] [PubMed] [Google Scholar]
- 35.Austin CA, Patel S, Ono K, et al. Site-specific DNA cleavage by mammalian DNA topoisomerase II induced by novel flavone and catechin derivatives. Biochem J 1992; 282: 883-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Slusarewicz P, Zhu K, Hedman T.Kinetic characterization and comparison of various protein crosslinking reagents for matrix modification. J Mater Sci Mater Med 2010; 21: 1175-81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Punathil T, Katiyar SK. Inhibition of Non-small Cell Lung Cancer Cell Migration by Grape Seed Proanthocyanidins Is Mediated Through the Inhibition of Nitric Oxide, Guanylate Cyclase, and ERK1/2. Mol Carcinog 2009; 48: 232-42 [DOI] [PubMed] [Google Scholar]
- 38.Akhtars, Meeran SM, Katiyar N, et al. Grape seed proanthocyanidins inhibit the growth of human non-small cell lung cancer xenografts by targeting insulin-like growth factor binding protein-3, tumor cell proliferation, and angiogenic factors. Clin Cancer Res 2009; 15: 821-31. [DOI] [PubMed]