Abstract
Our previous studies have shown that hyperbaric oxygen preconditioning (HBO-PC) induces tolerance to cerebral ischemia/reperfusion (I/R). This study aimed to investigate whether SirT1, a class III histone deacetylase, is involved in neuroprotection elicited by HBO-PC in animal and cell culture models of ischemia. Rats were subjected to middle cerebral artery occlusion for 120 minutes after HBO-PC (once a day for 5 days). Primary cultured cortical neurons were exposed to 2 hours of HBO-PC after 2 hours of oxygen–glucose deprivation (OGD). We showed that HBO-PC increased SirT1 protein and mRNA expression, promoted neurobehavioral score, reduced infarct volume, and improved morphology at 24 hours and 7 days after cerebral I/R. Neuroprotection of HBO-PC was attenuated by SirT1 inhibitor EX527 and SirT1 knockdown by short interfering RNA (siRNA), whereas it was mimicked by SirT1 activator resveratrol. Furthermore, HBO-PC enhanced SirT1 expression and cell viability and reduced lactate dehydrogenase release 24 hours after OGD/re-oxygenation. The neuroprotective effect of HBO-PC was emulated through upregulating SirT1 and, reversely, attenuated through downregulating SirT1. The modulation of SirT1 was made by adenovirus infection carrying SirT1 or SirT1 siRNA. Besides, SirT1 increased B-cell lymphoma 2 (Bcl-2) expression and decrease cleaved caspase 3. These results indicate that SirT1 mediates HBO-PC-induced tolerance to cerebral I/R through inhibition of apoptosis.
Keywords: cerebral ischemia/reperfusion, hyperbaric oxygen preconditioning, neuroprotection, oxygen–glucose deprivation, SirT1
Introduction
Ischemic stroke remains a major cause of morbidity and mortality worldwide, leading to severe neurologic disability in adults. There is still lack of approaches to effectively lessen brain damage induced by ischemia in clinic. Therefore, the development of novel treatment strategies is urgently needed to improve the prognosis of cerebral ischemia. We have shown previously that hyperbaric oxygen preconditioning (HBO-PC), which consists of exposure to 2.5 ATA (atmospheres absolute) pressure for 60 minutes per day for 5 consecutive days, induces ischemic tolerance to ischemia/reperfusion (I/R) in brain and spinal cord in both in vivo and in vitro experiments.1, 2 Recently, HBO-PC has been applied to improve myocardial function and reduce myocardial injury after coronary artery bypass graft surgery,3 suggesting that HBO-PC is a safe and feasible method and might be potentially promising to provide neuroprotective benefits for ischemic stroke in clinic. There are evidences that upregulation of hypoxia-inducible factor-1α or suppression of matrix metalloproteinase-9 may be involved in the neuroprotective effect of HBO-PC.4, 5 In addition, we have shown that the production of reactive oxygen species (ROS) at a nonlethal level or upregulated activity of antioxidant enzymes play an important role in HBO-PC-induced ischemic tolerance.6, 7 However, the precise mechanism underlying HBO-PC has not been fully elucidated and more evidence is needed for HBO-PC to be used clinically.
The SirT1 is a class III histone deacetylase belonging to mammalian sirtuin family that generates enzyme activity in the presence of NAD+.8 It is reported that increase of SirT1 activity promotes the lifespan in lower organisms, such as yeast, Caenorhabditis elegans, and Drosophila melanogaster.9, 10, 11 Furthermore, SirT1 is upregulated in response to stress-induced cell death in mammals, especially at oxidative stress and calorie restriction, whereas SirT1 knockout mice fail to extend the lifespan induced by calorie restriction.12 The SirT1 is also beneficial to central nervous system. A representative example is that SirT1 activity is responsible for axonal protection in Wallerian degeneration slow mice that show a markedly delay in axonal degeneration.13 Moreover, expression of SirT1 is increased to promote neuronal survival in the model of Alzheimer's disease or in primary neurons subjected to neurotoxic insult.14 These findings suggest that SirT1 may play an important role in neuroprotection.
Several studies have implicated the protective effect of SirT1 against ischemic insult in the heart. It was reported that cardiac I/R injury is aggravated in cardiac-specific SirT1 knockout mice in comparison with wild-type ones.15 In addition, SirT1 has a link to ischemic preconditioning. The SirT1 activity is increased during ischemic preconditioning and has a role in the protective mechanism of ischemic preconditioning in mice heart.16 Recently, SirT1 has also been found to be associated with ischemic damage in brain. Pretreatment with resveratrol, an activator of SirT1, produces neuroprotection against cerebral ischemia in the model of organotypic hippocampal slice culture.17 However, until now, no evidence has shown if SirT1 expression is in association with HBO-PC-induced ischemic tolerance to cerebral I/R injury. In the present study, we examined the expression of SirT1 after HBO-PC and assessed the potential role of SirT1 in neuroprotective effect of HBO-PC by upregulating or downregulating SirT1 expression in the rat model of focal cerebral ischemia induced by middle cerebral artery occlusion (MCAO) and primary cultured cortical neurons subjected to oxygen–glucose deprivation (OGD) injury.
Materials and Methods
Animals
All animal experiments were approved by the Ethics Committee for Animal Experimentation and were carried out in accordance with the NIH Guidelines for Care and Use of the Laboratory Animals. Sprague-Dawley rats, weighing 280 to 320 g, were obtained from the animal center of Fourth Military Medical University.
Hyperbaric Oxygen Preconditioning
The protocol for HBO-PC has been described in our previous studies.2, 6 Briefly, rats received 1 hour of HBO at 2.5 ATA in 100% oxygen each day for 5 consecutive days, or primary cortical neurons were subjected to HBO for 2 hours at 3.5 ATA in a hyperbaric chamber (Model No. NG90-IIC, Yantai Binglun, Yantai, China). Compression and decompression were performed at a rate of 0.2 atm/min. The oxygen concentration was monitored with a calibrated oxymeter. Accumulation of carbon dioxide was absorbed by calcium carbonate crystals. Chamber temperature was kept at 23 to 26°C in rats or 37°C in neurons.
Transient Focal Cerebral Ischemia
Focal cerebral ischemia was performed using the method of right MCAO with an intraluminal filament as described previously.18 At 2 hours after the induction of ischemia, the filament was slowly withdrawn. The CBF was monitored using laser Doppler flowmetry (Perimed AB, PeriFlux System 5000, Stockholm, Sweden) in the ipsilateral cortex (2 mm posterior and 5 mm lateral to bregma).
Neurobehavioral Evaluation and Infarction Assessment
At 24 hours or 7 days after reperfusion, an 18-point scoring system reported by Garcia et al19 was used to evaluate the neurologic deficits in rats. The minimum neurobehavioral score is 3 and the maximum is 18 that represents the rat is normal. Then, rats were decapitated. The brains were cut into six 2-mm-thick coronal sections and immersed in 1% 2,3,5-triphenyltetrazolium chloride (TTC) (Sigma-Aldrich, St Louis, MO, USA, T8877) at 37°C for 10 minutes. Unstained areas were defined as infarcts and were measured using image analysis software (Adobe Photoshop CS3, San Jose, CA, USA). The percentage of the infarct volume was calculated with the following formula: (total contralateral hemispheric volume−total ipsilateral hemispheric stained volume)/total contralateral hemispheric volume × 100.
Ischemic Penumbra Dissection
The ischemic penumbra was determined according to the methods described by Ashwal et al.20 Briefly, the brain was sectioned into three slices beginning 3 mm from the anterior tip of the frontal lobe. The front and back slices were 3 mm in thickness. The middle slice was 4 mm in thickness, which was cut longitudinally in the ischemic hemisphere 2 mm from the midline. A transverse diagonal cut was made at the 2 O'clock position to separate the core from the penumbra.
Western Blot Analysis
The ischemic penumbra of cerebral cortex or cultured neurons were homogenized, and the equivalent extracted protein was separated using sodium dodecyl sulfate–polyacrylamide gel electrophoresis and transferred onto a nitrocellulose filter membrane as previously described.18 The following primary antibodies were used: anti-SirT1 (1:1,000, Biochemical, Santa Cruz, CA, USA, sc-74465), anti-caspase-3 (1:1,000, Sigma, St Louis, MO, USA, C8487), or anti-Bcl-2 (1:500, Cell Signaling, Beverly, MA, USA, 2876). A β-actin antibody (1:500; Biosynthesis, Beijing, China, bs-0061R) was used as an internal control. The antigen–antibody complexes were observed using the enhanced chemiluminescence system (Thermo, Rockford, IL, USA, 32106). The density of each protein band was quantified using the Gelpro32 software (Media Cybernetics Inc., Rockville, MD, USA).
Quantitative Real-Time PCR
The anesthetized rats were decapitated and the brains were removed 3 hours after reperfusion. The total RNA was extracted from ischemic penumbra of cerebral cortex using Trizol reagent (Invitrogen, Carlsbad, CA, USA, 15596026). Total RNA (10 ng) was reversely transcribed into single-stranded complementary DNA with PrimeScript RT reagent Kit (TaKaRa, Dalian, China, DRR037S) following the manufacturer's instructions, and used for real-time PCR. Amplification and quantification were carried out with SYBR Premix Ex Taq II (TaKaRa, DRR081A) and MJ Research real-time PCR system (Bio-Rad, Hercules, CA, USA). Reactions were performed in a 25 μL reaction mixture consisting of 12.5 μL 2 × SYBR Green, 10 ng RNA, and 0.4 μmol/L of each primer, and run in triplicate using the under the following conditions: 95°C for 2 minutes, followed by 45 cycles of 95°C for 15 seconds, and 62°C for 1 minute. The primers used in quantitative real-time PCR were SirT1 (forward 5o-TCATTCTGACTGTGATGACGA-3′ and reverse 5anCTGCCACAGTGTCATATCCAA-3′), and glyceraldehyde 3-phosphate dehydrogenase (GAPDH, forward 5o-GGCACAGTCAAGGCTGAGAATG-3T and reverse 5anATGGTGGTGAAGACGCCAGTA-3A). Glyceraldehyde 3-phosphate dehydrogenase was used as endogenous control.
Double Immunofluorescence Staining
Anesthetized rats were transcardially perfused 3 hours after reperfusion, and brains were postfixed and dehydrated as previously described.18 Coronal serial sections were cut by a freezing microtome (Leica, Heidelberg, Germany, CM1850) at a thickness of 10 μm. The sections were incubated with a mixture of two primary antibodies: a mouse anti-rat neuronal nuclei (NeuN; 1:2,000, Millipore, Billerica, MA, USA, MAB377) and a rabbit polyclonal anti-SirT1 (1:200, Santa Cruz, sc-15404). A donkey anti-rabbit antibody that was conjugated with green-fluorescent Alexa Fluor 488 (1:800; Invitrogen, A21206) and a donkey anti-mouse antibody that was conjugated with red-fluorescent Alexa Fluor 594 (1:400; Invitrogen, A21203) were used to show immunoreactivity. Fluorescent signals were observed under confocal laser scanning microscopy (Olympus, Tokyo, Japan, FV1000).
Hematoxylin–Eosin Staining
The protocol for fixation of the rats and treatment of brain blocks was the same as described in double immunofluorescence staining. The brain blocks containing ischemic penumbra were embedded in paraffin wax and sectioned at 6 μm thickness. After dewaxing, the sections were stained with hematoxylin and eosin (HE) according to standard protocol, and observed under light microscope.
Intracerebroventricular Injection
Anesthetized rats were placed in a stereotaxic apparatus. A burr hole was drilled into the bone 1.5 mm lateral to and 1.0 mm posterior to bregma over the right hemisphere. A stainless-steel 26-gauge cannula (Plastic One, Roanoke, VA, USA, C315G) was slowly introduced through the burr hole into the right lateral ventricle (3.8 mm beneath the dural surface). The cannula was fixed using dental cement and four stainless-steel screws that were secured to the skull in advance. Reagent was infused into the right lateral ventricle at a rate of 0.5 μL/min.
Administration of SirT1 Inhibitor or Activator In Vivo
The SirT1 inhibitor EX527 (Tocris Bioscience, Bristol, UK, 2780) was first dissolved in dimethyl sulfoxide (DMSO) and then diluted to the final concentration with normal saline (15 μL, the final DMSO concentration <2%). Intracerebroventricular (i.c.v.) injection of EX527 at the dose of 1, 10, and 30 μg or the same volume of vehicle was performed 30 minutes before the onset of HBO every 2 days for 3 times. Resveratrol (Res, 50 mg/kg, Sigma, R5010), which was dissolved in 2 mL deionized water or the corresponding volume of vehicle, was administered intragastrically in rats for 15 days before MCAO. The dosage of resveratrol was adopted according to a published study.21
Transfection of Short Interfering RNA in Rat Brain
We performed in vivo short interfering RNA (siRNA) transfection in rat brain according to the method described by Chen et al.22 The transfection complex of SirT1 siRNA was prepared in the light of the manufacturer's instructions. Briefly, 5 μg SirT1 siRNA (Qiagen, Hilden, Germany, 1027417) was dissolved in 5 μL RNase-free water. Next, 5 μL SirT1 siRNA, 5 μL control siRNA (Qiagen, 1027280), and 5 μL Entranster-in vivo transfection reagent (Engreen, Beijing, China, 18668-11) were respectively diluted with 5 μL 10% glucose to get a final concentration of 5% glucose. Then, 10 μL Entranster-in vivo was added to 10 μL SirT1 siRNA or 10 μL control siRNA immediately. The solution was mixed for 15 minutes at room temperature. In ischemic hemisphere, 20 μL Entranster-in vivo–siRNA mixture was delivered into the lateral ventricle.
Primary Cortical Neuron Culture
Primary cortical neurons were obtained from embryonic day 17 Sprague-Dawley rats. Cortical neurons were dissociated and suspended in neurobasal media (Invitrogen, 12348017) containing B-27 supplement (Invitrogen, 17504044) and 0.5 mmol/L ℒ-glutamine (Sigma-Aldrich, G3126), and seeded onto plates coated with 0.01% poly-𝒟-lysine (Sigma-Aldrich, P7280) at a density of 3 × 105 cells/cm2 and maintained in an incubator with 95% air and 5% CO2 at 37°C. Half of the culture media was changed every 3 days. Neurons were used at 7 days after plating. The purity of the neuronal cultures was assessed by neuron-specific marker β-tubulin III (Sigma-Aldrich, T8578) and 4',6-diamidino-2-phenylindole (1 ng/μL; Sigma-Aldrich, D9542) staining showing the culture was higher than 95% neurons.
Oxygen–Glucose Deprivation
Oxygen–glucose deprivation was initiated after cortical neurons were cultured for 9 days. The neurons were first washed with phosphate-buffered saline three times and incubated in phosphate-buffered saline. Then, the neurons were placed in an incubator with constant 5% CO2 and 95% N2 atmosphere at 37°C for 2 hours. Finally, the neurons were incubated again in the culture media in the incubator with 5% CO2 for 24 hours.
Neuronal Survival Analysis
Primary cortical neurons were plated in 96-well plates. The cell viability was evaluated by 3-(4,5-22dimethylthiazol-2-yl)-2,5- diphenyltetrazolium bromide (MTT) assay. At 24 hours after re-oxygenation, the culture media was replaced with fresh culture media supplemented with 0.5 mg/mL MTT and incubated for 4 hours in the incubator. The supernatant was discarded and 100 μL DMSO was added into each well for 10 minutes to dissolve the formazan crystals. Absorbance was measured at 490 nm with an ELISA plate reader (TECAN, Männedorf, Switzerland). Cell viability was expressed as a percentage of the value of the control culture. Each experiment was repeated in triplicate using three independent cultures.
The neuron death was assessed by the lactate dehydrogenase (LDH) release using LDH assay kit (Jiancheng, Nanjing, China, A020-1). At 24 hours after re-oxygenation, the culture media were harvested and lysed with 1% Triton X-100 for 30 minutes at 37°C to release the intracellular LDH. In both the culture media and neuron lysates, LDH was detected according to the manufacturer's instructions. The absorbance was measured at 490 nm with a spectrophotometer (Bio-Rad Laboratories, Hercules, CA, USA). The LDH release is expressed as a percentage of LDH values of total neurons.
Adenovirus Infection In Vivo
Recombinant adenovirus coding for green fluorescent protein (GFP), including adenovirus expressing SirT1 (Ad-SirT1), control adenovirus (Ad-NC), and adenovirus carrying SirT1 interfering RNA (Ad-siRNA-SirT1) or control siRNA (Ad-siRNA-NC), were gifted from the Key Laboratory of Digestive Diseases, Xijing Hospital (Xi'an, China). The adenoviral stocks were amplified and purified to be 10 × 10 PFU/mL by Shanghai Genechem (Shanghai, China) and stored. At 7 days after cell culture, primary cortical neurons were infected with Ad-SirT1, Ad-NC, Ad-siRNA-SirT1, or Ad-siRNA-NC at different multiplicities of infection (MOIs 10, 50, and 100) for 2 hours in serum-free media. Then, the culture media were replaced with neurobasal media. Infected neurons were maintained in an incubator with 5% CO2 for 48 hours. Infection efficiency of recombinant adenovirus was calculated by the percentage of GFP-positive cells with an Olympus IX71-F22FL/PH inverted microscope. The optimal MOI was determined according to infection efficiency (>80%) and cell survival rate (>80%). Western blot was used to detect the effect of Ad-SirT1 and Ad-siRNA-SirT1 on the expression of SirT1.
Statistical Analysis
The statistical analyses were performed using SPSS 16.0 (SPSS Inc., Chicago, IL, USA) for Windows. All of the values were presented as mean±s.d., except for the neurobehavioral score, and were analyzed using Student's t-test or a one-way analysis of variance (ANOVA). The differences between two groups were tested with post hoc least significant difference (LSD) test. Neurobehavioral scores were expressed as the median (range) and were analyzed using a nonparametric method (Kruskal–Wallis test) followed by the Mann–Whitney U-test using Bonferroni correction. Statistical significance was defined as P<0.05.
Results
Hyperbaric Oxygen Preconditioning Upregulates the Expression of SirT1 in Neurons After Ischemia/Reperfusion
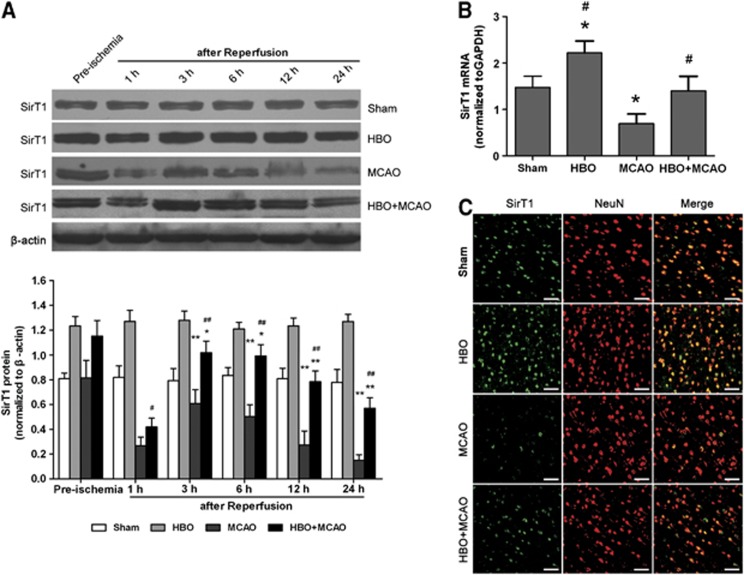
The level of SirT1 protein was detected by western blot in ischemic penumbra of cerebral cortex before the onset of ischemia, and at 1, 3, 6, 12, and 24 hours after reperfusion (Figure 1A). Compared with the sham group, the expression of SirT1 was significantly increased in the HBO group without ischemia challenge (P<0.01), whereas it was decreased in the MCAO group (P<0.01). Although the expression of SirT1 was also decreased beginning 3 hours after reperfusion in the HBO+MCAO group when compared with the preischemia state in same group, it was markedly higher than that in the MCAO group at the corresponding time point (P<0.05 at 1 hour after reperfusion; P<0.01 at 3, 6, 12, and 24 hours after reperfusion). In addition, the level of SirT1 mRNA was examined at the time point of 3 hours after reperfusion (Figure 1B). The results were similar to that of SirT1 protein. The expression of SirT1 mRNA in the HBO+MCAO group was higher than that of the MCAO group (P<0.01).
Figure 1.
Hyperbaric oxygen (HBO) preconditioning upregulates SirT1 protein and mRNA in ischemic penumbra of the brain. (A) Representative western blot bands and quantitative analysis of the time course of SirT1 protein expressions (n=5/time point/group). *P<0.05, **P<0.01 versus preischemia; #P<0.05, ##P<0.01 versus middle cerebral artery occlusion (MCAO). The expression of SirT1 was normalized to the expression of β-actin. (B) The expression of SirT1 mRNA was analyzed using quantitative real-time PCR 3 hours after reperfusion (n=5/group). *P<0.01 versus Sham; #P<0.01 versus MCAO. The expression of SirT1 was normalized to the expression of glyceraldehyde 3-phosphate dehydrogenase (GAPDH). Data are presented as mean±s.d., one-way analysis of variance (ANOVA) followed by the least significant difference (LSD) test. (C) Representative microphotographs showing the double immunofluorescence staining for SirT1 (green) and neuronal nuclei (NeuN; neuronal marker, red) to determine the cell identity for SirT1 expression in ischemic penumbra of brain at 3 hours after reperfusion (n=3/group). Scale bars=50 μm. MCAO: rats subjected to occlusion of middle cerebral artery for 120 minutes; HBO: rats subjected to HBO preconditioning (2.5 atmospheres absolute, 100% O2, 1 h/day, 5 days); HBO+MCAO: rats subjected to MCAO 24 hours after the end of HBO preconditioning. The color reproduction of this figure is available at the Journal of Cerebral Blood Flow and Metabolism journal online.
We further examined the expression and localization of SirT1 by double immunofluorescent staining with SirT1 (green) and NeuN (red), a neuronal-specific marker (Figure 1C). The confocal laser scanning microscope images showed that the expression of SirT1 was observed in the sham group and significantly increased in the HBO group throughout the hemisphere. A little expression of SirT1 was found in ischemic penumbra in the MCAO group, whereas HBO-PC increased SirT1 expression 3 hours after reperfusion. The expression of SirT1 protein was mostly expressed in neurons and the immunoreactive products are mainly located in their nuclei.
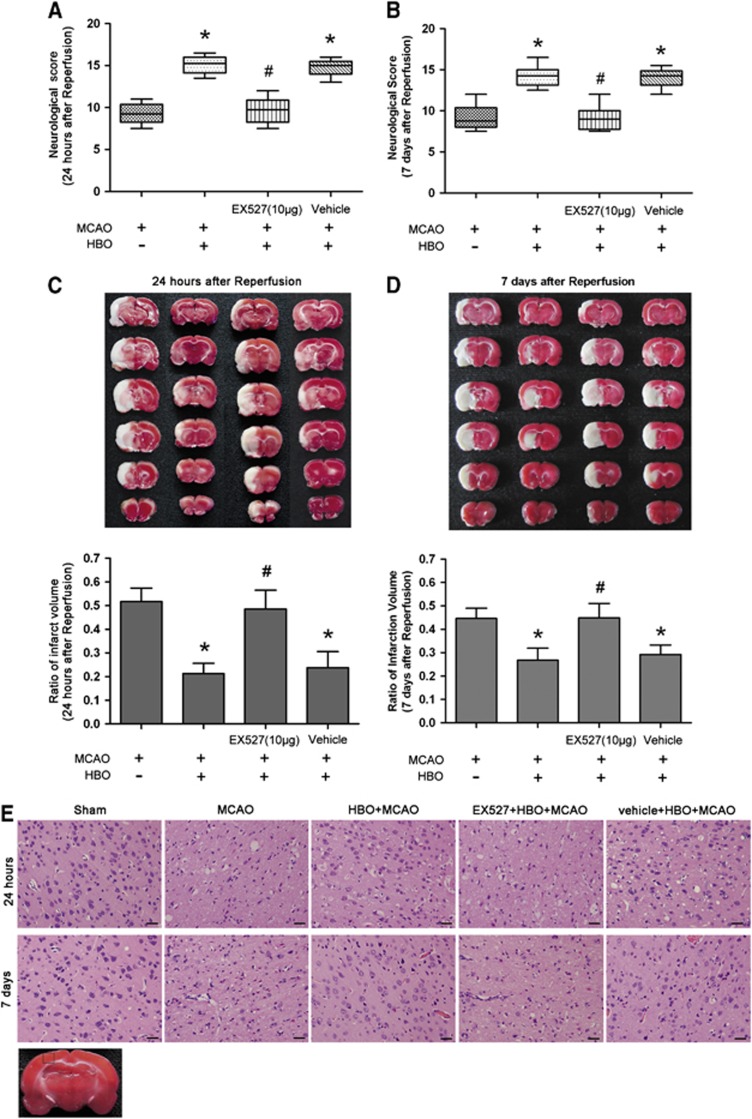
EX527 Treatment Attenuates Hyperbaric Oxygen Preconditioning-Induced Reduction of Infarct Volume and Improvement of Neurobehavioral Score and Morphology
To detect the role of SirT1 in neuroprotection of HBO-PC, rats received i.c.v. injections of SirT1 inhibitor EX527 or vehicle. At 24 hours and 7 days after reperfusion, HBO-PC significantly improved neurobehavioral score (P<0.01, HBO+MCAO versus MCAO). Treatment with EX527 (10 μg) reversed the beneficial effect of HBO-PC on neurologic function (P<0.01, 10 μg EX527+HBO+MCAO versus HBO+MCAO). The neurobehavioral score in EX527 (10 μg)+HBO+MCAO group was similar to that of the MCAO group. There were no significant differences in neurologic function between HBO+MCAO and vehicle+HBO+MCAO groups (Figures 2A and 2B). The HBO+MCAO group showed a smaller infarct volume compared with that of the MCAO group at 24 hours and 7 days after reperfusion (P<0.01). The infarct volume of EX527 (10 μg)+HBO+MCAO group was similar to that of the MCAO group and was larger than that of the HBO+MCAO group (P<0.01). The result of vehicle+HBO+MCAO group was not significantly different from that of the HBO+MCAO group (Figures 2C and 2D). The neuroprotective effect of HBO-PC in I/R injury remains for at least 7 days.
Figure 2.
The SirT1 inhibitor EX527 attenuates neuroprotection of hyperbaric oxygen (HBO) preconditioning in rat brain. EX527 (10 μg) or vehicle was delivered by intracerebroventricular injection 30 minutes before the onset of HBO preconditioning every 2 days for 3 times. Neurobehavioral score 24 hours (A) and 7 days (B) after reperfusion (n=8/group). Data are presented as the median (range), a nonparametric method (Kruskal–Wallis test) followed by the Mann–Whitney U-test by Bonferroni correction. Representative 2,3,5-triphenyltetrazolium chloride (TTC)-stained thick brain sections and quantitative evaluation of the infarction volume at 24 hours (C) and 7 days (D) after reperfusion (n=8/group). Data are presented as mean±s.d., one-way analysis of variance (ANOVA) followed by the least significant difference (LSD) test. *P<0.01 versus middle cerebral artery occlusion (MCAO); #P<0.01 versus HBO preconditioning. (E) Representative microphotographs showing hematoxylin and eosin (HE) staining to determine the cell damage by ischemia/reperfusion (I/R) in ischemic penumbra of brain 24 hours and 7 days after reperfusion (n=3/group). Scale bars=100 μm. MCAO: rats subjected to occlusion of middle cerebral artery for 120 minutes; HBO+MCAO: rats subjected to MCAO 24 hours after the end of HBO preconditioning.
Representative photomicrographs of HE staining at 24 hours and 7 days after reperfusion are shown in Figure 2E. Cortical cells lined regularly in sham group. The cell outline was clear and structure was compact, and the nucleolus was clearly visible. In MCAO and EX527 (10 μg)+HBO+MCAO groups, the number of cells was decreased and the cells were arranged irregularly in ischemic penumbra of cerebral cortex. Most of them were shrunken with triangulated pycnotic nucleus and eosinophilic cytoplasm. In contrast, these neuronal damages were substantially reduced in the HBO+MCAO and the vehicle+HBO+MCAO groups.
The effects of 1 and 30 μg EX527 in neuroprotection of HBO-PC were also examined at 24 hours after reperfusion. (Supplementary Figure S1). The neurobehavioral score and infarct volume in the EX527 (1 μg)+HBO+MCAO group were similar to that of the HBO+MCAO group. Treatment with 30 μg EX527 reversed the neuroprotective effect of HBO-PC (P<0.01, 30 μg EX527+HBO+MCAO versus HBO+MCAO), which was more significant than 10 μg EX527 (P<0.05, 30 μg EX527+HBO+MCAO versus 10 μg EX527+HBO+MCAO), suggesting that EX527 attenuates the neuroprotection of HBO-PC in a dose-dependent manner.
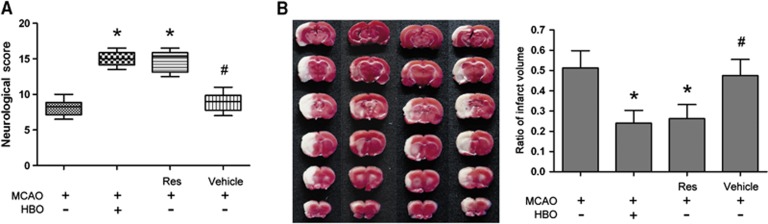
Resveratrol Mimics the Neuroprotective Effect of Hyperbaric Oxygen Preconditioning
Pretreatment with SirT1 activator resveratrol improved neurobehavioral score and reduced infarct volume 24 hours after reperfusion (P<0.01, Res+MCAO versus MCAO). The neuroprotective effect of resveratrol was similar to that of the HBO+MCAO group. The neurobehavioral score and infarct volume in the vehicle+MCAO group were not different from that of the MCAO group (Figures 3A and 3B).
Figure 3.
The SirT1 activator resveratrol (Res) mimics neuroprotection of hyperbaric oxygen (HBO) preconditioning in rat brain. Resveratrol or vehicle was administered intragastrically once a day for 15 days 24 hours before middle cerebral artery occlusion (MCAO). (A) Neurobehavioral score 24 hours after reperfusion (n=8/group). Data are presented as the median (range), a nonparametric method (Kruskal–Wallis test) followed by the Mann–Whitney U-test by Bonferroni correction. (B) Representative 2,3,5-triphenyltetrazolium chloride (TTC)-stained thick brain sections and quantitative evaluation of the infarction volume 24 hours after reperfusion (n=8/group). Data are presented as mean±s.d., one-way analysis of variance (ANOVA) followed by the least significant difference (LSD) test. *P<0.01 versus MCAO; #P<0.01 versus HBO preconditioning. MCAO: rats subjected to occlusion of middle cerebral artery for 120 minutes; HBO+MCAO: rats subjected to MCAO 24 hours after the end of HBO preconditioning.
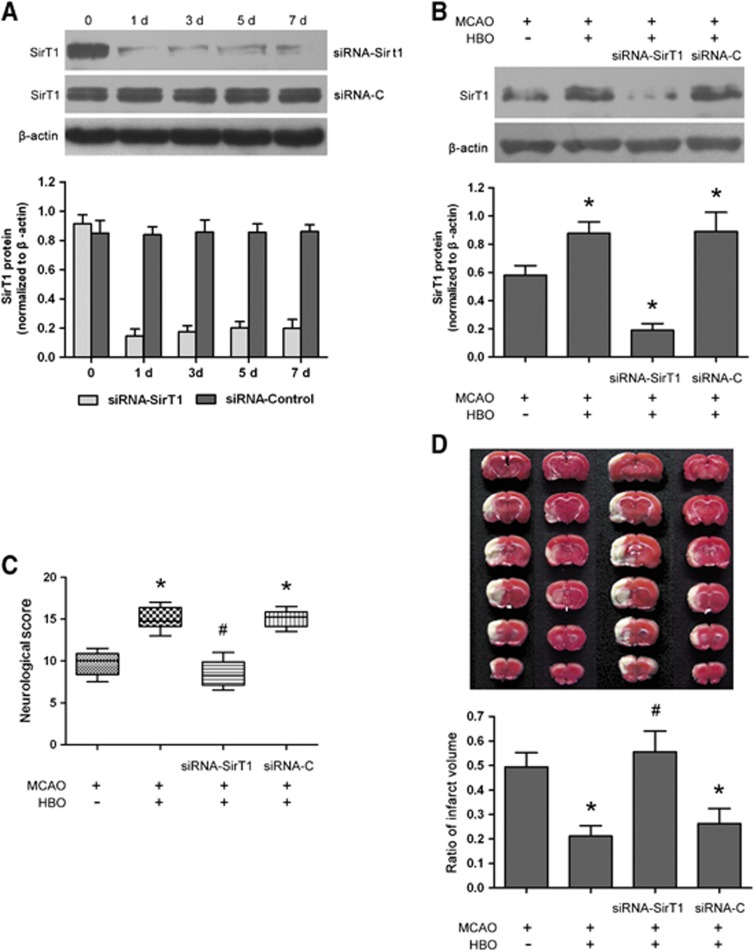
Downregulation of SirT1 by Short Interfering RNA Reverses Hyperbaric Oxygen Preconditioning-Induced Neuroprotection
To confirm the silencing efficiency of i.c.v. injection of siRNA in rats, the level of SirT1 protein was detected by western blot before the onset of injection and at 1, 3, 5, and 7 days after injection of SirT1 siRNA or control siRNA (Figure 4A). Compared with preinjection and control siRNA-treated rats, respectively, the expression of SirT1 was downregulated in SirT1 siRNA-treated rats beginning at 1 day and remaining at least 7 days after injection (P<0.01). There were no differences before and after injections in control siRNA-treated rats. Subsequently, rats were treated with a single i.c.v. injection of siRNA 24 hours before HBO-PC. Upregulation of SirT1 induced by HBO-PC was markedly inhibited by SirT1 siRNA 3 hours after reperfusion (P<0.01, siRNA-SirT1+HBO+MCAO versus HBO+MCAO). Treatment with control siRNA had no effect on the expression of SirT1 induced by HBO-PC (Figure 4B). Moreover, SirT1 siRNA treatment suppressed the improvement of neurobehavioral score and the reduction of infarct volume induced by HBO-PC 24 hours after reperfusion (P<0.01, siRNA-SirT1+HBO+MCAO versus HBO+MCAO), whereas control siRNA treatment had no effect on neuroprotection of HBO-PC (Figures 4C and 4D). In addition, when a single i.c.v. injection of SirT1 siRNA was administered 24 hours before ischemia, the cerebral damage caused by I/R was aggravated 24 hours after reperfusion (Supplementary Figure S2). The neurobehavioral score was significantly lower (P<0.05) and infarct volume was larger (P<0.01) in SirT1 siRNA-treated ischemic rats than in ischemic rats.
Figure 4.
Knockdown of SirT1 by short interfering RNA (siRNA) reverses neuroprotection of hyperbaric oxygen (HBO) preconditioning in rat brain. (A, B) Representative western blot bands and quantitative evaluation of SirT1 protein expression (n=5/group). Rats were treated with a single intracerebroventricular (i.c.v.) injection of 5 μg siRNA-SirT1 or siRNA-C. The expression of SirT1 was detected by immunoblots from day 1 to day 7 after siRNA injection (A). At 24 hours before HBO preconditioning, 5 μg siRNA-SirT1 or siRNA-C was delivered by a single i.c.v. injection. The expression of SirT1 was detected by immunoblots 24 hours after reperfusion (B). The expression of SirT1 was normalized to the expression of β-actin. Data are presented as mean±s.d., one-way analysis of variance (ANOVA) followed by the least significant difference (LSD) test. (C) Neurobehavioral score 24 hours after reperfusion (n=8/group). Data are presented as the median (range), a nonparametric method (Kruskal–Wallis test) followed by the Mann–Whitney U-test by Bonferroni correction. (D) Representative 2,3,5-triphenyltetrazolium chloride-stained thick brain sections and quantitative evaluation of the infarct volume 24 hours after reperfusion (n=8/group). Data are presented as mean±s.d., one-way ANOVA followed by (LSD test. *P<0.01 versus middle cerebral artery occlusion (MCAO); #P<0.01 versus HBO preconditioning. siRNA-SirT1, SirT1 short interfering RNA; siRNA-C, control short interfering RNA. MCAO: rats subjected to occlusion of middle cerebral artery for 120 minutes; HBO+MCAO: rats subjected to MCAO 24 hours after the end of HBO preconditioning.
Hyperbaric Oxygen Preconditioning Upregulates the Expression of SirT1 in Primary Cortical Neurons After Oxygen–Glucose Deprivation
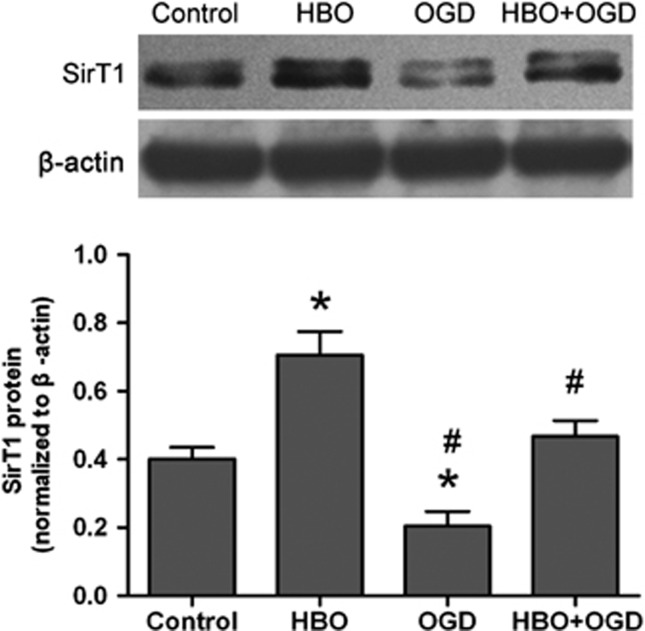
Our in vivo study results showed that SirT1 expression is associated with tolerance to cerebral ischemia by HBO-PC. These results were further tested in vitro using primary cortical neurons. Primary cortical neurons were exposed to 2 hours of OGD followed by 24 hours of re-oxygenation. The expression of SirT1 protein was detected 24 hours after re-oxygenation (Figure 5). SirT1 was significantly increased in the HBO group (P<0.01) and decreased in the OGD group (P<0.01) compared with the control group. In addition, SirT1 was significantly higher in the HBO+OGD group than in the OGD group (P<0.01). There were no statistical differences in the protein expression of SirT1 between HBO+OGD and control groups.
Figure 5.
Hyperbaric oxygen (HBO) preconditioning upregulates SirT1 protein after oxygen–glucose deprivation (OGD) injury in primary cortical neurons. Representative western blot bands and quantitative evaluation of SirT1 protein expression (n=3/group). The expression of SirT1 was detected by immunoblots 24 hours after re-oxygenation. The expression of SirT1 was normalized to the expression of β-actin. Data were presented as mean±s.d. of three repeated experiments, one-way analysis of variance (ANOVA) followed by the least significant difference (LSD) test. *P<0.01 versus control; #P<0.01 versus HBO preconditioning. OGD: neurons subjected to oxygen-glucose deprivation for 2 hours; HBO: neurons subjected to HBO preconditioning for 2 hours (3.5 atmospheres absolute, >98% O2); HBO +OGD: neurons subjected to OGD 24 hours after the end of HBO preconditioning.
Overexpression of SirT1 by Adenovirus Infection Emulates, Whereas Knockdown of SirT1 Abolishes, the Protective Effect of Hyperbaric Oxygen Preconditioning in Neurons Subjected to Oxygen–Glucose Deprivation
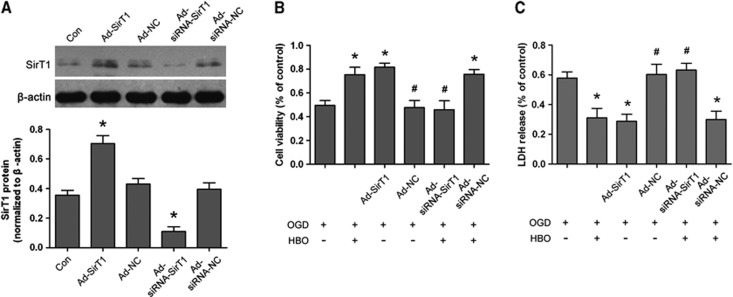
In the HBO+OGD group, cell viability was significantly increased (P<0.01) and LDH release was reduced (P<0.01) 24 hours after re-oxygenation compared with that of the OGD group (Supplementary Figure S3). The results of HBO-PC-induced neuroprotection were in accordance with our previous study in spinal cord neurons. At 48 hours after Ad-SirT1, Ad-NC, Ad-siRNA-SirT1, or Ad-siRNA-NC was infected into neurons, the percentage of GFP-positive cells and cell survival rate were measured at MOI of 10, 50, and 100 (data not shown). Optimal MOI of infection with Ad-SirT1 or Ad-siRNA-SirT1 was 50 or 100, respectively. The effect of Ad-SirT1 or Ad-siRNA-SirT1 on SirT1 expression was confirmed by western blot (Figure 6A). The level of SirT1 protein was significantly upregulated in Ad-SirT1-infected neurons compared with that of control neurons (P<0.01). There were no statistical differences in the expressions of SirT1 between Ad-NC-infected neurons and control neurons. Subsequently, Ad-SirT1 or Ad-NC was infected into neurons 48 hours before OGD injury. At 24 hours after re-oxygenation, the expression of SirT1 was upregulated in the Ad-SirT1+OGD group in comparison with the OGD group (P<0.01), and was similar to that of the HBO+OGD group. There were no statistical differences in the expressions of SirT1 between Ad-NC+OGD and OGD groups (Supplementary Figure S4). Cell viability was significantly higher and LDH release was markedly lower in the Ad-SirT1+OGD group than that of the OGD group (P<0.01; Figures 6B and 6C). The protective effect of Ad-SirT1 on neurons was similar to that of neuroprotection induced by HBO-PC. There were no differences in cell viability and LDH release between Ad-NC+OGD and OGD groups.
Figure 6.
Modulation of SirT1 by adenovirus infection with SirT1 or SirT1 short interfering RNA (siRNA) and effect of SirT1 in hyperbaric oxygen (HBO) preconditioning-induced neuroprotection in primary cortical neurons. (A) Representative western blot bands and quantitative evaluation of SirT1 protein expression. At 7 days after cell culture, Ad-SirT1 (multiplicity of infection (MOI) 50), Ad-NC, Ad-siRNA-SirT1 (MOI 100), or Ad-siRNA-NC were infected into neurons respectively for 48 hours. The expression of SirT1 was detected by immunoblots 48 hours after adenovirus infection. The expression of SirT1 was normalized to the expression of β-actin. *P<0.01 versus control. (B, C) Ad-SirT1 (MOI 50) or Ad-NC was infected into neurons 48 hours before oxygen–glucose deprivation (OGD), and Ad-siRNA-SirT1 (MOI 100) or Ad-siRNA-NC was infected into neurons 48 hours before HBO preconditioning. (B) Cell viability was measured using 3-(4,5-22dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay 24 hours after re-oxygenation. (C) Lactate dehydrogenase (LDH) release was measured 24 hours after re-oxygenation. *P<0.01 versus OGD; #P<0.01 versus HBO preconditioning in (B) and (C). Data are presented as mean±s.d. of three repeated experiments (n=3/group), one-way analysis of variance (ANOVA) followed by the least significant difference (LSD) test. Ad-SirT1, adenovirus expressing SirT1; Ad-NC, control adenovirus; Ad-siRNA-SirT1, adenovirus expressing siRNA for SirT1; Ad-siRNA-NC, adenovirus expressing control siRNA. OGD: neurons subjected to oxygen–glucose deprivation for 2 hours; HBO +OGD: neurons subjected to OGD 24 hours after the end of HBO preconditioning.
The expression of SirT1 protein was downregulated in Ad-siRNA-SirT1-infected neurons compared with that in control neurons at 48 hours after infection (P<0.01; Figure 6A). There were no differences in SirT1 expression between in Ad-siRNA-NC- infected and control neurons. At 48 hours after infection, neurons received HBO-PC for 2 hours and then OGD injury for 2 hours. The Ad-siRNA-SirT1 infection suppressed upregulation of SirT1 induced by HBO-PC 24 hours after re-oxygenation (P<0.01, Ad-siRNA-SirT1+HBO+OGD versus HBO+OGD), whereas Ad-siRNA-NC infection had no effect on SirT1 expression induced by HBO-PC (Ad-siRNA-NC+HBO+OGD versus HBO+OGD) (Supplementary Figure S4). Cell viability was significantly decreased (P<0.01), whereas LDH release was increased (P<0.01) in the Ad-siRNA-SirT1+HBO+OGD group 24 hours after re-oxygenation compared with that of the HBO+OGD group (Figures 6B and 6C). There were no significant differences in cell viability and LDH release between Ad-siRNA-SirT1+HBO+OGD and OGD groups. The Ad-siRNA-NC infection had no effect on neuroprotection of HBO-PC (Ad-siRNA-NC+HBO+OGD versus HBO+OGD).
Hyperbaric Oxygen Preconditioning Reduces Apoptosis After Oxygen–Glucose Deprivation Injury Mediated by SirT1
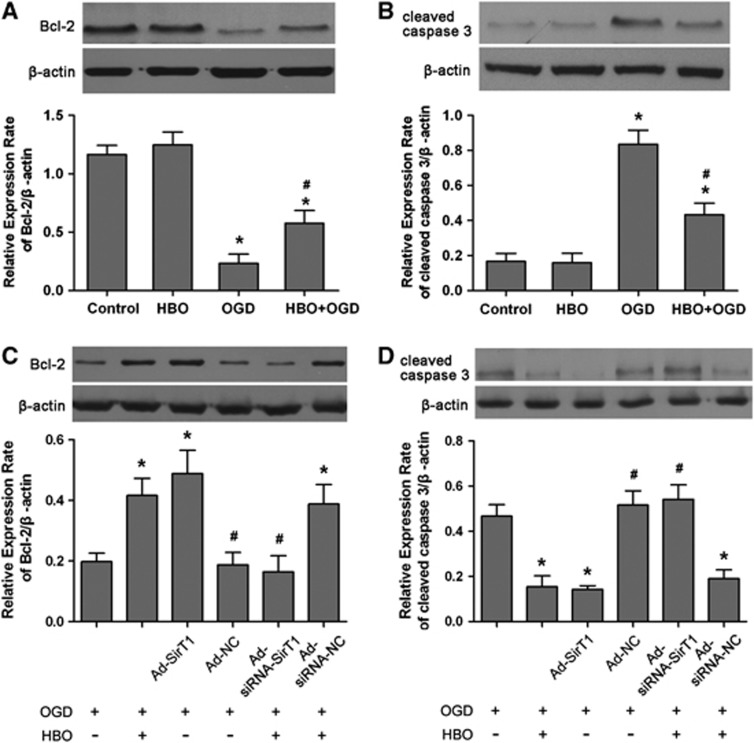
To investigate the molecular mechanism by which HBO-PC protects the brain from I/R injury via SirT1, we evaluated the expression of proteins involved in neuronal survival and death. At 24 hours after re-oxygenation, the expression of B-cell lymphoma 2 (Bcl-2) protein was significantly enhanced (P<0.01), whereas that of cleaved caspase-3 was decreased (P<0.01) in the HBO+OGD group compared with the OGD group (Figures 7A and 7B). Similarly, upregulation of SirT1 also increased the level of Bcl-2 protein (P<0.01) and decreased cleaved caspase-3 expression (P<0.01) at 24 hours after re-oxygenation in the Ad-SirT1+OGD group compared with that of the OGD group (Figures 7C and 7D). The results of the Ad-SirT1+OGD group were not significantly different from that of the HBO+OGD group. The Ad-NC had no effect on the expression of Bcl-2 and cleaved caspase-3 induced by HBO-PC after re-oxygenation. However, when upregulation of SirT1 induced by HBO-PC was inhibited by Ad-siRNA-SirT1, the expression of Bcl-2 was significantly downregulated and cleaved caspase-3 was upregulated 24 hours after re-oxygenation (P<0.01, Ad-siRNA-SirT1+HBO+OGD versus HBO+OGD). There were no statistical differences in the expression of Bcl-2 and cleaved caspase-3 between Ad-siRNA-SirT1+HBO+OGD and OGD groups.
Figure 7.
Modulation of B-cell lymphoma 2 (Bcl-2) and cleaved caspase 3 by hyperbaric oxygen (HBO) preconditioning and SirT1 in primary cortical neurons. Representative western blot bands and quantitative evaluation of Bcl-2 (A, C) and cleaved caspase 3 (B, D) expression. The expressions of Bcl-2 (A) and cleaved caspase (B) were detected by immunoblots 24 hours after re-oxygenation. Ad-SirT1 (multiplicity of infection (MOI) 50) or Ad-NC was infected into neurons 48 hours before oxygen–glucose deprivation (OGD), and Ad-siRNA-SirT1 (MOI 100) or Ad-siRNA-NC was infected into neurons 48 hours before HBO preconditioning after OGD injury. The expressions of Bcl-2 (C) and cleaved caspase (D) were detected by immunoblots 24 hours after re-oxygenation. The expression of SirT1 was normalized to the expression of β-actin. Data are presented as mean±s.d. of three repeated experiments (n=3/group), one-way analysis of variance (ANOVA) followed by the least significant difference (LSD) test. *P<0.01 versus OGD; #P<0.01 versus HBO preconditioning. Ad-SirT1, adenovirus expressing SirT1; Ad-NC, control adenovirus; Ad-siRNA-SirT1, adenovirus expressing short interfering RNA for SirT1; Ad-siRNA-NC, adenovirus expressing control siRNA. OGD: neurons subjected to oxygen–glucose deprivation for 2 hours; HBO +OGD: neurons subjected to OGD 24 hours after the end of HBO preconditioning.
Discussion
The present study reveals the novel findings that HBO-PC upregulates the expression of SirT1 protein and mRNA after focal cerebral ischemic injury. The ischemic tolerance to cerebral ischemia induced by HBO-PC was attenuated by SirT1 inhibitor EX527, while mimicked by pretreatment with SirT1 activator resveratrol. In addition, knockdown of SirT1 by siRNA reversed the neuroprotective effect of HBO-PC. In agreement with the in vivo results, HBO-PC reduced OGD-induced injury in primary cortical neurons and increased the protein expression of SirT1. Similar to HBO-PC, upregulation of SirT1 through adenovirus infection protected neurons from OGD injury. In contrast, downregulation of SirT1 by adenovirus siRNA infection abolished HBO-PC-induced neuroprotection. Besides, HBO-PC or upregulated SirT1 by Ad-SirT1 treatment promoted the expression of Bcl-2 protein as well as suppressed cleaved caspase 3 after neuronal OGD injury, whereas downregulated SirT1 by Ad-siRNA-SirT1 treatment abolished the modulatory effect of HBO-PC on Bcl-2 and cleaved caspase 3. Taking together, these data suggest that HBO-PC-induced ischemic tolerance against cerebral ischemic injury is in association with suppression of apoptosis mediated by upregulation of SirT1. Our study disclosed a novel mechanism underlying the neuroprotective effect of HBO-PC.
The knowledge about SirT1, acting as a longevity factor, initially comes from the studies in lifespan extension in organisms.9, 10, 11 There is evidence that SirT1 is also associated with life longevity in mammals and enhances mammalian cell survival under stress conditions via regulating the specific substrates.23, 24, 25 Several studies show that resveratrol has the protective effect against I/R injury in the heart and brain.26, 27 Data show that increase of SirT1 expression protects the heart from I/R.15 However, little is known about the effect of SirT1 in cerebral I/R injury and its role in neuroprotection of HBO-PC. The present experiment is, to our knowledge, the first study in this regard. We found that SirT1 protein and mRNA were both decreased in ischemic penumbra after I/R injury, whereas they were increased after treatment with HBO alone for 5 days in the brain. Moreover, HBO-PC substantially suppressed the reduction of SirT1 expression induced by I/R injury. In consistence, less number of SirT1-positive cells was observed after I/R injury, whereas HBO-PC increased the number of SirT1-positive cells in ischemic penumbra. We also found that SirT1 was mainly localized in the nuclei of neurons, indicating that the modulation of SirT1 expression by HBO-PC occurs in neuronal nuclei. Increasing evidence suggest that SirT1 localizes in both nuclei and cytoplasm, and shuttles between the two regions. Furthermore, the function of SirT1 varies with its localization within cells.28, 29 It is well known that SirT1 regulates the activities of transcription factors in nuclei and subsequently promotes cell survival. Several studies have shown that HBO-PC produces the neuroprotective effect against cerebral I/R injury via promoting neuron survival in ischemic penumbra,30 a region potentially destined for cellular necrosis but possibly to be rescued.31 Therefore, our observation indicate that upregulation of SirT1 in the neuronal nuclei may be involved in the neuroprotective effect of HBO-PC via modulation of activities of transcription factors.
Whereas EX527 inhibits the activation of SirT1 with high potency and specificity,32 resveratrol stimulates SirT1 activity.33 We showed that i.c.v. injection of EX527 attenuated neuroprotection of HBO-PC against I/R injury and its effect was in a dose-dependent manner. In contrast, pretreatment with resveratrol mimicked neuroprotection of HBO-PC. The latter results are in line with previously published studies showing that resveratrol reduces cerebral ischemic injury via upregulating endogenous SirT1 activity.17 These findings indirectly indicate that HBO-PC also activates SirT1 and neuroprotection of HBO-PC requires activation of SirT1. The contribution of SirT1 activity to ischemic preconditioning-induced cardioprotection during I/R injury has been previously reported in mice.16 However, there is evidence that the role of resveratrol in SirT1 activity depends on other molecules.34 Moreover, neuroprotection induced by resveratrol underlies many mechanisms, including antioxidant effect and improvement of brain energy metabolism.35, 36 Additionally, both of them have no effect on SirT1 expression. Therefore, these results did not provide the valid evidences for the association of upregulation of SirT1 expression with neuroprotection of HBO-PC. Thus, in the present study, i.c.v. injection of SirT1 siRNA and upregulating or downregulating SirT1 by adenovirus infection were conducted respectively in in vivo and in vitro models of ischemic injury to further confirm the role of SirT1 in neuroprotection of HBO-PC.
A single i.c.v. injection of SirT1 siRNA effectively downregulated the expression of SirT1 protein, implying that siRNA can successfully be transfected into brain tissue. We also found that the knockdown of SirT1 by SirT1 siRNA began at 1 day and remained at least for 7 days, suggesting that siRNA transfection can meet with the requirement for inhibition of increased SirT1 by HBO-PC for 5 days. The neuroprotective effect of HBO-PC was almost disappeared in rats subjected to treatment with SirT1 siRNA, indicating the pivotal role of SirT1 in neuroprotection of HBO-PC. In addition, we have verified the effect of SirT1 in ischemic rats, and found that downregulation of endogenous SirT1 expression by siRNA exacerbates cerebral injury, suggesting that SirT1 also plays an important and beneficial role in cerebral I/R injury.
In the in vitro study, we first showed that HBO-PC for 2 hours protects the primary cortical neurons from I/R damage caused by OGD. The result is in accordance with our previous study on cultured spinal cord neurons.6 Our results also showed that upregulated SirT1 emulated the neuroprotective effect of HBO-PC against OGD injury. On the contrary, neuroprotection of HBO-PC was abolished because of downregulation of SirT1. These data are consistent with in vivo experiments, thereby further supporting our deduction that neuroprotection induced by HBO preconditioning is dependent on upregulation of SirT1 expression.
The severity of cerebral I/R depends mainly on neuronal survival or death. It is generally acknowledged that most of the cells undergo necrosis in ischemic core, but apoptotic cell death occurs in ischemic penumbra. Therefore, we investigated whether the neuroprotective mechanism of HBO-PC mediated by SirT1 expression is associated with inhibition of apoptosis. The hypothesis was confirmed in this study, showing that not only HBO-PC but also upregulated SirT1 increased the protein expression of antiapoptotic Bcl-2, and decreased proapoptotic cleaved caspase 3 in neurons during OGD injury and, conversely, inhibition of enhanced SirT1 by HBO-PC suppressed Bcl-2 and promoted cleaved caspase 3 protein, suggesting that the mechanism of apoptosis is involved in neuroprotection of HBO-PC mediated by SirT1. Several studies have reported that SirT1 deacetylates p53, and p53 is also an apoptosis-inducible protein. The deacetylation of p53 by SirT1 leads to the inhibition of apoptosis.37 Thus, it is conceivable that SirT1 may regulate apoptosis through deacetylation of p53, taking part in neuroprotection of HBO-PC.
The pathway for SirT1-mediated neuroprotection of HBO-PC requires further exploration. In our previous studies, we have shown that HBO-PC moderately increases the level of endogenous ROS in central nervous system, which stimulates the increased production of endogenous antioxidant enzymes including heme oxygenase-1 (HO-1) and catalase (CAT). Furthermore, oxygen free radicals scavenge or antioxidant enzyme inhibitor attenuates neuroprotection of HBO-PC, indicating that ROS and antioxidant enzymes play a pivotal role in ischemic tolerance elicited by HBO-PC to I/R injury in the brain.6, 7 These findings are in line with another published study, suggesting that antioxidant enzymes, such as superoxide dismutase (SOD) and CAT, are critically involved in neuroprotection of HBO-PC.30 It is known that the modulation of SirT1 on physiologic function is mediated by transcription factors, including FoxO family and p53.24, 25 Meanwhile, the function of transcription factors relies on the modulation of SirT1. For example, STAT3 (signal transducer and activator of transcription 3), phosphorylation and its effect on liver were regulated by SirT1 in the nutritional status of mice.38 It is requisite for oxidative stress resistance in animals that SirT1 deacetylates FoxO.39 In addition, either activation or upregulation of FoxO can promote the expression of SOD and CAT in response to oxidative stress.40, 41 It has been reported that SirT1 conferred a protective effect on I/R injury in the heart through upregulation of antioxidants through activation of FoxO.15 Therefore, we infer that the promotion of antioxidant enzymes is also involved in ischemic tolerance elicited by HBO-PC against the subsequent I/R injury that is mediated by SirT1 upregulation. Further work is needed to explore the signaling pathways of SirT1 in neuroprotection of HBO-PC.
In summary, our study provides convincing evidence that HBO-PC increases the content of SirT1 in both rat brain subjected to I/R and neurons exposed to OGD, and inhibits apoptotic cell death during I/R injury. Downregulation of SirT1 reverses neuroprotection of HBO-PC and attenuates its action on modulation of antiapoptotic and proapoptotic proteins, whereas upregulation of SirT1 emulates the neuroprotective effect of HBO-PC. These data clearly indicate that SirT1 mediates neuroprotection of HBO-PC against I/R injury through inhibition of apoptosis.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Journal of Cerebral Blood Flow & Metabolism website (http://www.nature.com/jcbfm)
This work was supported by the National Science Foundation for Distinguished Young Scholars (Grant 30725039) and the key project for the National Natural Science Foundation of China (Grant 30930091) to LX.
Supplementary Material
References
- Dong H, Xiong L, Zhu Z, Chen S, Hou L, Sakabe T. Preconditioning with hyperbaric oxygen and hyperoxia induces tolerance against spinal cord ischemia in rabbits. Anesthesiology. 2002;96:907–912. doi: 10.1097/00000542-200204000-00018. [DOI] [PubMed] [Google Scholar]
- Xiong L, Zhu Z, Dong H, Hu W, Hou L, Chen S. Hyperbaric oxygen preconditioning induces neuroprotection against ischemia in transient not permanent middle cerebral artery occlusion rat model. Chin Med J (Engl) 2000;113:836–839. [PubMed] [Google Scholar]
- Yogaratnam JZ, Laden G, Guvendik L, Cowen M, Cale A, Griffin S. Hyperbaric oxygen preconditioning improves myocardial function, reduces length of intensive care stay, and limits complications post coronary artery bypass graft surgery. Cardiovasc Revasc Med. 2010;11:8–19. doi: 10.1016/j.carrev.2009.03.004. [DOI] [PubMed] [Google Scholar]
- Gu GJ, Li YP, Peng ZY, Xu JJ, Kang ZM, Xu WG, et al. Mechanism of ischemic tolerance induced by hyperbaric oxygen preconditioning involves upregulation of hypoxia-inducible factor-1alpha and erythropoietin in rats. J Appl Physiol. 2008;104:1185–1191. doi: 10.1152/japplphysiol.00323.2007. [DOI] [PubMed] [Google Scholar]
- Ostrowski RP, Jadhav V, Chen W, Zhang JH. Reduced matrix metalloproteinase-9 activity and cell death after global ischemia in the brain preconditioned with hyperbaric oxygen. Acta Neurochir Suppl. 2010;106:47–49. doi: 10.1007/978-3-211-98811-4_7. [DOI] [PubMed] [Google Scholar]
- Li Q, Li J, Zhang L, Wang B, Xiong L. Preconditioning with hyperbaric oxygen induces tolerance against oxidative injury via increased expression of heme oxygenase-1 in primary cultured spinal cord neurons. Life Sci. 2007;80:1087–1093. doi: 10.1016/j.lfs.2006.11.043. [DOI] [PubMed] [Google Scholar]
- Nie H, Xiong L, Lao N, Chen S, Xu N, Zhu Z. Hyperbaric oxygen preconditioning induces tolerance against spinal cord ischemia by upregulation of antioxidant enzymes in rabbits. J Cereb Blood Flow Metab. 2006;26:666–674. doi: 10.1038/sj.jcbfm.9600221. [DOI] [PubMed] [Google Scholar]
- Blander G, Guarente L. The Sir2 family of protein deacetylases. Annu Rev Biochem. 2004;73:417–435. doi: 10.1146/annurev.biochem.73.011303.073651. [DOI] [PubMed] [Google Scholar]
- Imai S, Armstrong CM, Kaeberlein M, Guarente L. Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature. 2000;403:795–800. doi: 10.1038/35001622. [DOI] [PubMed] [Google Scholar]
- Rogina B, Helfand SL. Sir2 mediates longevity in the fly through a pathway related to calorie restriction. Proc Natl Acad Sci USA. 2004;101:15998–16003. doi: 10.1073/pnas.0404184101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tissenbaum HA, Guarente L. Increased dosage of a sir-2 gene extends lifespan in Caenorhabditis elegans. Nature. 2001;410:227–230. doi: 10.1038/35065638. [DOI] [PubMed] [Google Scholar]
- Bordone L, Cohen D, Robinson A, Motta MC, van Veen E, Czopik A, et al. SIRT1 transgenic mice show phenotypes resembling calorie restriction. Aging Cell. 2007;6:759–767. doi: 10.1111/j.1474-9726.2007.00335.x. [DOI] [PubMed] [Google Scholar]
- Wang J, Zhai Q, Chen Y, Lin E, Gu W, McBurney MW, et al. A local mechanism mediates NAD-dependent protection of axon degeneration. J Cell Biol. 2005;170:349–355. doi: 10.1083/jcb.200504028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D, Nguyen MD, Dobbin MM, Fischer A, Sananbenesi F, Rodgers JT, et al. SIRT1 deacetylase protects against neurodegeneration in models for Alzheimer's disease and amyotrophic lateral sclerosis. EMBO J. 2007;26:3169–3179. doi: 10.1038/sj.emboj.7601758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu CP, Zhai P, Yamamoto T, Maejima Y, Matsushima S, Hariharan N, et al. Silent information regulator 1 protects the heart from ischemia/reperfusion. Circulation. 2010;122:2170–2182. doi: 10.1161/CIRCULATIONAHA.110.958033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadtochiy SM, Redman E, Rahman I, Brookes PS. Lysine deacetylation in ischaemic preconditioning: the role of SIRT1. Cardiovasc Res. 2011;89:643–649. doi: 10.1093/cvr/cvq287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raval AP, Dave KR, Perez-Pinzon MA. Resveratrol mimics ischemic preconditioning in the brain. J Cereb Blood Flow Metab. 2006;26:1141–1147. doi: 10.1038/sj.jcbfm.9600262. [DOI] [PubMed] [Google Scholar]
- Yan W, Zhang H, Bai X, Lu Y, Dong H, Xiong L. Autophagy activation is involved in neuroprotection induced by hyperbaric oxygen preconditioning against focal cerebral ischemia in rats. Brain Res. 2011;1402:109–121. doi: 10.1016/j.brainres.2011.05.049. [DOI] [PubMed] [Google Scholar]
- Garcia JH, Wagner S, Liu KF, Hu XJ. Neurological deficit and extent of neuronal necrosis attributable to middle cerebral artery occlusion in rats: statistical validation. Stroke. 1995;26:627–635. doi: 10.1161/01.str.26.4.627. [DOI] [PubMed] [Google Scholar]
- Ashwal S, Tone B, Tian HR, Cole DJ, Pearce WJ. Core and penumbral nitric oxide synthase activity during cerebral ischemia and reperfusion. Stroke. 1998;29:1037–1046. [PubMed] [Google Scholar]
- Dong W, Li N, Gao D, Zhen H, Zhang X, Li F. Resveratrol attenuates ischemic brain damage in the delayed phase after stroke and induces messenger RNA and protein express for angiogenic factors. J Vasc Surg. 2008;48:709–714. doi: 10.1016/j.jvs.2008.04.007. [DOI] [PubMed] [Google Scholar]
- Chen C, Hu Q, Yan J, Yang X, Shi X, Lei J, et al. Early inhibition of HIF-1alpha with small interfering RNA reduces ischemic-reperfused brain injury in rats. Neurobiol Dis. 2009;33:509–517. doi: 10.1016/j.nbd.2008.12.010. [DOI] [PubMed] [Google Scholar]
- Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunet A, Sweeney LB, Sturgill JF, Chua KF, Greer PL, Lin Y, et al. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science. 2004;303:2011–2015. doi: 10.1126/science.1094637. [DOI] [PubMed] [Google Scholar]
- Luo J, Nikolaev AY, Imai S, Chen D, Su F, Shiloh A, et al. Negative control of p53 by Sir2alpha promotes cell survival under stress. Cell. 2001;107:137–148. doi: 10.1016/s0092-8674(01)00524-4. [DOI] [PubMed] [Google Scholar]
- Chen CJ, Yu W, Fu YC, Wang X, Li JL, Wang W. Resveratrol protects cardiomyocytes from hypoxia-induced apoptosis through the SIRT1-FoxO1 pathway. Biochem Biophys Res Commun. 2009;378:389–393. doi: 10.1016/j.bbrc.2008.11.110. [DOI] [PubMed] [Google Scholar]
- Della-Morte D, Dave KR, DeFazio RA, Bao YC, Raval AP, Perez-Pinzon MA. Resveratrol pretreatment protects rat brain from cerebral ischemic damage via a sirtuin 1-uncoupling protein 2 pathway. Neuroscience. 2009;159:993–1002. doi: 10.1016/j.neuroscience.2009.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michishita E, Park JY, Burneskis JM, Barrett JC, Horikawa I. Evolutionarily conserved and nonconserved cellular localizations and functions of human SIRT proteins. Mol Biol Cell. 2005;16:4623–4635. doi: 10.1091/mbc.E05-01-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanno M, Sakamoto J, Miura T, Shimamoto K, Horio Y. Nucleocytoplasmic shuttling of the NAD+-dependent histone deacetylase SIRT1. J Biol Chem. 2007;282:6823–6832. doi: 10.1074/jbc.M609554200. [DOI] [PubMed] [Google Scholar]
- Li J, Liu W, Ding S, Xu W, Guan Y, Zhang JH, et al. Hyperbaric oxygen preconditioning induces tolerance against brain ischemia-reperfusion injury by upregulation of antioxidant enzymes in rats. Brain Res. 2008;1210:223–229. doi: 10.1016/j.brainres.2008.03.007. [DOI] [PubMed] [Google Scholar]
- Ginsberg MD. Adventures in the pathophysiology of brain ischemia: penumbra, gene expression, neuroprotection: the 2002 Thomas Willis Lecture. Stroke. 2003;34:214–223. doi: 10.1161/01.str.0000048846.09677.62. [DOI] [PubMed] [Google Scholar]
- Solomon JM, Pasupuleti R, Xu L, McDonagh T, Curtis R, DiStefano PS, et al. Inhibition of SIRT1 catalytic activity increases p53 acetylation but does not alter cell survival following DNA damage. Mol Cell Biol. 2006;26:28–38. doi: 10.1128/MCB.26.1.28-38.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG, et al. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003;425:191–196. doi: 10.1038/nature01960. [DOI] [PubMed] [Google Scholar]
- Pacholec M, Bleasdale JE, Chrunyk B, Cunningham D, Flynn D, Garofalo RS, et al. SRT1720, SRT2183, SRT1460, and resveratrol are not direct activators of SIRT1. J Biol Chem. 2010;285:8340–8351. doi: 10.1074/jbc.M109.088682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Yan Z, Zhu J, Yang J, He J. Neuroprotective effects of resveratrol on ischemic injury mediated by improving brain energy metabolism and alleviating oxidative stress in rats. Neuropharmacology. 2011;60:252–258. doi: 10.1016/j.neuropharm.2010.09.005. [DOI] [PubMed] [Google Scholar]
- Sinha K, Chaudhary G, Gupta YK. Protective effect of resveratrol against oxidative stress in middle cerebral artery occlusion model of stroke in rats. Life Sci. 2002;71:655–665. doi: 10.1016/s0024-3205(02)01691-0. [DOI] [PubMed] [Google Scholar]
- Alcendor RR, Kirshenbaum LA, Imai S, Vatner SF, Sadoshima J. Silent information regulator 2alpha, a longevity factor and class III histone deacetylase, is an essential endogenous apoptosis inhibitor in cardiac myocytes. Circ Res. 2004;95:971–980. doi: 10.1161/01.RES.0000147557.75257.ff. [DOI] [PubMed] [Google Scholar]
- Nie Y, Erion DM, Yuan Z, Dietrich M, Shulman GI, Horvath TL, et al. STAT3 inhibition of gluconeogenesis is downregulated by SirT1. Nat Cell Biol. 2009;11:492–500. doi: 10.1038/ncb1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daitoku H, Hatta M, Matsuzaki H, Aratani S, Ohshima T, Miyagishi M, et al. Silent information regulator 2 potentiates Foxo1-mediated transcription through its deacetylase activity. Proc Natl Acad Sci USA. 2004;101:10042–10047. doi: 10.1073/pnas.0400593101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balaban RS, Nemoto S, Finkel T. Mitochondria, oxidants, and aging. Cell. 2005;120:483–495. doi: 10.1016/j.cell.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Kops GJ, Dansen TB, Polderman PE, Saarloos I, Wirtz KW, Coffer PJ, et al. Forkhead transcription factor FOXO3a protects quiescent cells from oxidative stress. Nature. 2002;419:316–321. doi: 10.1038/nature01036. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.