Abstract
Rodent 13C magnetic resonance spectroscopy studies show that glutamatergic signaling requires high oxidative energy in the awake resting state and allowed calibration of functional magnetic resonance imaging (fMRI) signal in terms of energy relative to the resting energy. Here, we derived energy used for glutamatergic signaling in the awake resting human. We analyzed human data of electroencephalography (EEG), positron emission tomography (PET) maps of oxygen (CMRO2) and glucose (CMRglc) utilization, and calibrated fMRI from a variety of experimental conditions. CMRglc and EEG in the visual cortex were tightly coupled over several conditions, showing that the oxidative demand for signaling was four times greater than the demand for nonsignaling events in the awake state. Variations of CMRO2 and CMRglc from gray-matter regions and networks were within ±10% of means, suggesting that most areas required similar energy for ubiquitously high resting activity. Human calibrated fMRI results suggest that changes of fMRI signal in cognitive studies contribute at most ±10% CMRO2 changes from rest. The PET data of sleep, vegetative state, and anesthesia show metabolic reductions from rest, uniformly >20% across, indicating no region is selectively reduced when consciousness is lost. Future clinical investigations will benefit from using quantitative metabolic measures.
Keywords: astrocytes, baseline, field potentials, glutamate, multiunit activity, resting state
Introduction
The human brain consumes 20% of the body's energy at rest despite being only 2% of total body mass.1 Normally glucose oxidation is the source of energy production supporting brain function2 in which gray-matter signaling (i.e., events associated with neuronal firing) is dominated by glutamatergic neurons.3 Estimates of the fraction of resting brain energy devoted to signaling were originally quite low.4 But recent experimental studies in rodents and theoretical modeling have converged on the conclusion that majority of the resting energy consumption supports glutamatergic signaling and the energy demand changes linearly with pyramidal neuron firing rates and glutamate neurotransmitter release and reuptake.5, 6, 7, 8, 9 Therefore in rodent models it is possible, to a first order, to show that the resting energy is primarily dedicated to total glutamatergic signaling, and the changes in neuronal signaling relative to a well-defined resting activity level can be used to calibrate changes in energy consumption during functional magnetic resonance imaging (fMRI) experiments.10, 11
In the awake human brain, however, the fraction of resting energy usage devoted to glutamatergic signaling is less well understood. The magnitude of neuronal signaling in the human brain, and by inference the commensurate energy demand, underscores the functional relevance of resting activity and has profound implications for interpreting fMRI experiments in humans.12, 13, 14 Given the rapidly increasing use of resting-state fMRI in mapping networks—defined as a subset of cortical and subcortical gray-matter regions that function together15, 16—it is important to quantitatively establish if in the resting human the metabolic demand for neuronal signaling is high, as has been established for the rodent,5, 7, 8, 9 and to what extent metabolic demand varies across gray-matter regions.
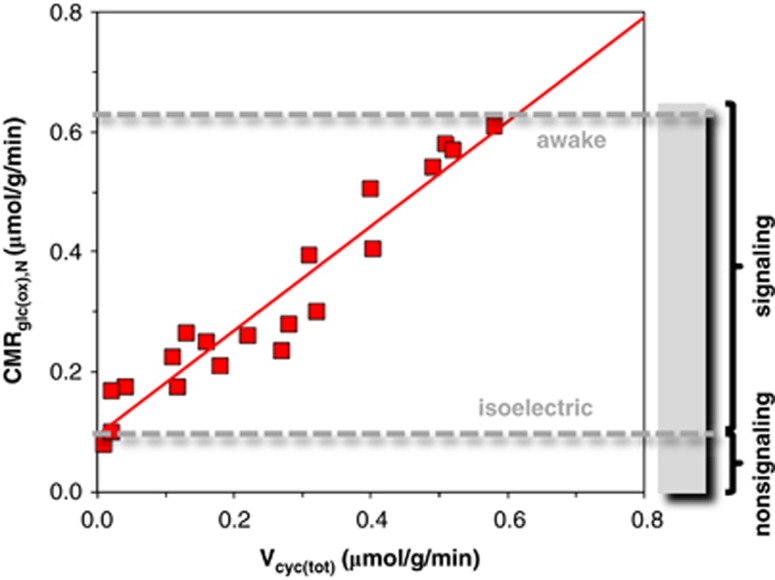
13C magnetic resonance spectroscopy (MRS) of 13C-labeled substrates (e.g., glucose and acetate) can measure rates of 13C label incorporation into cell-specific pools (e.g., glutamate and γ-amino butyric acid (GABA) are predominantly neuronal and glutamine is predominantly glial) thereby estimating metabolic fluxes17 of neuronal glucose oxidation (CMRglc(ox),N) and of total glutamate neurotransmitter cycling (Vcyc(tot)). Rat 13C MRS studies, over a wide range of activities (i.e., from isoelectric pentobarbital anesthesia under which there is no excitatory signaling to mildly anesthetized or awake states with higher signaling), showed a tight correlation between Vcyc(tot) and CMRglc(ox),N (Figure 1; Supplementary Table 1). This neurometabolic coupling in the rat somatosensory cortex, spanning from awake to anesthetized conditions (Figure 1), has significance for a wide range of cerebral activities. First, it showed a linear relationship between neuronal activity (as measured by glutamatergic function) and oxidative demand. Furthermore at the intercept where Vcyc(tot) decreases to zero, CMRglc(ox),N is ∼0.1 μmol/g per minute, which corresponds to at most ∼20% of total energy in the awake brain. Hence, in the awake resting rodent brain at least 80% of the neuronal energy demand is devoted to events associated with neuronal signaling.
Figure 1.
Summary of 13C magnetic resonance spectroscopy (MRS) results from the rat somatosensory cortex. Relationship between rates of glutamate neurotransmitter cycling (Vcyc(tot)) and glucose oxidation in neurons (CMRglc(ox),N) derived by 13C MRS. The different data points represent a variety of anesthetized and awake conditions (Supplementary Table 1). The results suggest that ∼80% of the resting energy consumption in the awake rat brain is dedicated to events associated with neuronal activity. The red line is the best-fit linear regression of the rat data (i.e., CMRglc(ox),N=0.9 Vcyc(tot)+0.1, R2=0.92).
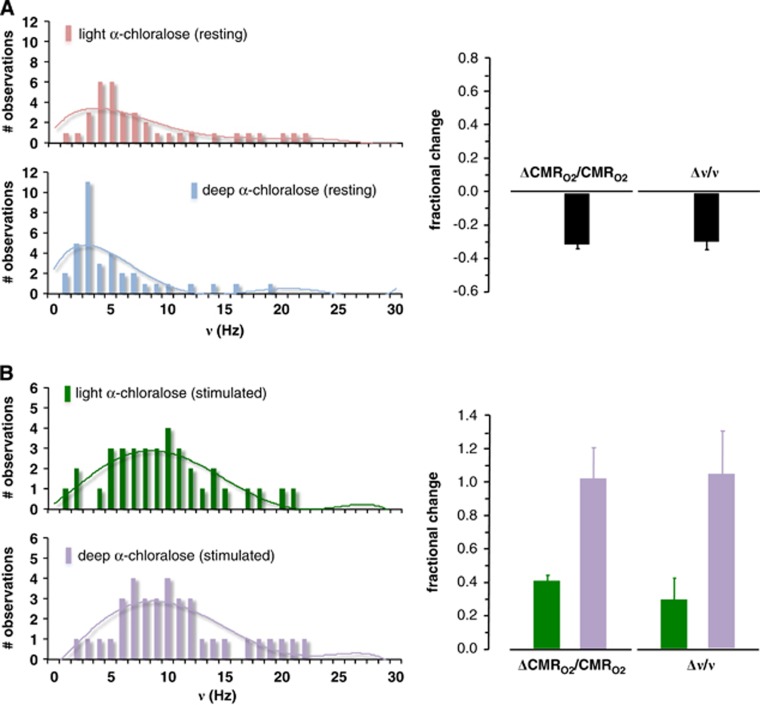
Because the blood oxygenation level-dependent (BOLD) contrast in fMRI is an indirect measure of neuronal activity,18 new methods have been developed for calibrating or converting the BOLD response into changes of oxidative energy demanded by neuronal signaling (for a historical perspective, see Hyder and Rothman19). Calibrated fMRI uses multimodal fMRI measurements of changes in BOLD signal, blood flow (CBF), and blood volume (CBV) to evaluate changes in oxidative demand (CMRO2), which is a fundamental parameter of brain function. Rat studies over a wide range of activities in the somatosensory cortex showed that values of (CMRO2) obtained from calibrated fMRI agreed with rates of pyramidal neuron firing (ν) measured by extracellular recordings. These studies, which measured changes in CMRO2 and ν during sensory stimulation from deeply and mildly anesthetized resting states, also show tight neurometabolic coupling (Figure 2).
Figure 2.
Summary of electrophysiology and calibrated functional magnetic resonance imaging (fMRI) results from the rat somatosensory cortex. Relationship between fractional changes in oxidative energy demand of neurons (ΔCMRO2/CMRO2) and pyramidal neuron firing rate (Δν/ν) obtained by calibrated fMRI and electrophysiology, respectively.7 Histograms, which represent firing rates of a population of neurons, are shown for (A) resting and (B) stimulated conditions from states achieved with different anesthetic doses. (A) As anesthetic dose increased the histograms shifted to lower firing rates such that both CMRO2 and ν decreased proportionately. (B) On sensory stimulation, the neuronal histograms shifted to higher firing rates such that both CMRO2 and ν increased consistently. The results suggest tight neurometabolic coupling.
These studies7 reported that as anesthetic dose increased the histograms, which represent firing rates of a population of neurons, shifted to lower firing rates so that both CMRO2 and ν decreased proportionately (Figure 2A). Moreover, on sensory stimulation from each of the anesthetized states the histograms shifted to higher firing rates so that both CMRO2 and ν increased consistently (Figure 2B). In agreement with the earlier 13C MRS results (Figure 1), these calibrated fMRI and electrophysiology results (Figure 2) showed a direct relationship between neuronal activities, as measured by multiunit firing of pyramidal neurons, and their oxidative demand. These results also showed that CMRO2 changes derived from calibrated fMRI are closely associated with glutamatergic function.20, 21
Results from other methods (e.g., electroencephalography (EEG), positron emission tomography (PET), 2-deoxyglucose (2DG) autoradiography) generally showed coupling between cortical activity and oxidative demand. 2DG autoradiographic studies in rats22 and PET studies in primates23 showed that anesthetic-dependent CMRglc decreased uniformly in the cerebral cortex. In humans, as discussed in the next section, EEG and PET data collected under different anesthetized states24 measured coupled decreases in electrical activity and energy metabolism in the cerebral cortex.
These converging results by independent methods, in addition to the results depicted in Figures 1 and 2, are consistent with the current understanding of molecular action of anesthetics,25 in which the dose-dependent potentiation of GABAergic systems affects the glutamatergic system by decreasing cortical activity/firing and energy demand. Glutamate and GABA, the major excitatory and inhibitory neurotransmitters, constitute >90% of cortical neurons in the adult mammalian brain.3
Results from rat studies suggest a tight neurometabolic coupling (i.e., nearly 1:1 relationship of ΔVcyc(tot):ΔCMRglc(ox),N and Δν:ΔCMRO2 as shown in Figures 1 and 2), with much smaller nonsignaling energy demand in the awake brain. But the question remains as to whether the same is true in the human brain. Moreover, it is not known if the energy demand for glutamatergic function in the awake resting human varies across brain regions. We used quantitative human brain imaging results from multiple modalities—EEG, PET, and calibrated fMRI—to estimate the fraction of resting energy consumption that supports glutamatergic signaling and to determine how gray-matter signaling varies regionally and under different conditions. The results of this meta-analysis, the potency of which is based on data collected from methods independent of 13C MRS and on data collected in other laboratories, suggest that the oxidative energy demand of glutamatergic signaling in the resting awake human brain is close to uniform in gray-matter space of the whole brain, a measurable property that could be of potential use in neurologic and psychiatric diagnosis.
Assessing the oxidative demand for excitatory signaling in the human brain
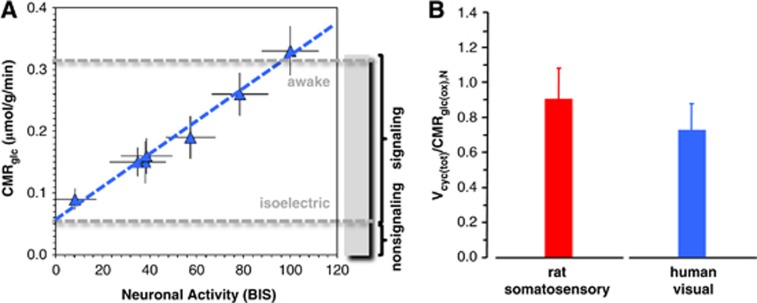
To derive evidence for an experimentally based relationship between neuronal oxidative demand and neuronal activity in the human brain, we analyzed human PET measurements of glucose utilization (CMRglc) under different conditions and compared them with human EEG measurements under similar conditions. These CMRglc and EEG data came from numerous behavioral conditions—awake, sleep, and different vegetative and sedated (i.e., anesthetized) states (Supplementary Table 2). All averaged CMRglc values were localized to the primary visual cortex. The EEG data were presented as bispectral index (BIS) values, ranging from 0 to 100, representing EEG silence (i.e., isoelectricity) to awake condition, respectively.26 Bispectral index is based on the weighted sum of several EEG parameters, spanning both time and frequency domains. The BIS measure is intended for monitoring the depth of anesthesia during surgeries where expert interpretation of the raw EEG data in terms of the different frequency bands, which change with overt behavioral variations, is not easily available.26, 27 While EEG is not as quantitative as microelectrode recordings, it has the advantage of noninvasively measuring small voltage fluctuations in vivo resulting from current flows within pyramidal neurons spanning cortical gray matter.28
As shown in Figure 3A, there is a close to linear dependence of BIS (measured by EEG) with CMRglc (measured by PET). The BIS and CMRglc values were obtained from different studies, but for the same experimental conditions. A best-fit of the data suggests that at isoelectricity (i.e., no neuronal signaling) CMRglc is reduced to ∼20% of the resting awake value, which means that at least 80% of total energy production in the human cerebral cortex is dedicated to excitatory signaling demands while the remaining fraction supports nonsignaling events. Another estimate of energy expenditure for minimal neuronal signaling can be obtained from comparing the CMRglc values in the persistent vegetative state (0.09±0.02 μmol/g per minute) with the resting awake state (0.34±0.04 μmol/g per minute). The comparison suggests that as much as 25% of the awake CMRglc value could be assigned to isoelectricity if it is assumed that the persistent vegetative state represents a state with minimal cortical activity. In other words, the finite intercept of the human PET and EEG data suggests nonnegligible energy consumption during isoelectric situations (Figure 3A), in congruence with 13C MRS values from rat brain (Figure 1), which are ∼20% to 25% of the energy in the awake state. Unfortunately, the 13C MRS data in the human are not as comprehensive as for the rat. While available human 13C MRS data are limited to the visual cortex with varying degrees of partial volume effects arising from white matter (Supplementary Table 1), the measured proportions of ΔVcyc(tot) and ΔCMRglc(ox),N agree quite well across both species (Supplementary Figure 1). Furthermore, as shown in Figure 3B, the high ratio of Vcyc(tot)/CMRglc(ox),N for the awake resting condition in the human suggests that events associated with glutamatergic neurotransmission consumes a majority of brain oxidative energy, similar to the rat. However, the absolute values of Vcyc(tot) and CMRglc(ox),N in the awake human and awake rat are significantly different, potentially because of lower metabolic rates in the human cerebral cortex.
Figure 3.
Summary of positron emission tomography (PET), electroencephalography (EEG), and 13C magnetic resonance spectroscopy (MRS) results from the human visual cortex. (A) Relationship between bispectral index (BIS) representing neuronal activity and glucose consumption (CMRglc) derived by EEG and PET, respectively. The different data points represent a variety of anesthetized and awake conditions (Supplementary Table 2). The dashed blue line represents the best-fit linear regression of all human data (i.e., CMRglc=0.003 BIS+0.06, R2=0.984), which is insignificantly different if the two vegetative state data points are removed (i.e., CMRglc=0.003 BIS+0.04, R2=0.986). The results suggest that ∼80% of the resting energy consumption in the awake human brain is dedicated to events associated with neuronal activity. (B) Relationship between rates of glutamate neurotransmitter cycling (Vcyc(tot)) and glucose oxidation in neurons (CMRglc(ox),N) derived by 13C MRS in awake rat and human brain, where the difference between species was insignificant (i.e., 0.89±0.17 versus 0.72±0.15 for rat and human, respectively). The data points were obtained from the somatosensory and visual cortices of rat and human, respectively (Supplementary Table 1; Supplementary Figure 1). The results suggest tight neurometabolic coupling.
Overall, these results suggest that metabolic energy demand for neuronal activity in the awake human is quite high and that there is tight neurometabolic coupling. However, a limitation of this analysis is that it depends on the quantitative accuracy with which BIS reflects various behavioral states.27, 29 While studies have shown that BIS values correlate well with other clinical sedation scores,26, 27 more studies are needed to assess the trends of BIS values across different sedation levels with different anesthetics. Clearly, the weakest data points in Figure 3A are the vegetative states because of possible tissue damage in cortical regions, which if included or removed do not significantly vary the linear regression tests (i.e., with or without the vegetative state data points, the slope is unchanged while the intercept changes from 0.06 to 0.04 μmol/g per minute). The similar values for these regressions argue that some minimal metabolism is present even in vegetative states, presumably for reduced signaling but other unknown factors could also be active. In the future, it should be possible to measure Vcyc(tot) and CMRglc(ox),N in humans by 13C MRS studies under different anesthetized states, as previously performed in rats. In some cases, such as in patients undergoing presurgical evaluation, it may be possible to directly measure neuronal firing30 and then relate those measurements to cerebral metabolism.
Regional oxidative demand for glutamatergic activity in the resting awake human brain
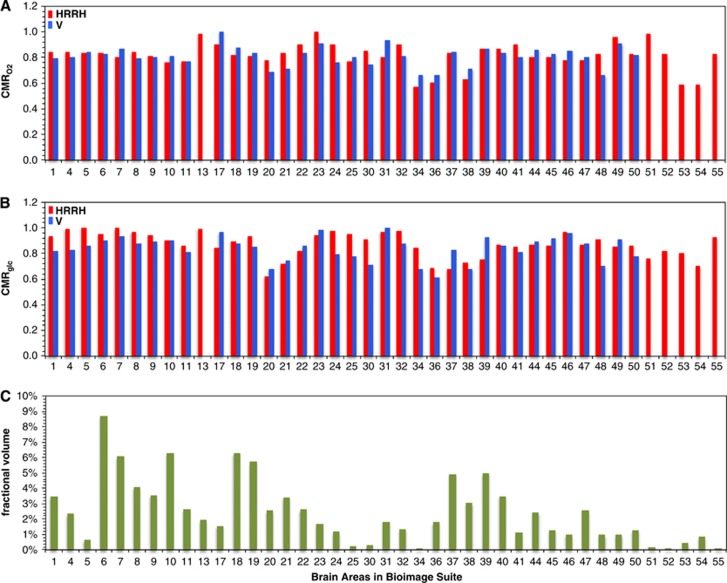
To determine whether awake resting energy and coupled neuronal activity are uniform across different regions, we analyzed regional distributions of CMRO2 and CMRglc in the normal human brain from PET data acquired under typical experimental settings of awake but eyes closed and ears covered (i.e., quiet experimental settings).31, 32, 33, 34, 35 The meta-analysis was composed of CMRO2 and CMRglc values from as few as 23 and as many as 46 distinct brain areas in 109 subjects. BioImage Suite (www.bioimagesuite.org)—an integrated image analysis software36—was used for reporting coordinates in standard brain templates to represent gray-matter regions in cortical and subcortical areas (Supplementary Figure 2; Supplementary Table 3). Four older data sets reported CMRO2 and CMRglc in absolute units,31, 32, 33, 34 whereas a more recent data set reported CMRO2 and CMRglc in relative units.35 The older and newer data, abbreviated as HRRH and V, were analyzed separately. From the HRRH data, we obtained one gray-matter mean absolute value of CMRglc and one mean gray-matter absolute value of CMRO2. The respective gray matter mean absolute values of CMRglc and CMRO2 from the HRRH data were then multiplied by the gray-matter relative values of CMRglc and CMRO2 from the V data. The oxygen-to-glucose index (OGI) was calculated from the CMRO2/CMRglc ratio. The left-right asymmetries in HRRH and V data were negligible (Supplementary Figure 3). Although the HRRH and V data had slightly different brain coverage, the pooled data sets allowed representation of the entire gray-matter space because missing regions in an individual data set were compensated by availability of coverage from other data sets (Supplementary Figure 4).
While deviations of ±10% from the mean CMRO2 and CMRglc values were observed across the majority of regions in HRRH and V data separately, the pooled data showed that regions with >10% deviations in CMRO2 and CMRglc corresponded to <10% of the entire gray-matter space (Figure 4). Some of the lowest metabolic values were found in the amygdala, hippocampus, entorhinal cortex, parahippocampal cortex, and temporopolar area, whereas some of the highest metabolic values were located in the insular cortex, cingulate cortex, putamen, and globus pallidus (Supplementary Figure 5).
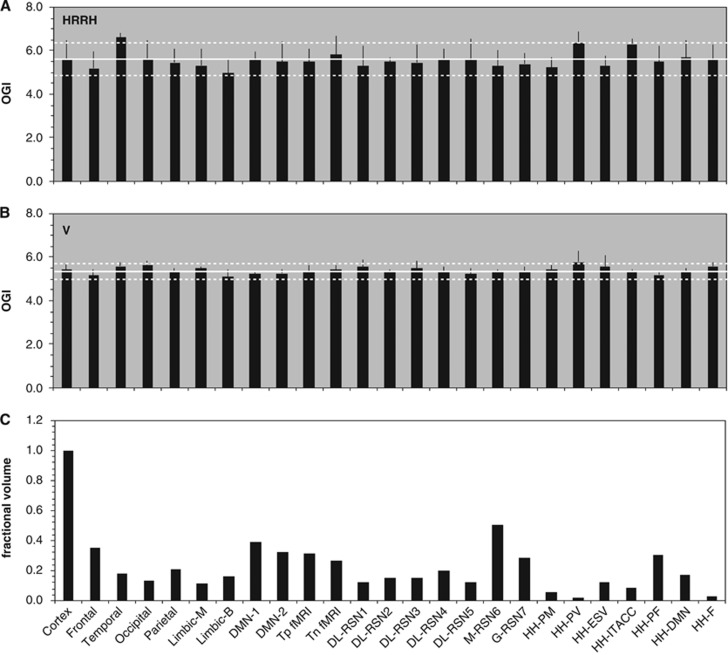
Figure 4.
Summary of oxidative demand across regions for the resting awake human brain. Comparison of relative values of (A) CMRO2, (B) CMRglc, and (C) fractional volume of 43 brain areas. First, the comparison of the two data sets shows that the general regional trends are similar for the meta-analysis of HRRH (red) and V (blue) data sets. Second, the comparison between CMRO2 and CMRglc shows that general oxidative demand is similar across regions. Third, the comparison of the metabolic and fractional volumes shows that for >85% of gray-matter regions depicted the metabolic variations are within ±10% of the gray-matter mean values. See Supplementary Table 3 for details of the brain areas. See Supplementary Figure 2 for visual inspection of where these areas are in the brain. See Supplementary Figure 3 for left-right asymmetries in the HRRH (red) and V (blue) data sets. See Supplementary Figure 4 for specific spatial uncertainties in the HRRH (red) and V (blue) data sets. See Supplementary Figure 5 for absolute values of (A) CMRO2, (B) CMRglc, and (C) oxygen-to-glucose index (OGI), respectively.
But how do these cortical CMRglc differences in the awake human PET data compare with awake primate PET data and awake rat 2DG data? Direct metabolic comparisons at rest across species should be made cautiously because the human PET data are usually from subjects in quiet settings (i.e., eyes closed and ears covered) without exposure to any drugs,31, 32, 33, 34, 35 whereas rat 2DG22 and primate PET23 data may be from variable experimental conditions (e.g., some level of thalamic activation when awake and reawakening from mild sedation). In 2DG studies, most cortical areas in mammals show <20% variation from the mean CMRglc values in the awake state, whereas PET data from human (reviewed here) and primate brains show <10% CMRglc variation across cortical and subcortical regions.
In the HRRH and V data, the respective mean CMRO2 values were 1.7±0.2 and 1.8±0.2 μmol/g per minute, whereas respective mean CMRglc values were 0.30±0.03 and 0.34±0.04 μmol/g per minute. These mean CMRO2 and CMRglc values corresponded to respective mean OGI values of 5.6±0.8 and 5.4±0.3 for the HRRH and V data sets, which are in good agreement with prior results.37, 38, 39, 40, 41 The slightly better regional stability in the V data (versus the HRRH data) was likely because of better spatial resolution in the V data, which were presumably less affected by partial volume effects arising from white matter, which has a much lower average metabolic rate than gray matter and a variable percentage within the image voxels can cause significant rate variations even with a constant rate of gray-matter metabolism. However in some small brain regions the metabolic values varied by as much as 50% from the gray-matter mean (Figure 4).
For example, in the HRRH data the lowest/highest metabolic values, in units of μmol/g per minute, were ∼1.2/2.0 for CMRO2 (gray-matter mean of 1.7 μmol/g per minute) and 0.21/0.34 for CMRglc (gray-matter mean of 0.30 μmol/g per minute), respectively. The lowest and highest CMRO2 values were located in parahippocampal gyrus and ventral posterior cingulate cortex (i.e., see # 34 and 23 in Supplementary Table 3). The lowest CMRglc value was located in inferior temporal gyrus, whereas the highest CMRglc values were found in areas like primary motor cortex, somatosensory association cortex, and insular cortex (i.e., see # 20, 4, 5, and 13 in Supplementary Table 3). But each of these regions corresponded to <2% of gray-matter space (see Supplementary Table 3). Similar trends were observed in the V data. Thus the majority of regions, which together represented >85% of gray-matter space, were within 10% of the mean CMRO2 and CMRglc values (Supplementary Figure 5).
We also examined whether there are differences in metabolic demands of brain networks, because considerable interest had been raised for the default mode network by reports that it has exceptionally high metabolic rate.42, 43, 44 Networks were identified as either classic or contemporary based on anatomic and functional boundaries, respectively (Supplementary Table 4). Classic networks consisted of homologous areas of major vascular branching, for example, frontal lobe, temporal lobe, occipital lobe, parietal lobe, and medial or basolateral limbic areas.45 Contemporary networks consisted of regions identified from resting-state fMRI experimental results.42, 46, 47, 48, 49, 50, 51, 52 Sizes of classic networks varied from ∼11% (medial limbic area) to 35% (frontal lobe) of gray-matter space, whereas sizes of contemporary networks varied substantially, for example, a frontal network (HH-F in Figure 5) was only 2.5% of gray-matter space,52 whereas a conscious resting-state network (M-RSN6 in Figure 5) was ∼50% of gray-matter space.50 All networks examined were found to be within ±10% of the mean OGI in gray matter (Figure 5), which are based on similar variations of CMRO2 and CMRglc across gray-matter space (Supplementary Figure 6).
Figure 5.
Summary of oxidative demand in various networks for the resting awake human brain. Comparison of (A) oxygen-to-glucose index (OGI) in HRRH data set, (B) OGI in V data set, and (C) fractional volume in 24 brain networks. First, the comparison of the two data sets shows that the general OGI trends across networks are similar. Second, the OGI is quite uniform across all networks. Third, the comparison of OGI and fractional volumes shows that small and large networks have similar oxidative energy demands. The horizontal white lines indicate mean and the standard deviations. See Supplementary Table 4 for details of the brain areas in each network and their origins. See Supplementary Figure 6 for absolute values of (A) CMRO2 and (B) CMRglc, respectively, for each network.
Dynamic variability of oxidative demand in the resting awake human brain
Since resting-state fMRI is used to identify networks based on spontaneous BOLD signal fluctuations, it is important to determine moment-to-moment metabolic demand of these fluctuations relative to the average resting level energy for the spontaneous neuronal activity in the awake state. Studies, to date, have not investigated this issue specifically because dynamic CMRO2 and CMRglc fluctuations are difficult to measure with MRS or PET methods. However, an estimate of CMRO2 fluctuations may be obtained from calibrated fMRI studies of the human cerebral cortex during sensory stimulation (for a recent review, see Hyder et al21).
In calibrated fMRI studies, the CMRO2 change is determined by combining BOLD signal measurements with CBF and CBV measurements (or indirectly from measured responses of changes in BOLD signal, CBF, and CBV with CO2 inhalation53, 54, 55). Based on these human studies at 1.5 to 3.0 T, an approximately ±1% fluctuation in the BOLD signal corresponds to at most ±10% variation in CMRO2 relative to the resting awake average.56, 57, 58 This is a maximum estimate because a considerable amount of the fluctuation amplitude of the BOLD signal may also be influenced by physiologic noise, motion, and electronic imperfections in the scanner.59, 60, 61 Furthermore, the BOLD signal fluctuations measured in resting-state fMRI, which are on the order of <0.1 Hz, do not account for the majority of spontaneous neuronal signal variations,62 suggesting lower than 10% variations in CMRO2. Therefore, we believe that the energy demand for the lower frequency signaling could be less than our current energy estimate for spontaneous fluctuations in the BOLD signal.58
Global reductions of oxidative demand and neuronal activity in the human brain
Since the regional and dynamic deviations in metabolism are small, a plausible hypothesis is that the energy demand in brain networks is uniformly high to support widespread signaling in the awake human.63 In other words, the less than ±10% variations of oxidative energy across gray-matter space do not necessarily indicate which regions are of greater or lesser importance given the similarly high metabolic activity in all regions. A test of this hypothesis is the extent to which oxidative metabolism, in relation to neuronal activity, is affected in states where consciousness is lost.
It is well known that a variety of sleep levels, states achieved with different anesthetics, or vegetative situations reduce cortical energy demand and electrical activity.64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76 In principle, if specific regions within the brain selectively reduce metabolic (and neuronal) activity when consciousness was lost, it might indicate that these regions have a preferentially important role in resting awake brain function. However, human PET studies with sleep, vegetative, and anesthetized states all show quite uniform CMRglc reductions—>20%—across gray-matter space, which seems to also parallel decreased EEG power (Figure 6). As shown previously in Figure 3, the decrease in CMRglc and EEG power (based on the BIS measure), is close to linear. Although a more detailed analysis will be required as was performed for CMRglc and CMRO2 studies in the resting awake state (Figures 4 and 5), present comparison of regional activity during states of altered consciousness do not show specific regions with selectively depressed activity.
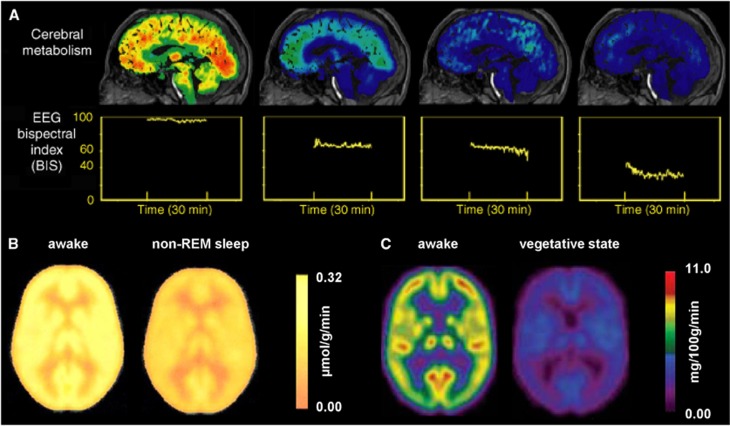
Figure 6.
Summary of global reduction in oxidative demand and neuronal activity in the human brain. Deep or nonrapid eye movement (non-REM) sleep states, different stages of the vegetative brain, and varying degrees of anesthesia depth show significant reductions in energy metabolism as measured by CMRglc with positron emission tomography (PET) and electrical activity as reflected with bispectral index (BIS) values from electroencephalography (EEG) recordings. (A) Different anesthetic depths, increasing from left to right, reduced CMRglc and BIS values compared with the awake brain.70, 71, 72, 73, 74 Reproduced with permission from Clin Pharmacol Ther.72 (B) Non-REM sleep shows CMRglc in the visual cortex to be reduced to 0.26±0.06 μmol/g per minute from 0.33±0.07 μmol/g per minute in the awake brain.64, 65 Reproduced with permission from Neuropsychopharmacology.64 Neuronal activity with BIS shows 100±12 versus 79±12 in awake versus non-REM sleep.66, 67 (C) Vegetative state shows CMRglc in the visual cortex to be reduced to 0.15±0.04 μmol/g per minute.68 Reproduced with permission from NeuroRehabilitation.68 Neuronal activity with BIS shows values of 100±12 versus 35±12 in awake versus persistent vegetative state.69
Conclusions
Our meta-analysis of cerebral energy consumption and its coupling with neuronal activity suggests that metabolic energy is quite uniform in gray-matter space of the human brain. This conclusion does not make any claims about uniformity of cellular density because the measured metabolic data from PET are susceptible to partial volume effects arising from either white matter or varying cortical thickness across the entire brain.77 We made no attempt to apply any partial volume corrections based on the cortical thickness alone, which would presumably reduce the heterogeneity of metabolic values across regions even further. Until individual voxel PET and MRI data are available on an individual subject basis, partial volume effects of gray versus white matter cannot be quantitatively addressed. This metabolic activity (i.e., CMRglc or CMRO2 with PET, CMRglc(ox),N with 13C MRS, CMRO2 with calibrated fMRI) changes in proportion to overall electrical activity (i.e., by BIS with EEG, neuronal firing rate with electrophysiology, Vcyc(tot) with 13C MRS), but it is reduced to ∼20% of resting awake levels in isoelectric conditions, indicating that the majority of metabolic demand is to support signaling and events downstream of signaling. Direct measurements by 13C MRS (i.e., Vcyc(tot) and CMRglc(ox),N) in human occipital lobe show that the coupling between metabolic activity and glutamate neurotransmitter cycling and neuronal firing rate is similar to the rodent somatosensory cortex (Figures 1 to 3), indicating that most of the metabolic activity is used to support mammalian brain glutamatergic signaling.78 The neurometabolic coupling for glutamatergic activity appears to be uniform across gray-matter space based on comparisons of CMRglc and CMRO2 data, all measured by PET, showing good regional agreement (Figures 4 to 6). The use of small average metabolic differences across regions as evidence that some networks have greater functional importance than others (e.g., default mode network42, 43, 44) does not seem justified given the much larger underlying activity that all networks and regions share. However, future studies with better spatial resolution are needed to resolve white-matter partial volume effects to represent metabolism in gray matter, especially across the cerebral cortex where gray-matter thickness varies considerably,77 such that regional differences can be more quantitatively assessed. The dependence of task-induced response on the resting brain activity strongly argues for the functional relevance of high metabolic demand of the resting awake state.12, 13, 14 The total resting brain activity is an independent parameter and when included in data analysis opens the way for new experimental paradigms that can delineate functions of the total energy. Additionally, the 13C MRS separation of neuronal and glial metabolic rates,79, 80 dissociating contributions from neuronal and glial populations across brain regions, is now seen to be another variable under experimental measure when studying networks in health and disease. Finally, given the importance of ubiquitously high oxidative energy demand for supporting conscious human behavior,63 we propose that clinical fMRI studies could take advantage of quantitative measures of metabolic energy when evaluating patients.
Acknowledgments
FH thanks Xenophon Papademetris for help with BioImage Suite (www.bioimagesuite.org).
The functional imaging group at Washington University at St Louis specifically Marcus Raichle and his colleagues are in direct conflict with this work and our conclusions based on their recent paper (Vaishnavi SN et al (2010) Regional aerobic glycolysis in the human brain. Proc Natl Acad Sci USA 107:17757–17762).
Footnotes
Supplementary Information accompanies the paper on the Journal of Cerebral Blood Flow & Metabolism website (http://www.nature.com/jcbfm)
This study was supported by National Institutes of Health Grants (R01 MH-067528 to FH, P30 NS-052519 to FH, and R01 AG-034953 to DLR).
Supplementary Material
References
- Aiello LC, Wheeler P. The expensive-tissue hypothesis—the brain and the digestive-system in human and primate evolution. Curr Anthropol. 1995;36:199–221. [Google Scholar]
- Siesjo BK. Brain Energy Metabolism. Wiley and Sons, Ltd.: New York, USA; 1978. [Google Scholar]
- Nicholls DG. Release of glutamate, aspartate, and gamma-aminobutyric acid from isolated nerve terminals. J Neurochem. 1989;52:331–341. doi: 10.1111/j.1471-4159.1989.tb09126.x. [DOI] [PubMed] [Google Scholar]
- Creutzfeldt O.Neurophysiological correlates of different functional states of the brainIn: Ingvar D, Lassen N, (eds).. Brain work the coupling of function, metabolism and blood flow in the brain alfred benzon symposium viii Academic Press: New York; 197521–46. [Google Scholar]
- Sibson NR, Dhankhar A, Mason GF, Rothman DL, Behar KL, Shulman RG. Stoichiometric coupling of brain glucose metabolism and glutamatergic neuronal activity. Proc Natl Acad Sci USA. 1998;95:316–321. doi: 10.1073/pnas.95.1.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attwell D, Laughlin SB. An energy budget for signaling in the grey matter of the brain. J Cereb Blood Flow Metab. 2001;21:1133–1145. doi: 10.1097/00004647-200110000-00001. [DOI] [PubMed] [Google Scholar]
- Smith AJ, Blumenfeld H, Behar KL, Rothman DL, Shulman RG, Hyder F. Cerebral energetics and spiking frequency: the neurophysiological basis of fMRI. Proc Natl Acad Sci USA. 2002;99:10765–10770. doi: 10.1073/pnas.132272199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyder F, Patel AB, Gjedde A, Rothman DL, Behar KL, Shulman RG. Neuronal-glial glucose oxidation and glutamatergic-gabaergic function. J Cereb Blood Flow Metab. 2006;26:865–877. doi: 10.1038/sj.jcbfm.9600263. [DOI] [PubMed] [Google Scholar]
- Maandag NJ, Coman D, Sanganahalli BG, Herman P, Smith AJ, Blumenfeld H, et al. Energetics of neuronal signaling and fMRI activity. Proc Natl Acad Sci USA. 2007;104:20546–20551. doi: 10.1073/pnas.0709515104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyder F, Rothman DL, Shulman RG. Total neuroenergetics support localized brain activity: Implications for the interpretation of fMRI. Proc Natl Acad Sci USA. 2002;99:10771–10776. doi: 10.1073/pnas.132272299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman RG, Rothman DL, Behar KL, Hyder F. Energetic basis of brain activity: Implications for neuroimaging. Trends Neurosci. 2004;27:489–495. doi: 10.1016/j.tins.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Shulman RG, Rothman DL. Interpreting functional imaging studies in terms of neurotransmitter cycling. Proc Natl Acad Sci USA. 1998;95:11993–11998. doi: 10.1073/pnas.95.20.11993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northoff G, Qin P, Nakao T. Rest-stimulus interaction in the brain: a review. Trends Neurosci. 2010;33:277–284. doi: 10.1016/j.tins.2010.02.006. [DOI] [PubMed] [Google Scholar]
- Hyder F, Rothman DL. Evidence for the importance of measuring total brain activity in neuroimaging. Proc Natl Acad Sci USA. 2011;108:5475–5476. doi: 10.1073/pnas.1102026108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ. Imaging neuroscience: principles or maps. Proc Natl Acad Sci USA. 1998;95:796–802. doi: 10.1073/pnas.95.3.796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal BB, Mennes M, Zuo XN, Gohel S, Kelly C, Smith SM, et al. Toward discovery science of human brain function. Proc Natl Acad Sci USA. 2010;107:4734–4739. doi: 10.1073/pnas.0911855107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman DL, De Feyter HM, de Graaf RA, Mason GF, Behar KL. 13c mrs studies of neuroenergetics and neurotransmitter cycling in humans. NMR Biomed. 2011;24:943–957. doi: 10.1002/nbm.1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa S, Lee TM, Kay AR, Tank DW. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc Natl Acad Sci USA. 1990;87:9868–9872. doi: 10.1073/pnas.87.24.9868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyder F, Rothman DL. Quantitative fMRI and oxidative neuroenergetics. Neuroimage. 2012;62:985–994. doi: 10.1016/j.neuroimage.2012.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyder F, Kida I, Behar KL, Kennan RP, Maciejewski PK, Rothman DL. Quantitative functional imaging of the brain: towards mapping neuronal activity by bold fMRI. NMR Biomed. 2001;14:413–431. doi: 10.1002/nbm.733. [DOI] [PubMed] [Google Scholar]
- Hyder F, Sanganahalli BG, Herman P, Coman D, Maandag NJ, Behar KL, et al. Neurovascular and neurometabolic couplings in dynamic calibrated fMRI: transient oxidative neuroenergetics for block-design and event-related paradigms Front Neuroenergetics 20102pii: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokoloff L, Reivich M, Kennedy C, Des Rosiers MH, Patlak CS, Pettigrew KD, et al. The [14c]deoxyglucose method for the measurement of local cerebral glucose utilization: theory, procedure, and normal values in the conscious and anesthetized albino rat. J Neurochem. 1977;28:897–916. doi: 10.1111/j.1471-4159.1977.tb10649.x. [DOI] [PubMed] [Google Scholar]
- Takamatsu H, Noda A, Kurumaji A, Murakami Y, Tatsumi M, Ichise R, et al. A pet study following treatment with a pharmacological stressor, FG7142, in conscious rhesus monkeys. Brain Res. 2003;980:275–280. doi: 10.1016/s0006-8993(03)02987-1. [DOI] [PubMed] [Google Scholar]
- Alkire MT. Quantitative eeg correlations with brain glucose metabolic rate during anesthesia in volunteers. Anesthesiology. 1998;89:323–333. doi: 10.1097/00000542-199808000-00007. [DOI] [PubMed] [Google Scholar]
- Franks NP. General anaesthesia: from molecular targets to neuronal pathways of sleep and arousal. Nat Rev Neurosci. 2008;9:370–386. doi: 10.1038/nrn2372. [DOI] [PubMed] [Google Scholar]
- Dominguez TE, Helfaer MA.Review of bispectral index monitoring in the emergency department and pediatric intensive care unit Pediatr Emerg Care 200622815–821.quiz 822-824. [DOI] [PubMed] [Google Scholar]
- Kent CD, Domino KB. Depth of anesthesia. Curr Opin Anaesthesiol. 2009;22:782–787. doi: 10.1097/ACO.0b013e3283326986. [DOI] [PubMed] [Google Scholar]
- Buzsaki G. Rhythms of the brain. Oxford University Press: New York, USA; 2006. [Google Scholar]
- Bennett C, Voss LJ, Barnard JP, Sleigh JW. Practical use of the raw electroencephalogram waveform during general anesthesia: the art and science. Anesth Analg. 2009;109:539–550. doi: 10.1213/ane.0b013e3181a9fc38. [DOI] [PubMed] [Google Scholar]
- Goldstein SR, Bak MJ, Oakley JC, Schmidt EM, Van Buren JM. An instrument for stable single cell recording from pulsating human cerebral cortex. Electroencephalogr Clin Neurophysiol. 1975;39:667–670. doi: 10.1016/0013-4694(75)90081-4. [DOI] [PubMed] [Google Scholar]
- Hatazawa J, Fujita H, Kanno I, Satoh T, Iida H, Miura S, et al. Regional cerebral blood flow, blood volume, oxygen extraction fraction, and oxygen utilization rate in normal volunteers measured by the autoradiographic technique and the single breath inhalation method. Ann Nucl Med. 1995;9:15–21. doi: 10.1007/BF03165003. [DOI] [PubMed] [Google Scholar]
- Horwitz B, Duara R, Rapoport SI. Intercorrelations of glucose metabolic rates between brain regions: Application to healthy males in a state of reduced sensory input. J Cereb Blood Flow Metab. 1984;4:484–499. doi: 10.1038/jcbfm.1984.73. [DOI] [PubMed] [Google Scholar]
- Roland PE, Eriksson L, Stone-Elander S, Widen L. Does mental activity change the oxidative metabolism of the brain. J Neurosci. 1987;7:2373–2389. [PMC free article] [PubMed] [Google Scholar]
- Rumsey JM, Duara R, Grady C, Rapoport JL, Margolin RA, Rapoport SI, et al. Brain metabolism in autism. Resting cerebral glucose utilization rates as measured with positron emission tomography. Arch Gen Psychiatry. 1985;42:448–455. doi: 10.1001/archpsyc.1985.01790280026003. [DOI] [PubMed] [Google Scholar]
- Vaishnavi SN, Vlassenko AG, Rundle MM, Snyder AZ, Mintun MA, Raichle ME. Regional aerobic glycolysis in the human brain. Proc Natl Acad Sci USA. 2010;107:17757–17762. doi: 10.1073/pnas.1010459107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacadie CM, Fulbright RK, Rajeevan N, Constable RT, Papademetris X. More accurate talairach coordinates for neuroimaging using non-linear registration. Neuroimage. 2008;42:717–725. doi: 10.1016/j.neuroimage.2008.04.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frackowiak RS, Herold S, Petty RK, Morgan-Hughes JA. The cerebral metabolism of glucose and oxygen measured with positron tomography in patients with mitochondrial diseases. Brain. 1988;111 (Pt 5:1009–1024. doi: 10.1093/brain/111.5.1009. [DOI] [PubMed] [Google Scholar]
- Herold S, Frackowiak RS, Le Couteur A, Rutter M, Howlin P. Cerebral blood flow and metabolism of oxygen and glucose in young autistic adults. Psychol Med. 1988;18:823–831. doi: 10.1017/s0033291700009752. [DOI] [PubMed] [Google Scholar]
- Madsen PL, Hasselbalch SG, Hagemann LP, Olsen KS, Bulow J, Holm S, et al. Persistent resetting of the cerebral oxygen/glucose uptake ratio by brain activation: Evidence obtained with the Kety-Schmidt technique. J Cereb Blood Flow Metab. 1995;15:485–491. doi: 10.1038/jcbfm.1995.60. [DOI] [PubMed] [Google Scholar]
- Shulman RG, Hyder F, Rothman DL. Lactate efflux and the neuroenergetic basis of brain function. NMR Biomed. 2001;14:389–396. doi: 10.1002/nbm.741. [DOI] [PubMed] [Google Scholar]
- Lindroos MM, Borra RJ, Parkkola R, Virtanen SM, Lepomaki V, Bucci M, et al. Cerebral oxygen and glucose metabolism in patients with mitochondrial m.3243a>G mutation. Brain. 2009;132:3274–3284. doi: 10.1093/brain/awp259. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci USA. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusnard DA, Raichle ME. Searching for a baseline: functional imaging and the resting human brain. Nat Rev Neurosci. 2001;2:685–694. doi: 10.1038/35094500. [DOI] [PubMed] [Google Scholar]
- Lin P, Hasson U, Jovicich J, Robinson S. A neuronal basis for task-negative responses in the human brain. Cereb Cortex. 2011;21:821–830. doi: 10.1093/cercor/bhq151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenfeld H. Neuroanatomy through clinical cases. Sinauer Associates, Inc.: Sunderland, MA; 2002. [Google Scholar]
- Shulman GI, Fiez JA, Corbetta M, Buckner RL, Miezin FM, Raichle ME, et al. Common blood flow changes across visual tasks: II. Decreases in cerebral cortex. J Cogn Neurosci. 1997;9:648–663. doi: 10.1162/jocn.1997.9.5.648. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain's default network: Anatomy, function, and relevance to disease. Ann NY Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci USA. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Luca M, Beckmann CF, De Stefano N, Matthews PM, Smith SM. fMRI resting state networks define distinct modes of long-distance interactions in the human brain. Neuroimage. 2006;29:1359–1367. doi: 10.1016/j.neuroimage.2005.08.035. [DOI] [PubMed] [Google Scholar]
- Mazoyer B, Zago L, Mellet E, Bricogne S, Etard O, Houde O, et al. Cortical networks for working memory and executive functions sustain the conscious resting state in man. Brain Res Bull. 2001;54:287–298. doi: 10.1016/s0361-9230(00)00437-8. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Kiviniemi V, Tervonen O, Vainionpaa V, Alahuhta S, Reiss AL, et al. Persistent default-mode network connectivity during light sedation. Hum Brain Mapp. 2008;29:839–847. doi: 10.1002/hbm.20537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel MP, Hulshoff Pol HE. Exploring the brain network: a review on resting-state fMRI functional connectivity. Eur Neuropsychopharmacol. 2010;20:519–534. doi: 10.1016/j.euroneuro.2010.03.008. [DOI] [PubMed] [Google Scholar]
- Buxton RB. Dynamic models of bold contrast. Neuroimage. 2012;62:953–961. doi: 10.1016/j.neuroimage.2012.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike GB. Quantitative functional MRI: concepts, issues and future challenges. Neuroimage. 2012;62:1234–1240. doi: 10.1016/j.neuroimage.2011.10.046. [DOI] [PubMed] [Google Scholar]
- Hoge RD. Calibrated fMRI. Neuroimage. 2012;62:930–937. doi: 10.1016/j.neuroimage.2012.02.022. [DOI] [PubMed] [Google Scholar]
- Gu H, Stein EA, Yang Y. Nonlinear responses of cerebral blood volume, blood flow and blood oxygenation signals during visual stimulation. Magn Reson Imaging. 2005;23:921–928. doi: 10.1016/j.mri.2005.09.007. [DOI] [PubMed] [Google Scholar]
- Wu CW, Gu H, Lu H, Stein EA, Chen JH, Yang Y. Mapping functional connectivity based on synchronized CMRO2 fluctuations during the resting state. Neuroimage. 2009;45:694–701. doi: 10.1016/j.neuroimage.2008.12.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyder F, Rothman DL. Neuronal correlate of bold signal fluctuations at rest: Err on the side of the baseline. Proc Natl Acad Sci USA. 2010;107:10773–10774. doi: 10.1073/pnas.1005135107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birn RM, Diamond JB, Smith MA, Bandettini PA. Separating respiratory-variation-related fluctuations from neuronal-activity-related fluctuations in fMRI. Neuroimage. 2006;31:1536–1548. doi: 10.1016/j.neuroimage.2006.02.048. [DOI] [PubMed] [Google Scholar]
- Chang C, Glover GH. Effects of model-based physiological noise correction on default mode network anti-correlations and correlations. Neuroimage. 2009;47:1448–1459. doi: 10.1016/j.neuroimage.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman P, Sanganahalli BG, Hyder F, Eke A. Fractal analysis of spontaneous fluctuations of the bold signal in rat brain. Neuroimage. 2011;58:1060–1069. doi: 10.1016/j.neuroimage.2011.06.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholvinck ML, Maier A, Ye FQ, Duyn JH, Leopold DA. Neural basis of global resting-state fMRI activity. Proc Natl Acad Sci USA. 2010;107:10238–10243. doi: 10.1073/pnas.0913110107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman RG, Hyder F, Rothman DL. Baseline brain energy supports the state of consciousness. Proc Natl Acad Sci USA. 2009;106:11096–11101. doi: 10.1073/pnas.0903941106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchsbaum MS, Hazlett EA, Wu J, Bunney WE. Positron emission tomography with deoxyglucose-F18 imaging of sleep. Neuropsychopharmacology. 2001;25 (5 Suppl:S50–S56. doi: 10.1016/S0893-133X(01)00339-6. [DOI] [PubMed] [Google Scholar]
- Heiss WD, Pawlik G, Herholz K, Wagner R, Wienhard K. Regional cerebral glucose metabolism in man during wakefulness, sleep, and dreaming. Brain Res. 1985;327:362–366. doi: 10.1016/0006-8993(85)91537-9. [DOI] [PubMed] [Google Scholar]
- Ozgoren M, Bayazit O, Kocaaslan S, Gokmen N, Oniz A. Brain function assessment in different conscious states. Nonlinear Biomed Phys. 2010;4 (Suppl 1:S6. doi: 10.1186/1753-4631-4-S1-S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tung A, Lynch JP, Roizen MF. Use of the bis monitor to detect onset of naturally occurring sleep. J Clin Monit Comput. 2002;17:37–42. doi: 10.1023/a:1015404803637. [DOI] [PubMed] [Google Scholar]
- Laureys S. Functional neuroimaging in the vegetative state. NeuroRehabilitation. 2004;19:335–341. [PubMed] [Google Scholar]
- Pandit JJ, Schmelzle-Lubiecki B, Goodwin M, Saeed N. Bispectral index-guided management of anaesthesia in permanent vegetative state. Anaesthesia. 2002;57:1190–1194. doi: 10.1046/j.1365-2044.2002.02891.x. [DOI] [PubMed] [Google Scholar]
- Alkire MT, Pomfrett CJ, Haier RJ, Gianzero MV, Chan CM, Jacobsen BP, et al. Functional brain imaging during anesthesia in humans: effects of halothane on global and regional cerebral glucose metabolism. Anesthesiology. 1999;90:701–709. doi: 10.1097/00000542-199903000-00011. [DOI] [PubMed] [Google Scholar]
- Alkire MT, Haier RJ, Barker SJ, Shah NK, Wu JC, Kao YJ.Cerebral metabolism during propofol anesthesia in humans studied with positron emission tomography Anesthesiology 199582393–403.discussion 327A. [DOI] [PubMed] [Google Scholar]
- Alkire MT. Probing the mind: Anesthesia and neuroimaging. Clin Pharmacol Ther. 2008;84:149–152. doi: 10.1038/clpt.2008.75. [DOI] [PubMed] [Google Scholar]
- Leslie K, Sleigh J, Paech MJ, Voss L, Lim CW, Sleigh C. Dreaming and electroencephalographic changes during anesthesia maintained with propofol or desflurane. Anesthesiology. 2009;111:547–555. doi: 10.1097/ALN.0b013e3181adf768. [DOI] [PubMed] [Google Scholar]
- Kaisti KK, Langsjo JW, Aalto S, Oikonen V, Sipila H, Teras M, et al. Effects of sevoflurane, propofol, and adjunct nitrous oxide on regional cerebral blood flow, oxygen consumption, and blood volume in humans. Anesthesiology. 2003;99:603–613. doi: 10.1097/00000542-200309000-00015. [DOI] [PubMed] [Google Scholar]
- Katoh T, Bito H, Sato S. Influence of age on hypnotic requirement, bispectral index, and 95% spectral edge frequency associated with sedation induced by sevoflurane. Anesthesiology. 2000;92:55–61. doi: 10.1097/00000542-200001000-00014. [DOI] [PubMed] [Google Scholar]
- Stullken EH, Milde JH, Michenfelder JD, Tinker JH. The nonlinear responses of cerebral metabolism to low concentrations of halothane, enflurane, isoflurane, and thiopental. Anesthesiology. 1977;46:28–34. doi: 10.1097/00000542-197701000-00007. [DOI] [PubMed] [Google Scholar]
- la Fougere C, Grant S, Kostikov A, Schirrmacher R, Gravel P, Schipper HM, et al. Where in-vivo imaging meets cytoarchitectonics: the relationship between cortical thickness and neuronal density measured with high-resolution [18f]flumazenil-pet. Neuroimage. 2011;56:951–960. doi: 10.1016/j.neuroimage.2010.11.015. [DOI] [PubMed] [Google Scholar]
- Hyder F, Rothman DL, Bennett MW.Cortical energy demands of signaling and nonsignaling components in brain are conserved across mammalian species and activity levels Proc Natl Acad Sci USA 2012 www.pnas.org/cgi/doi/10.1073/pnas.1214912110 . [DOI] [PMC free article] [PubMed]
- Lebon V, Petersen KF, Cline GW, Shen J, Mason GF, Dufour S, et al. Astroglial contribution to brain energy metabolism in humans revealed by 13c nuclear magnetic resonance spectroscopy: elucidation of the dominant pathway for neurotransmitter glutamate repletion and measurement of astrocytic oxidative metabolism. J Neurosci. 2002;22:1523–1531. doi: 10.1523/JNEUROSCI.22-05-01523.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boumezbeur F, Mason GF, de Graaf RA, Behar KL, Cline GW, Shulman GI, et al. Altered brain mitochondrial metabolism in healthy aging as assessed by in vivo magnetic resonance spectroscopy. J Cereb Blood Flow Metab. 2010;30:211–221. doi: 10.1038/jcbfm.2009.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.