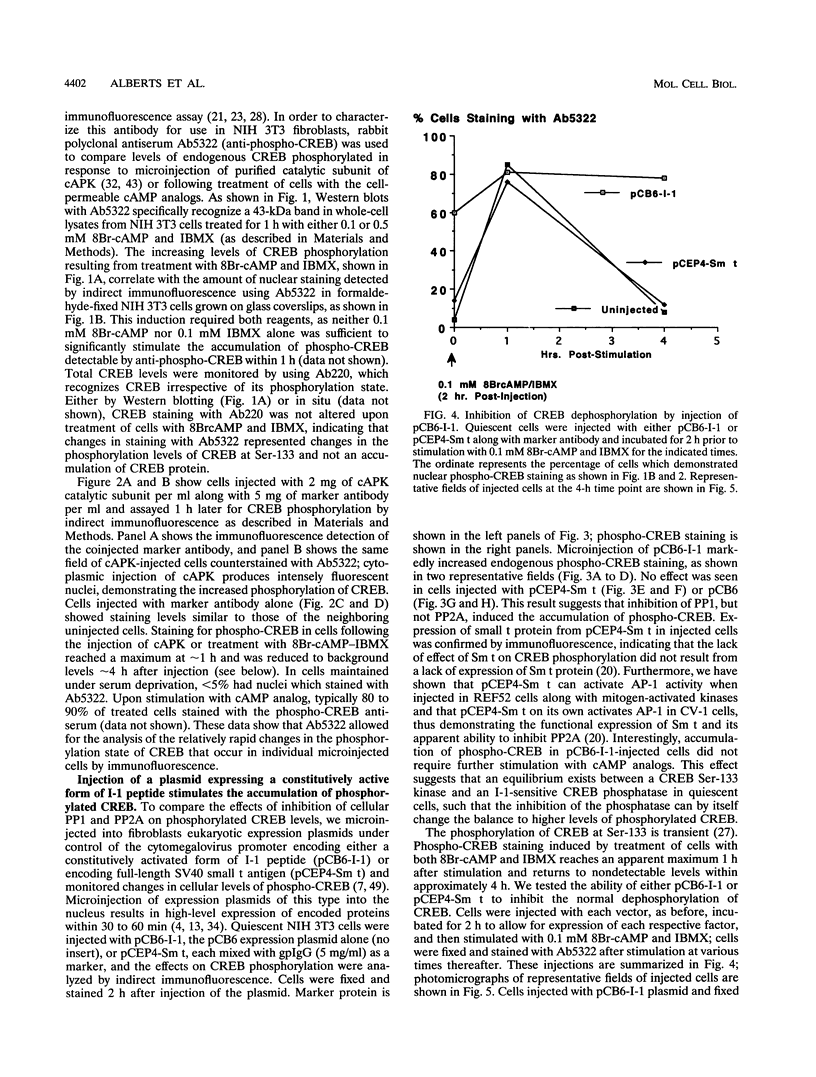
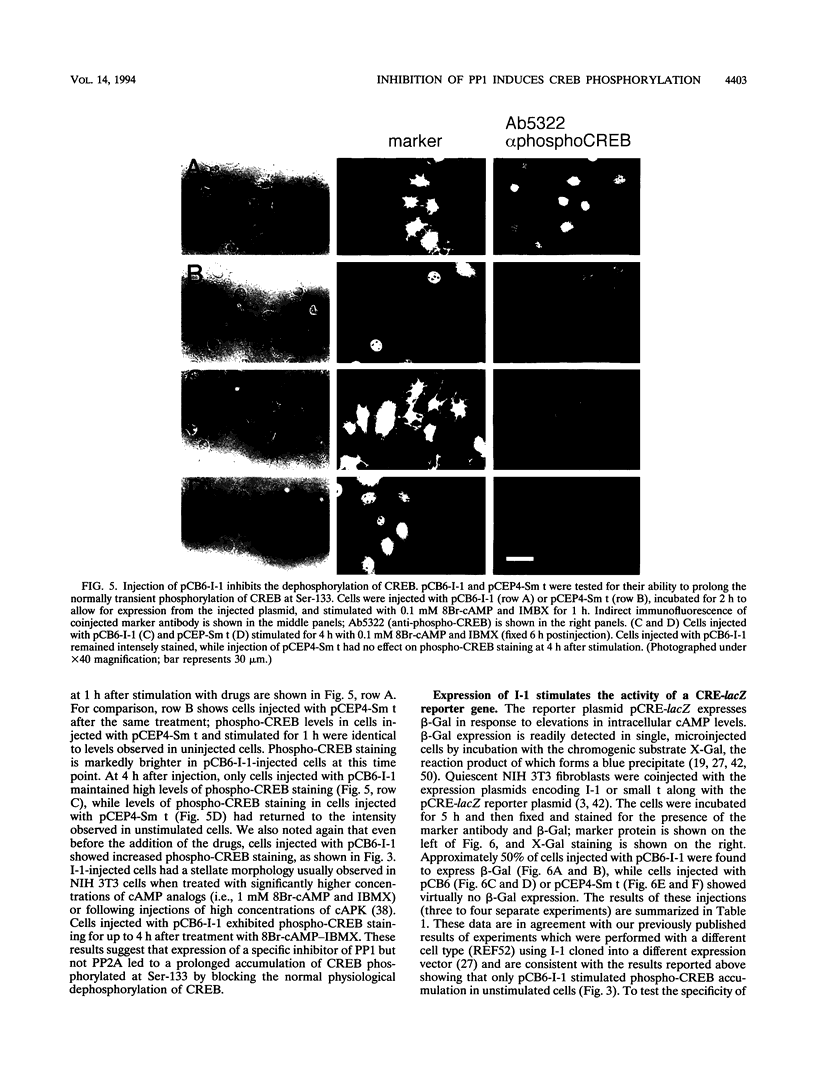
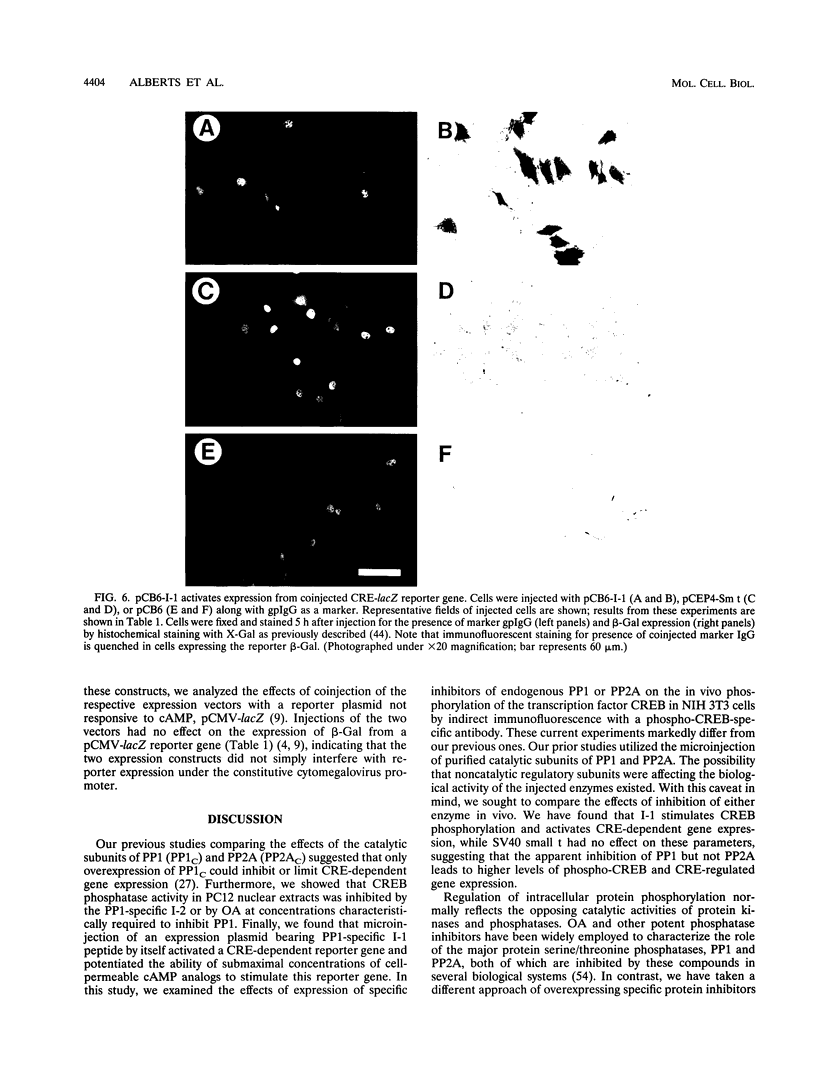
Abstract
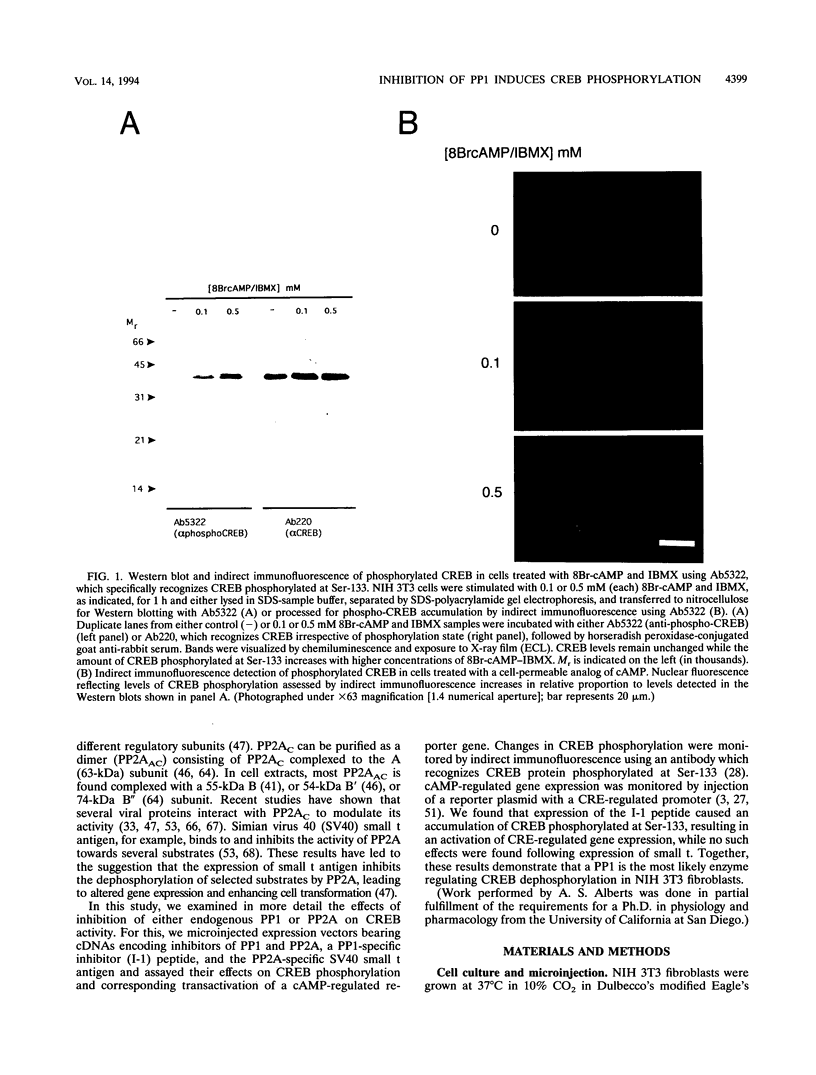
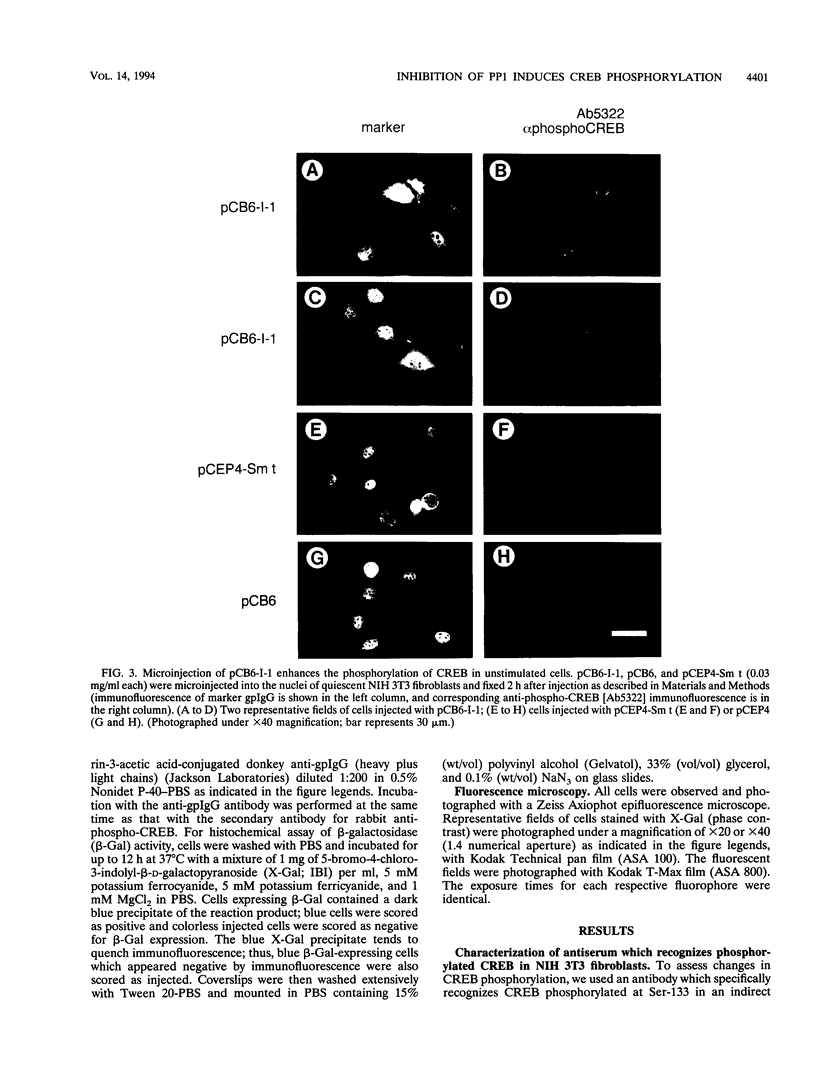
We have examined the activity and phosphorylation state of the cyclic AMP (cAMP) response element binding factor (CREB) in intact NIH 3T3 cells following microinjection of expression plasmids encoding regulatory proteins of type 1 (PP1) and 2A (PP2A) serine/threonine-specific protein phosphatases. Changes in CREB phosphorylation in the injected cells were monitored by indirect immunofluorescence using an affinity-purified antiserum (Ab5322) which specifically recognizes CREB phosphorylated at Ser-133, and changes in transcriptional activity of CREB were monitored by expression of a reporter gene regulated by cAMP. cAMP-stimulated phosphorylation in NIH 3T3 cells is normally transient, and as expected, after stimulation of cells with cell-permeable cAMP analogs, the level of phosphorylated CREB was found to initially increase and then return to a basal level within 4 h. Microinjection of an expression vector encoding a constitutively active form of inhibitor 1 (I-1), a PP1-specific inhibitor, by itself resulted in an apparent increase in phosphorylated CREB in unstimulated cells. Moreover, injection of the I-1 vector resulted in the prolonged appearance of phosphorylated CREB in cells after cAMP stimulation. In contrast, injection of a plasmid encoding simian virus 40 small t antigen, which interacts with PP2A to inhibit its activity towards several phosphoprotein substrates, had no effect on the phosphorylation state of CREB in stimulated or unstimulated NIH 3T3 cells. Consistent with these results, injection of the I-1 expression vector activated expression from a coinjected CRE-lacZ reporter plasmid, indicating that the increased phosphorylation of CREB also activated its transcriptional activity. These results provide further evidence for a role of a PP1 as the primary protein (Ser/Thr) phosphatase regulating the dephosphorylation of Ser-133 and thereby limiting the transcriptional activity of CREB.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aitken A., Cohen P. Isolation and characterisation of active fragments of protein phosphatase inhibitor-1 from rabbit skeletal muscle. FEBS Lett. 1982 Oct 4;147(1):54–58. doi: 10.1016/0014-5793(82)81010-7. [DOI] [PubMed] [Google Scholar]
- Alberts A. S., Arias J., Hagiwara M., Montminy M. R., Feramisco J. R. Recombinant cyclic AMP response element binding protein (CREB) phosphorylated on Ser-133 is transcriptionally active upon its introduction into fibroblast nuclei. J Biol Chem. 1994 Mar 11;269(10):7623–7630. [PubMed] [Google Scholar]
- Alberts A. S., Deng T., Lin A., Meinkoth J. L., Schönthal A., Mumby M. C., Karin M., Feramisco J. R. Protein phosphatase 2A potentiates activity of promoters containing AP-1-binding elements. Mol Cell Biol. 1993 Apr;13(4):2104–2112. doi: 10.1128/mcb.13.4.2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberts A. S., Frost J. A., Thorburn A. M. Rapid transcriptional assay for the expression of two distinct reporter genes by microinjection. DNA Cell Biol. 1993 Dec;12(10):935–943. doi: 10.1089/dna.1993.12.935. [DOI] [PubMed] [Google Scholar]
- Alberts A. S., Thorburn A. M., Shenolikar S., Mumby M. C., Feramisco J. R. Regulation of cell cycle progression and nuclear affinity of the retinoblastoma protein by protein phosphatases. Proc Natl Acad Sci U S A. 1993 Jan 15;90(2):388–392. doi: 10.1073/pnas.90.2.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson S., Davis D. L., Dahlbäck H., Jörnvall H., Russell D. W. Cloning, structure, and expression of the mitochondrial cytochrome P-450 sterol 26-hydroxylase, a bile acid biosynthetic enzyme. J Biol Chem. 1989 May 15;264(14):8222–8229. [PubMed] [Google Scholar]
- Beullens M., Van Eynde A., Bollen M., Stalmans W. Inactivation of nuclear inhibitory polypeptides of protein phosphatase-1 (NIPP-1) by protein kinase A. J Biol Chem. 1993 Jun 25;268(18):13172–13177. [PubMed] [Google Scholar]
- Boshart M., Weber F., Jahn G., Dorsch-Häsler K., Fleckenstein B., Schaffner W. A very strong enhancer is located upstream of an immediate early gene of human cytomegalovirus. Cell. 1985 Jun;41(2):521–530. doi: 10.1016/s0092-8674(85)80025-8. [DOI] [PubMed] [Google Scholar]
- Brautigan D. L., Sunwoo J., Labbé J. C., Fernandez A., Lamb N. J. Cell cycle oscillation of phosphatase inhibitor-2 in rat fibroblasts coincident with p34cdc2 restriction. Nature. 1990 Mar 1;344(6261):74–78. doi: 10.1038/344074a0. [DOI] [PubMed] [Google Scholar]
- Brindle P. K., Montminy M. R. The CREB family of transcription activators. Curr Opin Genet Dev. 1992 Apr;2(2):199–204. doi: 10.1016/s0959-437x(05)80274-6. [DOI] [PubMed] [Google Scholar]
- Capecchi M. R. High efficiency transformation by direct microinjection of DNA into cultured mammalian cells. Cell. 1980 Nov;22(2 Pt 2):479–488. doi: 10.1016/0092-8674(80)90358-x. [DOI] [PubMed] [Google Scholar]
- Chang L. S., Pater M. M., Hutchinson N. I., di Mayorca G. Transformation by purified early genes of simian virus 40. Virology. 1984 Mar;133(2):341–353. doi: 10.1016/0042-6822(84)90400-8. [DOI] [PubMed] [Google Scholar]
- Chrivia J. C., Kwok R. P., Lamb N., Hagiwara M., Montminy M. R., Goodman R. H. Phosphorylated CREB binds specifically to the nuclear protein CBP. Nature. 1993 Oct 28;365(6449):855–859. doi: 10.1038/365855a0. [DOI] [PubMed] [Google Scholar]
- Cobb M. H., Boulton T. G., Robbins D. J. Extracellular signal-regulated kinases: ERKs in progress. Cell Regul. 1991 Dec;2(12):965–978. doi: 10.1091/mbc.2.12.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen P. The structure and regulation of protein phosphatases. Annu Rev Biochem. 1989;58:453–508. doi: 10.1146/annurev.bi.58.070189.002321. [DOI] [PubMed] [Google Scholar]
- Fernandez A., Brautigan D. L., Mumby M., Lamb N. J. Protein phosphatase type-1, not type-2A, modulates actin microfilament integrity and myosin light chain phosphorylation in living nonmuscle cells. J Cell Biol. 1990 Jul;111(1):103–112. doi: 10.1083/jcb.111.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedmann T., Doolittle R. F., Walter G. Amino acid sequence homology between polyoma and SV40 tumour antigens deduced from nucleotide sequences. Nature. 1978 Jul 20;274(5668):291–293. doi: 10.1038/274291a0. [DOI] [PubMed] [Google Scholar]
- Gonzalez G. A., Menzel P., Leonard J., Fischer W. H., Montminy M. R. Characterization of motifs which are critical for activity of the cyclic AMP-responsive transcription factor CREB. Mol Cell Biol. 1991 Mar;11(3):1306–1312. doi: 10.1128/mcb.11.3.1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez G. A., Montminy M. R. Cyclic AMP stimulates somatostatin gene transcription by phosphorylation of CREB at serine 133. Cell. 1989 Nov 17;59(4):675–680. doi: 10.1016/0092-8674(89)90013-5. [DOI] [PubMed] [Google Scholar]
- Gonzalez G. A., Yamamoto K. K., Fischer W. H., Karr D., Menzel P., Biggs W., 3rd, Vale W. W., Montminy M. R. A cluster of phosphorylation sites on the cyclic AMP-regulated nuclear factor CREB predicted by its sequence. Nature. 1989 Feb 23;337(6209):749–752. doi: 10.1038/337749a0. [DOI] [PubMed] [Google Scholar]
- Gotoh Y., Nishida E., Sakai H. Okadaic acid activates microtubule-associated protein kinase in quiescent fibroblastic cells. Eur J Biochem. 1990 Nov 13;193(3):671–674. doi: 10.1111/j.1432-1033.1990.tb19385.x. [DOI] [PubMed] [Google Scholar]
- Guan K., Hakes D. J., Wang Y., Park H. D., Cooper T. G., Dixon J. E. A yeast protein phosphatase related to the vaccinia virus VH1 phosphatase is induced by nitrogen starvation. Proc Natl Acad Sci U S A. 1992 Dec 15;89(24):12175–12179. doi: 10.1073/pnas.89.24.12175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habener J. F. Cyclic AMP response element binding proteins: a cornucopia of transcription factors. Mol Endocrinol. 1990 Aug;4(8):1087–1094. doi: 10.1210/mend-4-8-1087. [DOI] [PubMed] [Google Scholar]
- Hagiwara M., Alberts A., Brindle P., Meinkoth J., Feramisco J., Deng T., Karin M., Shenolikar S., Montminy M. Transcriptional attenuation following cAMP induction requires PP-1-mediated dephosphorylation of CREB. Cell. 1992 Jul 10;70(1):105–113. doi: 10.1016/0092-8674(92)90537-m. [DOI] [PubMed] [Google Scholar]
- Hagiwara M., Brindle P., Harootunian A., Armstrong R., Rivier J., Vale W., Tsien R., Montminy M. R. Coupling of hormonal stimulation and transcription via the cyclic AMP-responsive factor CREB is rate limited by nuclear entry of protein kinase A. Mol Cell Biol. 1993 Aug;13(8):4852–4859. doi: 10.1128/mcb.13.8.4852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haystead T. A., Weiel J. E., Litchfield D. W., Tsukitani Y., Fischer E. H., Krebs E. G. Okadaic acid mimics the action of insulin in stimulating protein kinase activity in isolated adipocytes. The role of protein phosphatase 2a in attenuation of the signal. J Biol Chem. 1990 Sep 25;265(27):16571–16580. [PubMed] [Google Scholar]
- Hemmings H. C., Jr, Girault J. A., Nairn A. C., Bertuzzi G., Greengard P. Distribution of protein phosphatase inhibitor-1 in brain and peripheral tissues of various species: comparison with DARPP-32. J Neurochem. 1992 Sep;59(3):1053–1061. doi: 10.1111/j.1471-4159.1992.tb08347.x. [DOI] [PubMed] [Google Scholar]
- Hemmings H. C., Jr, Nairn A. C., Elliott J. I., Greengard P. Synthetic peptide analogs of DARPP-32 (Mr 32,000 dopamine- and cAMP-regulated phosphoprotein), an inhibitor of protein phosphatase-1. Phosphorylation, dephosphorylation, and inhibitory activity. J Biol Chem. 1990 Nov 25;265(33):20369–20376. [PubMed] [Google Scholar]
- Herberg F. W., Bell S. M., Taylor S. S. Expression of the catalytic subunit of cAMP-dependent protein kinase in Escherichia coli: multiple isozymes reflect different phosphorylation states. Protein Eng. 1993 Sep;6(7):771–777. doi: 10.1093/protein/6.7.771. [DOI] [PubMed] [Google Scholar]
- Joshi B., Rundell K. Association of simian virus 40 small-t antigen with the 61-kilodalton component of a cellular protein complex. J Virol. 1990 Nov;64(11):5649–5651. doi: 10.1128/jvi.64.11.5649-5651.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalderon D., Roberts B. L., Richardson W. D., Smith A. E. A short amino acid sequence able to specify nuclear location. Cell. 1984 Dec;39(3 Pt 2):499–509. doi: 10.1016/0092-8674(84)90457-4. [DOI] [PubMed] [Google Scholar]
- Kim S. J., Lafyatis R., Kim K. Y., Angel P., Fujiki H., Karin M., Sporn M. B., Roberts A. B. Regulation of collagenase gene expression by okadaic acid, an inhibitor of protein phosphatases. Cell Regul. 1990 Feb;1(3):269–278. doi: 10.1091/mbc.1.3.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuret J., Bell H., Cohen P. Identification of high levels of protein phosphatase-1 in rat liver nuclei. FEBS Lett. 1986 Jul 28;203(2):197–202. doi: 10.1016/0014-5793(86)80741-4. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lamb N. J., Fernandez A., Conti M. A., Adelstein R., Glass D. B., Welch W. J., Feramisco J. R. Regulation of actin microfilament integrity in living nonmuscle cells by the cAMP-dependent protein kinase and the myosin light chain kinase. J Cell Biol. 1988 Jun;106(6):1955–1971. doi: 10.1083/jcb.106.6.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C. Q., Yun Y. D., Hoeffler J. P., Habener J. F. Cyclic-AMP-responsive transcriptional activation of CREB-327 involves interdependent phosphorylated subdomains. EMBO J. 1990 Dec;9(13):4455–4465. doi: 10.1002/j.1460-2075.1990.tb07896.x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Liu J. S., Park E. A., Gurney A. L., Roesler W. J., Hanson R. W. Cyclic AMP induction of phosphoenolpyruvate carboxykinase (GTP) gene transcription is mediated by multiple promoter elements. J Biol Chem. 1991 Oct 5;266(28):19095–19102. [PubMed] [Google Scholar]
- Meinkoth J. L., Ji Y., Taylor S. S., Feramisco J. R. Dynamics of the distribution of cyclic AMP-dependent protein kinase in living cells. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9595–9599. doi: 10.1073/pnas.87.24.9595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinkoth J. L., Montminy M. R., Fink J. S., Feramisco J. R. Induction of a cyclic AMP-responsive gene in living cells requires the nuclear factor CREB. Mol Cell Biol. 1991 Mar;11(3):1759–1764. doi: 10.1128/mcb.11.3.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinkoth J., Alberts A. S., Feramisco J. R. Construction of mammalian cell lines with indicator genes driven by regulated promoters. Ciba Found Symp. 1990;150:47–56. doi: 10.1002/9780470513927.ch4. [DOI] [PubMed] [Google Scholar]
- Montminy M. R., Bilezikjian L. M. Binding of a nuclear protein to the cyclic-AMP response element of the somatostatin gene. Nature. 1987 Jul 9;328(6126):175–178. doi: 10.1038/328175a0. [DOI] [PubMed] [Google Scholar]
- Mumby M. C., Russell K. L., Garrard L. J., Green D. D. Cardiac contractile protein phosphatases. Purification of two enzyme forms and their characterization with subunit-specific antibodies. J Biol Chem. 1987 May 5;262(13):6257–6265. [PubMed] [Google Scholar]
- Mumby M. C., Walter G. Protein phosphatases and DNA tumor viruses: transformation through the back door? Cell Regul. 1991 Aug;2(8):589–598. doi: 10.1091/mbc.2.8.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumby M. C., Walter G. Protein serine/threonine phosphatases: structure, regulation, and functions in cell growth. Physiol Rev. 1993 Oct;73(4):673–699. doi: 10.1152/physrev.1993.73.4.673. [DOI] [PubMed] [Google Scholar]
- Patwardhan S., Gashler A., Siegel M. G., Chang L. C., Joseph L. J., Shows T. B., Le Beau M. M., Sukhatme V. P. EGR3, a novel member of the Egr family of genes encoding immediate-early transcription factors. Oncogene. 1991 Jun;6(6):917–928. [PubMed] [Google Scholar]
- Riabowol K. T., Fink J. S., Gilman M. Z., Walsh D. A., Goodman R. H., Feramisco J. R. The catalytic subunit of cAMP-dependent protein kinase induces expression of genes containing cAMP-responsive enhancer elements. Nature. 1988 Nov 3;336(6194):83–86. doi: 10.1038/336083a0. [DOI] [PubMed] [Google Scholar]
- Rose D. W., McCabe G., Feramisco J. R., Adler M. Expression of c-fos and AP-1 activity in senescent human fibroblasts is not sufficient for DNA synthesis. J Cell Biol. 1992 Dec;119(6):1405–1411. doi: 10.1083/jcb.119.6.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rundell K. Complete interaction of cellular 56,000- and 32,000-Mr proteins with simian virus 40 small-t antigen in productively infected cells. J Virol. 1987 Apr;61(4):1240–1243. doi: 10.1128/jvi.61.4.1240-1243.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheidtmann K. H., Mumby M. C., Rundell K., Walter G. Dephosphorylation of simian virus 40 large-T antigen and p53 protein by protein phosphatase 2A: inhibition by small-t antigen. Mol Cell Biol. 1991 Apr;11(4):1996–2003. doi: 10.1128/mcb.11.4.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schönthal A. Okadaic acid--a valuable new tool for the study of signal transduction and cell cycle regulation? New Biol. 1992 Jan;4(1):16–21. [PubMed] [Google Scholar]
- Schönthal A., Tsukitani Y., Feramisco J. R. Transcriptional and post-transcriptional regulation of c-fos expression by the tumor promoter okadaic acid. Oncogene. 1991 Mar;6(3):423–430. [PubMed] [Google Scholar]
- Shenolikar S., Nairn A. C. Protein phosphatases: recent progress. Adv Second Messenger Phosphoprotein Res. 1991;23:1–121. [PubMed] [Google Scholar]
- Sola M. M., Langan T., Cohen P. p34cdc2 phosphorylation sites in histone H1 are dephosphorylated by protein phosphatase 2A1. Biochim Biophys Acta. 1991 Sep 3;1094(2):211–216. doi: 10.1016/0167-4889(91)90011-l. [DOI] [PubMed] [Google Scholar]
- Sontag E., Fedorov S., Kamibayashi C., Robbins D., Cobb M., Mumby M. The interaction of SV40 small tumor antigen with protein phosphatase 2A stimulates the map kinase pathway and induces cell proliferation. Cell. 1993 Dec 3;75(5):887–897. doi: 10.1016/0092-8674(93)90533-v. [DOI] [PubMed] [Google Scholar]
- Sun H., Charles C. H., Lau L. F., Tonks N. K. MKP-1 (3CH134), an immediate early gene product, is a dual specificity phosphatase that dephosphorylates MAP kinase in vivo. Cell. 1993 Nov 5;75(3):487–493. doi: 10.1016/0092-8674(93)90383-2. [DOI] [PubMed] [Google Scholar]
- Thorburn A. M., Alberts A. S. Efficient expression of miniprep plasmid DNA after needle micro-injection into somatic cells. Biotechniques. 1993 Mar;14(3):356–358. [PubMed] [Google Scholar]
- Thévenin C., Kim S. J., Kehrl J. H. Inhibition of protein phosphatases by okadaic acid induces AP1 in human T cells. J Biol Chem. 1991 May 25;266(15):9363–9366. [PubMed] [Google Scholar]
- Tung H. Y., Alemany S., Cohen P. The protein phosphatases involved in cellular regulation. 2. Purification, subunit structure and properties of protein phosphatases-2A0, 2A1, and 2A2 from rabbit skeletal muscle. Eur J Biochem. 1985 Apr 15;148(2):253–263. doi: 10.1111/j.1432-1033.1985.tb08833.x. [DOI] [PubMed] [Google Scholar]
- Usui H., Imazu M., Maeta K., Tsukamoto H., Azuma K., Takeda M. Three distinct forms of type 2A protein phosphatase in human erythrocyte cytosol. J Biol Chem. 1988 Mar 15;263(8):3752–3761. [PubMed] [Google Scholar]
- Wadzinski B. E., Wheat W. H., Jaspers S., Peruski L. F., Jr, Lickteig R. L., Johnson G. L., Klemm D. J. Nuclear protein phosphatase 2A dephosphorylates protein kinase A-phosphorylated CREB and regulates CREB transcriptional stimulation. Mol Cell Biol. 1993 May;13(5):2822–2834. doi: 10.1128/mcb.13.5.2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter G., Carbone-Wiley A., Joshi B., Rundell K. Homologous cellular proteins associated with simian virus 40 small T antigen and polyomavirus medium T antigen. J Virol. 1988 Dec;62(12):4760–4762. doi: 10.1128/jvi.62.12.4760-4762.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter G., Ruediger R., Slaughter C., Mumby M. Association of protein phosphatase 2A with polyoma virus medium tumor antigen. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2521–2525. doi: 10.1073/pnas.87.7.2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S. I., Lickteig R. L., Estes R., Rundell K., Walter G., Mumby M. C. Control of protein phosphatase 2A by simian virus 40 small-t antigen. Mol Cell Biol. 1991 Apr;11(4):1988–1995. doi: 10.1128/mcb.11.4.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]