Abstract
The MAPK pathway has emerged as a central target for melanoma therapy due to its persistent activation in the majority of tumors. Several BRAF inhibitors aimed at curbing MAPK pathway activity are currently in advanced stages of clinical investigation. However, their therapeutic success is limited by the emergence of drug resistance, as responses are transient and tumors eventually recur. Understanding the mechanisms underlying resistance to BRAF inhibitors is essential to develop effective and long-lasting therapies for melanoma patients. Here, we briefly review recent pre-clinical studies providing insight into the molecular mechanisms of resistance to BRAF inhibitors and discuss potential strategies to treat drug resistant melanomas.
BRAF-V600E as a therapeutic target in melanoma (Introduction)
Current therapies for the treatment of metastatic melanoma, the most lethal form of skin cancer, offer limited clinical benefit. Patients with advanced disease have a poor prognosis and a 5-year survival rate of less than 20% (1). In the past decade however, the mitogen activated protein kinase (MAPK) pathway started taking center stage in melanoma therapy as it is commonly activated in tumors through mutations in BRAF, N-RAS, receptor tyrosine kinases (RTKs), G-coupled protein receptors, or by growth factor mediated stimulation (2, 3). The MAPK pathway regulates many key biological processes including proliferation, survival, and metastasis, thus curbing its activity is an attractive therapeutic endeavor (4). Early efforts were focused on the development of mutant BRAF inhibitors due to the presence of BRAF mutations in 50% of melanomas (5). The most common BRAF mutation (T1799A; BRAFV600E) causes constitutive kinase activity and hyper-activation of the MAPK pathway, providing a MAPK-relevant tumor-specific target. Pre-clinical and clinical studies have now demonstrated that targeting BRAF using RAF-selective inhibitors results in remarkable tumor shrinkage in BRAFV600E melanomas (4, 6-9). In addition, other activating mutations such as V600K/D/R also appear responsive to BRAF inhibitors (10). In a recent phase 3 trial in which patients with BRAFV600E melanomas were treated with the RAF inhibitor vemurafenib (PLX4032/RG7204) 48% had confirmed objective response rates and an increased overall survival (84%) compared to those treated with dacarbazine (64%) at 6 months (11). Despite these encouraging results, responses to RAF inhibitors are transient, resistance to these compounds develops, and tumors invariably recur. Understanding the molecular mechanisms of resistance to RAF inhibitors is now critical to maximize their clinical success, achieve complete durable responses, and improve patient outcomes.
Resistance to targeted agents, a frequent cause of therapy failure, can be mediated by diverse mechanisms including secondary mutations or epigenetic changes in the target gene, modifications in drug metabolism, and activation of compensatory pathways, leading to increased tumor cell survival. What mechanisms are at play as a result of RAF inhibition and when are they engaged is only now being unraveled.
Modeling Resistance to BRAF inhibitors (key findings)
Our group and others have been intensively investigating the molecular mechanisms underlying resistance to BRAF inhibitors using a variety of approaches (12-14). In our studies, we modeled the emergence of resistance to BRAF inhibitors by selecting a panel of BRAFV600E/PTEN+ melanoma cells which are highly sensitive to BRAF inhibition and chronically exposing them to increasing doses of SB-590885 (GlaxoSmithKline), a BRAF-selective inhibitor (15). Drug-resistant cells emerged approximately 6 months after persistent drug exposure and were able to proliferate and survive in the continuous presence of 1 μM SB-590885, unlike their parental counterparts. Importantly, chronic BRAF inhibition led to cross-resistance to several BRAF-selective inhibitors, including PLX4032, indicating that resistance is not likely to be easily overcome by switching to a new RAF inhibitor. All resistant clones were able to proliferate at normal rates, retained their anchorage independent growth, and were able to grow in a 3D-tumor-like microenvironment even in the presence of high doses of BRAF inhibitors.
Although a frequent mechanism of anti-cancer drug resistance is the development of secondary mutations in the target gene, we did not identify secondary mutations in BRAF in any of our resistant cell lines, all of which retained the BRAFV600E mutation. Biochemically, our resistant melanoma cells were able to reactivate the MAPK pathway in a BRAF-independent manner. While the parental (BRAF inhibitor-sensitive) cells rely on BRAF for MAPK activation, the BRAF-inhibitor resistant cells had elevated expression of CRAF and ARAF, and were able to dynamically use either of these two RAF isoforms to sustain MAPK activity and promote proliferation; nevertheless, the resistant cells were still sensitive to MEK inhibitors which target downstream of RAF (Figure 1). Treatment of BRAF-inhibitor resistant cells with various structurally different MEK inhibitors had mostly cytostatic effects, suggesting that additional bypass mechanisms could be promoting survival. Indeed, our resistant cells displayed differential activation of several RTKs, in particular IGF-1R. Although the parental melanoma cells, like all cells of melanocytic origin, express the IGF-1R receptor, some of our BRAF-resistant melanomas expressed higher surface levels of IGF-1R. We observed that treatment of parental cells with BRAF inhibitors led to a decrease in phospho-IGF-1R levels; however, phosphorylation of IGF-1R was sustained in our BRAF-inhibitor resistant cells. We further noted that enhanced IGF-1 mediated signaling was not due to amplification or mutations of the IGF-1R gene. Even though the precise mechanisms of enhanced IGF-1R expression and signaling in the context of chronic BRAF inhibition are not yet completely understood, our results suggest possible cross-talk between BRAF and RTKs, particularly IGF-1R-dependent networks, that requires further investigation.
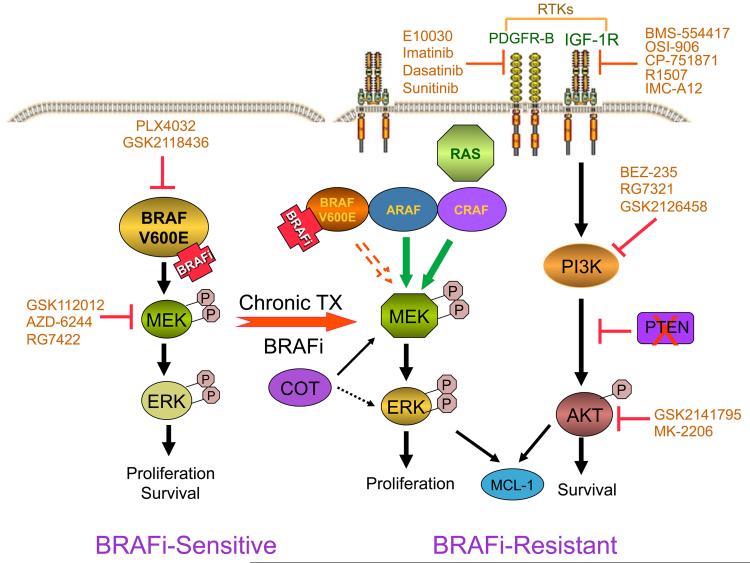
Figure 1.
Simplified schematic of signaling pathways driving resistance to BRAF inhibitors. In BRAF V600E mutant melanoma cells (left), BRAF inhibition causes growth arrest and apoptosis by blocking the MAPK pathway. These BRAF-inhibitor (BRAFi-) sensitive cells are highly dependent on BRAF for MAPK activation and survival. Following chronic BRAF inhibition, resistant cells (right) evolve an array of compensatory mechanisms, including RAF isoform switching, activation of RTKs such as IGF-1R, and engagement of the PI3K pathway to promote cell survival. PTEN loss leads to activation of the PI3K pathway. Understanding the signaling networks that drive drug resistance will enable the development of rational drug combinations to target melanomas refractory to BRAF inhibitors. Also shown are inhibitors that can be used to block the compensatory pathways identified so far.
IGF-1R plays an important role in survival and resistance to anti-cancer therapies (16) that could be mediated through activation of MAPK and PI3K signaling. In the context of resistance to BRAF inhibition, we found that IGF-1R promotes activation of PI3K and phosphorylation of AKT, but has no effect on the MAPK pathway. Pharmacological or genetic inhibition of IGF-1R inhibited only downstream PI3K/AKT signaling; exogenous IGF-1 increased PI3K-mediated signaling, but was not sufficient to induce resistance. Additionally, the MAPK and PI3K pathways appeared to jointly regulate the levels of the anti-apoptotic factor Mcl-1 to promote survival. Clearly, the MAPK and IGF-1R/PI3K/AKT signaling pathways cooperate to promote survival and expansion of the BRAF-inhibitor resistant cells. Validating this conclusion, we found that co-inhibition of these two pathways was more efficient in inducing apoptosis of BRAF-inhibitor resistant cells than inhibition of each individual pathway. Combination of MEK inhibitors, including GSK1120212 or AZD6244 with PI3K inhibitors such as GSK 2126458 or IGF-1R inhibitors led to striking cytotoxic effects in 3D BRAF-inhibitor resistant melanoma spheroids. Our findings strongly advocate the potential use of these combinatorial approaches to treat patients refractory to BRAF inhibitors.
To assess the clinical relevance of our studies, we compared paired biopsies (pre-treatment and post-relapse) in five patients with metastatic melanoma treated with PLX4032. All five patients initially responded to PLX4032 but relapsed after 4-15 months of treatment. Sequencing of all five paired tumor biopsies indicated that the mutation encoding BRAFV600E was present in all samples (pre-treatment and post-relapse), but no secondary mutations in BRAF, CRAF or RAS were identified. Through immunohistochemical analysis of these biopsies, we found increased levels of IGF-1R in the post-relapse samples of two patients, one of which also had increased levels of phosphorylated AKT. These findings are consistent with our in vitro data suggesting that enhanced IGF-1R expression and PI3K/AKT activity are associated with resistance to BRAF inhibitors in some patients. We also noted a homozygous loss of PTEN and increased pAKT levels in the post-relapse biopsy of one patient, suggesting that PTEN loss could also be linked to resistance to BRAF inhibitors in some patients; however, it is not likely to be the sole player. These findings need to be further investigated both in vitro and in clinical samples.
Multiple resistance mechanisms can bypass BRAF inhibition
As melanoma is a complex and heterogeneous disease comprised of biologically, genetically, and histopathologically distinct sub-types, it is not surprising that multiple mechanisms of resistance can compensate for chronic BRAF inhibition. Unlike other malignancies where development of secondary or “gatekeeper” mutations in the target gene is a common mechanism of resistance, so far no secondary mutations have been identified in BRAF-inhibitor resistant melanomas, despite the use of highly sensitive sequencing methods such as next generation targeted deep and ultra-deep sequencing (13, 14).
Notwithstanding that a diverse array of molecular mechanisms appear to be linked to acquired resistance to BRAF inhibitors, which poses a challenge for clinical translation, some common mechanistic themes have emerged (12-14). In most instances, re-activation of the MAPK pathway is required to circumvent chronic BRAF inhibition and resume proliferation; therefore, the MAPK pathway remains a good therapeutic target. Our studies support this observation and demonstrate that melanoma cells, which are initially addicted to BRAF, can switch and use one of the other RAF isoforms (A- or C-RAF) to reactivate the MAPK pathway and continue proliferating. Additionally, overexpression of CRAF or the MAP3K8 COT can also lead to MAPK reactivation (14). Alternatively, treatment with BRAF inhibitors could select for minor pre-existent NRAS mutant clones which do not respond to BRAF inhibitors but paradoxically hyperactivate the MAPK pathway (17-20). In fact, Nazarian et al. identified two different NRAS mutations (Q61K and Q61R) in 1/12 patients resistant to vemurafenib (13). Persistent addiction to MAPK, despite BRAF inhibition, renders the resistant cells partially sensitive to MEK inhibitors and provides the opportunity of using compounds such as GSK1120212 or AZD6244, which are in advanced stages of clinical investigation, to treat patients refractory to BRAF inhibitors.
Another emerging common theme is the activation of RTKs, in particular IGF-1R and PDGFR, possibly due to alterations in feedback loops or compensatory survival mechanisms. Increased expression of IGF-1R and PDGFR has been noted in 2/5 (12) and 4/11 (13) post-relapse patient samples, respectively. Both IGF-1R and PDGFR can activate PI3K/AKT signaling and modulate survival. Indeed, previous studies have shown that AKT can protect melanoma cells from PLX4720-mediated apoptosis (21). More recently, a MEK1-C121S mutation has been identified in one patient who developed resistance to PLX4032 (22). However, how frequent MEK or RAS mutations occur and how significant their contribution to resistance are, needs to be determined in a larger cohort of patients treated with BRAF inhibitors.
Designing next-generation therapies to overcome resistance to BRAF inhibitors (Implications - Future directions)
Melanomas appear to engage a number of strategies to bypass BRAF inhibition but how prevalent each of the compensatory mechanisms are, or whether other mechanisms will emerge in the clinical setting, is still being investigated. Collectively, all studies on resistance to BRAF inhibitors put forward new strategies to potentially override tumor recurrence (Figure 1). Reactivation of the MAPK pathway renders the resistant tumors susceptible to MEK or ERK inhibitors. While MEK inhibitors have shown mostly cytostatic effects or tumor stabilization in the clinical setting, using them in combination with BRAF or PI3K inhibitors may prove useful, as our results suggest. Resistance associated with activation of RTKs, including IGF-1R and PDGFR, or the serine/threonine kinase COT could be abrogated by a growing number of pharmacological inhibitors or blocking antibodies. Encouragingly, multiple RTK inhibitors, as well as anti-IGF-1R antibodies are now under clinical investigation and could be used in combination with RAF or MEK inhibitors. Targeting molecules downstream of the receptors is another option; PDGFR and IGF-1R can activate the PI3K pathway, thus blocking this survival mechanism may be achieved using pan-PI3K, AKT, or mTOR inhibitors (12). Meanwhile, resistance caused by RAS or MEK mutations are potentially more challenging as no effective inhibitors of RAS or mutant MEK are currently available; however, using combination therapies with MEK and PI3K or BRAF and MEK inhibitors may restrain these resistant tumors.
The complexity of bypass mechanisms, suggests that personalized second line treatment strategies may be needed. Despite this challenge, commonalities indicate that some therapeutic approaches could be effective in larger cohorts of patients, regardless of the specific resistance mechanism. For example, inhibitors of BRAF/MEK in combination with compounds targeting PI3K/AKT could potentially be used to treat tumors with MAPK reactivation and enhanced PI3K-mediated survival. Additionally, it is quite possible that novel inhibitors will be developed to prevent paradoxical MAPK pathway reactivation (G. Bollag, AACR 2011), or keep both the MAPK and PI3K pathways in check. The drug development field has not run out of options yet and we are optimistic about future developments.
Currently, several clinical trials are planned or in progress to help sustain the effects of BRAF inhibitors and prevent resistance, including trials combining selective BRAF and MEK inhibitors. However, to guide future trials, comprehensive pre-clinical studies, that consider the genetic and biological heterogeneity of melanoma, should be designed to identify the most effective, and least toxic combinations. Carefully designed phase I trials will also be required to determine the pharmacokinetic and pharmacodynamic profiles of the experimental agents given in combination.
The melanoma field has made tremendous progress over the last decade elucidating the biology of this deadly disease and the encouraging clinical results are proof that this hard work pays off. We have many options and agents available for combination treatments and the increasing knowledge of molecular mechanisms underlying drug resistance will facilitate the design of new effective long-lasting therapies. We are confident that this approach will result in sustained tumor growth inhibition and improved patient outcomes.
Acknowledgments
Grant Support Work in our laboratory is supported by grants from the National Cancer Institute (P01 CA114046, P01 CA025874, P30 CA010815) and the Dr. Miriam and Sheldon G. Adelson Medical Research Foundation.
References
- 1.Sun W, Schuchter LM. Metastatic melanoma. Curr Treat Options Oncol. 2001;2:193–202. doi: 10.1007/s11864-001-0033-5. [DOI] [PubMed] [Google Scholar]
- 2.Easty DJ, Gray SG, O’Byrne KJ, O’Donnell D, Bennett DC. Receptor tyrosine kinases and their activation in melanoma. Pigment Cell Melanoma Res. 2011;24:446–61. doi: 10.1111/j.1755-148X.2011.00836.x. [DOI] [PubMed] [Google Scholar]
- 3.Van Raamsdonk CD, Griewank KG, Crosby MB, Garrido MC, Vemula S, Wiesner T, et al. Mutations in GNA11 in uveal melanoma. N Engl J Med. 2010;363:2191–9. doi: 10.1056/NEJMoa1000584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vultur A, Villanueva J, Herlyn M. Targeting BRAF in advanced melanoma: a first step toward manageable disease. Clin Cancer Res. 2011;17:1658–63. doi: 10.1158/1078-0432.CCR-10-0174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–54. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 6.Lee JT, Li L, Brafford PA, van den Eijnden M, Halloran MB, Sproesser K, et al. PLX4032, a potent inhibitor of the B-Raf V600E oncogene, selectively inhibits V600E-positive melanomas. Pigment Cell Melanoma Res. 2010;23:820–7. doi: 10.1111/j.1755-148X.2010.00763.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang H, Higgins B, Kolinsky K, Packman K, Go Z, Iyer R, et al. RG7204 (PLX4032), a selective BRAFV600E inhibitor, displays potent antitumor activity in preclinical melanoma models. Cancer Res. 2010;70:5518–27. doi: 10.1158/0008-5472.CAN-10-0646. [DOI] [PubMed] [Google Scholar]
- 8.Flaherty KT, Puzanov I, Kim KB, Ribas A, McArthur GA, Sosman JA, et al. Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med. 2010;363:809–19. doi: 10.1056/NEJMoa1002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bollag G, Hirth P, Tsai J, Zhang J, Ibrahim PN, Cho H, et al. Clinical efficacy of a RAF inhibitor needs broad target blockade in BRAF-mutant melanoma. Nature. 2010;467:596–9. doi: 10.1038/nature09454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rubinstein JC, Sznol M, Pavlick AC, Ariyan S, Cheng E, Bacchiocchi A, et al. Incidence of the V600K mutation among melanoma patients with BRAF mutations, and potential therapeutic response to the specific BRAF inhibitor PLX4032. J Transl Med. 2010;8:67. doi: 10.1186/1479-5876-8-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chapman PB, Hauschild A, Robert C, Haanen JB, Ascierto P, Larkin J, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364:2507–16. doi: 10.1056/NEJMoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Villanueva J, Vultur A, Lee JT, Somasundaram R, Fukunaga-Kalabis M, Cipolla AK, et al. Acquired Resistance to BRAF Inhibitors Mediated by a RAF Kinase Switch in Melanoma Can Be Overcome by Cotargeting MEK and IGF-1R/PI3K. Cancer Cell. 2010;18:683–695. doi: 10.1016/j.ccr.2010.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nazarian RSH, Wang Q, Kong X, Koya RC, Lee H, Chen Z, et al. Melanomas acquire resistance to B-RAF(V600E) inhibition by RTK or N-RAS upregulation. Nature. 2010;468:973–7. doi: 10.1038/nature09626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johannessen CM, Boehm JS, Kim SY, Thomas SR, Wardwell L, Johnson LA, et al. COT drives resistance to RAF inhibition through MAP kinase pathway reactivation. Nature. 2010;468:968–72. doi: 10.1038/nature09627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.King AJ, Patrick DR, Batorsky RS, Ho ML, Do HT, Zhang SY, et al. Demonstration of a genetic therapeutic index for tumors expressing oncogenic BRAF by the kinase inhibitor SB-590885. Cancer Res. 2006;66:11100–5. doi: 10.1158/0008-5472.CAN-06-2554. [DOI] [PubMed] [Google Scholar]
- 16.Casa AJ, Dearth RK, Litzenburger BC, Lee AV, Cui X. The type I insulin-like growth factor receptor pathway: a key player in cancer therapeutic resistance. Front Biosci. 2008;13:3273–87. doi: 10.2741/2925. [DOI] [PubMed] [Google Scholar]
- 17.Poulikakos PI, Zhang C, Bollag G, Shokat KM, Rosen N. RAF inhibitors transactivate RAF dimers and ERK signalling in cells with wild-type BRAF. Nature. 2010;464:427–30. doi: 10.1038/nature08902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heidorn SJ, Milagre C, Whittaker S, Nourry A, Niculescu-Duvas I, Dhomen N, et al. Kinase-dead BRAF and oncogenic RAS cooperate to drive tumor progression through CRAF. Cell. 2010;140:209–21. doi: 10.1016/j.cell.2009.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaplan FM, Shao Y, Mayberry MM, Aplin AE. Hyperactivation of MEK-ERK1/2 signaling and resistance to apoptosis induced by the oncogenic B-RAF inhibitor, PLX4720, in mutant N-RAS melanoma cells. Oncogene. 2010;30:366–71. doi: 10.1038/onc.2010.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hatzivassiliou G, Song K, Yen I, Brandhuber BJ, Anderson DJ, Alvarado R, et al. RAF inhibitors prime wild-type RAF to activate the MAPK pathway and enhance growth. Nature. 2010;464:431–5. doi: 10.1038/nature08833. [DOI] [PubMed] [Google Scholar]
- 21.Shao Y, Aplin AE. Akt3-mediated resistance to apoptosis in B-RAF-targeted melanoma cells. Cancer Res. 2010;70:6670–81. doi: 10.1158/0008-5472.CAN-09-4471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wagle N, Emery C, Berger MF, Davis MJ, Sawyer A, Pochanard P, et al. Dissecting Therapeutic Resistance to RAF Inhibition in Melanoma by Tumor Genomic Profiling. J Clin Oncol. 2011 doi: 10.1200/JCO.2010.33.2312. published online on March 7. [DOI] [PMC free article] [PubMed] [Google Scholar]