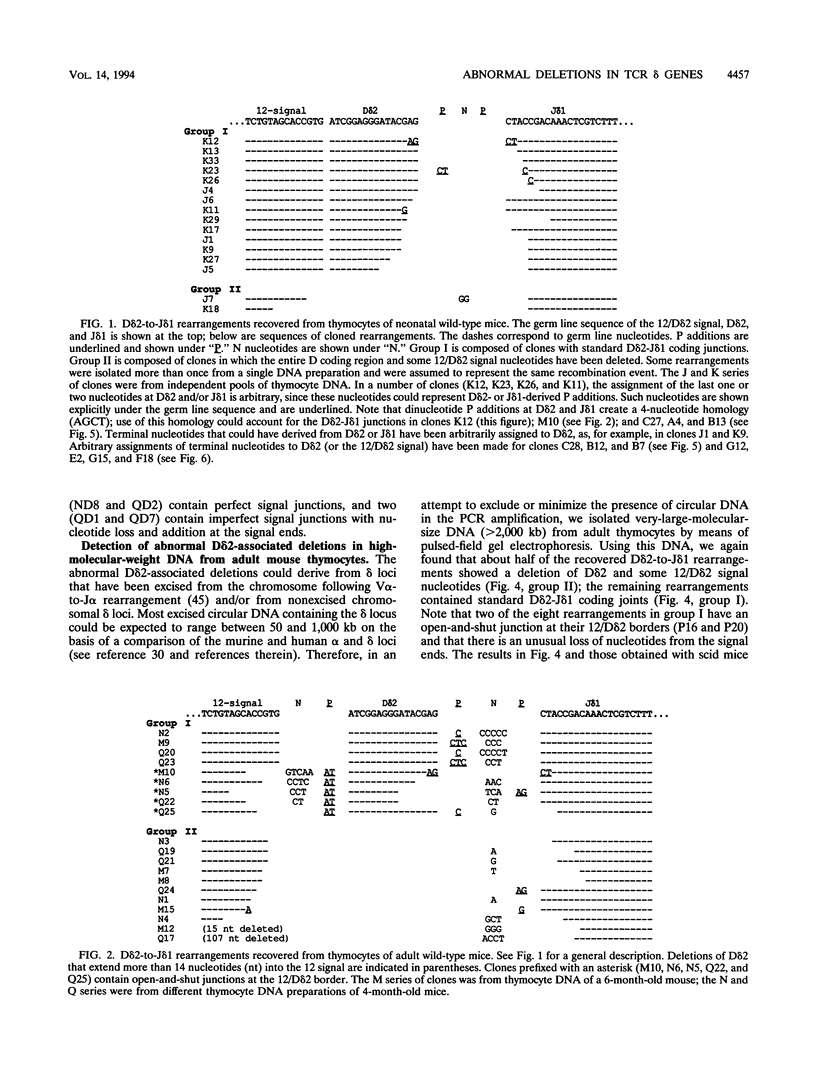
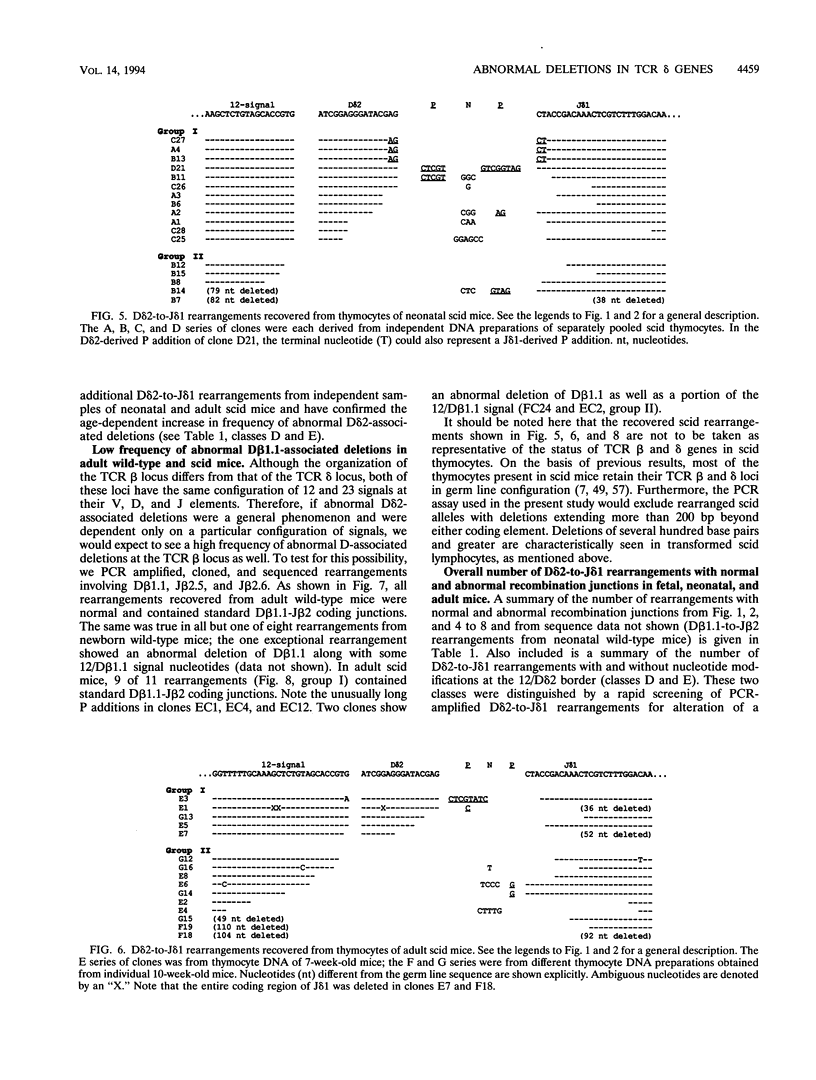
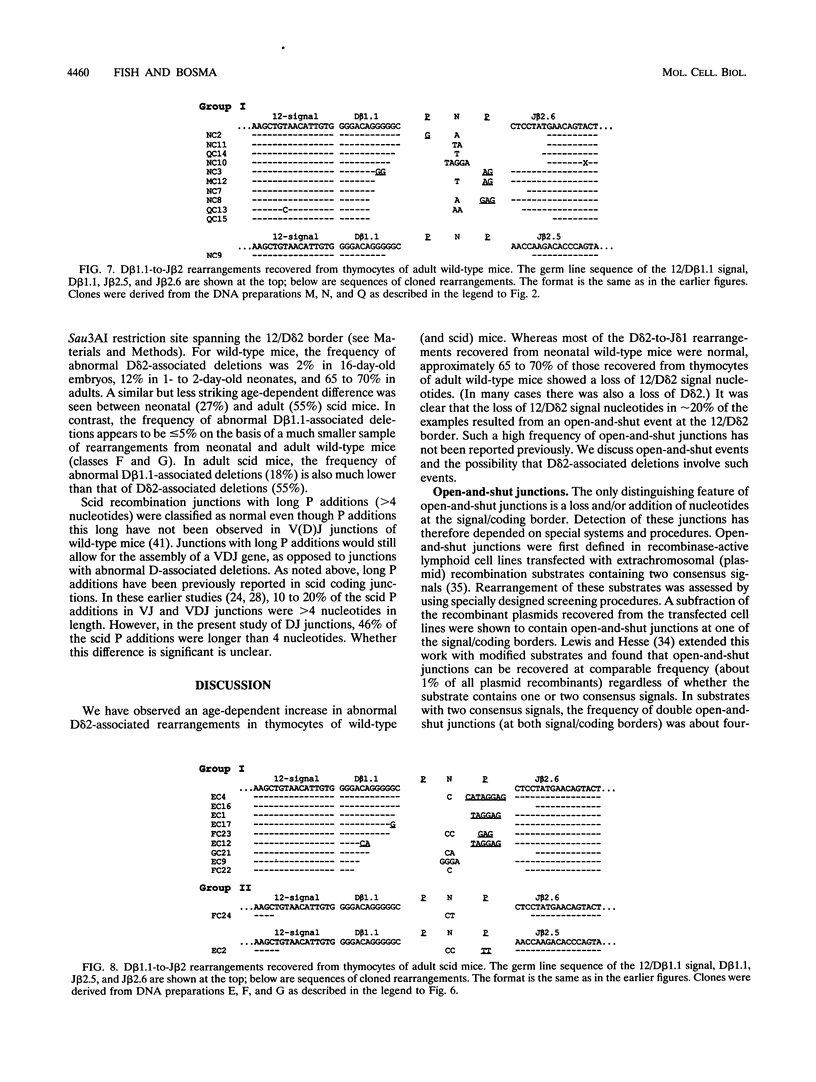
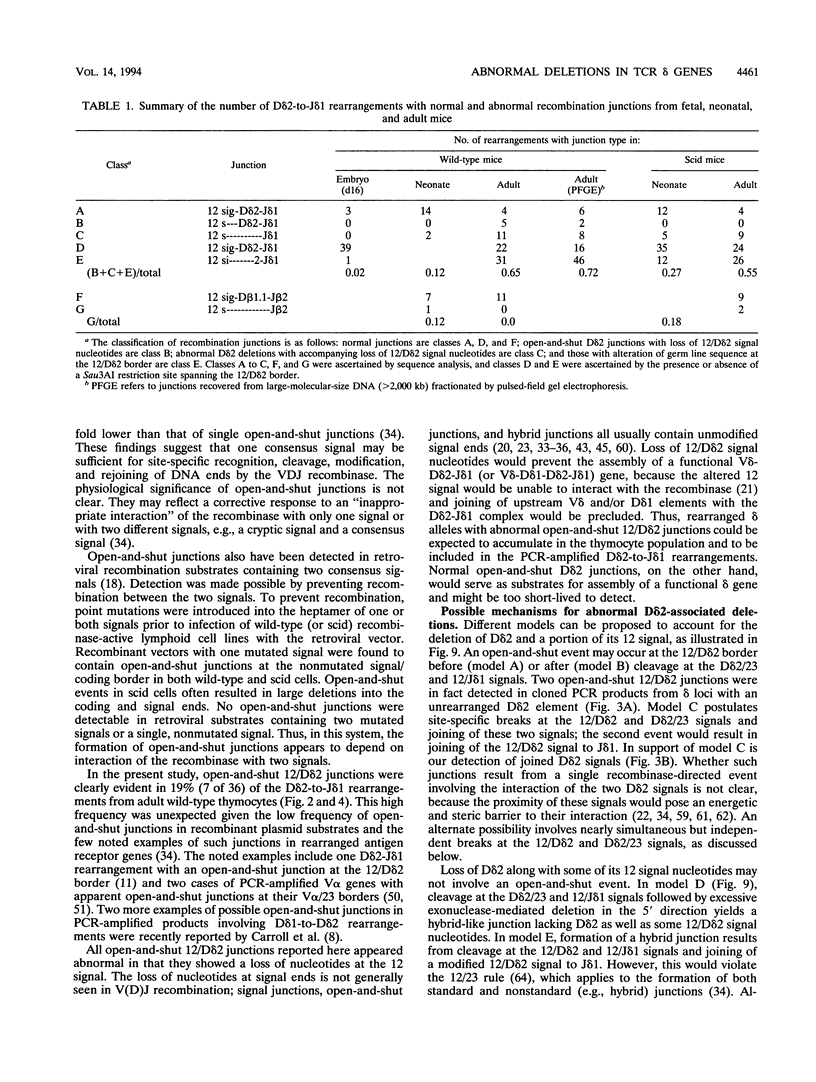
Abstract
Separate genetic elements (V, D, and J) encode the variable regions of lymphocyte antigen receptors. During early lymphocyte differentiation, these elements rearrange to form contiguous coding segments (VJ and VDJ) for a diverse array of variable regions. Rearrangement is mediated by a recombinase that recognizes short DNA sequences (signals) flanking V, D, and J elements. Signals flank both the 5' and 3' sides of each D element, thereby allowing assembly of a functional VDJ gene. However, in rearrangements involving the D delta 2 and J delta 1 elements of the mouse T-cell receptor delta (TCR delta) locus, we unexpectedly found that the D delta 2 element and a portion of its 5' signal are often deleted. Approximately 50% of recovered D delta 2 to J delta 1 rearrangements from thymocytes of adult wild-type mice showed such deletions. An additional 20% of the rearrangements contained standard D delta 2-J delta 1 coding junctions but showed some loss of nucleotides from the 5' D delta 2 signal. This loss was clearly associated with another event involving a site-specific cleavage at the 5' signal/coding border of D delta 2 and rejoining of the modified signal and coding ends. The abnormal loss of D delta 2 and a portion of the 5' D delta 2 signal was infrequently observed in D delta 2-to-J delta 1 rearrangements recovered from neonatal mice. The possible basis and significance of this age-dependent phenomenon are discussed.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alt F. W., Baltimore D. Joining of immunoglobulin heavy chain gene segments: implications from a chromosome with evidence of three D-JH fusions. Proc Natl Acad Sci U S A. 1982 Jul;79(13):4118–4122. doi: 10.1073/pnas.79.13.4118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asarnow D. M., Kuziel W. A., Bonyhadi M., Tigelaar R. E., Tucker P. W., Allison J. P. Limited diversity of gamma delta antigen receptor genes of Thy-1+ dendritic epidermal cells. Cell. 1988 Dec 2;55(5):837–847. doi: 10.1016/0092-8674(88)90139-0. [DOI] [PubMed] [Google Scholar]
- Barth R. K., Kim B. S., Lan N. C., Hunkapiller T., Sobieck N., Winoto A., Gershenfeld H., Okada C., Hansburg D., Weissman I. L. The murine T-cell receptor uses a limited repertoire of expressed V beta gene segments. Nature. 1985 Aug 8;316(6028):517–523. doi: 10.1038/316517a0. [DOI] [PubMed] [Google Scholar]
- Blin N., Stafford D. W. A general method for isolation of high molecular weight DNA from eukaryotes. Nucleic Acids Res. 1976 Sep;3(9):2303–2308. doi: 10.1093/nar/3.9.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogue M., Candéias S., Benoist C., Mathis D. A special repertoire of alpha:beta T cells in neonatal mice. EMBO J. 1991 Dec;10(12):3647–3654. doi: 10.1002/j.1460-2075.1991.tb04931.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Born W., Yagüe J., Palmer E., Kappler J., Marrack P. Rearrangement of T-cell receptor beta-chain genes during T-cell development. Proc Natl Acad Sci U S A. 1985 May;82(9):2925–2929. doi: 10.1073/pnas.82.9.2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll A. M., Bosma M. J. T-lymphocyte development in scid mice is arrested shortly after the initiation of T-cell receptor delta gene recombination. Genes Dev. 1991 Aug;5(8):1357–1366. doi: 10.1101/gad.5.8.1357. [DOI] [PubMed] [Google Scholar]
- Carroll A. M., Slack J. K., Mu X. V(D)J recombination generates a high frequency of nonstandard TCR D delta-associated rearrangements in thymocytes. J Immunol. 1993 Mar 15;150(6):2222–2230. [PubMed] [Google Scholar]
- Chien Y. H., Iwashima M., Kaplan K. B., Elliott J. F., Davis M. M. A new T-cell receptor gene located within the alpha locus and expressed early in T-cell differentiation. 1987 Jun 25-Jul 1Nature. 327(6124):677–682. doi: 10.1038/327677a0. [DOI] [PubMed] [Google Scholar]
- Chien Y. H., Iwashima M., Wettstein D. A., Kaplan K. B., Elliott J. F., Born W., Davis M. M. T-cell receptor delta gene rearrangements in early thymocytes. Nature. 1987 Dec 24;330(6150):722–727. doi: 10.1038/330722a0. [DOI] [PubMed] [Google Scholar]
- Elliott J. F., Rock E. P., Patten P. A., Davis M. M., Chien Y. H. The adult T-cell receptor delta-chain is diverse and distinct from that of fetal thymocytes. Nature. 1988 Feb 18;331(6157):627–631. doi: 10.1038/331627a0. [DOI] [PubMed] [Google Scholar]
- Feeney A. J. Junctional sequences of fetal T cell receptor beta chains have few N regions. J Exp Med. 1991 Jul 1;174(1):115–124. doi: 10.1084/jem.174.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feeney A. J. Lack of N regions in fetal and neonatal mouse immunoglobulin V-D-J junctional sequences. J Exp Med. 1990 Nov 1;172(5):1377–1390. doi: 10.1084/jem.172.5.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilfillan S., Dierich A., Lemeur M., Benoist C., Mathis D. Mice lacking TdT: mature animals with an immature lymphocyte repertoire. Science. 1993 Aug 27;261(5125):1175–1178. doi: 10.1126/science.8356452. [DOI] [PubMed] [Google Scholar]
- Gu H., Förster I., Rajewsky K. Sequence homologies, N sequence insertion and JH gene utilization in VHDJH joining: implications for the joining mechanism and the ontogenetic timing of Ly1 B cell and B-CLL progenitor generation. EMBO J. 1990 Jul;9(7):2133–2140. doi: 10.1002/j.1460-2075.1990.tb07382.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Güssow D., Clackson T. Direct clone characterization from plaques and colonies by the polymerase chain reaction. Nucleic Acids Res. 1989 May 25;17(10):4000–4000. doi: 10.1093/nar/17.10.4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haars R., Kronenberg M., Gallatin W. M., Weissman I. L., Owen F. L., Hood L. Rearrangement and expression of T cell antigen receptor and gamma genes during thymic development. J Exp Med. 1986 Jul 1;164(1):1–24. doi: 10.1084/jem.164.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrickson E. A., Liu V. F., Weaver D. T. Strand breaks without DNA rearrangement in V (D)J recombination. Mol Cell Biol. 1991 Jun;11(6):3155–3162. doi: 10.1128/mcb.11.6.3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrickson E. A., Schatz D. G., Weaver D. T. The scid gene encodes a trans-acting factor that mediates the rejoining event of Ig gene rearrangement. Genes Dev. 1988 Jul;2(7):817–829. doi: 10.1101/gad.2.7.817. [DOI] [PubMed] [Google Scholar]
- Hesse J. E., Lieber M. R., Gellert M., Mizuuchi K. Extrachromosomal DNA substrates in pre-B cells undergo inversion or deletion at immunoglobulin V-(D)-J joining signals. Cell. 1987 Jun 19;49(6):775–783. doi: 10.1016/0092-8674(87)90615-5. [DOI] [PubMed] [Google Scholar]
- Hesse J. E., Lieber M. R., Mizuuchi K., Gellert M. V(D)J recombination: a functional definition of the joining signals. Genes Dev. 1989 Jul;3(7):1053–1061. doi: 10.1101/gad.3.7.1053. [DOI] [PubMed] [Google Scholar]
- Horowitz D. S., Wang J. C. Torsional rigidity of DNA and length dependence of the free energy of DNA supercoiling. J Mol Biol. 1984 Feb 15;173(1):75–91. doi: 10.1016/0022-2836(84)90404-2. [DOI] [PubMed] [Google Scholar]
- Iwasato T., Yamagishi H. Novel excision products of T cell receptor gamma gene rearrangements and developmental stage specificity implied by the frequency of nucleotide insertions at signal joints. Eur J Immunol. 1992 Jan;22(1):101–106. doi: 10.1002/eji.1830220116. [DOI] [PubMed] [Google Scholar]
- Kienker L. J., Kuziel W. A., Tucker P. W. T cell receptor gamma and delta gene junctional sequences in SCID mice: excessive P nucleotide insertion. J Exp Med. 1991 Oct 1;174(4):769–773. doi: 10.1084/jem.174.4.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M. G., Schuler W., Bosma M. J., Marcu K. B. Abnormal recombination of Igh D and J gene segments in transformed pre-B cells of scid mice. J Immunol. 1988 Aug 15;141(4):1341–1347. [PubMed] [Google Scholar]
- Kleinfield R., Hardy R. R., Tarlinton D., Dangl J., Herzenberg L. A., Weigert M. Recombination between an expressed immunoglobulin heavy-chain gene and a germline variable gene segment in a Ly 1+ B-cell lymphoma. 1986 Aug 28-Sep 3Nature. 322(6082):843–846. doi: 10.1038/322843a0. [DOI] [PubMed] [Google Scholar]
- Komori T., Okada A., Stewart V., Alt F. W. Lack of N regions in antigen receptor variable region genes of TdT-deficient lymphocytes. Science. 1993 Aug 27;261(5125):1171–1175. doi: 10.1126/science.8356451. [DOI] [PubMed] [Google Scholar]
- Kotloff D. B., Bosma M. J., Ruetsch N. R. V(D)J recombination in peritoneal B cells of leaky scid mice. J Exp Med. 1993 Dec 1;178(6):1981–1994. doi: 10.1084/jem.178.6.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafaille J. J., DeCloux A., Bonneville M., Takagaki Y., Tonegawa S. Junctional sequences of T cell receptor gamma delta genes: implications for gamma delta T cell lineages and for a novel intermediate of V-(D)-J joining. Cell. 1989 Dec 1;59(5):859–870. doi: 10.1016/0092-8674(89)90609-0. [DOI] [PubMed] [Google Scholar]
- Lai E., Wilson R. K., Hood L. E. Physical maps of the mouse and human immunoglobulin-like loci. Adv Immunol. 1989;46:1–59. doi: 10.1016/s0065-2776(08)60650-1. [DOI] [PubMed] [Google Scholar]
- Landau N. R., Schatz D. G., Rosa M., Baltimore D. Increased frequency of N-region insertion in a murine pre-B-cell line infected with a terminal deoxynucleotidyl transferase retroviral expression vector. Mol Cell Biol. 1987 Sep;7(9):3237–3243. doi: 10.1128/mcb.7.9.3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis S. M., Hesse J. E. Cutting and closing without recombination in V(D)J joining. EMBO J. 1991 Dec;10(12):3631–3639. doi: 10.1002/j.1460-2075.1991.tb04929.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis S. M., Hesse J. E., Mizuuchi K., Gellert M. Novel strand exchanges in V(D)J recombination. Cell. 1988 Dec 23;55(6):1099–1107. doi: 10.1016/0092-8674(88)90254-1. [DOI] [PubMed] [Google Scholar]
- Lewis S., Gellert M. The mechanism of antigen receptor gene assembly. Cell. 1989 Nov 17;59(4):585–588. doi: 10.1016/0092-8674(89)90002-0. [DOI] [PubMed] [Google Scholar]
- Lewis S., Gifford A., Baltimore D. DNA elements are asymmetrically joined during the site-specific recombination of kappa immunoglobulin genes. Science. 1985 May 10;228(4700):677–685. doi: 10.1126/science.3158075. [DOI] [PubMed] [Google Scholar]
- Lieber M. R., Hesse J. E., Mizuuchi K., Gellert M. Lymphoid V(D)J recombination: nucleotide insertion at signal joints as well as coding joints. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8588–8592. doi: 10.1073/pnas.85.22.8588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallick C. A., Dudley E. C., Viney J. L., Owen M. J., Hayday A. C. Rearrangement and diversity of T cell receptor beta chain genes in thymocytes: a critical role for the beta chain in development. Cell. 1993 May 7;73(3):513–519. doi: 10.1016/0092-8674(93)90138-g. [DOI] [PubMed] [Google Scholar]
- Malynn B. A., Blackwell T. K., Fulop G. M., Rathbun G. A., Furley A. J., Ferrier P., Heinke L. B., Phillips R. A., Yancopoulos G. D., Alt F. W. The scid defect affects the final step of the immunoglobulin VDJ recombinase mechanism. Cell. 1988 Aug 12;54(4):453–460. doi: 10.1016/0092-8674(88)90066-9. [DOI] [PubMed] [Google Scholar]
- McCormack W. T., Tjoelker L. W., Carlson L. M., Petryniak B., Barth C. F., Humphries E. H., Thompson C. B. Chicken IgL gene rearrangement involves deletion of a circular episome and addition of single nonrandom nucleotides to both coding segments. Cell. 1989 Mar 10;56(5):785–791. doi: 10.1016/0092-8674(89)90683-1. [DOI] [PubMed] [Google Scholar]
- Meek K. D., Hasemann C. A., Capra J. D. Novel rearrangements at the immunoglobulin D locus. Inversions and fusions add to IgH somatic diversity. J Exp Med. 1989 Jul 1;170(1):39–57. doi: 10.1084/jem.170.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier J. T., Lewis S. M. P nucleotides in V(D)J recombination: a fine-structure analysis. Mol Cell Biol. 1993 Feb;13(2):1078–1092. doi: 10.1128/mcb.13.2.1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morzycka-Wroblewska E., Lee F. E., Desiderio S. V. Unusual immunoglobulin gene rearrangement leads to replacement of recombinational signal sequences. Science. 1988 Oct 14;242(4876):261–263. doi: 10.1126/science.3140378. [DOI] [PubMed] [Google Scholar]
- Okazaki K., Davis D. D., Sakano H. T cell receptor beta gene sequences in the circular DNA of thymocyte nuclei: direct evidence for intramolecular DNA deletion in V-D-J joining. Cell. 1987 May 22;49(4):477–485. doi: 10.1016/0092-8674(87)90450-8. [DOI] [PubMed] [Google Scholar]
- Okazaki K., Nishikawa S., Sakano H. Aberrant immunoglobulin gene rearrangement in scid mouse bone marrow cells. J Immunol. 1988 Aug 15;141(4):1348–1352. [PubMed] [Google Scholar]
- Okazaki K., Sakano H. Thymocyte circular DNA excised from T cell receptor alpha-delta gene complex. EMBO J. 1988 Jun;7(6):1669–1674. doi: 10.1002/j.1460-2075.1988.tb02994.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearse M., Wu L., Egerton M., Wilson A., Shortman K., Scollay R. A murine early thymocyte developmental sequence is marked by transient expression of the interleukin 2 receptor. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1614–1618. doi: 10.1073/pnas.86.5.1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrini J. H., Carroll A. M., Bosma M. J. T-cell receptor gene rearrangements in functional T-cell clones from severe combined immune deficient (scid) mice: reversion of the scid phenotype in individual lymphocyte progenitors. Proc Natl Acad Sci U S A. 1990 May;87(9):3450–3453. doi: 10.1073/pnas.87.9.3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reth M., Gehrmann P., Petrac E., Wiese P. A novel VH to VHDJH joining mechanism in heavy-chain-negative (null) pre-B cells results in heavy-chain production. 1986 Aug 28-Sep 3Nature. 322(6082):840–842. doi: 10.1038/322840a0. [DOI] [PubMed] [Google Scholar]
- Roth D. B., Menetski J. P., Nakajima P. B., Bosma M. J., Gellert M. V(D)J recombination: broken DNA molecules with covalently sealed (hairpin) coding ends in scid mouse thymocytes. Cell. 1992 Sep 18;70(6):983–991. doi: 10.1016/0092-8674(92)90248-b. [DOI] [PubMed] [Google Scholar]
- Roth M. E., Lacy M. J., McNeil L. K., Kranz D. M. Correction: selection of variable-joining region combinations in the T cell receptor. Science. 1989 Sep 8;245(4922):1032–1032. doi: 10.1126/science.2528208. [DOI] [PubMed] [Google Scholar]
- Roth M. E., Lacy M. J., McNeil L. K., Kranz D. M. Selection of variable-joining region combinations in the alpha chain of the T cell receptor. Science. 1988 Sep 9;241(4871):1354–1358. doi: 10.1126/science.2970673. [DOI] [PubMed] [Google Scholar]
- Rothenberg E., Triglia D. Clonal proliferation unlinked to terminal deoxynucleotidyl transferase synthesis in thymocytes of young mice. J Immunol. 1983 Apr;130(4):1627–1633. [PubMed] [Google Scholar]
- Schatz D. G., Oettinger M. A., Schlissel M. S. V(D)J recombination: molecular biology and regulation. Annu Rev Immunol. 1992;10:359–383. doi: 10.1146/annurev.iy.10.040192.002043. [DOI] [PubMed] [Google Scholar]
- Schuler W., Ruetsch N. R., Amsler M., Bosma M. J. Coding joint formation of endogenous T cell receptor genes in lymphoid cells from scid mice: unusual P-nucleotide additions in VJ-coding joints. Eur J Immunol. 1991 Mar;21(3):589–596. doi: 10.1002/eji.1830210309. [DOI] [PubMed] [Google Scholar]
- Schuler W., Schuler A., Bosma M. J. Defective V-to-J recombination of T cell receptor gamma chain genes in scid mice. Eur J Immunol. 1990 Mar;20(3):545–550. doi: 10.1002/eji.1830200313. [DOI] [PubMed] [Google Scholar]
- Schuler W., Schuler A., Lennon G. G., Bosma G. C., Bosma M. J. Transcription of unrearranged antigen receptor genes in scid mice. EMBO J. 1988 Jul;7(7):2019–2024. doi: 10.1002/j.1460-2075.1988.tb03041.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuler W., Weiler I. J., Schuler A., Phillips R. A., Rosenberg N., Mak T. W., Kearney J. F., Perry R. P., Bosma M. J. Rearrangement of antigen receptor genes is defective in mice with severe combined immune deficiency. Cell. 1986 Sep 26;46(7):963–972. doi: 10.1016/0092-8674(86)90695-1. [DOI] [PubMed] [Google Scholar]
- Sheehan K. M., Lieber M. R. V(D)J recombination: signal and coding joint resolution are uncoupled and depend on parallel synapsis of the sites. Mol Cell Biol. 1993 Mar;13(3):1363–1370. doi: 10.1128/mcb.13.3.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu T., Yamagishi H. Biased reading frames of pre-existing DH--JH coding joints and preferential nucleotide insertions at VH--DJH signal joints of excision products of immunoglobulin heavy chain gene rearrangements. EMBO J. 1992 Dec;11(13):4869–4875. doi: 10.1002/j.1460-2075.1992.tb05593.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shore D., Baldwin R. L. Energetics of DNA twisting. I. Relation between twist and cyclization probability. J Mol Biol. 1983 Nov 15;170(4):957–981. doi: 10.1016/s0022-2836(83)80198-3. [DOI] [PubMed] [Google Scholar]
- Shore D., Langowski J., Baldwin R. L. DNA flexibility studied by covalent closure of short fragments into circles. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4833–4837. doi: 10.1073/pnas.78.8.4833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siu G., Kronenberg M., Strauss E., Haars R., Mak T. W., Hood L. The structure, rearrangement and expression of D beta gene segments of the murine T-cell antigen receptor. 1984 Sep 27-Oct 3Nature. 311(5984):344–350. doi: 10.1038/311344a0. [DOI] [PubMed] [Google Scholar]
- Tonegawa S. Somatic generation of antibody diversity. Nature. 1983 Apr 14;302(5909):575–581. doi: 10.1038/302575a0. [DOI] [PubMed] [Google Scholar]


