Abstract
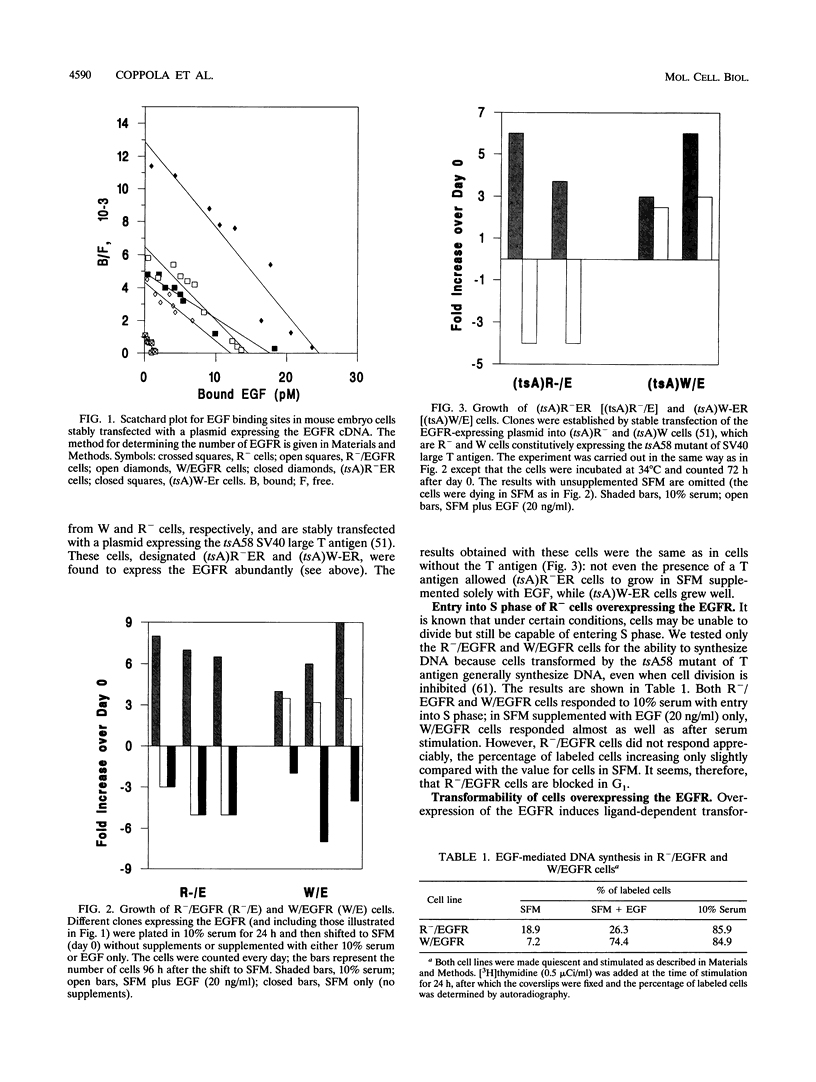
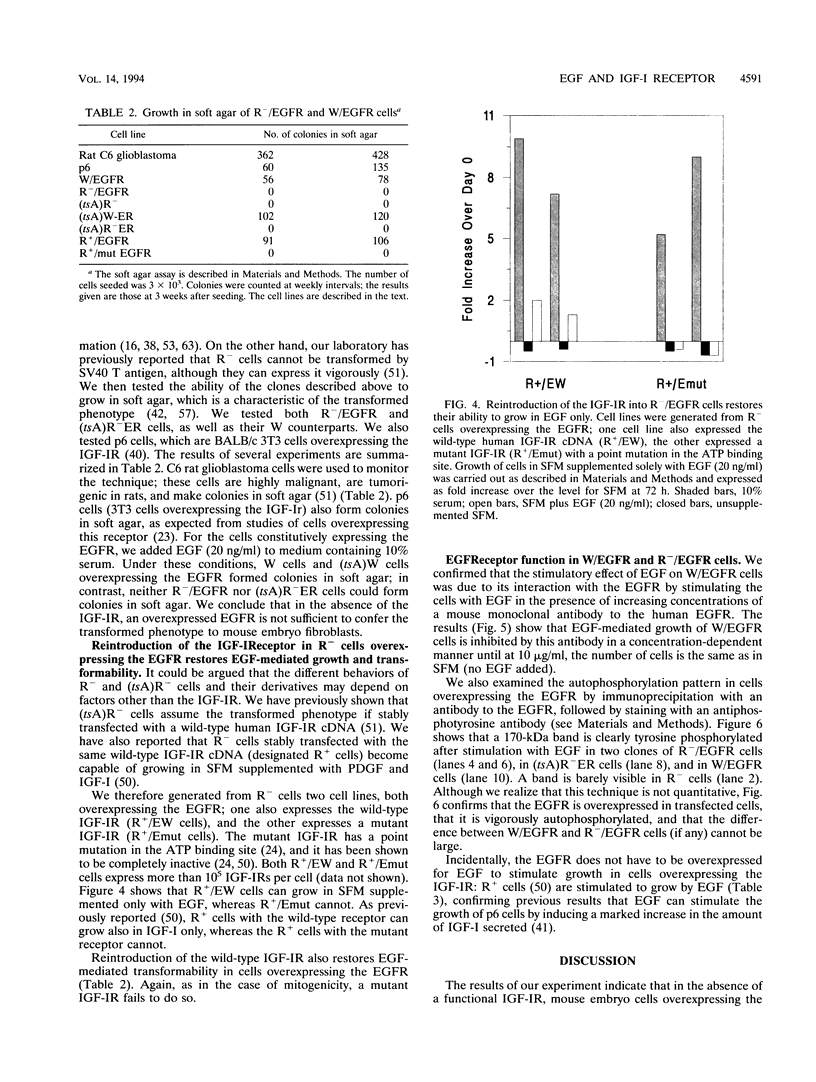
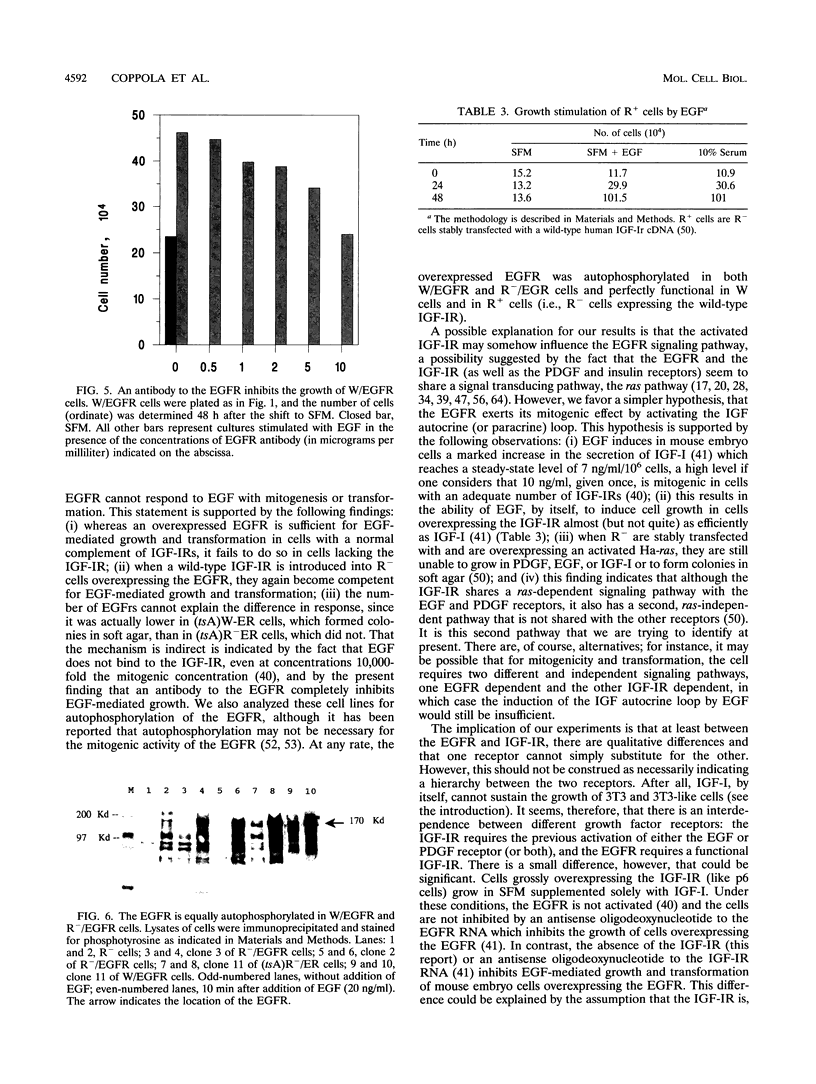
When wild-type mouse embryo cells are stably transfected with a plasmid constitutively overexpressing the epidermal growth factor (EGF) receptor (EGFR), the resulting cells can grow in serum-free medium supplemented solely with EGF. Supplementation with EGF also induces in these cells the transformed phenotype (growth in soft agar). However, when the same EGFR expression plasmid is introduced and overexpressed in cells derived from littermate embryos in which the insulin-like growth factor I (IGF-I) receptor genes have been disrupted by homologous recombination, the resulting cells are unable to grow or to be transformed by the addition of EGF. Reintroduction into these cells (null for the IGF-I receptor) of a wild-type (but not of a mutant) IGF-I receptor restores EGF-mediated growth and transformation. Our results indicate that at least in mouse embryo fibroblasts, the EGFR requires the presence of a functional IGF-I receptor for its mitogenic and transforming activities.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaronson S. A. Growth factors and cancer. Science. 1991 Nov 22;254(5035):1146–1153. doi: 10.1126/science.1659742. [DOI] [PubMed] [Google Scholar]
- Arteaga C. L. Interference of the IGF system as a strategy to inhibit breast cancer growth. Breast Cancer Res Treat. 1992;22(1):101–106. doi: 10.1007/BF01833338. [DOI] [PubMed] [Google Scholar]
- Arteaga C. L., Kitten L. J., Coronado E. B., Jacobs S., Kull F. C., Jr, Allred D. C., Osborne C. K. Blockade of the type I somatomedin receptor inhibits growth of human breast cancer cells in athymic mice. J Clin Invest. 1989 Nov;84(5):1418–1423. doi: 10.1172/JCI114315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arteaga C. L., Osborne C. K. Growth inhibition of human breast cancer cells in vitro with an antibody against the type I somatomedin receptor. Cancer Res. 1989 Nov 15;49(22):6237–6241. [PubMed] [Google Scholar]
- Baker J., Liu J. P., Robertson E. J., Efstratiadis A. Role of insulin-like growth factors in embryonic and postnatal growth. Cell. 1993 Oct 8;75(1):73–82. [PubMed] [Google Scholar]
- Baserga R., Rubin R. Cell cycle and growth control. Crit Rev Eukaryot Gene Expr. 1993;3(1):47–61. [PubMed] [Google Scholar]
- Baserga R., Sell C., Porcu P., Rubini M. The role of the IGF-I receptor in the growth and transformation of mammalian cells. Cell Prolif. 1994 Feb;27(2):63–71. doi: 10.1111/j.1365-2184.1994.tb01406.x. [DOI] [PubMed] [Google Scholar]
- Campa M. J., Glickman J. F., Yamamoto K., Chang K. J. The antibiotic azatyrosine suppresses progesterone or [Val12]p21 Ha-ras/insulin-like growth factor I-induced germinal vesicle breakdown and tyrosine phosphorylation of Xenopus mitogen-activated protein kinase in oocytes. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7654–7658. doi: 10.1073/pnas.89.16.7654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campisi J., Pardee A. B. Post-transcriptional control of the onset of DNA synthesis by an insulin-like growth factor. Mol Cell Biol. 1984 Sep;4(9):1807–1814. doi: 10.1128/mcb.4.9.1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemmons D. R. Multiple hormones stimulate the production of somatomedin by cultured human fibroblasts. J Clin Endocrinol Metab. 1984 May;58(5):850–856. doi: 10.1210/jcem-58-5-850. [DOI] [PubMed] [Google Scholar]
- Clemmons D. R., Shaw D. S. Variables controlling somatomedin production by cultured human fibroblasts. J Cell Physiol. 1983 May;115(2):137–142. doi: 10.1002/jcp.1041150206. [DOI] [PubMed] [Google Scholar]
- Cristofalo V. J., Phillips P. D., Sorger T., Gerhard G. Alterations in the responsiveness of senescent cells to growth factors. J Gerontol. 1989 Nov;44(6):55–62. doi: 10.1093/geronj/44.6.55. [DOI] [PubMed] [Google Scholar]
- Di Fiore P. P., Pierce J. H., Fleming T. P., Hazan R., Ullrich A., King C. R., Schlessinger J., Aaronson S. A. Overexpression of the human EGF receptor confers an EGF-dependent transformed phenotype to NIH 3T3 cells. Cell. 1987 Dec 24;51(6):1063–1070. doi: 10.1016/0092-8674(87)90592-7. [DOI] [PubMed] [Google Scholar]
- Egan S. E., Giddings B. W., Brooks M. W., Buday L., Sizeland A. M., Weinberg R. A. Association of Sos Ras exchange protein with Grb2 is implicated in tyrosine kinase signal transduction and transformation. Nature. 1993 May 6;363(6424):45–51. doi: 10.1038/363045a0. [DOI] [PubMed] [Google Scholar]
- Falco J. P., Taylor W. G., Di Fiore P. P., Weissman B. E., Aaronson S. A. Interactions of growth factors and retroviral oncogenes with mitogenic signal transduction pathways of Balb/MK keratinocytes. Oncogene. 1988 Jun;2(6):573–578. [PubMed] [Google Scholar]
- Fantl W. J., Escobedo J. A., Williams L. T. Mutations of the platelet-derived growth factor receptor that cause a loss of ligand-induced conformational change, subtle changes in kinase activity, and impaired ability to stimulate DNA synthesis. Mol Cell Biol. 1989 Oct;9(10):4473–4478. doi: 10.1128/mcb.9.10.4473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale N. W., Kaplan S., Lowenstein E. J., Schlessinger J., Bar-Sagi D. Grb2 mediates the EGF-dependent activation of guanine nucleotide exchange on Ras. Nature. 1993 May 6;363(6424):88–92. doi: 10.1038/363088a0. [DOI] [PubMed] [Google Scholar]
- Goldring M. B., Goldring S. R. Cytokines and cell growth control. Crit Rev Eukaryot Gene Expr. 1991;1(4):301–326. [PubMed] [Google Scholar]
- Gritz L., Davies J. Plasmid-encoded hygromycin B resistance: the sequence of hygromycin B phosphotransferase gene and its expression in Escherichia coli and Saccharomyces cerevisiae. Gene. 1983 Nov;25(2-3):179–188. doi: 10.1016/0378-1119(83)90223-8. [DOI] [PubMed] [Google Scholar]
- Kaleko M., Rutter W. J., Miller A. D. Overexpression of the human insulinlike growth factor I receptor promotes ligand-dependent neoplastic transformation. Mol Cell Biol. 1990 Feb;10(2):464–473. doi: 10.1128/mcb.10.2.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato H., Faria T. N., Stannard B., Roberts C. T., Jr, LeRoith D. Role of tyrosine kinase activity in signal transduction by the insulin-like growth factor-I (IGF-I) receptor. Characterization of kinase-deficient IGF-I receptors and the action of an IGF-I-mimetic antibody (alpha IR-3). J Biol Chem. 1993 Feb 5;268(4):2655–2661. [PubMed] [Google Scholar]
- Leof E. B., Lyons R. M., Cunningham M. R., O'Sullivan D. Mid-G1 arrest and epidermal growth factor independence of ras-transfected mouse cells. Cancer Res. 1989 May 1;49(9):2356–2361. [PubMed] [Google Scholar]
- Leof E. B., Wharton W., van Wyk J. J., Pledger W. J. Epidermal growth factor (EGF) and somatomedin C regulate G1 progression in competent BALB/c-3T3 cells. Exp Cell Res. 1982 Sep;141(1):107–115. doi: 10.1016/0014-4827(82)90073-8. [DOI] [PubMed] [Google Scholar]
- Li M., Bernard O. FDC-P1 myeloid cells engineered to express fibroblast growth factor receptor 1 proliferate and differentiate in the presence of fibroblast growth factor and heparin. Proc Natl Acad Sci U S A. 1992 Apr 15;89(8):3315–3319. doi: 10.1073/pnas.89.8.3315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N., Batzer A., Daly R., Yajnik V., Skolnik E., Chardin P., Bar-Sagi D., Margolis B., Schlessinger J. Guanine-nucleotide-releasing factor hSos1 binds to Grb2 and links receptor tyrosine kinases to Ras signalling. Nature. 1993 May 6;363(6424):85–88. doi: 10.1038/363085a0. [DOI] [PubMed] [Google Scholar]
- Liu J. P., Baker J., Perkins A. S., Robertson E. J., Efstratiadis A. Mice carrying null mutations of the genes encoding insulin-like growth factor I (Igf-1) and type 1 IGF receptor (Igf1r). Cell. 1993 Oct 8;75(1):59–72. [PubMed] [Google Scholar]
- Lu K. H., Levine R. A., Campisi J. c-ras-Ha gene expression is regulated by insulin or insulinlike growth factor and by epidermal growth factor in murine fibroblasts. Mol Cell Biol. 1989 Aug;9(8):3411–3417. doi: 10.1128/mcb.9.8.3411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matuoka K., Shibasaki F., Shibata M., Takenawa T. Ash/Grb-2, a SH2/SH3-containing protein, couples to signaling for mitogenesis and cytoskeletal reorganization by EGF and PDGF. EMBO J. 1993 Sep;12(9):3467–3473. doi: 10.1002/j.1460-2075.1993.tb06021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick F. Signal transduction. How receptors turn Ras on. Nature. 1993 May 6;363(6424):15–16. doi: 10.1038/363015a0. [DOI] [PubMed] [Google Scholar]
- McCubrey J. A., Steelman L. S., Mayo M. W., Algate P. A., Dellow R. A., Kaleko M. Growth-promoting effects of insulin-like growth factor-1 (IGF-1) on hematopoietic cells: overexpression of introduced IGF-1 receptor abrogates interleukin-3 dependency of murine factor-dependent cells by a ligand-dependent mechanism. Blood. 1991 Aug 15;78(4):921–929. [PubMed] [Google Scholar]
- Medema R. H., Bos J. L. The role of p21ras in receptor tyrosine kinase signaling. Crit Rev Oncog. 1993;4(6):615–661. [PubMed] [Google Scholar]
- Mohammadi M., Dionne C. A., Li W., Li N., Spivak T., Honegger A. M., Jaye M., Schlessinger J. Point mutation in FGF receptor eliminates phosphatidylinositol hydrolysis without affecting mitogenesis. Nature. 1992 Aug 20;358(6388):681–684. doi: 10.1038/358681a0. [DOI] [PubMed] [Google Scholar]
- Moroni M. C., Willingham M. C., Beguinot L. EGF-R antisense RNA blocks expression of the epidermal growth factor receptor and suppresses the transforming phenotype of a human carcinoma cell line. J Biol Chem. 1992 Feb 5;267(4):2714–2722. [PubMed] [Google Scholar]
- Myers M. G., Jr, Sun X. J., Cheatham B., Jachna B. R., Glasheen E. M., Backer J. M., White M. F. IRS-1 is a common element in insulin and insulin-like growth factor-I signaling to the phosphatidylinositol 3'-kinase. Endocrinology. 1993 Apr;132(4):1421–1430. doi: 10.1210/endo.132.4.8384986. [DOI] [PubMed] [Google Scholar]
- Pietrzkowski Z., Lammers R., Carpenter G., Soderquist A. M., Limardo M., Phillips P. D., Ullrich A., Baserga R. Constitutive expression of insulin-like growth factor 1 and insulin-like growth factor 1 receptor abrogates all requirements for exogenous growth factors. Cell Growth Differ. 1992 Apr;3(4):199–205. [PubMed] [Google Scholar]
- Pietrzkowski Z., Sell C., Lammers R., Ullrich A., Baserga R. Roles of insulinlike growth factor 1 (IGF-1) and the IGF-1 receptor in epidermal growth factor-stimulated growth of 3T3 cells. Mol Cell Biol. 1992 Sep;12(9):3883–3889. doi: 10.1128/mcb.12.9.3883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrzkowski Z., Sell C., Lammers R., Ullrich A., Baserga R. Roles of insulinlike growth factor 1 (IGF-1) and the IGF-1 receptor in epidermal growth factor-stimulated growth of 3T3 cells. Mol Cell Biol. 1992 Sep;12(9):3883–3889. doi: 10.1128/mcb.12.9.3883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randazzo P. A., Morey V. A., Polishook A. K., Jarett L. Characterization of the growth of murine fibroblasts that express human insulin receptors. I. The effect of insulin in the absence of other growth factors. Exp Cell Res. 1990 Sep;190(1):25–30. doi: 10.1016/0014-4827(90)90139-2. [DOI] [PubMed] [Google Scholar]
- Resnicoff M., Sell C., Rubini M., Coppola D., Ambrose D., Baserga R., Rubin R. Rat glioblastoma cells expressing an antisense RNA to the insulin-like growth factor-1 (IGF-1) receptor are nontumorigenic and induce regression of wild-type tumors. Cancer Res. 1994 Apr 15;54(8):2218–2222. [PubMed] [Google Scholar]
- Roussel M. F., Cleveland J. L., Shurtleff S. A., Sherr C. J. Myc rescue of a mutant CSF-1 receptor impaired in mitogenic signalling. Nature. 1991 Sep 26;353(6342):361–363. doi: 10.1038/353361a0. [DOI] [PubMed] [Google Scholar]
- Rozakis-Adcock M., Fernley R., Wade J., Pawson T., Bowtell D. The SH2 and SH3 domains of mammalian Grb2 couple the EGF receptor to the Ras activator mSos1. Nature. 1993 May 6;363(6424):83–85. doi: 10.1038/363083a0. [DOI] [PubMed] [Google Scholar]
- Rubini M., Werner H., Gandini E., Roberts C. T., Jr, LeRoith D., Baserga R. Platelet-derived growth factor increases the activity of the promoter of the insulin-like growth factor-1 (IGF-1) receptor gene. Exp Cell Res. 1994 Apr;211(2):374–379. doi: 10.1006/excr.1994.1101. [DOI] [PubMed] [Google Scholar]
- Scher C. D., Shepard R. C., Antoniades H. N., Stiles C. D. Platelet-derived growth factor and the regulation of the mammalian fibroblast cell cycle. Biochim Biophys Acta. 1979 Aug 10;560(2):217–241. doi: 10.1016/0304-419x(79)90020-9. [DOI] [PubMed] [Google Scholar]
- Sell C., Dumenil G., Deveaud C., Miura M., Coppola D., DeAngelis T., Rubin R., Efstratiadis A., Baserga R. Effect of a null mutation of the insulin-like growth factor I receptor gene on growth and transformation of mouse embryo fibroblasts. Mol Cell Biol. 1994 Jun;14(6):3604–3612. doi: 10.1128/mcb.14.6.3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sell C., Rubini M., Rubin R., Liu J. P., Efstratiadis A., Baserga R. Simian virus 40 large tumor antigen is unable to transform mouse embryonic fibroblasts lacking type 1 insulin-like growth factor receptor. Proc Natl Acad Sci U S A. 1993 Dec 1;90(23):11217–11221. doi: 10.1073/pnas.90.23.11217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvennoinen O., Schindler C., Schlessinger J., Levy D. E. Ras-independent growth factor signaling by transcription factor tyrosine phosphorylation. Science. 1993 Sep 24;261(5129):1736–1739. doi: 10.1126/science.8378775. [DOI] [PubMed] [Google Scholar]
- Soler C., Beguinot L., Sorkin A., Carpenter G. Tyrosine phosphorylation of ras GTPase-activating protein does not require association with the epidermal growth factor receptor. J Biol Chem. 1993 Oct 15;268(29):22010–22019. [PubMed] [Google Scholar]
- Stewart A. J., Johnson M. D., May F. E., Westley B. R. Role of insulin-like growth factors and the type I insulin-like growth factor receptor in the estrogen-stimulated proliferation of human breast cancer cells. J Biol Chem. 1990 Dec 5;265(34):21172–21178. [PubMed] [Google Scholar]
- Stiles C. D., Capone G. T., Scher C. D., Antoniades H. N., Van Wyk J. J., Pledger W. J. Dual control of cell growth by somatomedins and platelet-derived growth factor. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1279–1283. doi: 10.1073/pnas.76.3.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobe K., Matuoka K., Tamemoto H., Ueki K., Kaburagi Y., Asai S., Noguchi T., Matsuda M., Tanaka S., Hattori S. Insulin stimulates association of insulin receptor substrate-1 with the protein abundant Src homology/growth factor receptor-bound protein 2. J Biol Chem. 1993 May 25;268(15):11167–11171. [PubMed] [Google Scholar]
- Travali S., Reiss K., Ferber A., Petralia S., Mercer W. E., Calabretta B., Baserga R. Constitutively expressed c-myb abrogates the requirement for insulinlike growth factor 1 in 3T3 fibroblasts. Mol Cell Biol. 1991 Feb;11(2):731–736. doi: 10.1128/mcb.11.2.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsarfaty I., Rong S., Resau J. H., Rulong S., da Silva P. P., Vande Woude G. F. The Met proto-oncogene mesenchymal to epithelial cell conversion. Science. 1994 Jan 7;263(5143):98–101. doi: 10.1126/science.7505952. [DOI] [PubMed] [Google Scholar]
- Ullrich A., Gray A., Tam A. W., Yang-Feng T., Tsubokawa M., Collins C., Henzel W., Le Bon T., Kathuria S., Chen E. Insulin-like growth factor I receptor primary structure: comparison with insulin receptor suggests structural determinants that define functional specificity. EMBO J. 1986 Oct;5(10):2503–2512. doi: 10.1002/j.1460-2075.1986.tb04528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentinis B., Porcu P. L., Quinn K., Baserga R. The role of the insulin-like growth factor I receptor in the transformation by simian virus 40 T antigen. Oncogene. 1994 Mar;9(3):825–831. [PubMed] [Google Scholar]
- Velu T. J., Vass W. C., Lowy D. R., Beguinot L. Functional heterogeneity of proto-oncogene tyrosine kinases: the C terminus of the human epidermal growth factor receptor facilitates cell proliferation. Mol Cell Biol. 1989 Apr;9(4):1772–1778. doi: 10.1128/mcb.9.4.1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters S. B., Yamauchi K., Pessin J. E. Functional expression of insulin receptor substrate-1 is required for insulin-stimulated mitogenic signaling. J Biol Chem. 1993 Oct 25;268(30):22231–22234. [PubMed] [Google Scholar]
- Werner H., Re G. G., Drummond I. A., Sukhatme V. P., Rauscher F. J., 3rd, Sens D. A., Garvin A. J., LeRoith D., Roberts C. T., Jr Increased expression of the insulin-like growth factor I receptor gene, IGF1R, in Wilms tumor is correlated with modulation of IGF1R promoter activity by the WT1 Wilms tumor gene product. Proc Natl Acad Sci U S A. 1993 Jun 15;90(12):5828–5832. doi: 10.1073/pnas.90.12.5828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westermark B., Heldin C. H. Platelet-derived growth factor in autocrine transformation. Cancer Res. 1991 Oct 1;51(19):5087–5092. [PubMed] [Google Scholar]
- Yamasaki H., Prager D., Gebremedhin S., Melmed S. Human insulin-like growth factor I receptor 950tyrosine is required for somatotroph growth factor signal transduction. J Biol Chem. 1992 Oct 15;267(29):20953–20958. [PubMed] [Google Scholar]
- Yoshinouchi M., Baserga R. The role of the IGF-1 receptor in the stimulation of cells by short pulses of growth factors. Cell Prolif. 1993 Mar;26(2):139–146. doi: 10.1111/j.1365-2184.1993.tb00014.x. [DOI] [PubMed] [Google Scholar]
- de la Luna S., Soria I., Pulido D., Ortín J., Jiménez A. Efficient transformation of mammalian cells with constructs containing a puromycin-resistance marker. Gene. 1988;62(1):121–126. doi: 10.1016/0378-1119(88)90585-9. [DOI] [PubMed] [Google Scholar]



