Abstract
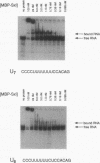
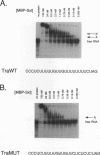
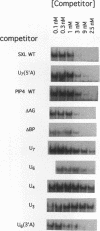
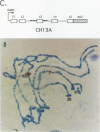
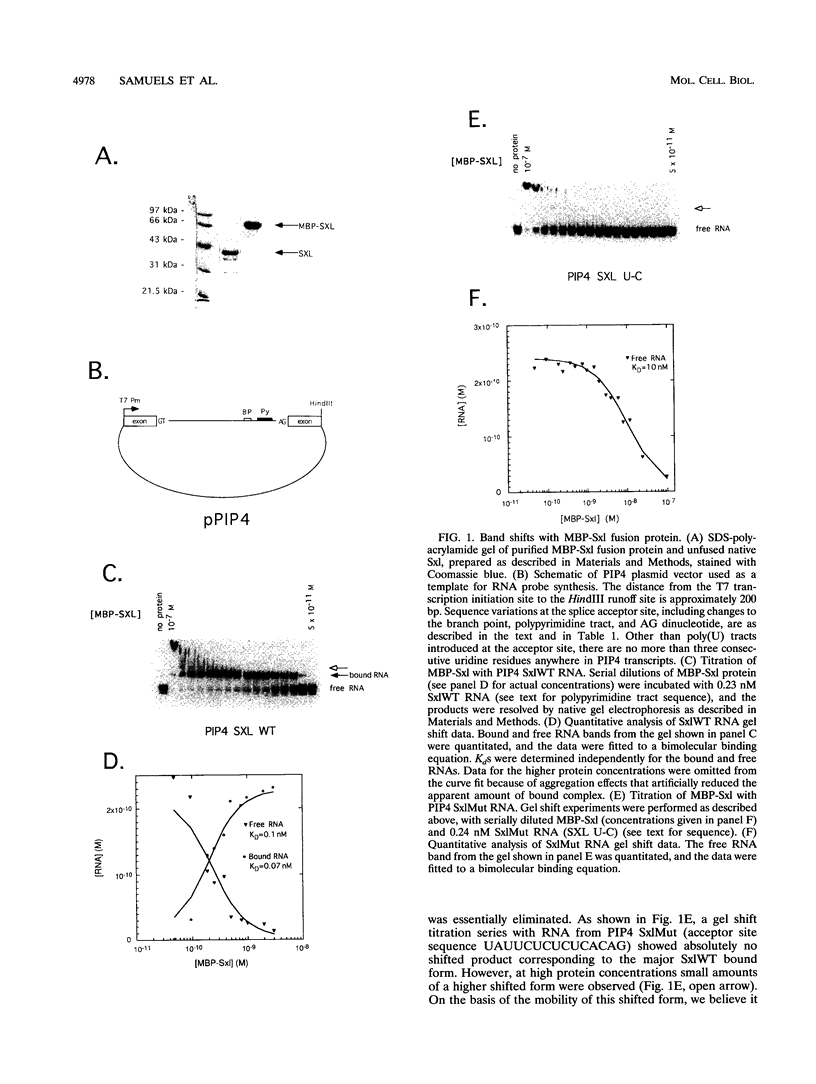
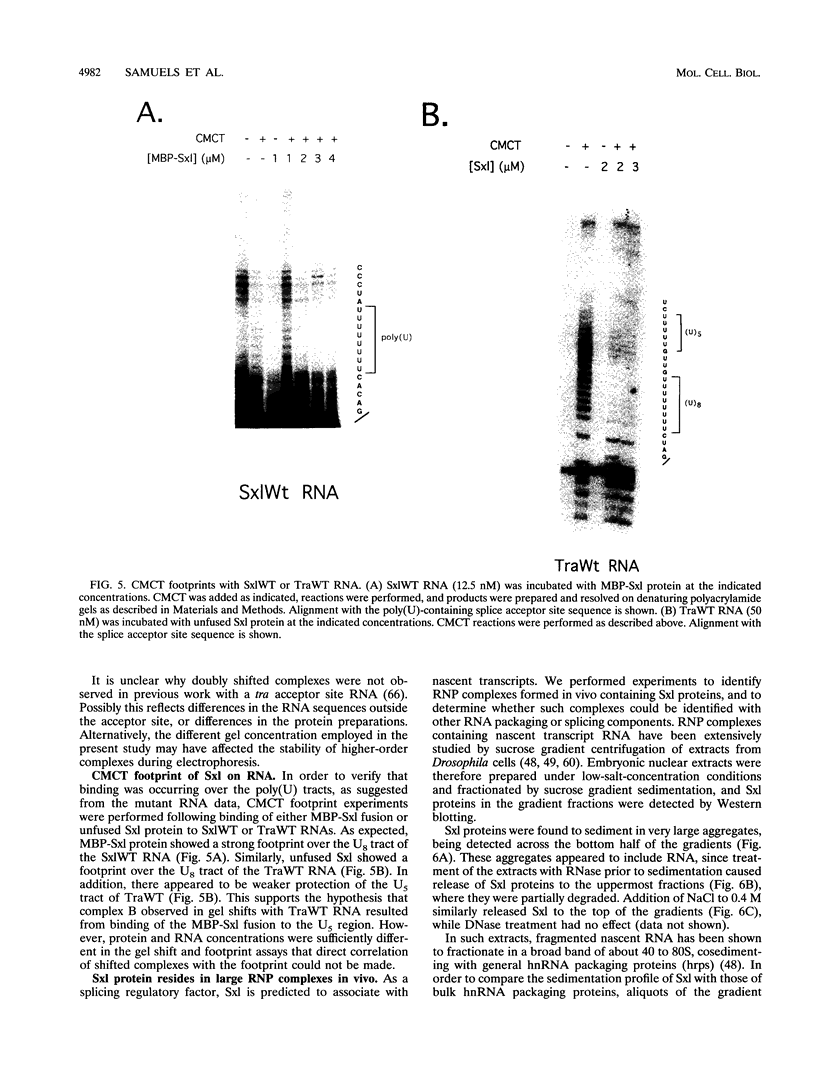
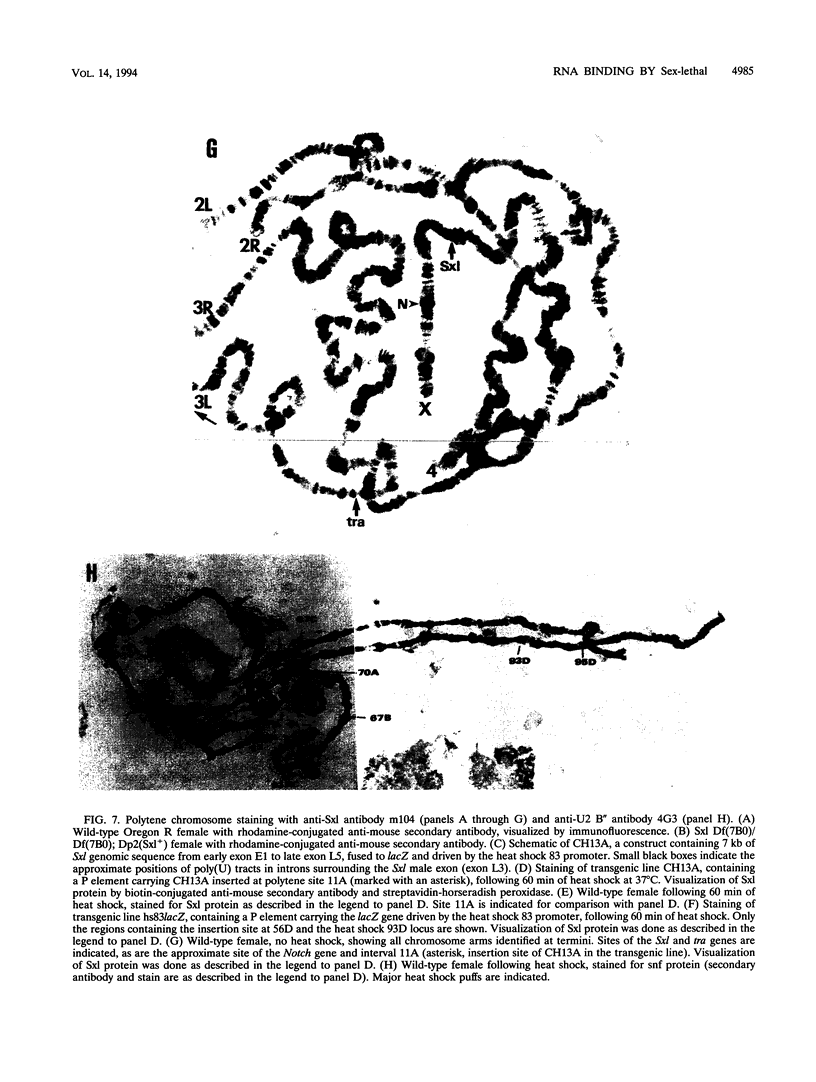
Sxl has been proposed to regulate splicing of specific target genes by directly interacting with their pre-mRNAs. We have therefore examined the RNA-binding properties of Sxl protein in vitro and in vivo. Gel shift and UV cross-linking assays with a purified recombinant MBP-Sxl fusion protein demonstrated preferential binding to RNAs containing poly(U) tracts, and the protein footprinted over the poly(U) region. The protein did not appear to recognize either branch point or AG dinucleotide sequences, but an adenosine residue at the 5' end of the poly(U) tract enhanced binding severalfold. MBP-Sxl formed two shifted complexes on a tra regulated acceptor site RNA; the doubly shifted form may have been stabilized by protein-protein interactions. Consistent with its proposed role in pre-mRNA processing, in nuclear extracts Sxl was found in large ribonucleoprotein (RNP) complexes which sedimented significantly faster than bulk heterogeneous nuclear RNP and small nuclear RNPs. Anti-Sxl staining of polytene chromosomes showed Sxl protein at a number of chromosomal locations, among which was the Sxl locus itself. Sxl protein could also be targeted to a new chromosomal site carrying a transgene containing splicing regulatory sequences from the Sxl gene, following transcriptional induction. After prolonged heat shock, all Sxl protein was restricted to the heat-induced puff at the hs93D locus. In contrast, a presumptive small nuclear RNP protein was observed at several heat puffs following shock.
Full text
PDF












Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amero S. A., Raychaudhuri G., Cass C. L., van Venrooij W. J., Habets W. J., Krainer A. R., Beyer A. L. Independent deposition of heterogeneous nuclear ribonucleoproteins and small nuclear ribonucleoprotein particles at sites of transcription. Proc Natl Acad Sci U S A. 1992 Sep 15;89(18):8409–8413. doi: 10.1073/pnas.89.18.8409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker B. S. Sex in flies: the splice of life. Nature. 1989 Aug 17;340(6234):521–524. doi: 10.1038/340521a0. [DOI] [PubMed] [Google Scholar]
- Bandziulis R. J., Swanson M. S., Dreyfuss G. RNA-binding proteins as developmental regulators. Genes Dev. 1989 Apr;3(4):431–437. doi: 10.1101/gad.3.4.431. [DOI] [PubMed] [Google Scholar]
- Bell L. R., Horabin J. I., Schedl P., Cline T. W. Positive autoregulation of sex-lethal by alternative splicing maintains the female determined state in Drosophila. Cell. 1991 Apr 19;65(2):229–239. doi: 10.1016/0092-8674(91)90157-t. [DOI] [PubMed] [Google Scholar]
- Bell L. R., Maine E. M., Schedl P., Cline T. W. Sex-lethal, a Drosophila sex determination switch gene, exhibits sex-specific RNA splicing and sequence similarity to RNA binding proteins. Cell. 1988 Dec 23;55(6):1037–1046. doi: 10.1016/0092-8674(88)90248-6. [DOI] [PubMed] [Google Scholar]
- Bentley R. C., Keene J. D. Recognition of U1 and U2 small nuclear RNAs can be altered by a 5-amino-acid segment in the U2 small nuclear ribonucleoprotein particle (snRNP) B" protein and through interactions with U2 snRNP-A' protein. Mol Cell Biol. 1991 Apr;11(4):1829–1839. doi: 10.1128/mcb.11.4.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boggs R. T., Gregor P., Idriss S., Belote J. M., McKeown M. Regulation of sexual differentiation in D. melanogaster via alternative splicing of RNA from the transformer gene. Cell. 1987 Aug 28;50(5):739–747. doi: 10.1016/0092-8674(87)90332-1. [DOI] [PubMed] [Google Scholar]
- Bopp D., Bell L. R., Cline T. W., Schedl P. Developmental distribution of female-specific Sex-lethal proteins in Drosophila melanogaster. Genes Dev. 1991 Mar;5(3):403–415. doi: 10.1101/gad.5.3.403. [DOI] [PubMed] [Google Scholar]
- Burd C. G., Matunis E. L., Dreyfuss G. The multiple RNA-binding domains of the mRNA poly(A)-binding protein have different RNA-binding activities. Mol Cell Biol. 1991 Jul;11(7):3419–3424. doi: 10.1128/mcb.11.7.3419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler B., Pirrotta V., Irminger-Finger I., Nöthiger R. The sex-determining gene tra of Drosophila: molecular cloning and transformation studies. EMBO J. 1986 Dec 20;5(13):3607–3613. doi: 10.1002/j.1460-2075.1986.tb04689.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey J. Gel retardation. Methods Enzymol. 1991;208:103–117. doi: 10.1016/0076-6879(91)08010-f. [DOI] [PubMed] [Google Scholar]
- Cline T. W. Autoregulatory functioning of a Drosophila gene product that establish es and maintains the sexually determined state. Genetics. 1984 Jun;107(2):231–277. doi: 10.1093/genetics/107.2.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobianchi F., Karpel R. L., Williams K. R., Notario V., Wilson S. H. Mammalian heterogeneous nuclear ribonucleoprotein complex protein A1. Large-scale overproduction in Escherichia coli and cooperative binding to single-stranded nucleic acids. J Biol Chem. 1988 Jan 15;263(2):1063–1071. [PubMed] [Google Scholar]
- Dangli A., Bautz E. K. Differential distribution of nonhistone proteins from polytene chromosomes of Drosophila melanogaster after heat shock. Chromosoma. 1983;88(3):201–207. doi: 10.1007/BF00285621. [DOI] [PubMed] [Google Scholar]
- Dangli A., Grond C., Kloetzel P., Bautz E. K. Heat-shock puff 93 D from Drosophila melanogaster: accumulation of a RNP-specific antigen associated with giant particles of possible storage function. EMBO J. 1983;2(10):1747–1751. doi: 10.1002/j.1460-2075.1983.tb01652.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flickinger T. W., Salz H. K. The Drosophila sex determination gene snf encodes a nuclear protein with sequence and functional similarity to the mammalian U1A snRNP protein. Genes Dev. 1994 Apr 15;8(8):914–925. doi: 10.1101/gad.8.8.914. [DOI] [PubMed] [Google Scholar]
- Garbe J. C., Bendena W. G., Pardue M. L. Sequence evolution of the Drosophila heat shock locus hsr omega. I. The nonrepeated portion of the gene. Genetics. 1989 Jun;122(2):403–415. doi: 10.1093/genetics/122.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbe J. C., Pardue M. L. Heat shock locus 93D of Drosophila melanogaster: a spliced RNA most strongly conserved in the intron sequence. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1812–1816. doi: 10.1073/pnas.83.6.1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill S. C., von Hippel P. H. Calculation of protein extinction coefficients from amino acid sequence data. Anal Biochem. 1989 Nov 1;182(2):319–326. doi: 10.1016/0003-2697(89)90602-7. [DOI] [PubMed] [Google Scholar]
- Gorman M., Kuroda M. I., Baker B. S. Regulation of the sex-specific binding of the maleless dosage compensation protein to the male X chromosome in Drosophila. Cell. 1993 Jan 15;72(1):39–49. doi: 10.1016/0092-8674(93)90048-u. [DOI] [PubMed] [Google Scholar]
- Govind S., Whalen A. M., Steward R. In vivo self-association of the Drosophila rel-protein dorsal. Proc Natl Acad Sci U S A. 1992 Sep 1;89(17):7861–7865. doi: 10.1073/pnas.89.17.7861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habets W. J., Hoet M. H., De Jong B. A., Van der Kemp A., Van Venrooij W. J. Mapping of B cell epitopes on small nuclear ribonucleoproteins that react with human autoantibodies as well as with experimentally-induced mouse monoclonal antibodies. J Immunol. 1989 Oct 15;143(8):2560–2566. [PubMed] [Google Scholar]
- Hodgkin J. Drosophila sex determination: a cascade of regulated splicing. Cell. 1989 Mar 24;56(6):905–906. doi: 10.1016/0092-8674(89)90619-3. [DOI] [PubMed] [Google Scholar]
- Horabin J. I., Schedl P. Regulated splicing of the Drosophila sex-lethal male exon involves a blockage mechanism. Mol Cell Biol. 1993 Mar;13(3):1408–1414. doi: 10.1128/mcb.13.3.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horabin J. I., Schedl P. Sex-lethal autoregulation requires multiple cis-acting elements upstream and downstream of the male exon and appears to depend largely on controlling the use of the male exon 5' splice site. Mol Cell Biol. 1993 Dec;13(12):7734–7746. doi: 10.1128/mcb.13.12.7734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue K., Hoshijima K., Sakamoto H., Shimura Y. Binding of the Drosophila sex-lethal gene product to the alternative splice site of transformer primary transcript. Nature. 1990 Mar 29;344(6265):461–463. doi: 10.1038/344461a0. [DOI] [PubMed] [Google Scholar]
- Kelley R. L. Initial organization of the Drosophila dorsoventral axis depends on an RNA-binding protein encoded by the squid gene. Genes Dev. 1993 Jun;7(6):948–960. doi: 10.1101/gad.7.6.948. [DOI] [PubMed] [Google Scholar]
- Maine E. M., Salz H. K., Schedl P., Cline T. W. Sex-lethal, a link between sex determination and sexual differentiation in Drosophila melanogaster. Cold Spring Harb Symp Quant Biol. 1985;50:595–604. doi: 10.1101/sqb.1985.050.01.072. [DOI] [PubMed] [Google Scholar]
- Matunis E. L., Matunis M. J., Dreyfuss G. Association of individual hnRNP proteins and snRNPs with nascent transcripts. J Cell Biol. 1993 Apr;121(2):219–228. doi: 10.1083/jcb.121.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matunis E. L., Matunis M. J., Dreyfuss G. Characterization of the major hnRNP proteins from Drosophila melanogaster. J Cell Biol. 1992 Jan;116(2):257–269. doi: 10.1083/jcb.116.2.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matunis M. J., Matunis E. L., Dreyfuss G. Isolation of hnRNP complexes from Drosophila melanogaster. J Cell Biol. 1992 Jan;116(2):245–255. doi: 10.1083/jcb.116.2.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeown M., Belote J. M., Boggs R. T. Ectopic expression of the female transformer gene product leads to female differentiation of chromosomally male Drosophila. Cell. 1988 Jun 17;53(6):887–895. doi: 10.1016/s0092-8674(88)90369-8. [DOI] [PubMed] [Google Scholar]
- McKeown M. Sex differentiation: the role of alternative splicing. Curr Opin Genet Dev. 1992 Apr;2(2):299–303. doi: 10.1016/s0959-437x(05)80288-6. [DOI] [PubMed] [Google Scholar]
- Moazed D., Stern S., Noller H. F. Rapid chemical probing of conformation in 16 S ribosomal RNA and 30 S ribosomal subunits using primer extension. J Mol Biol. 1986 Feb 5;187(3):399–416. doi: 10.1016/0022-2836(86)90441-9. [DOI] [PubMed] [Google Scholar]
- Nagai K., Thøgersen H. C. Synthesis and sequence-specific proteolysis of hybrid proteins produced in Escherichia coli. Methods Enzymol. 1987;153:461–481. doi: 10.1016/0076-6879(87)53072-5. [DOI] [PubMed] [Google Scholar]
- Nagoshi R. N., McKeown M., Burtis K. C., Belote J. M., Baker B. S. The control of alternative splicing at genes regulating sexual differentiation in D. melanogaster. Cell. 1988 Apr 22;53(2):229–236. doi: 10.1016/0092-8674(88)90384-4. [DOI] [PubMed] [Google Scholar]
- O'Neil M. T., Belote J. M. Interspecific comparison of the transformer gene of Drosophila reveals an unusually high degree of evolutionary divergence. Genetics. 1992 May;131(1):113–128. doi: 10.1093/genetics/131.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton J. G., Porro E. B., Galceran J., Tempst P., Nadal-Ginard B. Cloning and characterization of PSF, a novel pre-mRNA splicing factor. Genes Dev. 1993 Mar;7(3):393–406. doi: 10.1101/gad.7.3.393. [DOI] [PubMed] [Google Scholar]
- Rastelli L., Chan C. S., Pirrotta V. Related chromosome binding sites for zeste, suppressors of zeste and Polycomb group proteins in Drosophila and their dependence on Enhancer of zeste function. EMBO J. 1993 Apr;12(4):1513–1522. doi: 10.1002/j.1460-2075.1993.tb05795.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raychaudhuri G., Haynes S. R., Beyer A. L. Heterogeneous nuclear ribonucleoprotein complexes and proteins in Drosophila melanogaster. Mol Cell Biol. 1992 Feb;12(2):847–855. doi: 10.1128/mcb.12.2.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risau W., Symmons P., Saumweber H., Frasch M. Nonpackaging and packaging proteins of hnRNA in Drosophila melanogaster. Cell. 1983 Jun;33(2):529–541. doi: 10.1016/0092-8674(83)90434-8. [DOI] [PubMed] [Google Scholar]
- Roscigno R. F., Weiner M., Garcia-Blanco M. A. A mutational analysis of the polypyrimidine tract of introns. Effects of sequence differences in pyrimidine tracts on splicing. J Biol Chem. 1993 May 25;268(15):11222–11229. [PubMed] [Google Scholar]
- Ryseck R. P., Walldorf U., Hoffmann T., Hovemann B. Heat shock loci 93D of Drosophila melanogaster and 48B of Drosophila hydei exhibit a common structural and transcriptional pattern. Nucleic Acids Res. 1987 Apr 24;15(8):3317–3333. doi: 10.1093/nar/15.8.3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto H., Inoue K., Higuchi I., Ono Y., Shimura Y. Control of Drosophila Sex-lethal pre-mRNA splicing by its own female-specific product. Nucleic Acids Res. 1992 Nov 11;20(21):5533–5540. doi: 10.1093/nar/20.21.5533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salz H. K., Cline T. W., Schedl P. Functional changes associated with structural alterations induced by mobilization of a P element inserted in the Sex-lethal gene of Drosophila. Genetics. 1987 Oct;117(2):221–231. doi: 10.1093/genetics/117.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salz H. K., Maine E. M., Keyes L. N., Samuels M. E., Cline T. W., Schedl P. The Drosophila female-specific sex-determination gene, Sex-lethal, has stage-, tissue-, and sex-specific RNAs suggesting multiple modes of regulation. Genes Dev. 1989 May;3(5):708–719. doi: 10.1101/gad.3.5.708. [DOI] [PubMed] [Google Scholar]
- Samuels M. E., Schedl P., Cline T. W. The complex set of late transcripts from the Drosophila sex determination gene sex-lethal encodes multiple related polypeptides. Mol Cell Biol. 1991 Jul;11(7):3584–3602. doi: 10.1128/mcb.11.7.3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherly D., Boelens W., Dathan N. A., van Venrooij W. J., Mattaj I. W. Major determinants of the specificity of interaction between small nuclear ribonucleoproteins U1A and U2B'' and their cognate RNAs. Nature. 1990 Jun 7;345(6275):502–506. doi: 10.1038/345502a0. [DOI] [PubMed] [Google Scholar]
- Schuldt C., Kloetzel P. M., Bautz E. K. Molecular organization of RNP complexes containing P11 antigen in heat-shocked and non-heat-shocked Drosophila cells. Eur J Biochem. 1989 Apr 15;181(1):135–142. doi: 10.1111/j.1432-1033.1989.tb14704.x. [DOI] [PubMed] [Google Scholar]
- Soeller W. C., Poole S. J., Kornberg T. In vitro transcription of the Drosophila engrailed gene. Genes Dev. 1988 Jan;2(1):68–81. doi: 10.1101/gad.2.1.68. [DOI] [PubMed] [Google Scholar]
- Sosnowski B. A., Belote J. M., McKeown M. Sex-specific alternative splicing of RNA from the transformer gene results from sequence-dependent splice site blockage. Cell. 1989 Aug 11;58(3):449–459. doi: 10.1016/0092-8674(89)90426-1. [DOI] [PubMed] [Google Scholar]
- Steinmann-Zwicky M., Amrein H., Nöthiger R. Genetic control of sex determination in Drosophila. Adv Genet. 1990;27:189–237. doi: 10.1016/s0065-2660(08)60026-7. [DOI] [PubMed] [Google Scholar]
- Studier F. W., Rosenberg A. H., Dunn J. J., Dubendorff J. W. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 1990;185:60–89. doi: 10.1016/0076-6879(90)85008-c. [DOI] [PubMed] [Google Scholar]
- Swanson M. S., Dreyfuss G. Classification and purification of proteins of heterogeneous nuclear ribonucleoprotein particles by RNA-binding specificities. Mol Cell Biol. 1988 May;8(5):2237–2241. doi: 10.1128/mcb.8.5.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valcárcel J., Singh R., Zamore P. D., Green M. R. The protein Sex-lethal antagonizes the splicing factor U2AF to regulate alternative splicing of transformer pre-mRNA. Nature. 1993 Mar 11;362(6416):171–175. doi: 10.1038/362171a0. [DOI] [PubMed] [Google Scholar]
- Wharton K. A., Johansen K. M., Xu T., Artavanis-Tsakonas S. Nucleotide sequence from the neurogenic locus notch implies a gene product that shares homology with proteins containing EGF-like repeats. Cell. 1985 Dec;43(3 Pt 2):567–581. doi: 10.1016/0092-8674(85)90229-6. [DOI] [PubMed] [Google Scholar]
- Zamore P. D., Green M. R. Identification, purification, and biochemical characterization of U2 small nuclear ribonucleoprotein auxiliary factor. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9243–9247. doi: 10.1073/pnas.86.23.9243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamore P. D., Patton J. G., Green M. R. Cloning and domain structure of the mammalian splicing factor U2AF. Nature. 1992 Feb 13;355(6361):609–614. doi: 10.1038/355609a0. [DOI] [PubMed] [Google Scholar]
- Zink B., Paro R. In vivo binding pattern of a trans-regulator of homoeotic genes in Drosophila melanogaster. Nature. 1989 Feb 2;337(6206):468–471. doi: 10.1038/337468a0. [DOI] [PubMed] [Google Scholar]
- di Guan C., Li P., Riggs P. D., Inouye H. Vectors that facilitate the expression and purification of foreign peptides in Escherichia coli by fusion to maltose-binding protein. Gene. 1988 Jul 15;67(1):21–30. doi: 10.1016/0378-1119(88)90004-2. [DOI] [PubMed] [Google Scholar]