Abstract
ATP-binding cassette protein G1 (ABCG1) is important for the formation of HDL. However, the biochemical properties of ABCG1 have not been reported, and the mechanism of how ABCG1 is involved in HDL formation remains unclear. We established a procedure to express and purify human ABCG1 using the suspension-adapted human cell FreeStyle293-F. ABCG1, fused at the C terminus with green fluorescent protein and Flag-peptide, was solubilized with n-dodecyl-β-D-maltoside and purified via a single round of Flag-M2 antibody affinity chromatography. The purified ABCG1 was reconstituted in liposome of various lipid compositions, and the ATPase activity was analyzed. ABCG1 reconstituted in egg lecithin showed ATPase activity (150 nmol/min/mg), which was inhibited by beryllium fluoride. The ATPase activity of ABCG1, reconstituted in phosphatidylserine liposome, was stimulated by cholesterol and choline phospholipids (especially sphingomyelin), and the affinity for cholesterol was increased by the addition of sphingomyelin. These results suggest that ABCG1 is an active lipid transporter and possesses different binding sites for cholesterol and sphingomyelin, which may be synergistically coupled.
Keywords: ABC transporter, cholesterol homeostasis, high density lipoprotein
Cholesterol is an important component of cellular membranes and a source of various steroid hormones. However, excess cholesterol is toxic for cells and becomes a risk factor for atherosclerosis. Therefore, cholesterol level is strictly regulated by synthesis, intake, storage as an esterified form, and efflux. The generation of HDL is the only pathway to remove excess cholesterol from peripheral tissues and thus protect from atherosclerosis (1, 2).
Two ATP binding cassette (ABC) transporters are involved in HDL generation. ABCA1 transports free cholesterol and phosphatidylcholine to lipid-free apolipoprotein A-1 and generates lipid-poor preHDL. ABCG1 is predicted to further transfer free cholesterol to lipid-poor HDL (3, 4). Indeed, mice lacking ABCG1 accumulate lipids in macrophages and hepatocytes when fed a high-fat and a high-cholesterol diet (5). Moreover, the knockout of both ABCA1 and ABCG1 in mice results in dramatic foam cell formation and the acceleration of atherosclerosis (6). These data suggest that ABCG1 plays a critical role in cellular cholesterol homeostasis.
We have reported that ABCG1 mediates the efflux of cholesterol and choline phospholipids, especially sphingomyelin (SM) (7), and that cholesterol efflux by ABCG1 is dependent on the cellular SM level (8). However, it is unclear whether ABCG1 selectively transports cholesterol and SM. Furthermore, the biochemical analysis of ABCG1 with the purified protein have not been reported, although Cserepes et al. (9) reported some biochemical properties by using crude membranes from insect cells. To address these issues, we established a procedure to purify ABCG1 and then analyzed its ATPase activity. Our results suggest that ABCG1 is an active lipid transporter and possesses synergistically coupled binding sites for cholesterol and SM.
MATERIALS AND METHODS
Materials
L-α-lecithin from egg yolk, cholesterol, Na2ATP, phosphatidylserine (PS), FLAG M2-agarose and FLAG peptide (Sigma-Aldrich); FreeStyle 293-F cells, FreeStyle 293 expression medium, and OPTI-MEM1 (Invitrogen); phosphatidylcholine (PC) and SM (Avanti Polar Lipids); phosphatidylethanolamine (PE) (Wako Pure Chemical Industries Ltd); anti-ABCG1 rabbit polyclonal antibody (H65) (Santa Cruz Biotechnology); and protease inhibitor (Roche Applied Science) were obtained.
Cell culture
Suspension-adapted HEK293 cells (FreeStyle293-F) were cultivated in 293 expression medium containing 25 μg/ml of gentamicin to avoid contamination of microorganisms. Cells were maintained in 100 ml Erlenmeyer flasks and shaken at 130 rpm in an atmosphere of 8% CO2; they were passaged every 2 days. The cell density was maintained at between 5 × 105 and 2 × 106 cells/ml. Large-scale culture was performed in a 3-l spinner flask with the cell culture controller Cellmaster Model 1700 (Wakenyaku). The temperature, dissolved oxygen, and agitation speed were maintained at 37°C, 3.0 ppm, and 100 rpm, respectively. One day before transfection, cells were seeded at a cell density of 0.5 × 106 cells/ml, resulting in a cell density of 1.0 × 106 cells/ml and log-phase growth on the day of transfection.
Transfection vector and reagent
The human ABCG1 cDNA (7) was fused at the C terminus with tandemly repeated (eight times) glycine-threonine-serine sequence, green fluorescent protein (GFP), FLAG tag, and 10 histidine residues in pcDNA3.1. A stock solution (1 mg/ml) of polyethyleneimine “MAX” (Polysciences) was prepared in H2O, sterilized by filtration (0.2 μm), and stored at 4°C. For the transfection in the large-scale culture, polyethyleneimine (7.5 mg) and DNA (3 mg) were separately dissolved in 150 ml of OPTI-MEM1 and then mixed and incubated for 15 min before being added to the cell culture. The cells were harvested 48 h after transfection.
Cellular lipid release assay
Cells were subcultured in 100 ml Erlenmeyer flasks at a cell density of 0.5 × 106 cells/ml and shaken at 130 rpm. After incubation for 24 h, the cells were transfected as described above. After another 24 h, the cells were incubated with 293 expression medium containing 0.02% BSA and 20 μg/ml HDL. The lipid content in the medium was determined after 24 h of incubation by fluorescence enzyme assay (10). HDL cholesterol in the media of no cell control was subtracted.
Crude membrane preparation
Cells were resuspended in buffer A containing 50 mM Tris-HCl (pH 7.5), 10% (v/v) glycerol, 150 mM NaCl, 1 mM EDTA, 1 mM 2-mercaptoethanol, and protease inhibitors (complete EDTA-free; Roche Applied Science). The cell suspension was sonicated for 2 min (output 7, 24 rounds of sonication for 5 s at an interval of 30 s) with a probe sonicator (Misonix Inc.) and centrifuged at 1,500 g for 10 min to remove unbroken cells and nuclei. The supernatant was treated with 0.5 M NaCl to remove the peripherally anchored proteins and centrifuged at 45,000 g for 100 min. The crude pellet was resuspended in ice-cold buffer A. The crude membrane suspension was passed through a homogenizer and stored at −80°C.
Fluorescence size exclusion chromatography analysis
Crude membranes were resuspended in buffer (50 mM Tris [pH 7.4], 150 mM NaCl, 10% glycerol, and protease inhibitor) and solubilized with detergents at 4°C. Insoluble materials were removed by centrifugation (15,000 g for 10 min), and the supernatant was loaded onto a Superose 6 column (10/300; Amersham Biosciences) pre-equilibrated with SEC buffer (50 mM Tris [pH 7.5], 150 mM NaCl, 10% glycerol, 2 mM dithiothreitol, and 0.03% C12E8). GFP fluorescence was detected with an in-line fluorescence detector (Ex 480 nm/Em 510 nm).
Protein purification
All purification steps were performed at 0–4°C. The crude membranes resuspended in buffer A containing 0.8% n-dodecyl-β-D-maltoside (DDM) (Anatrace) and protease inhibitors were kept on ice for 30 min with occasional gentle mixing. The insoluble materials were removed by centrifugation (45,000 g, 30 min). The solubilized proteins were incubated with FLAG M2-agarose beads (Sigma-Aldrich) for 12 h at 4°C. The beads were washed four times with 10× bed volume of buffer A containing 0.8% DDM and then washed three times with 10× bed volume of buffer A containing 0.05% DDM. Proteins were eluted with 10× bed volume of buffer A containing 0.05% DDM and 150 μg/ml of 1× and 3× FLAG peptides. The eluate was concentrated by ultrafiltration (100 kDa cut-off PES membrane; Sartorius Stedim Biotech) to 0.1–0.3 mg/ml protein.
Reconstitution of purified ABCG1 into liposome
Lipids dissolved in chloroform and dried by evaporation were resuspended in reaction buffer (50 mM Tris-HCl [pH 7.5], 0.1 mM EGTA) at 10 mg/ml. The lipid suspension was flash-frozen in liquid N2 and slowly thawed at room temperature. This step was repeated five times to make large multilamellar vesicles. The vesicles were extruded 15 times through a 200-nm polycarbonate filter to form large unilamellar vesicles. To a 10 μl aliquot of vesicles, 75 μl of the reaction buffer, 10 μl of 100 mM dithiothreitol, and 5 μl of 0.1% DDM were sequentially added to destabilize the liposomes. Purified protein (1 μg) was added to 0.1 ml of detergent-destabilized liposomes (11), and the mixture was incubated under gentle agitation for 30 min. To remove the detergent, a 50% slurry of Bio-Beads was added in a stepwise fashion: 50 μl for 30 min, 50 μl for 30 min, and 50 μl for 40 min. After reconstitution, protein concentrations were determined by measuring the GFP fluorescence.
Measurement of ATPase activity
The ATPase reaction was performed as reported previously (12) with minor modifications. Reconstituted protein was incubated in 20 μl of reaction buffer (50 mM Tris/HCl [pH 7.5], 0.1 mM EGTA, 3 mM Na2ATP, 5 mM MgCl2) at 37°C for 10 min. The reaction was stopped by adding 20 μl of 10 mM EDTA. To analyze the effect of methyl-β-cyclodextrin (MβCD)-conjugated cholesterol on ATPase activity, the Michaelis–Menten equation was computer-fitted to the experimental data:
where Vcmax is the enhanced activity, V0 is the basal activity, and [S] is the concentration of MβCD-conjugated cholesterol,. Fitting was carried out using KaleidaGraph software, and values for V0, Vcmax, and Km were extracted. Vmax values included the basal activity and the Vcmax value.
Preparation of the cholesterol-MβCD complex
Cholesterol dissolved in ethanol was added to MβCD solution at 80°C, and the mixture was stirred until the initially precipitated cholesterol was completely dissolved. The solution (1 mM cholesterol, 10 mM MβCD) was stored at room temperature. Proteoliposome was mixed with cholesterol-MβCD complex at room temperature and incubated for 10 min. The cholesterol content in liposomes was determined using a fluorescence enzyme assay (10).
Statistical analysis
Experiments were performed in triplicate, and the data are presented as means ± SD. The statistical significance between mean values was determined by unpaired t-test. A P value less than 0.05 was considered statistically significant.
RESULTS
Expression of human ABCG1 in FreeStyle 293-F cells
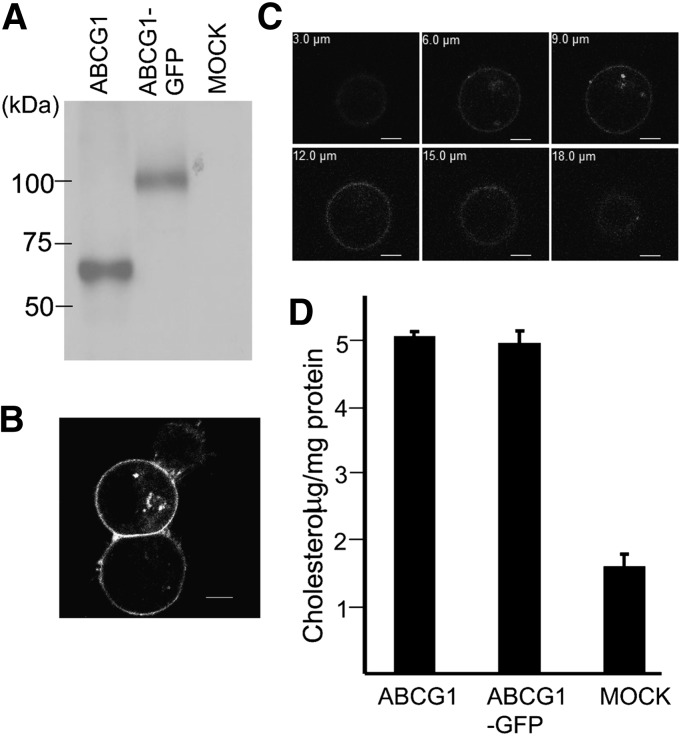
Human ABCG1 was fused at the C terminus with GFP and Flag-peptide (ABCG1-GFP) for the purification and stability screening. ABCG1-GFP was expressed in FreeStyle 293-F cells at a level comparable to ABCG1 (Fig. 1A). Similar to wild-type ABCG1 expressed in HEK293 cells, ABCG1-GFP was localized mainly at the plasma membrane and some in the vesicles (7) (Fig. 1B, C). We found that HDL-dependent cholesterol efflux by ABCG1-GFP was also comparable to that by ABCG1 (Fig. 1D). These results suggest that ABCG1-GFP is as active as ABCG1 and that FreeStyle 293-F cells can be used as a host for expressing ABCG1.
Fig. 1.
Subcellular localization and cholesterol efflux activity of ABCG1-GFP. A: Western blot of ABCG1 and ABCG1-GFP expressed in FreeStyle 293-F cells. B: Subcellular localization of ABCG1-GFP in FreeStyle 293-F cells. ABCG1-GFP was visualized by confocal fluorescence microscopy (Ex 488 nm/Em 505 nm). C: Z-stack images of a cell expressing ABCG1-GFP. D: Cholesterol efflux activity of ABCG1 and ABCG1-GFP. ABCG1-GFP was transiently expressed in FreeStyle 293-F cells, and cholesterol efflux was analyzed in the presence of HDL. Experiments were done twice independently (different transfection), and similar results were obtained.
Optimal detergent for purification
Selection of the optimal detergent is important for purifying membrane proteins to maintain activity during purification procedures (13, 14). To examine the stability of ABCG1-GFP in detergents, we analyzed the molecular size and monodispersity by fluorescence size exclusion chromatography (FSEC) analysis (15). A crude membrane was solubilized with various detergents, and the proteins were separated by gel filtration (Fig. 2). ABCG1-GFP was solubilized by most detergents examined and mainly eluted at around 280 kDa with a symmetrical peak shape. The molecular weight was consistent with the estimated one of the ABCG1-GFP dimer (200 kDa) containing detergent micelles, suggesting that ABCG1-GFP kept a stable dimer complex in most detergents examined. The smaller peaks were GFP cleaved from the fusion protein and endogenous fluorescent proteins in FreeStyle 293-F cells (supplementary Fig. I). Among the examined detergents, DDM and Fos-choline-14 showed the highest efficacy in solubilizing ABCG1-GFP.
Fig. 2.
Screening of optimal detergent for solubilizing ABCG1. A crude membrane containing ABCG1-GFP was solubilized with 1% n-dodecyl-β-D-maltoside (DDM), Fos-choline-14 (FC14), 6-cyclohexyl-1-hexyl-β-d-maltoside (CYMAL6), C12E8, lauryldimethylamine-N-oxide (LDAO), TritonX-100, Tween20, 2% 3-[(3-cholamidopropyl)-dimethylammonio]-propane sulfonate (CHAPS), or n-octyl-β-D-glucoside (OG) and separated by a Superose 6 10/300 GL column. Column void volume is indicated by V0. Molecular weight of the standards are thyroglobulin (bovine), 670,000; γ-globulin (bovine), 158,000; ovalbumin (chicken), 44,000; and myoglobin (horse), 17,000.
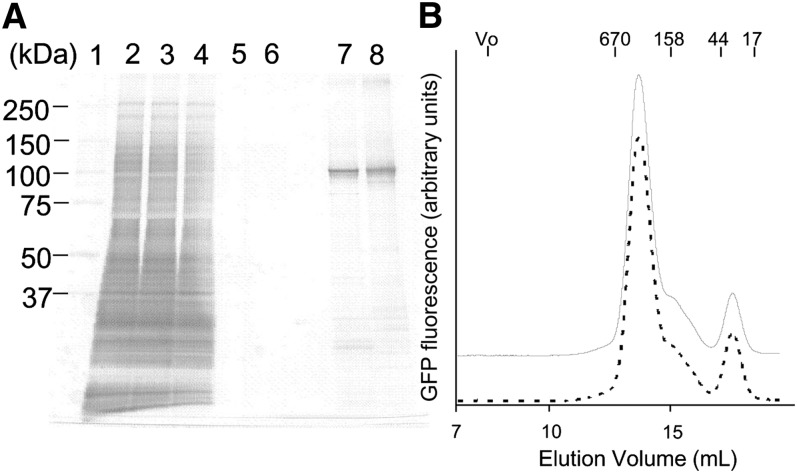
Purification of ABCG1
ABCG1-GFP and ABCG1(KM)-GFP, in which a lysine residue critical for ATP hydrolysis was replaced with methionine, were expressed in FreeStyle 293-F cells in large-scale culture using a bioreactor. Crude membranes were solubilized with DDM (Fig. 3) or Fos-choline-14 (supplementary Fig. II), and ABCG1 was purified with a single round of Flag-M2 antibody affinity chromatography. Judging from the silver-stained SDS-PAGE gel, the purity of purified proteins was estimated between 80 to 90% (Fig. 3A). To assess the oligomeric state, purified ABCG1-GFP and ABCG1(KM)-GFP were analyzed by FSEC (Fig. 3B). The main peak was eluted at 280 kDa, and a small shoulder was observed at around 163 kDa. A small peak was also observed at 47 kDa, which was likely a cleaved GFP moiety. The elution profile of ABCG1(KM)-GFP was similar to that of the wild-type, suggesting that a major fraction of the purified protein kept the dimer structure while a fraction was degraded. The elution pattern of purified ABCG1-GFP solubilized with Fos-choline-14 was similar to that with DDM (supplementary Fig. II).
Fig. 3.
Purification of human ABCG1 expressed in FreeStyle 293-F cells. A: Silver staining of SDS/PAGE (5–20% gradient gel). Lane 1, size marker; lane 2, crude membranes; lane 3, crude membrane proteins solubilized with 0.8% DDM; lane 4, unbound proteins to FLAG agarose; lane 5, washout proteins with 0.8% DDM; lane 6, washout proteins with 0.05% DDM; lane 7, purified ABCG1-GFP (0.3 μg); lane 8, purified ABCG1(KM)-GFP (0.3 μg). Equal volumes of sample were loaded in lanes 2–6. B: FSEC analysis of the purified ABCG1-GFP (solid line) and ABCG1(KM)-GFP (dotted line).
Enzymatic parameters of the ATPase activity of purified ABCG1
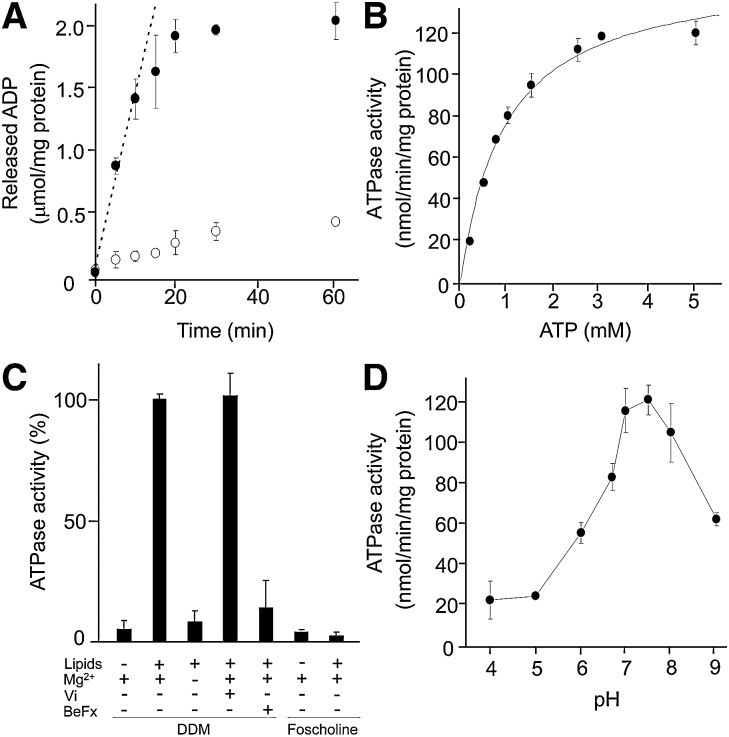
Purified ABCG1-GFP was reconstituted into egg lecithin liposome, and the kinetic parameters of ATP hydrolysis reaction were examined. ABCG1-GFP showed ATPase activity and the amount of released ADP linearly increased within approximately 10 min (Fig. 4A). Therefore, the ATPase activity was analyzed at 10 min in the following experiments. The replacement of the lysine residue in Walker A motif with methionine largely impaired the ATPase activity. The ATPase activity of ABCG1-GFP followed Michaelis–Menten kinetics, and Km value and maximum velocity were calculated as 0.95 ± 0.12 mM and 150 ± 6.9 nmol/min/mg, respectively (Fig. 4B). Without reconstitution ABCG1-GFP did not show ATPase activity (Fig. 4C), suggesting that the lipid bilayer environment is important for the function of ABCG1 as observed with MDR1 (16, 17). Interestingly, ABCG1-GFP purified with Fos-choline-14 did not show ATPase activity even after reconstitution (Fig. 4C), although it was purified as a dimer (supplementary Fig. II).
Fig. 4.
ATPase activity of purified ABCG1. A: Time-dependence of the ATPase reaction of purified ABCG1-GFP. Purified ABCG1-GFP (closed circles) or ABCG1(KM)-GFP (open circles) was reconstituted in egg lecithin liposomes and mixed with 3 mM ATP and 5 mM MgCl2. After incubation at 37°C for the indicated time, the reaction was stopped by the addition of EDTA, and the amounts of released ADP were quantified. B: ATP dependence of the ATPase reaction of the purified ABCG1-GFP. Reconstituted ABCG1-GFP was incubated at 37°C for 10 min with varied concentrations of ATP in the presence of a 2 mM excess of MgCl2 over the concentration of ATP. The Michaelis–Menten equation was fitted to the experimental data. C: ATPase activity of DDM-purified and Fos-Choline-14-purified ABCG1-GFP before and after reconstitution and the effects of phosphate analogs and magnesium ion. Detergent solubilized or reconstituted ABCG1-GFP was incubated with 3 mM ATP and 5 mM MgCl2 at 37°C for 10 min in the absence or presence of 3 mM ortho-vanadate (Vi) or beryllium fluoride (BeFx). D: pH dependence of the ATPase activity in 50 mM GTA buffer (16.7 mM 3,3-dimethyl-glutaric acid, 16.7 mM Tris [hydroxymethyl] aminomethane, and 16.7 mM 2-amino-2-methyl-1,3-propanediol) containing 0.1 mM EGTA, 3 mM ATP, and 5 mM MgCl2. The reactions were carried at 37°C for 10 min.
The effects of phosphate analogs on ATPase activity
Typical ABC transporters release γ phosphate before ADP after ATP hydrolysis and form a stable catalytically inactive complex when excess phosphate analogs are present (18). To analyze the mode of ATP hydrolysis of ABCG1, ATPase activity was measured in the presence of 3 mM ortho-vanadate or beryllium fluoride (Fig. 4C). Beryllium fluoride effectively inhibited the ATPase activity, whereas ortho-vanadate had no inhibitory effect.
pH dependency of the ATPase activity
The pH dependency of ATPase activity was analyzed in GTA buffer with increasing pH (Fig. 4D). The ATPase activity of ABCG1-GFP increased sharply with increasing pH. The maximal activity was observed at pH 7.5, and then the activity decreased sharply with increasing pH.
The effect of various lipids on the ATPase activity
We previously reported that cholesterol and phospholipids, especially SM, were exported from ABCG1-expressed cells to the medium (7). Because the ATPase activities of transporters are generally stimulated by its transport substrates, we analyzed the ATPase activity of purified ABCG1 reconstituted in PS liposomes containing various concentrations of cholesterol, SM, PC, or PE (Fig. 5A). The ATPase activity reconstituted in PS liposomes was quite low (31.9 ± 5.8 nmol/min/mg) compared with that in egg lecithin (Fig. 4B). ABCG1 showed higher ATPase activity in liposomes containing cholesterol, SM (18:0), or 1,2-distearoyl-sn-glycero-3-phosphocholine (DSPC) (18:0-18:0), and it increased in a dose-dependent manner, whereas 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine showed no effect (Fig. 5A). The half maximal values (in molar ratios) for cholesterol, SM, and DSPC were 9.5%, 8.3%, and 14%, respectively. These results suggest that ABCG1 selectively interacts with cholesterol and phospholipids with the choline head group and that the affinity for SM is about 1.7-fold higher than that for PC.
Fig. 5.
Lipid dependence of ATPase activity. A: ATPase activity of ABCG1-GFP reconstituted in PS liposomes containing various amounts of cholesterol (closed diamonds), 18:0 SM (triangles), DSPC (squares), or 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine DOPE (open circles). B: The effects of acyl chain structures. ABCG1-GFP was reconstituted in PS liposomes containing 30% PC of various acyl chains. *P < 0.05 as compared with PC (18:0, 18:0) containing PS liposome. C: Synergistic effect of sphingomyelin and cholesterol. Purified ABCG1-GFP was reconstituted in a PS liposome (open diamonds) containing 2.5% 18:0 SM (closed circles) or 5% DSPC (squares). Various concentrations of MβCD-conjugated cholesterol were added. We confirmed that cholesterol was equally incorporated into liposomes and increased linearly with the increasing concentrations of MβCD-cholesterol. Reactions were done in the presence of 3 mM ATP and 5 mM MgCl2 at 37°C.
The effect of acyl chain structures on the ATPase activity
To determine which moiety of phospholipids affect the ATPase activity, ABCG1 was reconstituted in PS liposomes containing 30% PC with different acyl chain structures (Fig. 5B). ABCG1 showed equally high ATPase activity in liposomes containing 1,2-dioleoyl-sn-glycero-3-phosphocholine (18:1–18:1), DSPC (18:0–18:0), and 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (16:0–18:1), whereas it showed lower activity in the 1,2-dimyristoyl-sn-glycero-3-phosphocholine (14:0–14:0) containing liposome. These results suggest that the acyl chain should be longer than 16, but the structure (saturated or unsaturated) of acyl chains does not affect the ATPase activity.
The synergistic stimulation of ATPase activity by SM and cholesterol
It has been reported that several ABC transporters possess multiple substrate-binding sites (19–21). To examine whether ABCG1 has different binding sites for cholesterol and SM and whether their binding to ABCG1 affects each other, ABCG1-GFP was reconstituted into a PS liposome containing 2.5% SM or 5% PC, and different concentrations of cholesterol were added in the MβCD-conjugate form (Fig. 5C). Cholesterol added to the PS liposome stimulated ATPase activity in a dose-dependent manner, and the Km for cholesterol and Vmax of ATPase were calculated as 0.10 ± 0.01 mM and 155.2 ± 12.6 nmol/min/mg, respectively. The ATPase activities of ABCG1 reconstituted in PS liposomes containing 2.5% SM or 5% PC were similar. When cholesterol was added to the PC-containing liposome, the Km for cholesterol and Vmax of ATPase were 0.10 ± 0.01 mM and 147.6 ± 4.5 nmol/min/mg, respectively. Interestingly, in the SM-containing liposome, the Km values for cholesterol were shifted to a lower concentration at 0.06 ± 0.01 mM, whereas the Vmax of ATPase was not affected and was 153.3 ± 1.9 nmol/min/mg.
DISCUSSION
In this study, we analyzed the biochemical properties of human ABCG1 via the purified protein. To purify human ABCG1, we used FreeStyle 293-F suspension culture-adapted cells as a host because they can be cultured in large scale via a bioreactor. We also expected that human cells would be best suited for expressing human membrane proteins. GFP and Flag-tag were fused at the C terminus of ABCG1 for the detergent screening and purification. We first examined the effects of the C-terminal fusion of ABCG1. GFP-fused ABCG1 was localized mainly at the plasma membrane. The level of cholesterol efflux was comparable to that by the wild-type ABCG1, and it was even higher than that by ABCG1 expressed in HEK293 cells (7). These results suggest that FreeStyle 293-F cells can be used for the expression of functional ABCG1, that the suspension culture does not affect the activity of ABCG1, and that GFP fusion at the C terminus does not affect the localization or the function of ABCG1.
Because ABCG1 is a half-size ABC transporter and dimer formation is required for the function, we were concerned with the stability of dimer form of ABCG1 in detergents. However, the ABCG1 dimer was rather stable in most detergents examined. This suggests that the dimer form of ABCG1 is stable once the complex is assembled in the endoplasmic reticulum. In FSEC analysis, Fos-Choline-14 showed high efficacy for solubilizing ABCG1, even higher than DDM. However, ABCG1, solubilized and purified with Fos-Choline-14, did not show any ATPase activity, indicating that Fos-choline-14 irreversibly inactivates ABCG1 during the purification procedures without destroying the dimer form. Fos-choline-14, having a structure similar to PC, may tightly interact with ABCG1 and inactivate it. It will be interesting to investigate the binding site of Fos-choline-14 in ABCG1 and the mechanism of inactivation in future studies.
ABCG1 purified with DDM showed ATPase activity after reconstitution in egg lecithin liposomes. ABCG1 did not show any ATPase activity without reconstitution, indicating that the lipid bilayer environment is crucial for the function of ABCG1. The ATPase activity (about 150 nmol/min/mg) of ABCG1 was lower than that of ABCA1, MDR1, and TAP (450, 1700, and 1920 nmol/min/mg, respectively) (13, 22, 23) but was similar to that of ABCA4 and ABCG5/G8 (92 and 260 nmol/min/mg, respectively) (24, 25). Thus, the ATPase activity of ABCG1 is high enough to function as an active transporter.
ATPase activity was inhibited by beryllium fluoride but not by ortho-vanadate (Fig. 4C), even though both are phosphate analogs; this selectivity is similar to that of ABCG5/8 and ABCA1 (22, 26). In contrast, many ABC transporters, including MDR1, are inhibited by both analogs. It has been reported that the geometry around phosphate analogs is slightly different between beryllium fluoride and ortho-vanadate in crystal structures of the maltose transporter (27). It is not clear why some ABC transporters can be inhibited by both analogs while others are inhibited by only one. The geometry around the ATP binding site may be slightly different in each ABC transporter. The ATPase activity of ABCG1 showed a narrower optimal pH range compared with other ABC transporters (26, 28, 29), suggesting a difference in the environment of the ATP binding site.
We have reported that ABCG1 mediates the efflux of cholesterol and choline phospholipids, especially SM, from cells (7). Substrate transport is coupled with ATP hydrolysis, and substrate binding is predicted to stimulate ATP hydrolysis in an ATP-dependent transporter. Therefore, we examined whether the ATPase activity of ABCG1 is stimulated by specific lipids by reconstituting ABCG1 in PS liposomes containing various concentrations of cholesterol, SM, PC, or PE (Fig. 5A). Cholesterol, SM, and PC stimulated ATPase activity about 3-fold, and the half maximal values in molar ratios for cholesterol, SM, and PC were 9.5%, 8.3%, and 14%, respectively. These results suggest that ABCG1 selectively interacts with cholesterol and choline phospholipids, especially SM. We first speculated that the high affinity of SM was due to the saturated acyl chain of SM. However, the structure (saturated or unsaturated) of acyl chains did not affect the ATPase activity if they were longer than 16 (Fig. 5B). These results suggest that the choline head group and a free hydroxyl group of SM, which does not exist in PC, are important for interaction with ABCG1.
Because MDR1 was reported to possess more than two substrate binding sites that were synergistically coupled (19, 20), we examined whether ABCG1 was similar. We reconstituted ABCG1-GFP into PS liposomes containing SM or PC and different concentrations of cholesterol and found that SM lowered the Km value for cholesterol without affecting the Vmax value of the ATPase. This suggests that ABCG1 possesses different binding sites for SM and cholesterol and that SM binding accelerates cholesterol binding. Interestingly, this synergistic binding was not observed between PC and cholesterol. It is known that cholesterol and SM spontaneously interact and form liquid-ordered microdomains (30). However, cholesterol stimulated the ATPase activity of ABCG1 more efficiently in SM-containing liposome than in PC-containing liposome even at concentrations lower than 0.05 mM, which are lower than the concentration required for microdomain formation (30). We speculate that the binding sites for SM and cholesterol of ABCG1 are synergistically coupled. However, it is also possible that the interaction of SM and cholesterol within the drug binding sites causes a pseudo-synergistic coupling of the binding sites. Alternatively, SM and cholesterol changed the membrane fluidity of liposomes, which might affect the ATPase activity of ABCG1. The mechanism of substrate recognition by ABCG1 is to be investigated further.
In summary, we showed for the first time that the ATPase activity of ABCG1 is cooperatively stimulated by SM and cholesterol. We have reported that cholesterol efflux by ABCG1 is dependent on the cellular SM level (8). We propose that cholesterol efflux by ABCG1 is regulated by SM levels in the plasma membrane via the modification of its ATPase activity.
Supplementary Material
Footnotes
Abbreviations:
- BeFx
- beryllium fluoride
- DDM
- n-dodecyl-β-D-maltoside
- DSPC
- 1,2-distearoyl-sn-glycero-3-phosphocholine
- FSEC
- fluorescence size exclusion chromatography
- GFP
- green fluorescent protein
- MβCD
- methyl-β-cyclodextrin
- MDR1
- multidrug resistance 1
- OG
- n-octyl-β-D-glucoside
- PC
- phosphatidylcholine
- PE
- phosphatidylethanolamine
- PS
- phosphatidylserine
- POPC
- 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine
This work was supported by a grant-in-aid for scientific research (S) from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan; by the Japan New Energy and Industrial Technology Development Organization (NEDO); by the Program for Promotion of Basic and Applied Researches for Innovations in Bio-oriented Industry (BRAIN) of Japan; and by the World Premier International Research Center Initiative, MEXT, Japan.
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of two figures.
REFERENCES
- 1.Murphy A. J., Westerterp M., Yvan-Charvet L., Tall A. R. 2012. Anti-atherogenic mechanisms of high density lipoprotein: effects on myeloid cells. Biochim. Biophys. Acta. 1821: 513–521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nagao K., Tomioka M., Ueda K. 2011. Function and regulation of ABCA1–membrane meso-domain organization and reorganization. FEBS J. 278: 3190–3203 [DOI] [PubMed] [Google Scholar]
- 3.Gelissen I. C., Harris M., Rye K. A., Quinn C., Brown A. J., Kockx M., Cartland S., Packianathan M., Kritharides L., Jessup W. 2006. ABCA1 and ABCG1 synergize to mediate cholesterol export to apoA-I. Arterioscler. Thromb. Vasc. Biol. 26: 534–540 [DOI] [PubMed] [Google Scholar]
- 4.Vaughan A. M., Oram J. F. 2006. ABCA1 and ABCG1 or ABCG4 act sequentially to remove cellular cholesterol and generate cholesterol-rich HDL. J. Lipid Res. 47: 2433–2443 [DOI] [PubMed] [Google Scholar]
- 5.Baldan A., Pei L., Lee R., Tarr P., Tangirala R. K., Weinstein M. M., Frank J., Li A. C., Tontonoz P., Edwards P. A. 2006. Impaired development of atherosclerosis in hyperlipidemic Ldlr−/− and ApoE−/− mice transplanted with Abcg1−/− bone marrow. Arterioscler. Thromb. Vasc. Biol. 26: 2301–2307 [DOI] [PubMed] [Google Scholar]
- 6.Yvan-Charvet L., Ranalletta M., Wang N., Han S., Terasaka N., Li R., Welch C., Tall A. R. 2007. Combined deficiency of ABCA1 and ABCG1 promotes foam cell accumulation and accelerates atherosclerosis in mice. J. Clin. Invest. 117: 3900–3908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kobayashi A., Takanezawa Y., Hirata T., Shimizu Y., Misasa K., Kioka N., Arai H., Ueda K., Matsuo M. 2006. Efflux of sphingomyelin, cholesterol, and phosphatidylcholine by ABCG1. J. Lipid Res. 47: 1791–1802 [DOI] [PubMed] [Google Scholar]
- 8.Sano O., Kobayashi A., Nagao K., Kumagai K., Kioka N., Hanada K., Ueda K., Matsuo M. 2007. Sphingomyelin-dependence of cholesterol efflux mediated by ABCG1. J. Lipid Res. 48: 2377–2384 [DOI] [PubMed] [Google Scholar]
- 9.Cserepes J., Szentpetery Z., Seres L., Ozvegy-Laczka C., Langmann T., Schmitz G., Glavinas H., Klein I., Homolya L., Varadi A., et al. 2004. Functional expression and characterization of the human ABCG1 and ABCG4 proteins: indications for heterodimerization. Biochem. Biophys. Res. Commun. 320: 860–867 [DOI] [PubMed] [Google Scholar]
- 10.Amundson D. M., Zhou M. 1999. Fluorometric method for the enzymatic determination of cholesterol. J. Biochem. Biophys. Methods. 38: 43–52 [DOI] [PubMed] [Google Scholar]
- 11.Geertsma E. R., Nik Mahmood N. A., Schuurman-Wolters G. K., Poolman B. 2008. Membrane reconstitution of ABC transporters and assays of translocator function. Nat. Protoc. 3: 256–266 [DOI] [PubMed] [Google Scholar]
- 12.Kimura Y., Kioka N., Kato H., Matsuo M., Ueda K. 2007. Modulation of drug-stimulated ATPase activity of human MDR1/P-glycoprotein by cholesterol. Biochem. J. 401: 597–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Herget M., Kreissig N., Kolbe C., Scholz C., Tampe R., Abele R. 2009. Purification and reconstitution of the antigen transport complex TAP: a prerequisite for determination of peptide stoichiometry and ATP hydrolysis. J. Biol. Chem. 284: 33740–33749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Galian C., Manon F., Dezi M., Torres C., Ebel C., Levy D., Jault J. M. 2011. Optimized purification of a heterodimeric ABC transporter in a highly stable form amenable to 2-D crystallization. PLoS ONE. 6: e19677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kawate T., Gouaux E. 2006. Fluorescence-detection size-exclusion chromatography for precrystallization screening of integral membrane proteins. Structure. 14: 673–681 [DOI] [PubMed] [Google Scholar]
- 16.Kimura Y., Shibasaki S., Morisato K., Ishizuka N., Minakuchi H., Nakanishi K., Matsuo M., Amachi T., Ueda M., Ueda K. 2004. Microanalysis for MDR1 ATPase by high-performance liquid chromatography with a titanium dioxide column. Anal. Biochem. 326: 262–266 [DOI] [PubMed] [Google Scholar]
- 17.Doige C. A., Yu X., Sharom F. J. 1993. The effects of lipids and detergents on ATPase-active P-glycoprotein. Biochim. Biophys. Acta. 1146: 65–72 [DOI] [PubMed] [Google Scholar]
- 18.Urbatsch I. L., Tyndall G. A., Tombline G., Senior A. E. 2003. P-glycoprotein catalytic mechanism: studies of the ADP-vanadate inhibited state. J. Biol. Chem. 278: 23171–23179 [DOI] [PubMed] [Google Scholar]
- 19.Shapiro A. B., Ling V. 1997. Positively cooperative sites for drug transport by P-glycoprotein with distinct drug specificities. Eur. J. Biochem. 250: 130–137 [DOI] [PubMed] [Google Scholar]
- 20.Buxbaum E. 1999. Co-operative binding sites for transported substrates in the multiple drug resistance transporter Mdr1. Eur. J. Biochem. 265: 64–70 [DOI] [PubMed] [Google Scholar]
- 21.Loe D. W., Deeley R. G., Cole S. P. 2000. Verapamil stimulates glutathione transport by the 190-kDa multidrug resistance protein 1 (MRP1). J. Pharmacol. Exp. Ther. 293: 530–538 [PubMed] [Google Scholar]
- 22.Takahashi K., Kimura Y., Kioka N., Matsuo M., Ueda K. 2006. Purification and ATPase activity of human ABCA1. J. Biol. Chem. 281: 10760–10768 [DOI] [PubMed] [Google Scholar]
- 23.Urbatsch I. L., Wilke-Mounts S., Gimi K., Senior A. E. 2001. Purification and characterization of N-glycosylation mutant mouse and human P-glycoproteins expressed in Pichia pastoris cells. Arch. Biochem. Biophys. 388: 171–177 [DOI] [PubMed] [Google Scholar]
- 24.Tsybovsky Y., Wang B., Quazi F., Molday R. S., Palczewski K. 2011. Posttranslational modifications of the photoreceptor-specific ABC transporter ABCA4. Biochemistry. 50: 6855–6866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johnson B. J., Lee J. Y., Pickert A., Urbatsch I. L. 2010. Bile acids stimulate ATP hydrolysis in the purified cholesterol transporter ABCG5/G8. Biochemistry. 49: 3403–3411 [DOI] [PubMed] [Google Scholar]
- 26.Wang Z., Stalcup L. D., Harvey B. J., Weber J., Chloupkova M., Dumont M. E., Dean M., Urbatsch I. L. 2006. Purification and ATP hydrolysis of the putative cholesterol transporters ABCG5 and ABCG8. Biochemistry. 45: 9929–9939 [DOI] [PubMed] [Google Scholar]
- 27.Oldham M. L., Chen J. 2011. Snapshots of the maltose transporter during ATP hydrolysis. Proc. Natl. Acad. Sci. USA. 108: 15152–15156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Urbatsch I. L., al-Shawi M. K., Senior A. E. 1994. Characterization of the ATPase activity of purified Chinese hamster P-glycoprotein. Biochemistry. 33: 7069–7076 [DOI] [PubMed] [Google Scholar]
- 29.Mao Q., Leslie E. M., Deeley R. G., Cole S. P. 1999. ATPase activity of purified and reconstituted multidrug resistance protein MRP1 from drug-selected H69AR cells. Biochim. Biophys. Acta. 1461: 69–82 [DOI] [PubMed] [Google Scholar]
- 30.Ramstedt B., Slotte J. P. 2006. Sphingolipids and the formation of sterol-enriched ordered membrane domains. Biochim. Biophys. Acta. 1758: 1945–1956 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.