Abstract
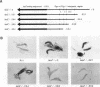
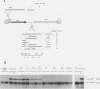
Pig-1 and Sgs-4 are a pair of closely linked and divergently transcribed Drosophila melanogaster genes, which are both expressed in larval salivary glands but at different times during development. While Sgs-4 is expressed at high levels only at the end of the third instar, Pig-1 exhibits a major peak of expression during late second and early third instar. Thus, Pig-1 expression declines as Sgs-4 expression is induced. In this paper, we show that three adjacent elements located within the short region between these genes can account for the switch from Pig-1 to Sgs-4 expression. A 170-bp segment acts as an enhancer to direct Sgs-4 expression in late-third-instar salivary glands. A 64-bp sequence located just upstream from the enhancer can modify its temporal specificity so that it works throughout the third instar. Expression induced at mid-third instar by a combination of these two elements can be repressed by a negative regulatory sequence located still further upstream. We present evidence suggesting that the changing interactions between these regulatory elements and the Sgs-4 and Pig-1 promoters lead to the correct pattern of expression of the two genes.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atchison M. L. Enhancers: mechanisms of action and cell specificity. Annu Rev Cell Biol. 1988;4:127–153. doi: 10.1146/annurev.cb.04.110188.001015. [DOI] [PubMed] [Google Scholar]
- Barnett S. W., Flynn K., Webster M. K., Beckendorf S. K. Noncoordinate expression of Drosophila glue genes: Sgs-4 is expressed at many stages and in two different tissues. Dev Biol. 1990 Aug;140(2):362–373. doi: 10.1016/0012-1606(90)90086-x. [DOI] [PubMed] [Google Scholar]
- Basler K., Siegrist P., Hafen E. The spatial and temporal expression pattern of sevenless is exclusively controlled by gene-internal elements. EMBO J. 1989 Aug;8(8):2381–2386. doi: 10.1002/j.1460-2075.1989.tb08367.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckendorf S. K., Kafatos F. C. Differentiation in the salivary glands of Drosophila melanogaster: characterization of the glue proteins and their developmental appearance. Cell. 1976 Nov;9(3):365–373. doi: 10.1016/0092-8674(76)90081-7. [DOI] [PubMed] [Google Scholar]
- Bieberich C. J., Utset M. F., Awgulewitsch A., Ruddle F. H. Evidence for positive and negative regulation of the Hox-3.1 gene. Proc Natl Acad Sci U S A. 1990 Nov;87(21):8462–8466. doi: 10.1073/pnas.87.21.8462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan M. D., Weiner A. J., Goralski T. J., Mahowald A. P. The follicle cells are a major site of vitellogenin synthesis in Drosophila melanogaster. Dev Biol. 1982 Jan;89(1):225–236. doi: 10.1016/0012-1606(82)90309-8. [DOI] [PubMed] [Google Scholar]
- Chen C. N., Malone T., Beckendorf S. K., Davis R. L. At least two genes reside within a large intron of the dunce gene of Drosophila. Nature. 1987 Oct 22;329(6141):721–724. doi: 10.1038/329721a0. [DOI] [PubMed] [Google Scholar]
- Choi O. R., Engel J. D. Developmental regulation of beta-globin gene switching. Cell. 1988 Oct 7;55(1):17–26. doi: 10.1016/0092-8674(88)90005-0. [DOI] [PubMed] [Google Scholar]
- Corbin V., Maniatis T. Role of transcriptional interference in the Drosophila melanogaster Adh promoter switch. Nature. 1989 Jan 19;337(6204):279–282. doi: 10.1038/337279a0. [DOI] [PubMed] [Google Scholar]
- Corbin V., Maniatis T. The role of specific enhancer-promoter interactions in the Drosophila Adh promoter switch. Genes Dev. 1989 Dec;3(12B):2191–2120. doi: 10.1101/gad.3.12b.2191. [DOI] [PubMed] [Google Scholar]
- Crowley T. E., Mathers P. H., Meyerowitz E. M. A trans-acting regulatory product necessary for expression of the Drosophila melanogaster 68C glue gene cluster. Cell. 1984 Nov;39(1):149–156. doi: 10.1016/0092-8674(84)90200-9. [DOI] [PubMed] [Google Scholar]
- DiBello P. R., Withers D. A., Bayer C. A., Fristrom J. W., Guild G. M. The Drosophila Broad-Complex encodes a family of related proteins containing zinc fingers. Genetics. 1991 Oct;129(2):385–397. doi: 10.1093/genetics/129.2.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer J. A., Maniatis T. Drosophila Adh: a promoter element expands the tissue specificity of an enhancer. Cell. 1988 May 6;53(3):451–461. doi: 10.1016/0092-8674(88)90165-1. [DOI] [PubMed] [Google Scholar]
- Fischer J. A., Maniatis T. Regulatory elements involved in Drosophila Adh gene expression are conserved in divergent species and separate elements mediate expression in different tissues. EMBO J. 1986 Jun;5(6):1275–1289. doi: 10.1002/j.1460-2075.1986.tb04357.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley K. P., Engel J. D. Individual stage selector element mutations lead to reciprocal changes in beta- vs. epsilon-globin gene transcription: genetic confirmation of promoter competition during globin gene switching. Genes Dev. 1992 May;6(5):730–744. doi: 10.1101/gad.6.5.730. [DOI] [PubMed] [Google Scholar]
- Garabedian M. J., Hung M. C., Wensink P. C. Independent control elements that determine yolk protein gene expression in alternative Drosophila tissues. Proc Natl Acad Sci U S A. 1985 Mar;82(5):1396–1400. doi: 10.1073/pnas.82.5.1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garabedian M. J., Shepherd B. M., Wensink P. C. A tissue-specific transcription enhancer from the Drosophila yolk protein 1 gene. Cell. 1986 Jun 20;45(6):859–867. doi: 10.1016/0092-8674(86)90560-x. [DOI] [PubMed] [Google Scholar]
- Garfinkel M. D., Pruitt R. E., Meyerowitz E. M. DNA sequences, gene regulation and modular protein evolution in the Drosophila 68C glue gene cluster. J Mol Biol. 1983 Aug 25;168(4):765–789. doi: 10.1016/s0022-2836(83)80074-6. [DOI] [PubMed] [Google Scholar]
- Graves B. J., Eisenberg S. P., Coen D. M., McKnight S. L. Alternate utilization of two regulatory domains within the Moloney murine sarcoma virus long terminal repeat. Mol Cell Biol. 1985 Aug;5(8):1959–1968. doi: 10.1128/mcb.5.8.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guay P. S., Guild G. M. The ecdysone-induced puffing cascade in Drosophila salivary glands: a Broad-Complex early gene regulates intermolt and late gene transcription. Genetics. 1991 Sep;129(1):169–175. doi: 10.1093/genetics/129.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann A., Garfinkel M. D., Meyerowitz E. M. cis-acting sequences required for expression of the divergently transcribed Drosophila melanogaster Sgs-7 and Sgs-8 glue protein genes. Mol Cell Biol. 1991 Jun;11(6):2971–2979. doi: 10.1128/mcb.11.6.2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann A., Korge G. Upstream sequences of dosage-compensated and non-compensated alleles of the larval secretion protein gene Sgs-4 in Drosophila. Chromosoma. 1987;96(1):1–7. doi: 10.1007/BF00285876. [DOI] [PubMed] [Google Scholar]
- Jongens T. A., Fowler T., Shermoen A. W., Beckendorf S. K. Functional redundancy in the tissue-specific enhancer of the Drosophila Sgs-4 gene. EMBO J. 1988 Aug;7(8):2559–2567. doi: 10.1002/j.1460-2075.1988.tb03105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korge G. Chromosome puff activity and protein synthesis in larval salivary glands of Drosophila melanogaster. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4550–4554. doi: 10.1073/pnas.72.11.4550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan S. K., Garabedian M. J., Wensink P. C. DNA regions that regulate the ovarian transcriptional specificity of Drosophila yolk protein genes. Genes Dev. 1989 Sep;3(9):1453–1461. doi: 10.1101/gad.3.9.1453. [DOI] [PubMed] [Google Scholar]
- Logan S. K., Wensink P. C. Ovarian follicle cell enhancers from the Drosophila yolk protein genes: different segments of one enhancer have different cell-type specificities that interact to give normal expression. Genes Dev. 1990 Apr;4(4):613–623. doi: 10.1101/gad.4.4.613. [DOI] [PubMed] [Google Scholar]
- Mariani B. D., Lingappa J. R., Kafatos F. C. Temporal regulation in development: negative and positive cis regulators dictate the precise timing of expression of a Drosophila chorion gene. Proc Natl Acad Sci U S A. 1988 May;85(9):3029–3033. doi: 10.1073/pnas.85.9.3029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin P., Martin A., Osmani A., Sofer W. A transient expression assay for tissue-specific gene expression of alcohol dehydrogenase in Drosophila. Dev Biol. 1986 Oct;117(2):574–580. doi: 10.1016/0012-1606(86)90326-x. [DOI] [PubMed] [Google Scholar]
- McNabb S. L., Beckendorf S. K. Cis-acting sequences which regulate expression of the Sgs-4 glue protein gene of Drosophila. EMBO J. 1986 Sep;5(9):2331–2340. doi: 10.1002/j.1460-2075.1986.tb04501.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyerowitz E. M., Hogness D. S. Molecular organization of a Drosophila puff site that responds to ecdysone. Cell. 1982 Jan;28(1):165–176. doi: 10.1016/0092-8674(82)90386-5. [DOI] [PubMed] [Google Scholar]
- Miyazaki J., Appella E., Ozato K. Negative regulation of the major histocompatibility class I gene in undifferentiated embryonal carcinoma cells. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9537–9541. doi: 10.1073/pnas.83.24.9537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori N., Stein R., Sigmund O., Anderson D. J. A cell type-preferred silencer element that controls the neural-specific expression of the SCG10 gene. Neuron. 1990 Apr;4(4):583–594. doi: 10.1016/0896-6273(90)90116-w. [DOI] [PubMed] [Google Scholar]
- Muskavitch M. A., Hogness D. S. Molecular analysis of a gene in a developmentally regulated puff of Drosophila melanogaster. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7362–7366. doi: 10.1073/pnas.77.12.7362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak T. J., Rothenberg E. V. Differential transient and long-term expression of DNA sequences introduced into T-lymphocyte lines. DNA. 1986 Dec;5(6):439–451. doi: 10.1089/dna.1.1986.5.439. [DOI] [PubMed] [Google Scholar]
- Pattatucci A. M., Kaufman T. C. The homeotic gene Sex combs reduced of Drosophila melanogaster is differentially regulated in the embryonic and imaginal stages of development. Genetics. 1991 Oct;129(2):443–461. doi: 10.1093/genetics/129.2.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posakony J. W., Fischer J. A., Maniatis T. Identification of DNA sequences required for the regulation of Drosophila alcohol dehydrogenase gene expression. Cold Spring Harb Symp Quant Biol. 1985;50:515–520. doi: 10.1101/sqb.1985.050.01.063. [DOI] [PubMed] [Google Scholar]
- Romano C. P., Bienz-Tadmor B., Mariani B. D., Kafatos F. C. Both early and late Drosophila chorion gene promoters confer correct temporal, tissue and sex specificity on a reporter Adh gene. EMBO J. 1988 Mar;7(3):783–790. doi: 10.1002/j.1460-2075.1988.tb02876.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin G. M., Spradling A. C. Genetic transformation of Drosophila with transposable element vectors. Science. 1982 Oct 22;218(4570):348–353. doi: 10.1126/science.6289436. [DOI] [PubMed] [Google Scholar]
- Rubin G. M., Spradling A. C. Vectors for P element-mediated gene transfer in Drosophila. Nucleic Acids Res. 1983 Sep 24;11(18):6341–6351. doi: 10.1093/nar/11.18.6341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudkin G. T. Replication in polytene chromosomes. Results Probl Cell Differ. 1972;4:59–85. doi: 10.1007/978-3-540-37164-9_3. [DOI] [PubMed] [Google Scholar]
- Shermoen A. W., Jongens J., Barnett S. W., Flynn K., Beckendorf S. K. Developmental regulation by an enhancer from the Sgs-4 gene of Drosophila. EMBO J. 1987 Jan;6(1):207–214. doi: 10.1002/j.1460-2075.1987.tb04740.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shore E. M., Guild G. M. Larval salivary gland secretion proteins in Drosophila structural analysis of the Sgs-5 gene. J Mol Biol. 1986 Jul 20;190(2):149–158. doi: 10.1016/0022-2836(86)90288-3. [DOI] [PubMed] [Google Scholar]
- Thummel C. S., Boulet A. M., Lipshitz H. D. Vectors for Drosophila P-element-mediated transformation and tissue culture transfection. Gene. 1988 Dec 30;74(2):445–456. doi: 10.1016/0378-1119(88)90177-1. [DOI] [PubMed] [Google Scholar]
- Tolias P. P., Kafatos F. C. Functional dissection of an early Drosophila chorion gene promoter: expression throughout the follicular epithelium is under spatially composite regulation. EMBO J. 1990 May;9(5):1457–1464. doi: 10.1002/j.1460-2075.1990.tb08262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twell D., Yamaguchi J., Wing R. A., Ushiba J., McCormick S. Promoter analysis of genes that are coordinately expressed during pollen development reveals pollen-specific enhancer sequences and shared regulatory elements. Genes Dev. 1991 Mar;5(3):496–507. doi: 10.1101/gad.5.3.496. [DOI] [PubMed] [Google Scholar]
- Vacher J., Tilghman S. M. Dominant negative regulation of the mouse alpha-fetoprotein gene in adult liver. Science. 1990 Dec 21;250(4988):1732–1735. doi: 10.1126/science.1702902. [DOI] [PubMed] [Google Scholar]