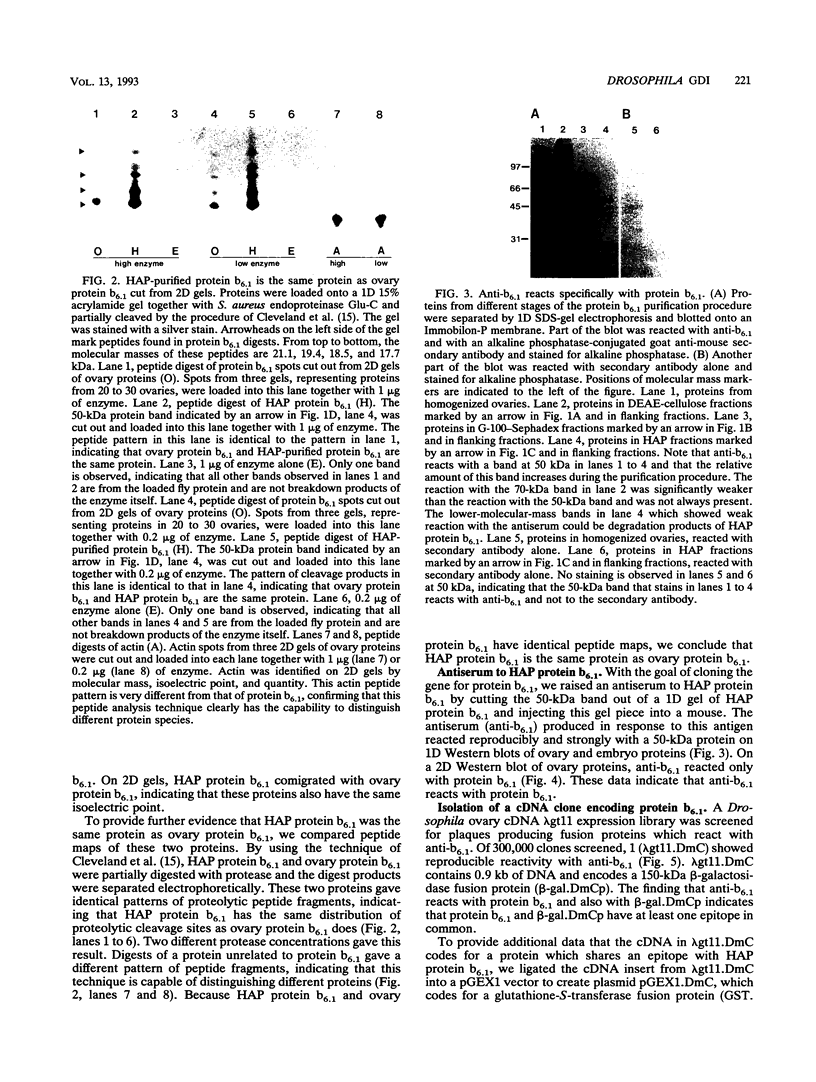
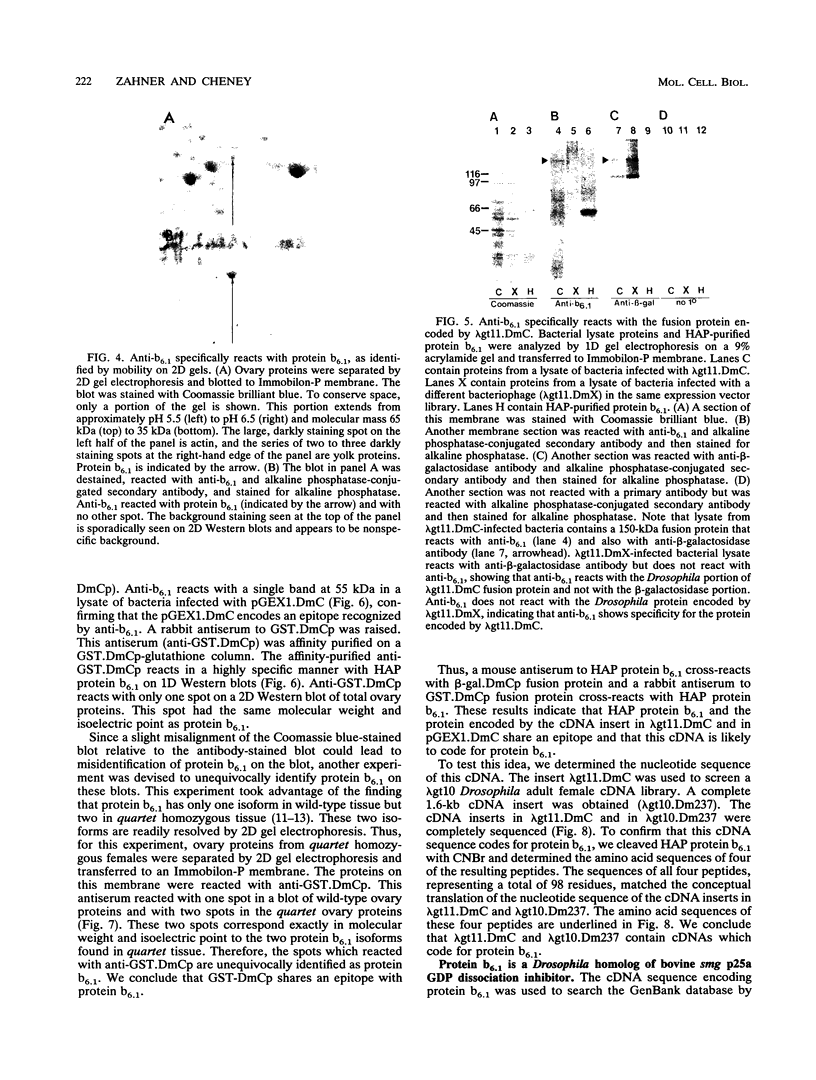
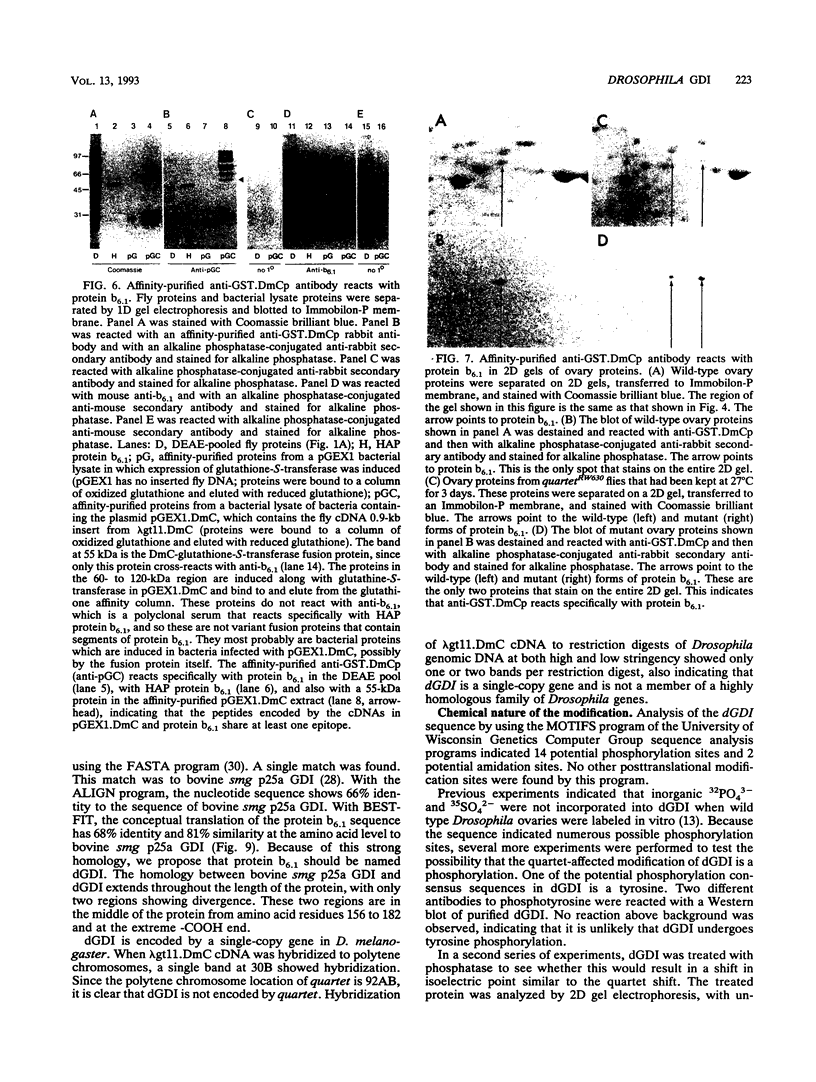
Abstract
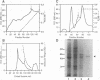
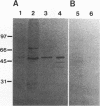
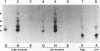
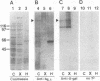
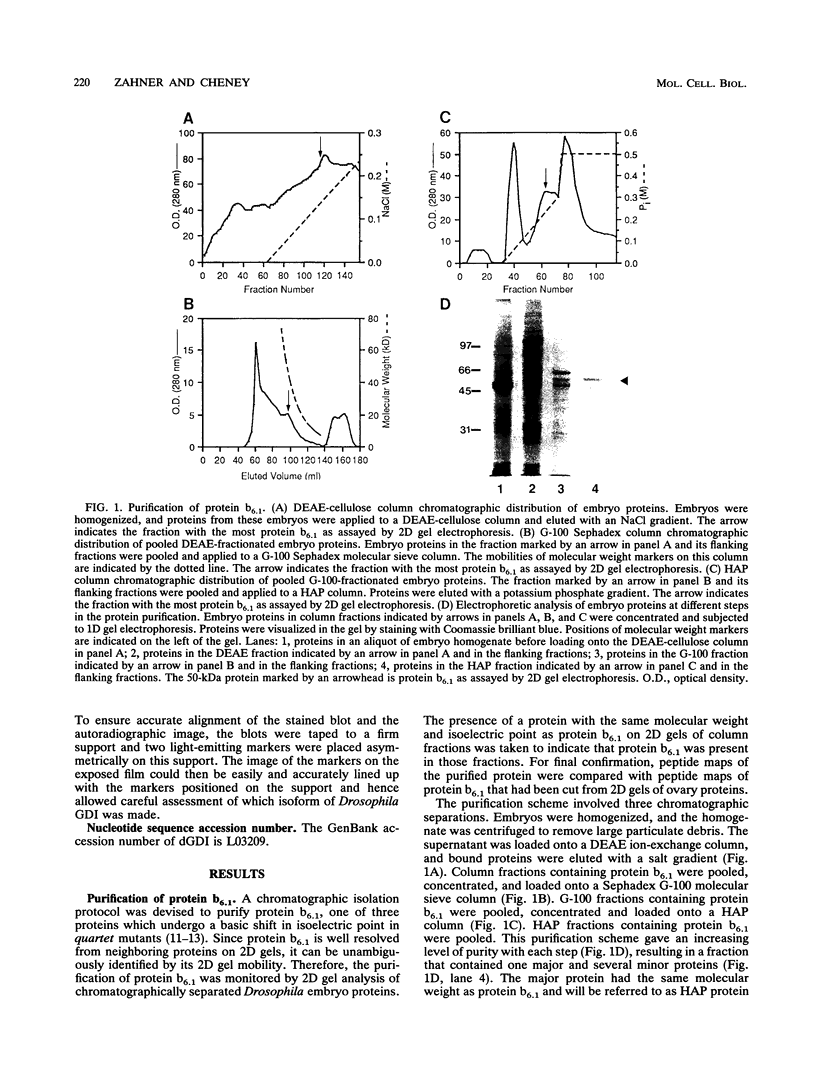
The Drosophila developmental mutation quartet causes late larval lethality and small imaginal discs and, when expressed in the adult female, has a lethal effect on early embryogenesis. These developmental defects are associated with mitotic defects, which include a low mitotic index in larval brains and incomplete separation of chromosomes in mitosis in the early embryo. quartet mutations also have a biochemical effect, i.e., a basic shift in isoelectric point in three proteins. We have purified one of these proteins, raised an antibody to it, and isolated and sequenced its cDNA. At the amino acid level, the sequence shows 68% identity and 81% similarity to bovine smg p25a GDP dissociation inhibitor (GDI), a regulator of ras-like small GTPases of the rab/SEC4/YPT1 subfamily. The correlation between a basic shift in isoelectric point in Drosophila GDI in quartet mutant tissue and the quartet developmental phenotype raises the possibility that a posttranslational modification of GDI is necessary for its function and that GDI function is essential for development.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abo A., Pick E., Hall A., Totty N., Teahan C. G., Segal A. W. Activation of the NADPH oxidase involves the small GTP-binding protein p21rac1. Nature. 1991 Oct 17;353(6345):668–670. doi: 10.1038/353668a0. [DOI] [PubMed] [Google Scholar]
- Bailly E., McCaffrey M., Touchot N., Zahraoui A., Goud B., Bornens M. Phosphorylation of two small GTP-binding proteins of the Rab family by p34cdc2. Nature. 1991 Apr 25;350(6320):715–718. doi: 10.1038/350715a0. [DOI] [PubMed] [Google Scholar]
- Balch W. E. Small GTP-binding proteins in vesicular transport. Trends Biochem Sci. 1990 Dec;15(12):473–477. doi: 10.1016/0968-0004(90)90301-q. [DOI] [PubMed] [Google Scholar]
- Biggs J., Hersperger E., Steeg P. S., Liotta L. A., Shearn A. A Drosophila gene that is homologous to a mammalian gene associated with tumor metastasis codes for a nucleoside diphosphate kinase. Cell. 1990 Nov 30;63(5):933–940. doi: 10.1016/0092-8674(90)90496-2. [DOI] [PubMed] [Google Scholar]
- Biggs J., Tripoulas N., Hersperger E., Dearolf C., Shearn A. Analysis of the lethal interaction between the prune and Killer of prune mutations of Drosophila. Genes Dev. 1988 Oct;2(10):1333–1343. doi: 10.1101/gad.2.10.1333. [DOI] [PubMed] [Google Scholar]
- Borner C., Filipuzzi I., Wartmann M., Eppenberger U., Fabbro D. Biosynthesis and posttranslational modifications of protein kinase C in human breast cancer cells. J Biol Chem. 1989 Aug 15;264(23):13902–13909. [PubMed] [Google Scholar]
- Bourne H. R., Sanders D. A., McCormick F. The GTPase superfamily: a conserved switch for diverse cell functions. Nature. 1990 Nov 8;348(6297):125–132. doi: 10.1038/348125a0. [DOI] [PubMed] [Google Scholar]
- Bourne H. R., Sanders D. A., McCormick F. The GTPase superfamily: conserved structure and molecular mechanism. Nature. 1991 Jan 10;349(6305):117–127. doi: 10.1038/349117a0. [DOI] [PubMed] [Google Scholar]
- Cheney C. M., Lang T. J. Defects in protein modification precede developmental defects in l(3)c21RRW630, a temperature-sensitive Drosophila developmental mutant. Dev Biol. 1986 Mar;114(1):34–41. doi: 10.1016/0012-1606(86)90381-7. [DOI] [PubMed] [Google Scholar]
- Cheney C. M., Lang T. J. Developmental and protein modification defects caused by mutations in the Drosophila gene l(3)c21R. Dev Biol. 1988 Dec;130(2):551–557. doi: 10.1016/0012-1606(88)90350-8. [DOI] [PubMed] [Google Scholar]
- Cheney C. M., Miller K. G., Lang T. J., Shearn A. Specific protein modifications are altered in a temperature-sensitive Drosophila developmental mutant. Proc Natl Acad Sci U S A. 1984 Oct;81(20):6422–6426. doi: 10.1073/pnas.81.20.6422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheney C. M., Shearn A. Developmental regulation of Drosophila imaginal disc proteins: synthesis of a heat shock protein under non-heat-shock conditions. Dev Biol. 1983 Feb;95(2):325–330. doi: 10.1016/0012-1606(83)90033-7. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Dearolf C. R., Hersperger E., Shearn A. Developmental consequences of awdb3, a cell-autonomous lethal mutation of Drosophila induced by hybrid dysgenesis. Dev Biol. 1988 Sep;129(1):159–168. doi: 10.1016/0012-1606(88)90170-4. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Fukumoto Y., Kaibuchi K., Hori Y., Fujioka H., Araki S., Ueda T., Kikuchi A., Takai Y. Molecular cloning and characterization of a novel type of regulatory protein (GDI) for the rho proteins, ras p21-like small GTP-binding proteins. Oncogene. 1990 Sep;5(9):1321–1328. [PubMed] [Google Scholar]
- Fyrberg E. A., Donady J. J. Actin Heterogeneity in primary embryonic culture cells from Drosophila melanogaster. Dev Biol. 1979 Feb;68(2):487–502. doi: 10.1016/0012-1606(79)90220-3. [DOI] [PubMed] [Google Scholar]
- Hall A. The cellular functions of small GTP-binding proteins. Science. 1990 Aug 10;249(4969):635–640. doi: 10.1126/science.2116664. [DOI] [PubMed] [Google Scholar]
- Hancock J. F., Cadwallader K., Marshall C. J. Methylation and proteolysis are essential for efficient membrane binding of prenylated p21K-ras(B). EMBO J. 1991 Mar;10(3):641–646. doi: 10.1002/j.1460-2075.1991.tb07992.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson-Schlitz D., Lim J. K. Cytogenetics of Notch mutations arising in the unstable X chromosome Uc of Drosophila melanogaster. Genetics. 1987 Apr;115(4):701–709. doi: 10.1093/genetics/115.4.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston P. A., Archer B. T., 3rd, Robinson K., Mignery G. A., Jahn R., Südhof T. C. rab3A attachment to the synaptic vesicle membrane mediated by a conserved polyisoprenylated carboxy-terminal sequence. Neuron. 1991 Jul;7(1):101–109. doi: 10.1016/0896-6273(91)90078-e. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Langer-Safer P. R., Levine M., Ward D. C. Immunological method for mapping genes on Drosophila polytene chromosomes. Proc Natl Acad Sci U S A. 1982 Jul;79(14):4381–4385. doi: 10.1073/pnas.79.14.4381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui Y., Kikuchi A., Araki S., Hata Y., Kondo J., Teranishi Y., Takai Y. Molecular cloning and characterization of a novel type of regulatory protein (GDI) for smg p25A, a ras p21-like GTP-binding protein. Mol Cell Biol. 1990 Aug;10(8):4116–4122. doi: 10.1128/mcb.10.8.4116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pentz E. S., Shearn A. Analysis of the autonomy of imaginal disc defects in a small-disc mutant of Drosophila melanogaster. Dev Biol. 1979 May;70(1):149–170. doi: 10.1016/0012-1606(79)90013-7. [DOI] [PubMed] [Google Scholar]
- Pollard K. M., Chan E. K., Grant B. J., Sullivan K. F., Tan E. M., Glass C. A. In vitro posttranslational modification of lamin B cloned from a human T-cell line. Mol Cell Biol. 1990 May;10(5):2164–2175. doi: 10.1128/mcb.10.5.2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poodry C. A. shibire, a neurogenic mutant of Drosophila. Dev Biol. 1990 Apr;138(2):464–472. doi: 10.1016/0012-1606(90)90212-2. [DOI] [PubMed] [Google Scholar]
- Ruggieri R., McCormick F. Ras and the awd couple. Nature. 1991 Oct 3;353(6343):390–391. doi: 10.1038/353390a0. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki T., Kaibuchi K., Kabcenell A. K., Novick P. J., Takai Y. A mammalian inhibitory GDP/GTP exchange protein (GDP dissociation inhibitor) for smg p25A is active on the yeast SEC4 protein. Mol Cell Biol. 1991 May;11(5):2909–2912. doi: 10.1128/mcb.11.5.2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki T., Kikuchi A., Araki S., Hata Y., Isomura M., Kuroda S., Takai Y. Purification and characterization from bovine brain cytosol of a protein that inhibits the dissociation of GDP from and the subsequent binding of GTP to smg p25A, a ras p21-like GTP-binding protein. J Biol Chem. 1990 Feb 5;265(4):2333–2337. [PubMed] [Google Scholar]
- Smith D. B., Johnson K. S. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene. 1988 Jul 15;67(1):31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Takai Y., Kaibuchi K., Kikuchi A., Kawata M. Small GTP-binding proteins. Int Rev Cytol. 1992;133:187–230. doi: 10.1016/s0074-7696(08)61861-6. [DOI] [PubMed] [Google Scholar]
- Teng D. H., Engele C. M., Venkatesh T. R. A product of the prune locus of Drosophila is similar to mammalian GTPase-activating protein. Nature. 1991 Oct 3;353(6343):437–440. doi: 10.1038/353437a0. [DOI] [PubMed] [Google Scholar]
- Ueda T., Kikuchi A., Ohga N., Yamamoto J., Takai Y. Purification and characterization from bovine brain cytosol of a novel regulatory protein inhibiting the dissociation of GDP from and the subsequent binding of GTP to rhoB p20, a ras p21-like GTP-binding protein. J Biol Chem. 1990 Jun 5;265(16):9373–9380. [PubMed] [Google Scholar]
- Ueda T., Takeyama Y., Ohmori T., Ohyanagi H., Saitoh Y., Takai Y. Purification and characterization from rat liver cytosol of a GDP dissociation inhibitor (GDI) for liver 24K G, a ras p21-like GTP-binding protein, with properties similar to those of smg p25A GDI. Biochemistry. 1991 Jan 29;30(4):909–917. doi: 10.1021/bi00218a005. [DOI] [PubMed] [Google Scholar]
- Wang L. L., Spudich J. A. A 45,000-mol-wt protein from unfertilized sea urchin eggs severs actin filaments in a calcium-dependent manner and increases the steady-state concentration of nonfilamentous actin. J Cell Biol. 1984 Sep;99(3):844–851. doi: 10.1083/jcb.99.3.844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahner J. E., Cheney C. M. Quartet: a Drosophila developmental mutation affecting chromosome separation in mitosis. Dev Genet. 1990;11(1):27–40. doi: 10.1002/dvg.1020110105. [DOI] [PubMed] [Google Scholar]