Abstract
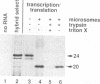
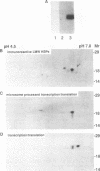
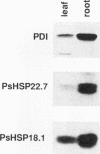
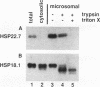
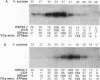
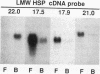
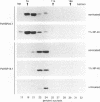
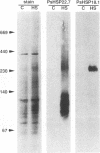
Three related gene families of low-molecular-weight (LMW) heat shock proteins (HSPs) have been characterized in plants. We describe a fourth LMW HSP family, represented by PsHSP22.7 from Pisum sativum and GmHSP22.0 from Glycine max, and demonstrate that this family of proteins is endomembrane localized. PsHSP22.7 and GmHSP22.0 are 76.7% identical at the amino acid level. Both proteins have amino-terminal signal peptides and carboxyl-terminal sequences characteristic of endoplasmic reticulum (ER) retention signals. The two proteins closely resemble class I cytoplasmic LMW HSPs, suggesting that they evolved from the cytoplasmic proteins through the addition of the signal peptide and ER retention motif. The endomembrane localization of these proteins was confirmed by cell fractionation. The polypeptide product of PsHSP22.7 mRNA was processed to a smaller-M(r) form by canine pancreatic microsomes; in vivo, GmHSP22.0 polysomal mRNA was found to be predominantly membrane bound. In vitro-processed PsHSP22.7 corresponded in mass and pI to one of two proteins detected in ER fractions from heat-stressed plants by using anti-PsHSP22.7 antibodies. Like other LMW HSPs, PsHSP22.7 was observed in higher-molecular-weight structures with apparent masses of between 80 and 240 kDa. The results reported here indicate that members of this new class of LMW HSPs are most likely resident ER proteins and may be similar in function to related LMW HSPs in the cytoplasm. Along with the HSP90 and HSP70 classes of HSPs, this is the third category of HSPs localized to the ER.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson L. -O., Borg H., Mikaelsson M. Molecular weight estimations of proteins by electrophoresis in polyacrylamide gels of graded porosity. FEBS Lett. 1972 Feb 1;20(2):199–202. doi: 10.1016/0014-5793(72)80793-2. [DOI] [PubMed] [Google Scholar]
- Andres D. A., Dickerson I. M., Dixon J. E. Variants of the carboxyl-terminal KDEL sequence direct intracellular retention. J Biol Chem. 1990 Apr 15;265(11):5952–5955. [PubMed] [Google Scholar]
- Arrigo A. P., Welch W. J. Characterization and purification of the small 28,000-dalton mammalian heat shock protein. J Biol Chem. 1987 Nov 15;262(32):15359–15369. [PubMed] [Google Scholar]
- Bowles D. J., Kauss H. Characterization, enzymatic and lectin properties of isolated membranes from Phaseolus aureus. Biochim Biophys Acta. 1976 Sep 7;443(3):360–374. doi: 10.1016/0005-2736(76)90456-9. [DOI] [PubMed] [Google Scholar]
- Chen Q., Lauzon L. M., DeRocher A. E., Vierling E. Accumulation, stability, and localization of a major chloroplast heat-shock protein. J Cell Biol. 1990 Jun;110(6):1873–1883. doi: 10.1083/jcb.110.6.1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q., Vierling E. Analysis of conserved domains identifies a unique structural feature of a chloroplast heat shock protein. Mol Gen Genet. 1991 May;226(3):425–431. doi: 10.1007/BF00260655. [DOI] [PubMed] [Google Scholar]
- Collier N. C., Heuser J., Levy M. A., Schlesinger M. J. Ultrastructural and biochemical analysis of the stress granule in chicken embryo fibroblasts. J Cell Biol. 1988 Apr;106(4):1131–1139. doi: 10.1083/jcb.106.4.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conner T. W., Goekjian V. H., LaFayette P. R., Key J. L. Structure and expression of two auxin-inducible genes from Arabidopsis. Plant Mol Biol. 1990 Oct;15(4):623–632. doi: 10.1007/BF00017836. [DOI] [PubMed] [Google Scholar]
- Cooper P., Ho T. H. Intracellular localization of heat shock proteins in maize. Plant Physiol. 1987 Aug;84(4):1197–1203. doi: 10.1104/pp.84.4.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauwalder M., Whaley W. G., Kephart J. E. Phosphatases and differentiation of the Golgi apparatus. J Cell Sci. 1969 Mar;4(2):455–497. doi: 10.1242/jcs.4.2.455. [DOI] [PubMed] [Google Scholar]
- Derocher A. E., Helm K. W., Lauzon L. M., Vierling E. Expression of a Conserved Family of Cytoplasmic Low Molecular Weight Heat Shock Proteins during Heat Stress and Recovery. Plant Physiol. 1991 Aug;96(4):1038–1047. doi: 10.1104/pp.96.4.1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman R. B. Protein disulfide isomerase: multiple roles in the modification of nascent secretory proteins. Cell. 1989 Jun 30;57(7):1069–1072. doi: 10.1016/0092-8674(89)90043-3. [DOI] [PubMed] [Google Scholar]
- Gantt J. S., Key J. L. Coordinate expression of ribosomal protein mRNAs following auxin treatment of soybean hypocotyls. J Biol Chem. 1985 May 25;260(10):6175–6181. [PubMed] [Google Scholar]
- Gething M. J., Sambrook J. Transport and assembly processes in the endoplasmic reticulum. Semin Cell Biol. 1990 Feb;1(1):65–72. [PubMed] [Google Scholar]
- Ghosh S., Gepstein S., Heikkila J. J., Dumbroff E. B. Use of a scanning densitometer or an ELISA plate reader for measurement of nanogram amounts of protein in crude extracts from biological tissues. Anal Biochem. 1988 Mar;169(2):227–233. doi: 10.1016/0003-2697(88)90278-3. [DOI] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lindquist S., Craig E. A. The heat-shock proteins. Annu Rev Genet. 1988;22:631–677. doi: 10.1146/annurev.ge.22.120188.003215. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro S., Pelham H. R. An Hsp70-like protein in the ER: identity with the 78 kd glucose-regulated protein and immunoglobulin heavy chain binding protein. Cell. 1986 Jul 18;46(2):291–300. doi: 10.1016/0092-8674(86)90746-4. [DOI] [PubMed] [Google Scholar]
- Nagao R. T., Czarnecka E., Gurley W. B., Schöffl F., Key J. L. Genes for low-molecular-weight heat shock proteins of soybeans: sequence analysis of a multigene family. Mol Cell Biol. 1985 Dec;5(12):3417–3428. doi: 10.1128/mcb.5.12.3417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Normington K., Kohno K., Kozutsumi Y., Gething M. J., Sambrook J. S. cerevisiae encodes an essential protein homologous in sequence and function to mammalian BiP. Cell. 1989 Jun 30;57(7):1223–1236. doi: 10.1016/0092-8674(89)90059-7. [DOI] [PubMed] [Google Scholar]
- Nover L., Scharf K. D., Neumann D. Cytoplasmic heat shock granules are formed from precursor particles and are associated with a specific set of mRNAs. Mol Cell Biol. 1989 Mar;9(3):1298–1308. doi: 10.1128/mcb.9.3.1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R. Control of protein exit from the endoplasmic reticulum. Annu Rev Cell Biol. 1989;5:1–23. doi: 10.1146/annurev.cb.05.110189.000245. [DOI] [PubMed] [Google Scholar]
- Roberts B. E., Paterson B. M. Efficient translation of tobacco mosaic virus RNA and rabbit globin 9S RNA in a cell-free system from commercial wheat germ. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2330–2334. doi: 10.1073/pnas.70.8.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose M. D., Misra L. M., Vogel J. P. KAR2, a karyogamy gene, is the yeast homolog of the mammalian BiP/GRP78 gene. Cell. 1989 Jun 30;57(7):1211–1221. doi: 10.1016/0092-8674(89)90058-5. [DOI] [PubMed] [Google Scholar]
- Rossi J. M., Lindquist S. The intracellular location of yeast heat-shock protein 26 varies with metabolism. J Cell Biol. 1989 Feb;108(2):425–439. doi: 10.1083/jcb.108.2.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schöffl F., Key J. L. An analysis of mRNAs for a group of heat shock proteins of soybean using cloned cDNAs. J Mol Appl Genet. 1982;1(4):301–314. [PubMed] [Google Scholar]
- Sticher L., Biswas A. K., Bush D. S., Jones R. L. Heat Shock Inhibits alpha-Amylase Synthesis in Barley Aleurone without Inhibiting the Activity of Endoplasmic Reticulum Marker Enzymes. Plant Physiol. 1990 Feb;92(2):506–513. doi: 10.1104/pp.92.2.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studier F. W., Rosenberg A. H., Dunn J. J., Dubendorff J. W. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 1990;185:60–89. doi: 10.1016/0076-6879(90)85008-c. [DOI] [PubMed] [Google Scholar]
- Susek R. E., Lindquist S. L. hsp26 of Saccharomyces cerevisiae is related to the superfamily of small heat shock proteins but is without a demonstrable function. Mol Cell Biol. 1989 Nov;9(11):5265–5271. doi: 10.1128/mcb.9.11.5265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vierling E., Harris L. M., Chen Q. The major low-molecular-weight heat shock protein in chloroplasts shows antigenic conservation among diverse higher plant species. Mol Cell Biol. 1989 Feb;9(2):461–468. doi: 10.1128/mcb.9.2.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter P., Blobel G. Preparation of microsomal membranes for cotranslational protein translocation. Methods Enzymol. 1983;96:84–93. doi: 10.1016/s0076-6879(83)96010-x. [DOI] [PubMed] [Google Scholar]
- von Heijne G. Patterns of amino acids near signal-sequence cleavage sites. Eur J Biochem. 1983 Jun 1;133(1):17–21. doi: 10.1111/j.1432-1033.1983.tb07424.x. [DOI] [PubMed] [Google Scholar]
- von Heijne G. Signal sequences. The limits of variation. J Mol Biol. 1985 Jul 5;184(1):99–105. doi: 10.1016/0022-2836(85)90046-4. [DOI] [PubMed] [Google Scholar]