Abstract
We here report the results of a Phase I/IIa open-label four dose-escalation clinical study assessing the safety, tolerability, and possible therapeutic efficacy of a single intramuscular administration of DVC1-0101, a new gene transfer vector based on a nontransmissible recombinant Sendai virus (rSeV) expressing the human fibroblast growth factor-2 (FGF-2) gene (rSeV/dF-hFGF2), in patients with peripheral arterial disease (PAD). Gene transfer was done in 12 limbs of 12 patients with rest pain, and three of them had ischemic ulcer(s). No cardiovascular or other serious adverse events (SAEs) caused by gene transfer were detected in the patients over a 6-month follow-up. No infectious viral particles, as assessed by hemagglutination activity, were detected in any patient during the study. No representative elevation of proinflammatory cytokines or plasma FGF-2 was seen. Significant and continuous improvements in Rutherford category, absolute claudication distance (ACD), and rest pain were observed (P < 0.05 to 0.01). To the best of our knowledge, this is the first clinical trial of the use of a gene transfer vector based on rSeV. The single intramuscular administration of DVC1-0101 to PAD patients was safe and well tolerated, and resulted in significant improvements of limb function. Larger pivotal studies are warranted as a next step.
Introduction
Peripheral arterial disease (PAD) is a typical phenotype of progressive and systemic atherosclerosis, and causes disability of limb function (intermittent claudication (IC)) as well as serious pain or limb loss (critical limb ischemia (CLI)). PAD is estimated to affect more than 8.4 million individuals in the United States,1 and its prevalence is increasing in Asian countries, including Japan. Even with the current standard therapies, subjects with severe PAD are left with immobility, intractable ischemia, ulceration, impaired wound healing, or the necessity of amputation. Therefore, the development of new therapeutics to rescue the limbs from critical ischemia, normalize limb function, and prevent the progression of PAD is urgently needed.
In contrast to interventional revascularization and bypasses that target the large- and medium-sized arteries, therapeutic angiogenesis modalities target the microvessels.2 Soon after the discovery of angiogenic growth factors, investigators began to test proteins3,4 and genes5,6,7,8 for their ability to stimulate neovessels in subjects with PAD. However, the majority of the results of clinical trials of therapeutic angiogenesis have been disappointing, and importantly, the positive findings obtained in the small early trials were not confirmed in the larger, well-controlled multicenter trials. Nonetheless, since a number of preclinical evaluations in animal studies support the concept of therapeutic angiogenesis, the critical factors that affect its efficacy in clinical settings should be reconsidered in order to more fully investigate and test this intriguing therapy.
Among the published angiogenic trials for IC, only the TRAFFIC study—a well-controlled clinical trial for intraarterial injection of human recombinant basic fibroblast growth factor (bFGF/FGF-2)—could provide proof of concept; namely, a single-dose injection of bFGF/FGF-2 significantly improved the peak walking time of IC patients at day 90.4 However, the TRAFFIC trial was terminated, because of the relatively limited clinical outcome as well as the mild-to-moderate adverse events (AEs), including hypotension and proteinuria, which may have been due to the systemic leakage of FGF-2. Therefore, a higher and sustained protein concentration of FGF-2 in local target muscles might be more effective and may have fewer side effects in clinical settings.
Recombinant Sendai virus (rSeV) vector is a powerful gene transfer agent9 for the cytoplasmic transcription of therapeutic genes. We previously showed that, in the ischemic muscles of mice, rSeV administration led to high-level expression of an angiogenic factor, FGF-2, with the levels being as much as 300-fold higher than at baseline.10 At a higher dose, the ischemic murine muscles underwent severe congestion associated with markedly dilated postcapillary veins (Y. Yonemitsu et al., unpublished observation), providing the first potential evidence of a toxic dose among the preclinical evaluations of angiogenic gene therapies. Using this system, we demonstrated that: (i) rSeV-mediated overexpression of FGF-2 showed better efficacy for limb salvage in mouse models with surgically induced severe limb ischemia than did administration of vascular endothelial growth factor (VEGF);10 (ii) the biological and therapeutic effects of FGF-2 were dependent on the inducible expression of endogenous VEGF and hepatocyte growth factor (HGF) at an early phase during the angiogenic cascade11 via a platelet-derived growth factor receptor-α/p70S6-mediated mechanism;12 (iii) FGF-2 gene transfer induced not only angiogenesis but also lymphangiogenesis via the VEGF-C/VEGFR3 system;13,14 and (iv) FGF-2 also stimulates transient inflammatory/arteriogenic responses via a monocyte/macrophage chemoattractant protein-1–mediated mechanism.15 These results and the clinical information obtained by the TRAFFIC study strongly suggested that intramuscular injection of rSeV expressing FGF-2 could be an effective method for treating PAD patients.
Our new gene drug product, DVC1-0101, was designed as a RNA drug to induce cytoplasmic expression of native human FGF-2 in vivo to improve the walking performance of PAD patients. DVC1-0101 was based on a first-generation vector established by deleting the fusion (F)-gene (rSeV/dF) to render it nontransmissible.16 We here report the results of the first-in-man, Phase I and IIa, open-labeled, single-center, dose-escalation clinical study of DVC1-0101 to treat PAD patients. This clinical study was designed to evaluate the safety and determine an effective dose of DVC1-0101 in PAD patients in whom revascularization was not considered a suitable option.
Results
Baseline patient characteristics
The Japanese Guidelines for Clinical Trials of Gene Therapy issued by the Ministry of Health, Labor, and Welfare (MHLW) stipulate that only “no-option” patients should be enrolled as subjects in first-in-man gene therapy studies. Therefore, the Governmental Review Board recommended that this trial target cases of CLI without indications for any standard vascular interventions. In addition, because DVC1-0101 was designed to improve the walking performance of PAD patients, we made an effort to recruit subjects who could be tested on a treadmill.
A total of 18 patients with PAD who met the inclusion criteria were screened, and among them, 12 patients who did not meet the exclusion criteria were enrolled (Table 1). A total of 12 limbs, one limb per patient, were treated once with DVC1-0101 in a four dose-escalation fashion (Supplementary Table S1).
Table 1. Patients' baseline characteristics (n = 12).
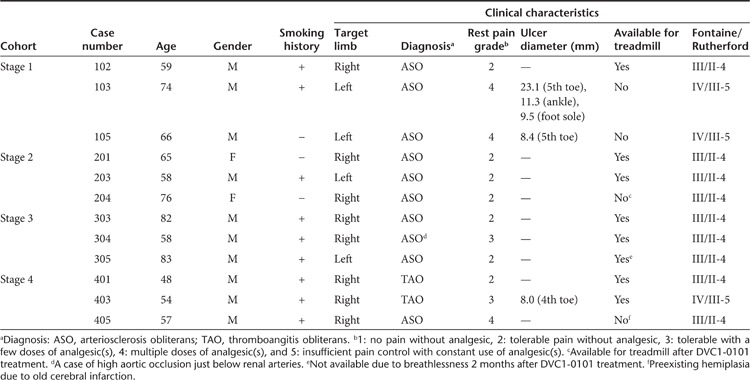
As shown in Table 1, the cohort consisted of 10 males and 2 females (mean age, 65 years; range, 48–82), including 10 cases of arteriosclerosis obliterans and 2 of thromboangitis obliterans (TAO, Buerger's disease; both cases were in Stage 4). Three patients had single (cases 103 and 403) or multiple (case 105) ischemic ulcers, and eight patients had relatively mild-to-moderate rest pain that did not prevent their taking a treadmill test. Nine patients had a smoking history, and enrollment was restricted to the patients who had ceased smoking at least 1 month before screening.
Primary end point: safety
Survival. Although one patient (case 105, Stage 1) was lost due to acute-on-chronic progression of preexisting interstitial pneumonitis ~2 years after treatment, no other death occurred over a 1-year period (the observation period ranged from 1 year and 1 month to 4 years and 8 months).
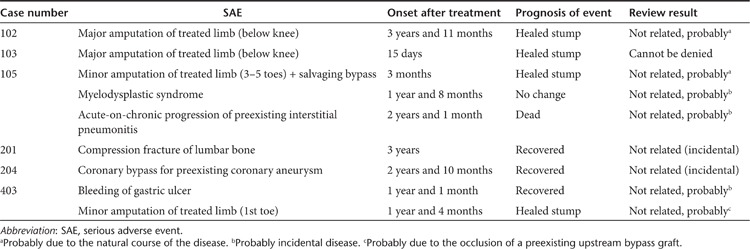
AEs. In the clinical protocol used, serious AEs (SAEs) are reported and reviewed by the Data Safety and Monitoring Board and followed by the Health Sciences Council of the Japan MHLW for 5 years after the protocol, and other AEs are carefully followed for 6 months after the gene transfer date. Nine SAEs occurred in six patients during the observation period (Table 2). No cardiovascular events or other SAEs that could be definitively attributed to gene transfer were observed.
Table 2. Serious adverse events (SAEs) reviewed by the Data Safety and Monitoring Board and Health Sciences Council of the Japan Ministry of Health, Labor, and Welfare.
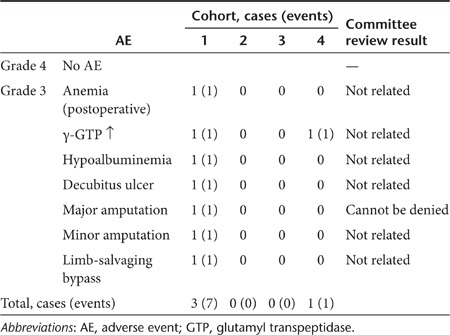
A total of 136 AEs were observed (pretreatment = 16 events; post-treatment = 120 events) over the 6 months after the gene transfer. Among these, as shown in Table 3, there was no AE categorized as grade 4, and seven of eight AEs related to amputation in Stage 1 were grade 3 according to the Common Terminology Criteria for Adverse Events (CTCAE version 4.03, published by the U.S. National Cancer Institute, 14 June 2010: http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_8.5×11.pdf). Another AE at Stage 4 was due to alcoholic liver damage. No dose-response relationship was observed.
Table 3. Adverse events (AEs) categorized as grade 3 or 4 within 6 months of gene transfer.
Virus shedding. Urine and whole blood samples were collected from all of the patients and used to monitor the virus shedding in individuals by measuring the levels of genome copies using nested real-time reverse transcription-PCR as well as hemagglutination activity suggesting infectious activity of DVC1-0101 (assessed by the hemagglutination activity of chicken RBCs). As shown in Supplementary Table S2, neither the viral RNA genome nor infectious activity of DVC1-0101 was detected in urine samples. Three patients (cases 303, 401, and 405) exhibited transient persistence of the viral genome; however, no infectious activity was found in any samples, including those that tested positive for the RNA genome.
Proinflammatory reactions. All 12 patients were administered methylprednisolone hemisuccinate (Solu-medrol; 125 mg/day) via intravenous drip on day −1, day 0 (just before the vector injection), and day 1 in order to avoid an unexpected evocation of the innate immune response and allergic reactions to vectors. Administration of this cortisone has been shown to suppress the innate immune response but not antigen-specific acquired immune responses against the rSeV under experimental conditions.17 The patients' laboratory parameters of systemic inflammatory reaction related to the vector injection and treatment are provided in Supplementary Figure S1a. In most of the patients, the WBC count increased transiently soon after the gene transfer, and recovered to baseline by day 3. Since no corresponding increase of C-reactive protein or other proinflammatory cytokines was found, the increase of WBC counts was thought to be a result of the methylprednisolone treatment. In contrast, the levels of C-reactive protein and interleukin-6 were increased on days 7–14 on average; this finding was attributed largely to the marked elevation of these parameters in case 103, who underwent a major amputation after showing a septic reaction to a methicillin-resistant Staphylococcus aureus infection. Other proinflammatory cytokines, including interleukin-1β and tumor necrosis factor-α, did not show significant change during the trial.
Circulating angiogenic factors. To monitor the possible leakage into the blood circulation of exogenously expressed FGF-2 and its inducible and endogenous downstream angiogenic factors, VEGF and HGF,12 we subjected the plasma and serum to analysis with specific ELISA systems. We used samples within 7 days of their collection, because our previous studies demonstrated that the expression of exogenous gene products peaked at 2 days and declined at around 7–14 days after gene transfer using multiple genes and vector constructs.10,18
As shown in Supplementary Figure S1b, there was no significant elevation in any angiogenic factors during the trial.
Other examinations related to safety. All patients underwent a retinal examination by independent ophthalmologists, cancer screening for tumor markers, and computed tomography scans of the brain and whole body as part of a follow-up examination 6 months after the gene transfer. No newly developed lesion was detected in any examination.
Secondary end point: efficacy-related parameters
Since CLI patients without indications for standard vascular interventions were eligible for this trial, we monitored the time course of clinical symptoms (Rutherford classification, rest pain, and ulcer healing) and other surrogates possibly related to the hemodynamics, including ankle-brachial pressure index and toe pressure index, laser Doppler perfusion images (LDPIs), foot-pad temperature assessed by thermography, and pulse-volume recording (PVR), at each patient visit for 6 months after the gene transfer. In addition, a flat treadmill test at 2.4 km/hour, terminated at 300 m to minimize the risk for cardiovascular complications, was added for the eight patients who were able to take the test (n = 6 for arteriosclerosis obliterans and n = 2 for TAO, Table 1).
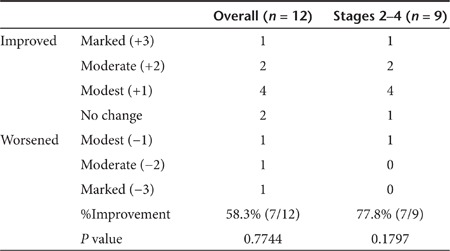
Improvement of clinical staging (Rutherford classification). All CLI patients enrolled in this trial were categorized as Rutherford grade19 II-4 or III-5. At the visit 6 months after the gene transfer, each patient's Rutherford category was re-evaluated and scored as +/−1 (improved/worsened one category), +/−2 (improved/worsened two categories), and +/−3 (improved/worsened more than three categories). The overall improvement rate was 58.3% (n = 7/12, P = 0.7744), and the improvement for Stages 2–4 was 77.8% (n = 7/9, P = 0.1797), as shown in Table 4.
Table 4. Changes of Rutherford categories at 6 months after gene transfer.
Rest pain. Rest pain was scored based on the frequency of intake of analgesics, in most cases nonsteroidal anti-inflammatory drugs, within 24 hours of the interview, and was categorized as follows: 1, completely pain-free without analgesic; 2, feel pain but no need for analgesic; 3, feel pain and sometimes need analgesic(s); 4, use analgesic(s) constantly to control the pain; and 5, uncontrollable pain despite continuous use of analgesic(s). Two patients (cases 103 and 105) were excluded because of major and minor amputation, respectively.
Overall (n = 10), significant pain reduction was seen and continued for over 6 months (P < 0.01 or P < 0.05, Wilcoxon's signed-rank test), and six patients were completely pain-free during our observation (Supplementary Figure S2a).
Ischemic ulcers. The time course of a preexisting ischemic ulcer was recorded in three patients (cases 103 and 105 at Stage 1, and case 403 at Stage 4). No improvement in the size of the referenced ulcers was observed in the two Stage 1 patients, and complete healing was observed in the Stage 4 patient.
Ankle-brachial pressure index and toe pressure index. To avoid examiner-dependent biases, ankle-brachial pressure index and toe pressure index were measured by an automatic oscillometric system (VS-1500A; Fukuda Denshi, Tokyo, Japan). When the blood pressure of the target limb was too low to be detected, the theoretical lower limit, 10 mmHg, was used for calculation. As shown in Supplementary Figure S2b, no significant change was observed at any time point up to 6 months after the gene transfer.
LDPI and foot-pad temperature. We measured the blood flow ratio of both legs of each patient using a LDPI analyzer (Moor Instruments, Devon, UK). To minimize data variables due to ambient light and temperature, the LDPI index was expressed as the ratio of the blood flow in the treated limb to that in the contralateral limb. As shown in Supplementary Figure S2c (left graph), no significant change was observed as of 1 month after gene transfer.
The foot skin temperature of both limbs was measured by independent and blinded physicians (dermatologists in the hospital). The measurements were performed at constant room temperature (25 °C), and a 60-minute interval was allocated for patient acclimatization after the removal of his or her socks and shoes. As shown in Supplementary Figure S2c (right graph), a significant decrease in the differences of foot-pad temperature (°C = treated limb − untreated limb) was observed at 1 month after the gene transfer (P < 0.05, paired t-test).
PVR. As shown in Supplementary Table S3, 10 patients (excluding the two amputated cases) underwent constant PVR measurements. Seven of the 10 patients (cases 102, 201, 204, 304, 305, 403, and 405) showed negative PVR values at pretreatment, and six subjects (excluding case 102) occasionally exhibited significant appearance of PVR values after the gene transfer.
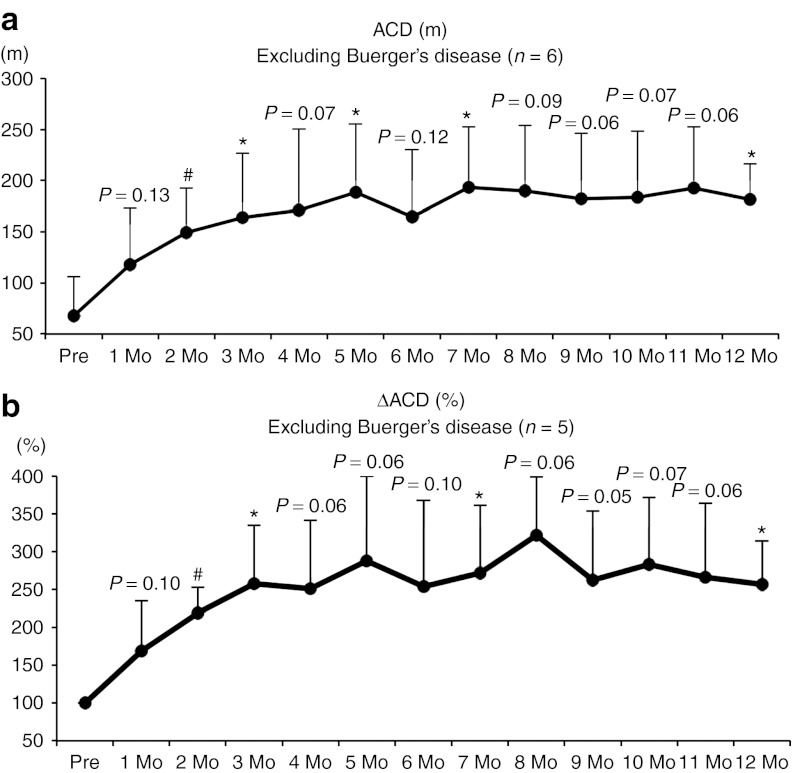
Walking ability assessed by treadmill. Eight patients (n = 6 for arteriosclerosis obliterans and n = 2 for TAO) were eligible for the flat treadmill test; the test was performed at 2.4 km/hour and terminated at 300 m to minimize the risk of cardiovascular complications. Regarding the absolute claudication distance (ACD)-based walking performance, all six of the patients who did not have TAO demonstrated steady and significant improvement (Figure 1a). Changes of ACD (ΔACD) were also observed in five of these patients (one patient was excluded due to a lack of pretreatment data because of very severe rest pain), and showed similar results (Figure 1b). Even when the data included the two cases of Buerger's disease (TAO), the trends of ACD and ΔACD were not influenced (Supplementary Figure S3a,b), suggesting that intramuscular injections of DVC1-0101 may be contributed to the improvement of walking performance in patients with PAD.
Figure 1.
Time course of walking activity examined by treadmill. (a) Time course of absolute claudication distance (ACD; m, n = 6). (b) Change of ACD (ΔACD; %, n = 5). *P < 0.05, #P < 0.01. The data for the two patients with Buerger's disease were excluded. Mo, month.
Case report
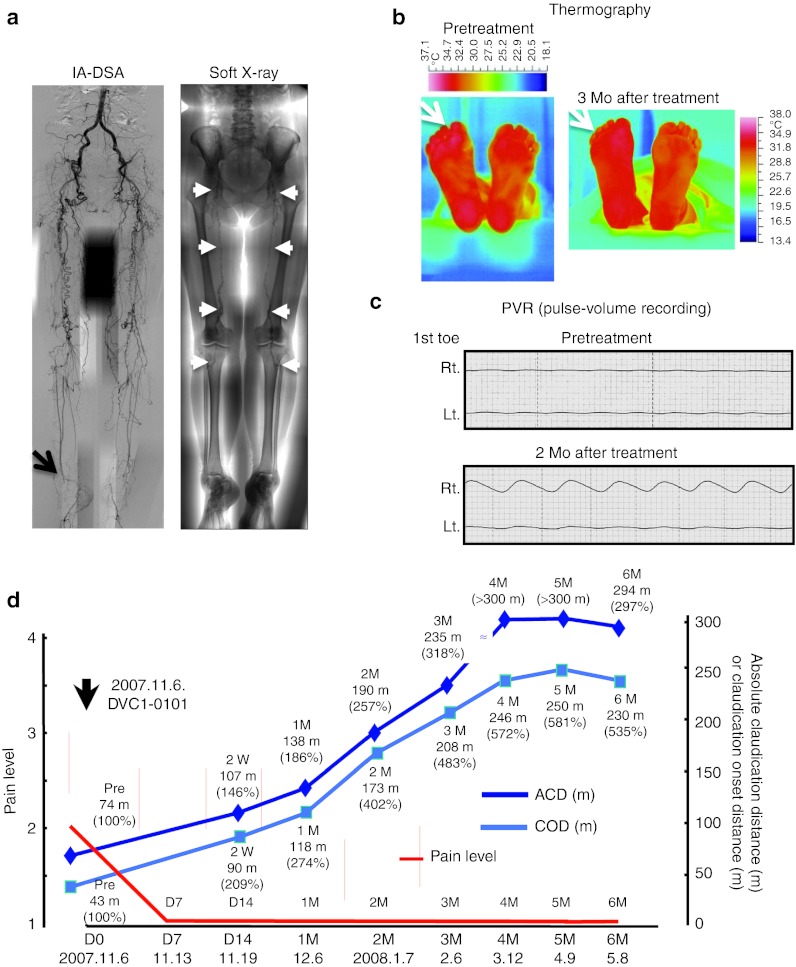
Figure 2 presents the findings for case 201, a 65-year-old Japanese woman at Stage 2 with both legs affected and with ~200 m of IC since 1993 due to PAD. Her symptoms had been stable with beraprost and cilostazol treatment for the past 14 years, and then she developed rest pain in the right foot, which was treated with nonsteroidal anti-inflammatory drugs. Her angiogram demonstrated complete obstruction of the whole superficial femoral to popliteal arteries and all main arteries at the ankle level in both limbs (Figure 2a, left panel, arrow). Severe calcification throughout the superficial femoral to popliteal arteries on soft X-ray (Figure 2a, right panel, white arrows) and insufficient size of both saphenous veins suggested the difficulty of surgical management; therefore, she was enrolled in the present trial on 22 October 2007.
Figure 2.
The case of a 65-year-old Japanese woman (case no. 201 at Stage 2). Both limbs were affected, with ~200 m of intermittent claudication since 1993 due to PAD. Her symptoms had been stable for 14 years, and then she developed rest pain in the right foot, which was treated by NSAIDs. (a) Her angiogram (left panel) demonstrated complete obstruction of the whole superficial femoral to popliteal arteries and all main arteries at the ankle level in both limbs. Severe calcification was seen throughout the superficial femoral to popliteal arteries on soft X-ray (right panel, white arrows). (b) A thermographic examination. A right limb-specific increase of foot temperature (white arrows) is apparent at 3 months after the gene transfer. (c) A pulse-volume recording (PVR). Right first toe-specific pulsation was seen. (d) Clinical course. Her rest pain completely disappeared within 1 week after the gene transfer, and her treadmill examination demonstrated the linear increase of absolute claudication distance (ACD) and claudication onset distance (COD) at 4 months after the gene transfer. These improvements of clinical symptoms have been maintained at 5 years post-gene transfer. IA-DSA, intraarterial-digital subtraction angiography; Mo, month; NSAID, nonsteroidal anti-inflammatory drug; PAD, peripheral arterial disease.
She received DVC1-0101 at 30 sites of the right leg (10 sites at the upper and lower thigh and calf, respectively, for a total of 1.63 × 108 cell infection units (ciu)). Her experimental course was uneventful. A thermographic examination demonstrated a right leg-specific increase of foot temperature (Figure 2b, white arrows), and a PVR showed right first toe-specific pulsation as well (Figure 2c). Her rest pain completely disappeared within 1 week after the gene transfer, and her treadmill examination demonstrated a linear increase of ACD and claudication onset distance within 4 months after the gene transfer. These improvements of clinical symptoms have been maintained nearly 5 years later.
Discussion
We here report the first-in-human Phase I/IIa dose-escalating clinical study of a new hFGF-2 gene expressing rSeV-based RNA gene drug, DVC1-0101, to treat PAD patients. The key findings of this study were: (i) the intramuscular administration of DVC1-0101, up to 5 × 109 ciu/60 kg, to an ischemic limb of CLI patients was feasible and well tolerated, and demonstrated no drug-related SAE within the 6-month study period; (ii) even though three patients transiently exhibited a significant amount of viral genome in whole blood, significant hemagglutinating activity was shown in neither urine nor whole blood; (iii) neither a serious inflammatory reaction nor significant leakage of angiogenic factors to the circulation was detected during the experimental course, except for one patient (case 103) who showed infected progressive gangrene; and (iv) representative and stable improvement was seen in the rest pain scores and ACD values. These findings suggest that DVC1-0101 may be safe and effective for the treatment of PAD patients, and indicate that further investigations are warranted.
Since the pioneering preclinical report describing the increase of capillary and collateral vessels after the administration of an angiogenic growth factor (VEGF) in preclinical animal models,2 the clinical benefits of therapeutic angiogenesis to treat PAD have been intensively examined worldwide with the use of various proteins and genes. The majority of the early clinical studies on IC and CLI have suggested the possible clinical efficacy of therapeutic angiogenesis; however, almost all of the double-blinded and placebo-controlled trials of protein- or gene-based therapeutic angiogenesis have failed to show definitive clinical benefit.
At the initial stage of the development of DVC1-0101, we hypothesized that the peak expression and local concentration of the therapeutic gene in ischemic muscle might be a key factor for determining the efficacy; therefore, we used rSeV as the vector. In fact, using a severe ischemic limb model of balb/c nu/nu mice, we discovered that the combination of rSeV and FGF-2 showed a higher limb-salvaging effect compared with other combinations (i.e., rSeV expressing FGF-1, VEGF, or angiopoietin-1, as well as plasmid DNA expressing VEGF, angiopoietin-1, or HGF; Y. Yonemitsu et al., unpublished data). In addition, a preclinical dose-efficacy study demonstrated that 14.3- to 68.8-fold increase (compared with the baseline) in the local FGF-2 content that was obtained by administering 3 × 106 to 1 × 107 ciu/30 g mouse had the maximum limb-salvaging effect.17 Such strong expression in muscles is likely to be the main reason for the safety advantage of rSeV in clinical settings, because the requirement of a lesser amount of vectors can avoid the vector-related activation of innate immunity, in addition to minimizing the leakage of vector particles into the blood circulation. The results of the present study support this notion.
Regarding the efficacy parameters, we employed a number of measurements, including hemodynamic surrogates, because there has been no definitive report indicating which parameter(s) are sensitive and optimal for determining the clinical outcomes of angiogenic trials. Importantly, the present findings suggest that DVC1-0101 may improve PAD patients' walking performance as examined by treadmill; however, we have to be cautious when interpreting this data, since it has been considered that the measurement of walking function in a number of previous angiogenesis trials was compromised by a placebo effect. First, the data in Figure 1 were analyzed to suggest the possible efficacy in improving the walking function. Therefore, additional placebo-controlled studies with sufficient number of patients would be needed. Second, the placebo group usually exhibits ~30% of improvement of walking ability in therapeutic angiogenesis trials.4,5,20 Such a placebo effect may be explained, for example, by some effects due to injection trauma for gene transfer and to immune-related responses against vectors. This may not be likely, however, because a similar level of placebo-related improvements was also observed by pharmacotherapies, including treatment with the first-line drug cilostazol.20 In addition, the improvement of walking performance by DVC1-0101 obtained in this study reached ~240–300% (Figure 1b), suggesting much stronger activity than that seen by cilostazol.21 Since walking performance based on a treadmill test is recognized as a feasible end point in PAD trials for IC,21,22 the next well-organized placebo-controlled study may make it clear whether DVC1-0101 is truly effective for patients with IC.
In summary, we observed no major adverse consequences of DVC1-0101 treatment during over the months of follow-up period after 30 direct intramuscular injections to patients with CLI. The dose-escalation schema were completed as planned. Further studies with large numbers of patients and a placebo control will be needed to establish both the safety and efficacy of DVC1-0101.
Materials and Methods
DVC1-0101 (rSeV/dF-hFGF2): an F-gene–deleted nontransmissible rSeV vector expressing the human FGF-2 gene. DVC1-0101 is an F-gene–deleted nontransmissible rSeV based on a Z-strain encoding negative-stranded full-length complementary RNA of human FGF-2 at the upstream of gene encoding N-protein (rSeV/dF-hFGF2). The DVC1-0101 used in the present study was constructed at BioReliance (Starling, UK) in accordance with good manufacturing practice regulations that meet the good manufacturing practice standards of the USA, European Union, and Japan. In brief, LLC-MK2 cells were transfected with a plasmid mixture containing each plasmid: pSeV+18/dF-hFGF2, pGEM-NP, pGEM-P, and pGEM-L. The transfected cells were maintained for 3 hours, then washed and incubated for 60 hours in minimum essential medium containing ara-C. The cells were collected and lysed, and the lysate solution was incubated on the stably F-expressing LLC-MK2 cells in a 24-well plate. Twenty-four hours later, the cells were washed and incubated in minimum essential medium containing ara-C and trypsin. Purification was achieved using column chromatography and filtration to concentrate the vector. The virus yield is expressed in ciu, as described previously.16
Study design. The trial was conducted as a single-center Phase I/IIa, open-labeled, dose-escalating study investigating the safety, bioactivity, and potential clinical benefits of single intramuscular injections of DVC1-0101. The study design, protocol, and informed consent forms were approved by the institutional ethical committees, the Health Sciences Council of the Japan MHLW, and Biosafety Committees according to national regulations. A Data Safety and Monitoring Board provided an independent review of the safety data and of accordance of the defined inclusion criteria for each patient. This study was conducted, recorded, and reported in compliance with the principles of good clinical practice regulations with the support of the Clinical and Translational Research Center of Kyushu University Hospital and an external Contract Research Organization (EPS, Tokyo, Japan).
Patient cohort. Patients were enrolled when they (i) had chronic CLI including rest pain or ischemic ulcer that was resistant to standard medication for at least 2 weeks, (ii) were not candidates for surgical or catheter revascularization based on usual practice standards, (iii) did not have cancer or a history of cancer within the prior 5 years, (iv) did not have any significant vital organ dysfunction, (v) did not have any immunosuppressive agent, (vi) did not have any active inflammatory disease, and (vii) did not have proliferative or unstable retinopathy. The patient eligibility criteria and possible ethical discussion points of this trial based on the Declaration of Helsinki were confirmed by an independent committee for gene therapy clinical trials and the institutional review board at Kyushu University, which was approved by the MHLW. Patients participated in the trial on an outpatient basis, except for ~2 weeks just before and after the gene transfer. The baseline characteristics of the patients are summarized in Table 1.
Treatment and follow-up. On day 0, 30 intramuscular injections were performed in a single leg under bolus epidural anesthesia (0.5 ml/injection = 15 ml/patient). The sites of injection differed at each administration, and were selected according to angiogram findings and the investigator's discretion (Supplementary Table S1). Patient follow-up to collect the assessments was done monthly up to 6 months (±7 days at each visit), and the follow-up to monitor the occurrence of SAEs was continued to 5 years after the gene transfer. Rest pain was scored based on the frequency of intake of analgesics within 24 hours of each interview (mainly concerning nonsteroidal anti-inflammatory drugs) and was categorized as 1: completely pain-free without analgesic, 2: feel pain but no need for analgesic, 3: feel pain and sometimes need analgesic(s), 4: use analgesic(s) constantly to control the pain, and 5: uncontrollable pain even though constant use of analgesic(s).
Virus shedding. Hemagglutinating activity was determined using chicken RBCs as described previously.23 For the detection of genome copies, total RNA was extracted from whole blood or urine samples at each time point. The target viral sequence was first amplified by reverse transcription--PCR using the following primer set: forward, 5′-ATCACTGCCACCCAGAAGACT-3′ reverse, 5′-ACCAGGAAATGAG-CTTGACAA-3′. Real-time monitoring of the amplification of target genes was done by a Sequence Detection System, model 7000 (Applied Biosystems, Tokyo, Japan), according to the manufacturer's instructions for Taq-Man methods. The oligonucleotide sequences of the PCR primers and TaqMan probes were as follows: forward, 5′-ACTTGGATCCAAAACAGGACCTGGG-3′ reverse, 5′-TGTA TCGAAG-GTGCTCAACAACCCG-3′ and probe: 5′-FAM-CATCGCGG CCGCAGATCTTCAC-GATGGC-TAMRA-3′. The detection limit was 0.1 genome copy/µl for all assays.
ELISA. Angiogenic factors in serum and plasma were determined using Quantikine Immunoassay systems for human FGF-2, VEGF, and HGF (R&D Systems, Minneapolis, MN) according to the manufacturer's instructions. Data are expressed as pg/ml.
Statistical analysis. All data are expressed as means ± SD. The data were examined using paired t-tests, when appropriate, in comparison with baseline values. A probability value of P < 0.05 was considered significant. All data were analyzed by the independent Data Center at Kyushu University Hospital.
SUPPLEMENTARY MATERIAL Figure S1. Time course of proinflammatory markers and circulating angiogenic factors. Figure S2. Time course of rest pain and hemodynamic surrogates. Figure S3. Time course of walking activity examined by treadmill. Table S1. Patients cohort. Table S2. Virus shedding. Table S3. Pulse-volume recording.
Acknowledgments
The authors thank Keiko Kikutake, Michi Onizuka, and Nozomi Kataoka for their clinical research coordination, Akiko Kanaya for her help with drug preservation, Mio Yoneda-Maehara for her help with the trial paperwork, and Rio Yamauchi, Takeshi Maruoka, and Minoru Ido at EPS Co. for their help with the trial management. KN International Co., Ltd. assisted with the preparation of the manuscript. This study was supported in part by a grant for Translational Research from Japanese Ministry of Health, Labor, and Welfare to Y.Y. Dr Yonemitsu is a member of the Scientific Advisory Board of DNAVEC Corporation. The other authors declared no conflict of interest.
Supplementary Material
References
- Weitz JI, Byrne J, Clagett GP, Farkouh ME, Porter JM, Sackett DL.et al. (1996Diagnosis and treatment of chronic arterial insufficiency of the lower extremities: a critical review Circulation 943026–3049. [DOI] [PubMed] [Google Scholar]
- Takeshita S, Zheng LP, Brogi E, Kearney M, Pu LQ, Bunting S.et al. (1994Therapeutic angiogenesis. A single intraarterial bolus of vascular endothelial growth factor augments revascularization in a rabbit ischemic hind limb model J Clin Invest 93662–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarous DF, Unger EF, Epstein SE, Stine A, Arevalo JL, Chew EY.et al. (2000Basic fibroblast growth factor in patients with intermittent claudication: results of a phase I trial J Am Coll Cardiol 361239–1244. [DOI] [PubMed] [Google Scholar]
- Lederman RJ, Mendelsohn FO, Anderson RD, Saucedo JF, Tenaglia AN, Hermiller JB, TRAFFIC Investigators et al. Therapeutic angiogenesis with recombinant fibroblast growth factor-2 for intermittent claudication (the TRAFFIC study): a randomised trial. Lancet. 2002;359:2053–2058. doi: 10.1016/s0140-6736(02)08937-7. [DOI] [PubMed] [Google Scholar]
- Rajagopalan S, Mohler ER., 3rd, , Lederman RJ, Mendelsohn FO, Saucedo JF, Goldman CK.et al. (2003Regional angiogenesis with vascular endothelial growth factor in peripheral arterial disease: a phase II randomized, double-blind, controlled study of adenoviral delivery of vascular endothelial growth factor 121 in patients with disabling intermittent claudication Circulation 1081933–1938. [DOI] [PubMed] [Google Scholar]
- Comerota AJ, Throm RC, Miller KA, Henry T, Chronos N, Laird J.et al. (2002Naked plasmid DNA encoding fibroblast growth factor type 1 for the treatment of end-stage unreconstructible lower extremity ischemia: preliminary results of a phase I trial J Vasc Surg 35930–936. [DOI] [PubMed] [Google Scholar]
- Nikol S, Baumgartner I, Van Belle E, Diehm C, Visoná A, Capogrossi MC, TALISMAN 201 investigators et al. Therapeutic angiogenesis with intramuscular NV1FGF improves amputation-free survival in patients with critical limb ischemia. Mol Ther. 2008;16:972–978. doi: 10.1038/mt.2008.33. [DOI] [PubMed] [Google Scholar]
- Belch J, Hiatt WR, Baumgartner I, Driver IV, Nikol S, Norgren L, TAMARIS Committees and Investigators et al. Effect of fibroblast growth factor NV1FGF on amputation and death: a randomised placebo-controlled trial of gene therapy in critical limb ischaemia. Lancet. 2011;377:1929–1937. doi: 10.1016/S0140-6736(11)60394-2. [DOI] [PubMed] [Google Scholar]
- Yonemitsu Y, Kitson C, Ferrari S, Farley R, Griesenbach U, Judd D.et al. (2000Efficient gene transfer to airway epithelium using recombinant Sendai virus Nat Biotechnol 18970–973. [DOI] [PubMed] [Google Scholar]
- Masaki I, Yonemitsu Y, Yamashita A, Sata S, Tanii M, Komori K.et al. (2002Angiogenic gene therapy for experimental critical limb ischemia: acceleration of limb loss by overexpression of vascular endothelial growth factor 165 but not of fibroblast growth factor-2 Circ Res 90966–973. [DOI] [PubMed] [Google Scholar]
- Onimaru M, Yonemitsu Y, Tanii M, Nakagawa K, Masaki I, Okano S.et al. (2002Fibroblast growth factor-2 gene transfer can stimulate hepatocyte growth factor expression irrespective of hypoxia-mediated downregulation in ischemic limbs Circ Res 91923–930. [DOI] [PubMed] [Google Scholar]
- Tsutsumi N, Yonemitsu Y, Shikada Y, Onimaru M, Tanii M, Okano S.et al. (2004Essential role of PDGFRalpha-p70S6K signaling in mesenchymal cells during therapeutic and tumor angiogenesis in vivo: role of PDGFRalpha during angiogenesis Circ Res 941186–1194. [DOI] [PubMed] [Google Scholar]
- Kubo H, Cao R, Brakenhielm E, Mäkinen T, Cao Y., and, Alitalo K. Blockade of vascular endothelial growth factor receptor-3 signaling inhibits fibroblast growth factor-2-induced lymphangiogenesis in mouse cornea. Proc Natl Acad Sci USA. 2002;99:8868–8873. doi: 10.1073/pnas.062040199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onimaru M, Yonemitsu Y, Fujii T, Tanii M, Nakano T, Nakagawa K.et al. (2009VEGF-C regulates lymphangiogenesis and capillary stability by regulation of PDGF-B Am J Physiol Heart Circ Physiol 297H1685–H1696. [DOI] [PubMed] [Google Scholar]
- Fujii T, Yonemitsu Y, Onimaru M, Tanii M, Nakano T, Egashira K.et al. (2006Nonendothelial mesenchymal cell-derived MCP-1 is required for FGF-2-mediated therapeutic neovascularization: critical role of the inflammatory/arteriogenic pathway Arterioscler Thromb Vasc Biol 262483–2489. [DOI] [PubMed] [Google Scholar]
- Li HO, Zhu YF, Asakawa M, Kuma H, Hirata T, Ueda Y.et al. (2000A cytoplasmic RNA vector derived from nontransmissible Sendai virus with efficient gene transfer and expression J Virol 746564–6569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda Y, Yonemitsu Y, Sakamoto T, Ishibashi T, Ueno H, Kato A.et al. (2002Recombinant Sendai virus-mediated gene transfer into adult rat retinal tissue: efficient gene transfer by brief exposure Exp Eye Res 7539–48. [DOI] [PubMed] [Google Scholar]
- Shoji T, Yonemitsu Y, Komori K, Tanii M, Itoh H, Sata S.et al. (2003Intramuscular gene transfer of FGF-2 attenuates endothelial dysfunction and inhibits intimal hyperplasia of vein grafts in poor-runoff limbs of rabbit Am J Physiol Heart Circ Physiol 285H173–H182. [DOI] [PubMed] [Google Scholar]
- Rutherford RB, Baker JD, Ernst C, Johnston KW, Porter JM, Ahn S.et al. (1997Recommended standards for reports dealing with lower extremity ischemia: revised version J Vasc Surg 26517–538. [DOI] [PubMed] [Google Scholar]
- Grossman PM, Mendelsohn F, Henry TD, Hermiller JB, Litt M, Saucedo JF.et al. (2007Results from a phase II multicenter, double-blind placebo-controlled study of Del-1 (VLTS-589) for intermittent claudication in subjects with peripheral arterial disease Am Heart J 153874–880. [DOI] [PubMed] [Google Scholar]
- Regensteiner JG, Ware JE, Jr, McCarthy WJ, Zhang P, Forbes WP, Heckman J.et al. (2002Effect of cilostazol on treadmill walking, community-based walking ability, and health-related quality of life in patients with intermittent claudication due to peripheral arterial disease: meta-analysis of six randomized controlled trials J Am Geriatr Soc 501939–1946. [DOI] [PubMed] [Google Scholar]
- Labs KH, Dormandy JA, Jaeger KA, Stuerzebecher CS., and, Hiatt WR. Transatlantic Conference on Clinical Trial Guidelines in Peripheral Arterial Disease: clinical trial methodology. Basel PAD Clinical Trial Methodology Group. Circulation. 1999;100:e75–e81. doi: 10.1161/01.cir.100.17.e75. [DOI] [PubMed] [Google Scholar]
- Yonemitsu Y., and, Kaneda Y.1999Hemagglutinating virus of Japan liposome-mediated gene delivery to vascular cells Baker AH.ed.). Molecular Biology of Vascular Diseases. Methods in Molecular Medicine Humana Press: Totowa, NJ; pp. 295–306. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.