Abstract
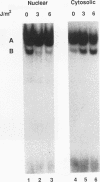
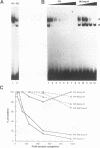
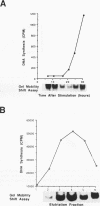
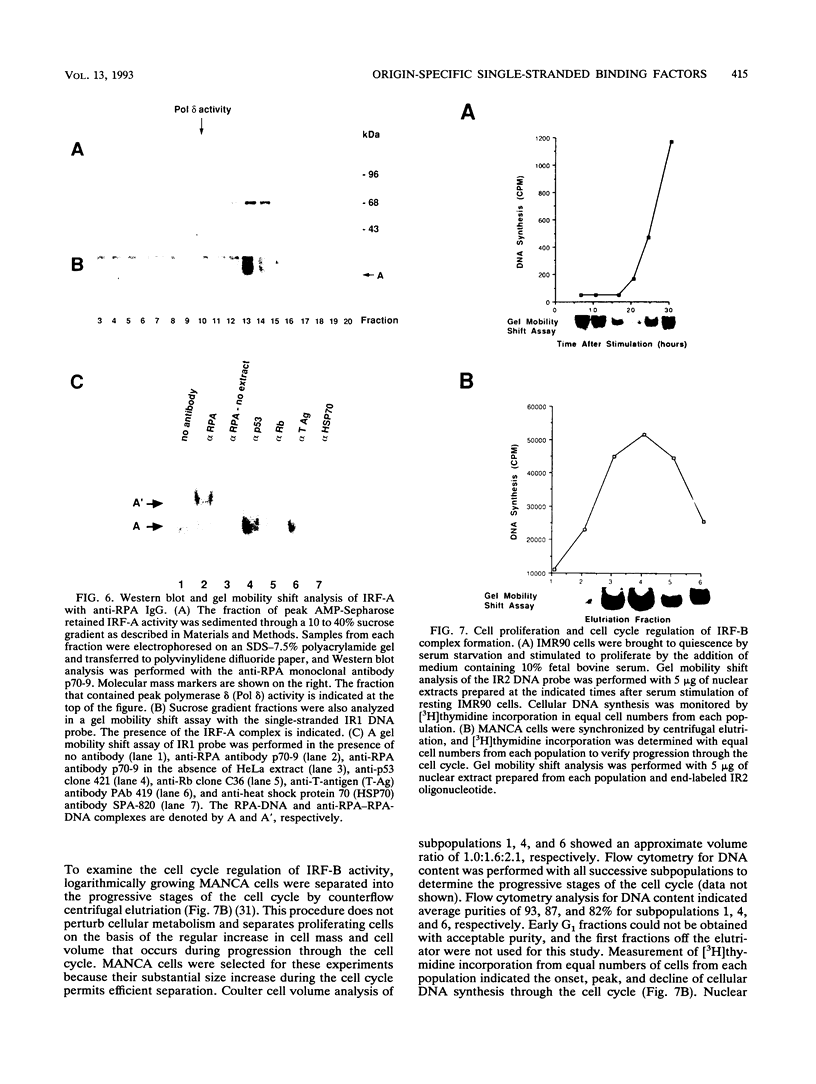
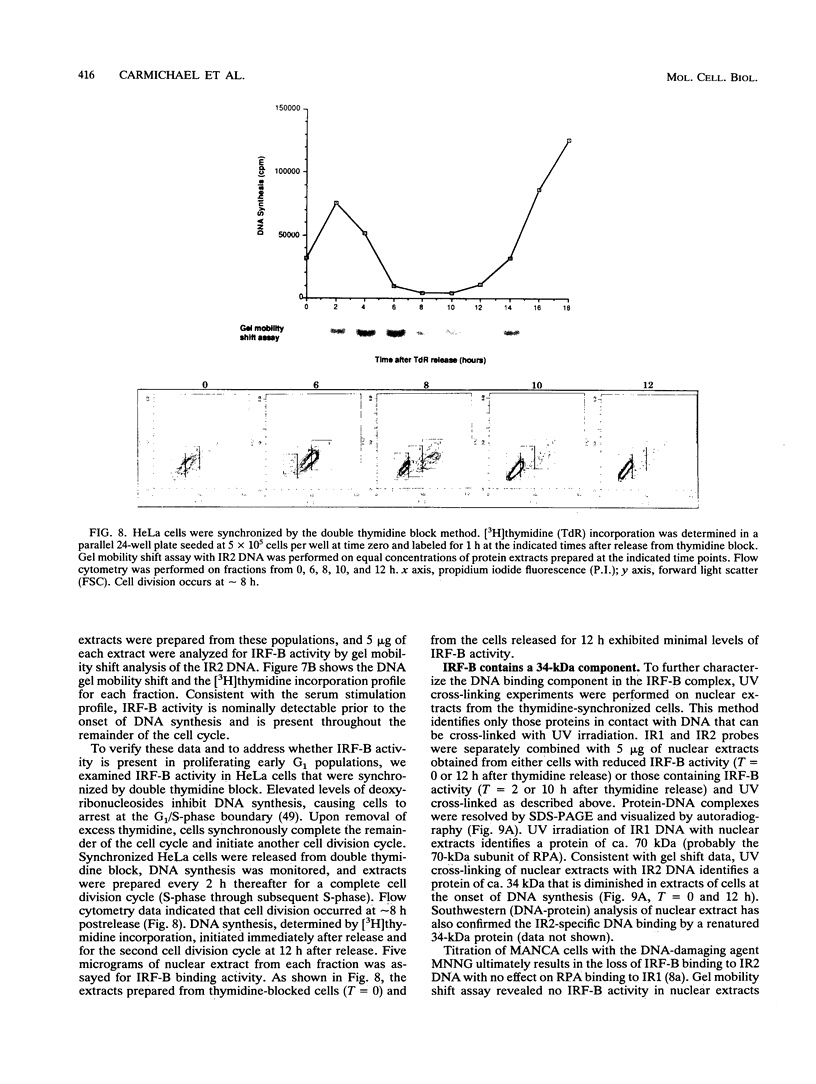
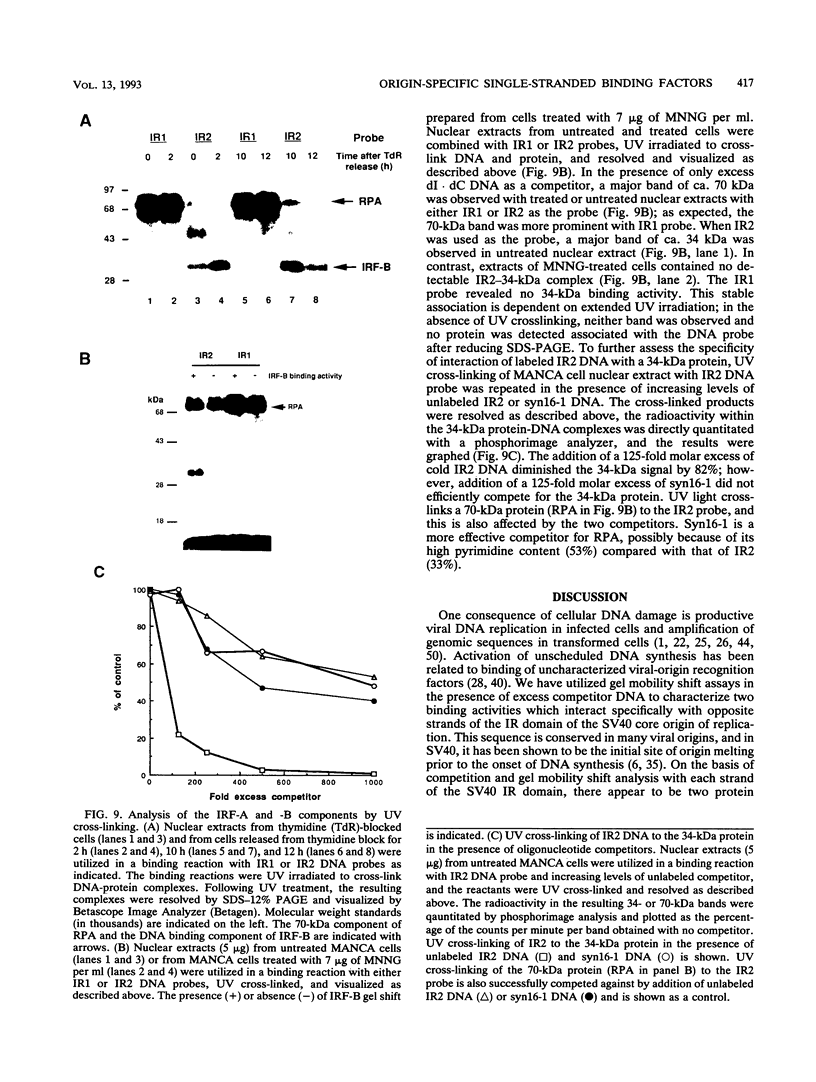
The inverted repeat domain (IR domain) within the simian virus 40 origin of replication is the site of initial DNA melting prior to the onset of DNA synthesis. The domain had previously been shown to be bound by a cellular factor in response to DNA damage. We demonstrate that two distinct cellular components bind opposite strands of the IR domain. Replication protein A (RPA), previously identified as a single-stranded DNA binding protein required for origin-specific DNA replication in vitro, is shown to have a preference for the pyrimidine-rich strand. A newly described component, IR factor B (IRF-B), specifically recognizes the opposite strand. IRF-B binding activity in nuclear extract varies significantly with cell proliferation and the cell cycle, so that binding of IRF-B to the IR domain is negatively correlated with the onset of DNA synthesis. Loss of IRF-B binding from the nucleus also occurs in response to cellular DNA damage. UV cross-linking indicates that the core binding component of IRF-B is a protein of ca. 34 kDa. We propose that RPA and IRF-B bind opposite strands of the IR domain and together may function in the regulation of origin activation.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aladjem M. I., Koltin Y., Lavi S. Enhancement of copper resistance and CupI amplification in carcinogen-treated yeast cells. Mol Gen Genet. 1988 Jan;211(1):88–94. doi: 10.1007/BF00338397. [DOI] [PubMed] [Google Scholar]
- Baer R., Bankier A. T., Biggin M. D., Deininger P. L., Farrell P. J., Gibson T. J., Hatfull G., Hudson G. S., Satchwell S. C., Séguin C. DNA sequence and expression of the B95-8 Epstein-Barr virus genome. Nature. 1984 Jul 19;310(5974):207–211. doi: 10.1038/310207a0. [DOI] [PubMed] [Google Scholar]
- Bergemann A. D., Johnson E. M. The HeLa Pur factor binds single-stranded DNA at a specific element conserved in gene flanking regions and origins of DNA replication. Mol Cell Biol. 1992 Mar;12(3):1257–1265. doi: 10.1128/mcb.12.3.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berko-Flint Y., Karby S., Hassin D., Lavi S. Carcinogen-induced DNA amplification in vitro: overreplication of the simian virus 40 origin region in extracts from carcinogen-treated CO60 cells. Mol Cell Biol. 1990 Jan;10(1):75–83. doi: 10.1128/mcb.10.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borowiec J. A., Hurwitz J. Localized melting and structural changes in the SV40 origin of replication induced by T-antigen. EMBO J. 1988 Oct;7(10):3149–3158. doi: 10.1002/j.1460-2075.1988.tb03182.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Chang L. M. DNA polymerases from bakers' yeast. J Biol Chem. 1977 Mar 25;252(6):1873–1880. [PubMed] [Google Scholar]
- Chodosh L. A., Carthew R. W., Sharp P. A. A single polypeptide possesses the binding and transcription activities of the adenovirus major late transcription factor. Mol Cell Biol. 1986 Dec;6(12):4723–4733. doi: 10.1128/mcb.6.12.4723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross F., Roberts J., Weintraub H. Simple and complex cell cycles. Annu Rev Cell Biol. 1989;5:341–396. doi: 10.1146/annurev.cb.05.110189.002013. [DOI] [PubMed] [Google Scholar]
- Crute J. J., Wahl A. F., Bambara R. A. Purification and characterization of two new high molecular weight forms of DNA polymerase delta. Biochemistry. 1986 Jan 14;25(1):26–36. doi: 10.1021/bi00349a005. [DOI] [PubMed] [Google Scholar]
- Cunningham T. P., Pipas J. M. Simian agent 12 is a BK virus-like papovavirus which replicates in monkey cells. J Virol. 1985 May;54(2):483–492. doi: 10.1128/jvi.54.2.483-492.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Din S., Brill S. J., Fairman M. P., Stillman B. Cell-cycle-regulated phosphorylation of DNA replication factor A from human and yeast cells. Genes Dev. 1990 Jun;4(6):968–977. doi: 10.1101/gad.4.6.968. [DOI] [PubMed] [Google Scholar]
- Ehrfeld A., Planas-Bohne F., Lücke-Huhle C. Amplification of oncogenes and integrated SV40 sequences in mammalian cells by the decay of incorporated iodine-125. Radiat Res. 1986 Oct;108(1):43–51. [PubMed] [Google Scholar]
- Fairman M. P., Stillman B. Cellular factors required for multiple stages of SV40 DNA replication in vitro. EMBO J. 1988 Apr;7(4):1211–1218. doi: 10.1002/j.1460-2075.1988.tb02933.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogel M., Sachs L. Induction of virus synthesis in polyoma transformed cells by ultraviolet light and mitomycin C. Virology. 1970 Jan;40(1):174–177. doi: 10.1016/0042-6822(70)90391-0. [DOI] [PubMed] [Google Scholar]
- Frisque R. J., Bream G. L., Cannella M. T. Human polyomavirus JC virus genome. J Virol. 1984 Aug;51(2):458–469. doi: 10.1128/jvi.51.2.458-469.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamatake R. K., Hasegawa H., Clark A. B., Bebenek K., Kunkel T. A., Sugino A. Purification and characterization of DNA polymerase II from the yeast Saccharomyces cerevisiae. Identification of the catalytic core and a possible holoenzyme form of the enzyme. J Biol Chem. 1990 Mar 5;265(7):4072–4083. [PubMed] [Google Scholar]
- Kim C., Snyder R. O., Wold M. S. Binding properties of replication protein A from human and yeast cells. Mol Cell Biol. 1992 Jul;12(7):3050–3059. doi: 10.1128/mcb.12.7.3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinberger T., Etkin S., Lavi S. Carcinogen-mediated methotrexate resistance and dihydrofolate reductase amplification in Chinese hamster cells. Mol Cell Biol. 1986 Jun;6(6):1958–1964. doi: 10.1128/mcb.6.6.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lambert M. E., Gattoni-Celli S., Kirschmeier P., Weinstein I. B. Benzo[a]pyrene induction of extrachromosomal viral DNA synthesis in rat cells transformed by polyoma virus. Carcinogenesis. 1983;4(5):587–593. doi: 10.1093/carcin/4.5.587. [DOI] [PubMed] [Google Scholar]
- Lambert M. E., Pellegrini S., Gattoni-Celli S., Weinstein I. B. Carcinogen induced asynchronous replication of polyoma DNA is mediated by a trans-acting factor. Carcinogenesis. 1986 Jun;7(6):1011–1017. doi: 10.1093/carcin/7.6.1011. [DOI] [PubMed] [Google Scholar]
- Lavi S. Carcinogen-mediated amplification of viral DNA sequences in simian virus 40-transformed Chinese hamster embryo cells. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6144–6148. doi: 10.1073/pnas.78.10.6144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lücke-Huhle C., Herrlich P. Alpha-radiation-induced amplification of integrated SV40 sequences is mediated by a trans-acting mechanism. Int J Cancer. 1987 Jan 15;39(1):94–98. doi: 10.1002/ijc.2910390117. [DOI] [PubMed] [Google Scholar]
- Lücke-Huhle C., Mai S., Herrlich P. UV-induced early-domain binding factor as the limiting component of simian virus 40 DNA amplification in rodent cells. Mol Cell Biol. 1989 Nov;9(11):4812–4818. doi: 10.1128/mcb.9.11.4812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lücke-Huhle C., Pech M., Herrlich P. Selective gene amplification in mammalian cells after exposure to 60Co gamma rays, 241Am alpha particles, or uv light. Radiat Res. 1986 Jun;106(3):345–355. [PubMed] [Google Scholar]
- Marraccino R. L., Wahl A. F., Keng P. C., Lord E. M., Bambara R. A. Cell cycle dependent activities of DNA polymerases alpha and delta in Chinese hamster ovary cells. Biochemistry. 1987 Dec 1;26(24):7864–7870. doi: 10.1021/bi00398a049. [DOI] [PubMed] [Google Scholar]
- Morris G. F., Mathews M. B. Regulation of proliferating cell nuclear antigen during the cell cycle. J Biol Chem. 1989 Aug 15;264(23):13856–13864. [PubMed] [Google Scholar]
- Nomura S., Oishi M. UV Irradiation induces an activity which stimulates Simian virus 40 rescue upon cell fusion. Mol Cell Biol. 1984 Jun;4(6):1159–1162. doi: 10.1128/mcb.4.6.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh S. J., Chittenden T., Levine A. J. Identification of cellular factors that bind specifically to the Epstein-Barr virus origin of DNA replication. J Virol. 1991 Jan;65(1):514–519. doi: 10.1128/jvi.65.1.514-519.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons R., Anderson M. E., Tegtmeyer P. Three domains in the simian virus 40 core origin orchestrate the binding, melting, and DNA helicase activities of T antigen. J Virol. 1990 Feb;64(2):509–518. doi: 10.1128/jvi.64.2.509-518.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riordan J. R., Deuchars K., Kartner N., Alon N., Trent J., Ling V. Amplification of P-glycoprotein genes in multidrug-resistant mammalian cell lines. 1985 Aug 29-Sep 4Nature. 316(6031):817–819. doi: 10.1038/316817a0. [DOI] [PubMed] [Google Scholar]
- Roberts J. M., D'Urso G. An origin unwinding activity regulates initiation of DNA replication during mammalian cell cycle. Science. 1988 Sep 16;241(4872):1486–1489. doi: 10.1126/science.2843984. [DOI] [PubMed] [Google Scholar]
- Ronai Z. A., Lambert M. E., Johnson M. D., Okin E., Weinstein I. B. Induction of asynchronous replication of polyoma DNA in rat cells by ultraviolet irradiation and the effects of various inhibitors. Cancer Res. 1987 Sep 1;47(17):4565–4570. [PubMed] [Google Scholar]
- Ronai Z. A., Weinstein I. B. Identification of a UV-induced trans-acting protein that stimulates polyomavirus DNA replication. J Virol. 1988 Mar;62(3):1057–1060. doi: 10.1128/jvi.62.3.1057-1060.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronai Z. A., Weinstein I. B. Identification of ultraviolet-inducible proteins that bind to a TGACAACA sequence in the polyoma virus regulatory region. Cancer Res. 1990 Sep 1;50(17):5374–5381. [PubMed] [Google Scholar]
- Roninson I. B., Abelson H. T., Housman D. E., Howell N., Varshavsky A. Amplification of specific DNA sequences correlates with multi-drug resistance in Chinese hamster cells. Nature. 1984 Jun 14;309(5969):626–628. doi: 10.1038/309626a0. [DOI] [PubMed] [Google Scholar]
- Schimke R. T. Gene amplification in cultured animal cells. Cell. 1984 Jul;37(3):705–713. doi: 10.1016/0092-8674(84)90406-9. [DOI] [PubMed] [Google Scholar]
- Schimke R. T. Gene amplification, drug resistance, and cancer. Cancer Res. 1984 May;44(5):1735–1742. [PubMed] [Google Scholar]
- Schlehofer J. R., Ehrbar M., zur Hausen H. Vaccinia virus, herpes simplex virus, and carcinogens induce DNA amplification in a human cell line and support replication of a helpervirus dependent parvovirus. Virology. 1986 Jul 15;152(1):110–117. doi: 10.1016/0042-6822(86)90376-4. [DOI] [PubMed] [Google Scholar]
- Stark G. R., Wahl G. M. Gene amplification. Annu Rev Biochem. 1984;53:447–491. doi: 10.1146/annurev.bi.53.070184.002311. [DOI] [PubMed] [Google Scholar]
- Stow N. D., McMonagle E. C. Characterization of the TRS/IRS origin of DNA replication of herpes simplex virus type 1. Virology. 1983 Oct 30;130(2):427–438. doi: 10.1016/0042-6822(83)90097-1. [DOI] [PubMed] [Google Scholar]
- Syväoja J., Suomensaari S., Nishida C., Goldsmith J. S., Chui G. S., Jain S., Linn S. DNA polymerases alpha, delta, and epsilon: three distinct enzymes from HeLa cells. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6664–6668. doi: 10.1073/pnas.87.17.6664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tlsty T. D., Brown P. C., Schimke R. T. UV radiation facilitates methotrexate resistance and amplification of the dihydrofolate reductase gene in cultured 3T6 mouse cells. Mol Cell Biol. 1984 Jun;4(6):1050–1056. doi: 10.1128/mcb.4.6.1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tlsty T. D., White A., Sanchez J. Suppression of gene amplification in human cell hybrids. Science. 1992 Mar 13;255(5050):1425–1427. doi: 10.1126/science.1542791. [DOI] [PubMed] [Google Scholar]
- Traut W., Fanning E. Sequence-specific interactions between a cellular DNA-binding protein and the simian virus 40 origin of DNA replication. Mol Cell Biol. 1988 Feb;8(2):903–911. doi: 10.1128/mcb.8.2.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treger J. M., Hauser J., Dixon K. Molecular analysis of enhanced replication of UV-damaged simian virus 40 DNA in UV-treated mammalian cells. Mol Cell Biol. 1988 Jun;8(6):2428–2434. doi: 10.1128/mcb.8.6.2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl A. F., Geis A. M., Spain B. H., Wong S. W., Korn D., Wang T. S. Gene expression of human DNA polymerase alpha during cell proliferation and the cell cycle. Mol Cell Biol. 1988 Nov;8(11):5016–5025. doi: 10.1128/mcb.8.11.5016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein I. B. The origins of human cancer: molecular mechanisms of carcinogenesis and their implications for cancer prevention and treatment--twenty-seventh G.H.A. Clowes memorial award lecture. Cancer Res. 1988 Aug 1;48(15):4135–4143. [PubMed] [Google Scholar]
- Wobbe C. R., Weissbach L., Borowiec J. A., Dean F. B., Murakami Y., Bullock P., Hurwitz J. Replication of simian virus 40 origin-containing DNA in vitro with purified proteins. Proc Natl Acad Sci U S A. 1987 Apr;84(7):1834–1838. doi: 10.1073/pnas.84.7.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wold M. S., Kelly T. Purification and characterization of replication protein A, a cellular protein required for in vitro replication of simian virus 40 DNA. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2523–2527. doi: 10.1073/pnas.85.8.2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakobson B., Hrynko T. A., Peak M. J., Winocour E. Replication of adeno-associated virus in cells irradiated with UV light at 254 nm. J Virol. 1989 Mar;63(3):1023–1030. doi: 10.1128/jvi.63.3.1023-1030.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Lubbe J. L., Abrahams P. J., van Drunen C. M., van der Eb A. J. Enhanced induction of SV40 replication from transformed rat cells by fusion with UV-irradiated normal and repair-deficient human fibroblasts. Mutat Res. 1986 Mar;165(2):47–56. doi: 10.1016/0167-8817(86)90059-3. [DOI] [PubMed] [Google Scholar]