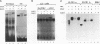Abstract
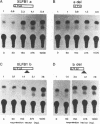
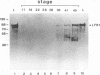
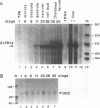
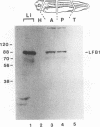
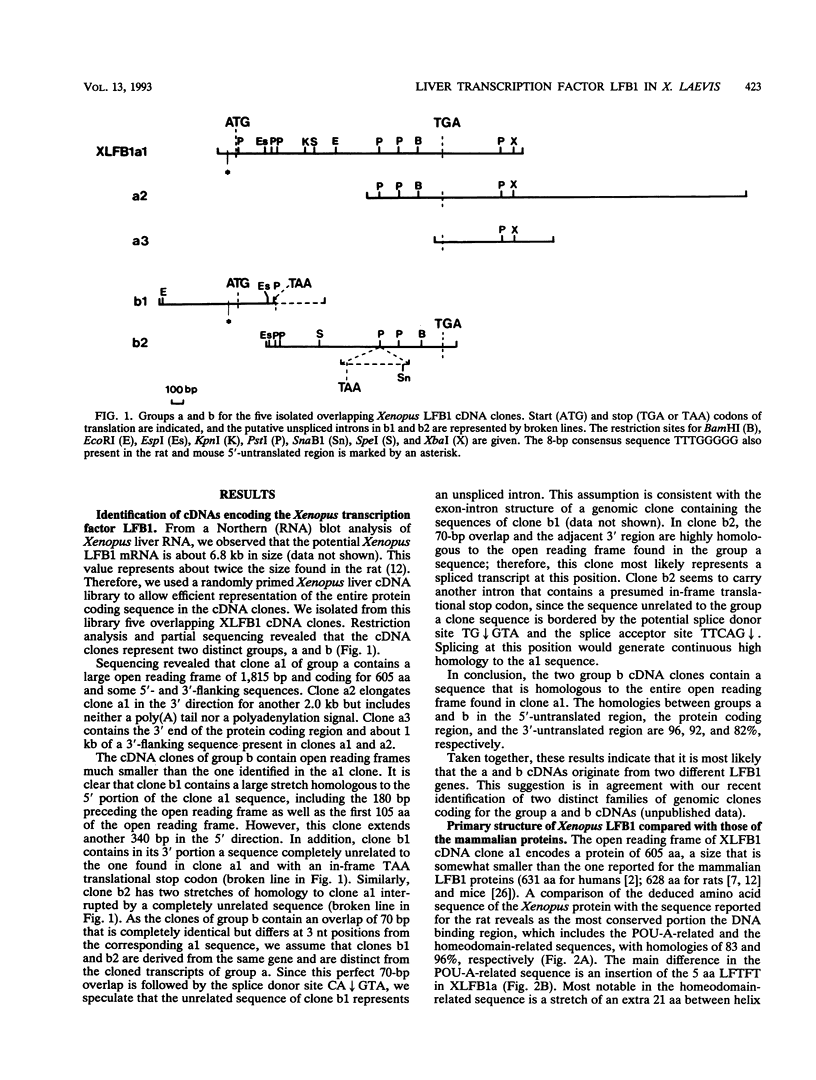
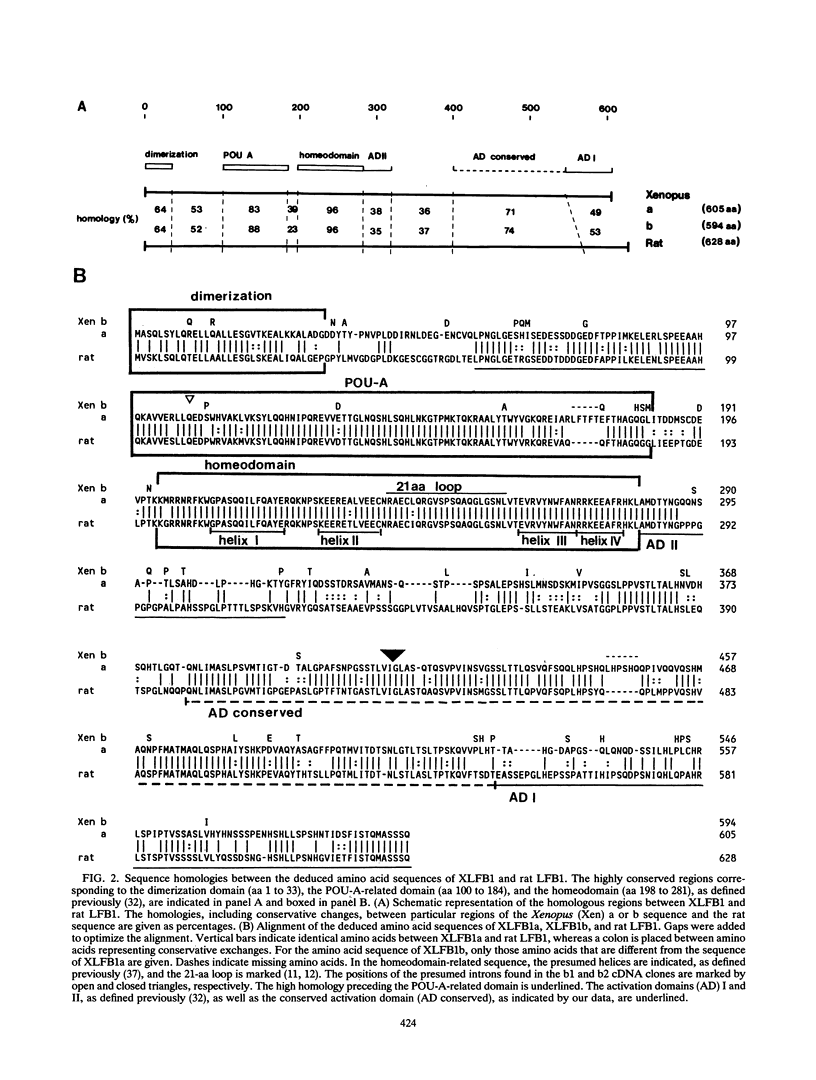
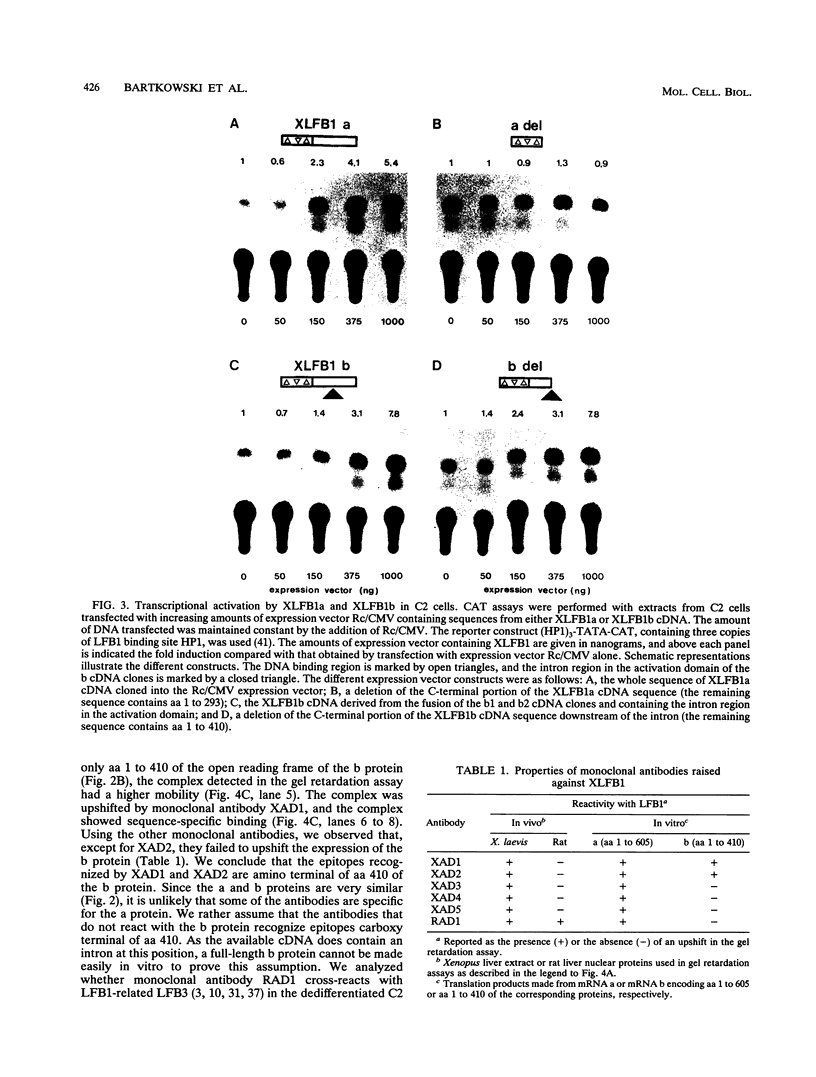
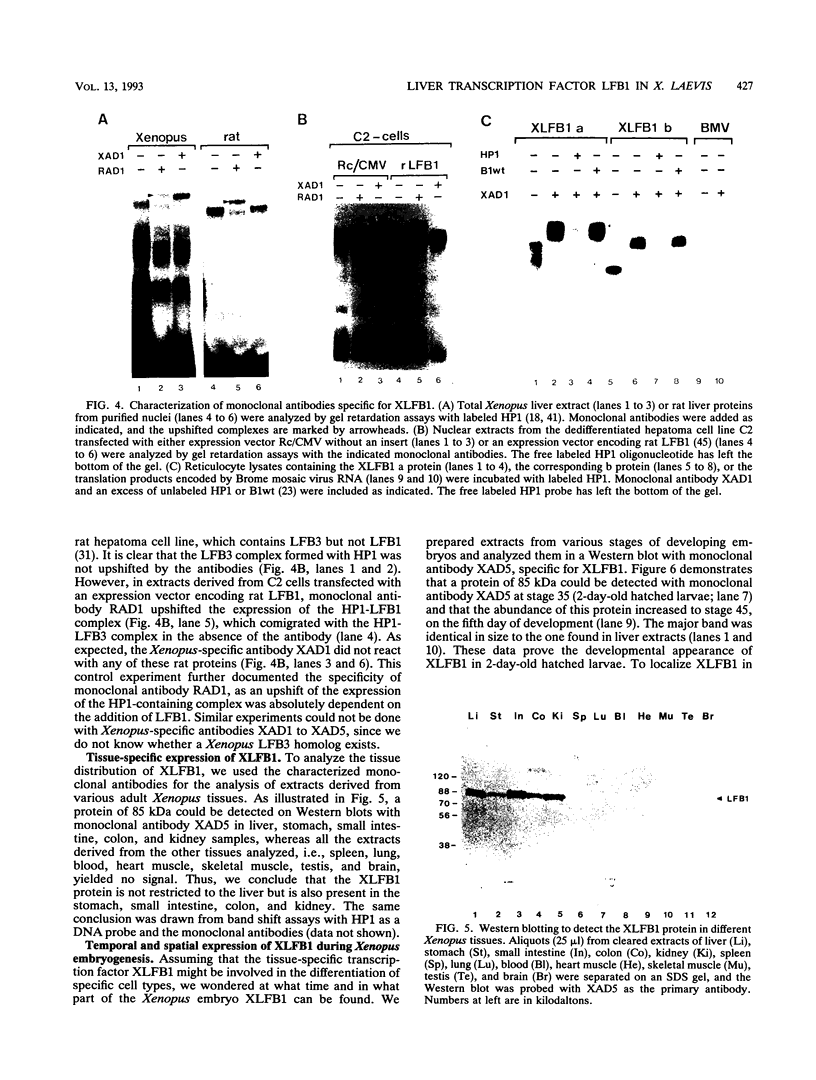
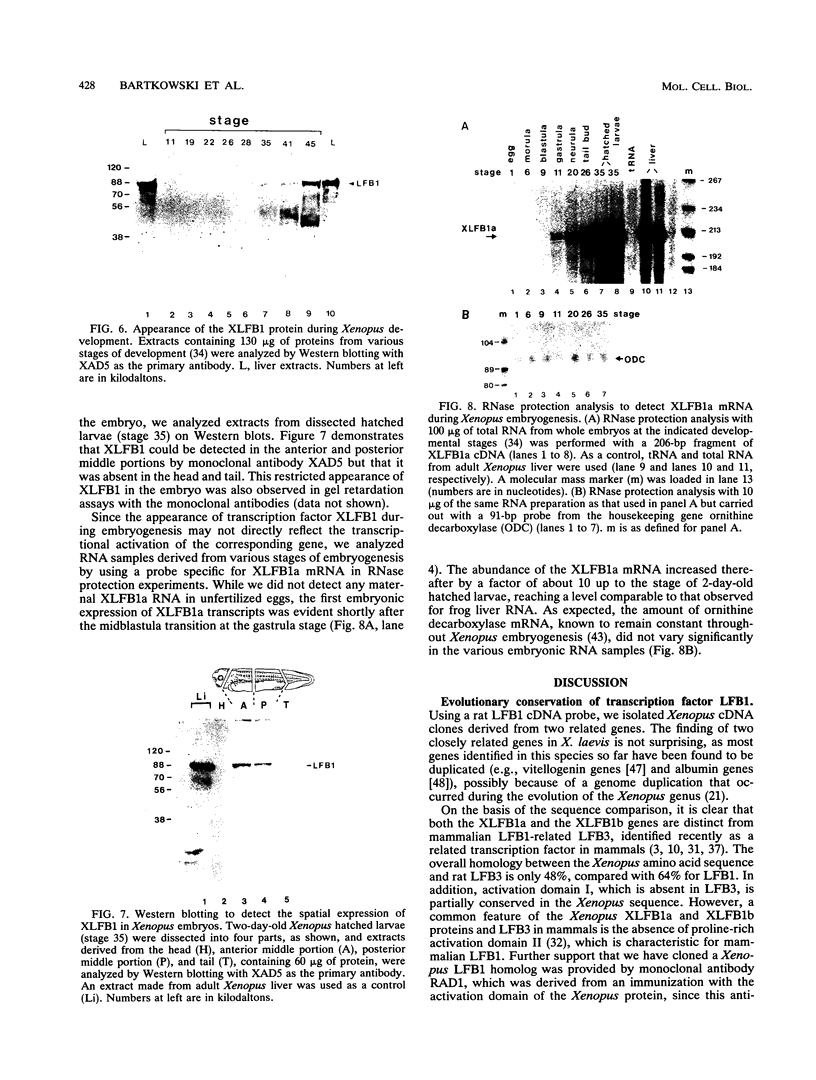
The transcription factor LFB1 (HNF1) was initially identified as a regulator of liver-specific gene expression in mammals. It interacts with the promoter element HP1, which is functionally conserved between mammals and amphibians, suggesting that a homologous factor, XLFB1, also exists in Xenopus laevis. To study the role of LFB1 in early development, we isolated two groups of cDNAs coding for this factor from a Xenopus liver cDNA library by using a rat LFB1 cDNA probe. A comparison of the primary structures of the Xenopus and mammalian proteins shows that the myosin-like dimerization helix, the POU-A-related domain, the homeo-domain-related region, and the serine/threonine-rich activation domain are conserved between X. laevis and mammals, suggesting that all these features typical for LFB1 are essential for function. Using monoclonal antibodies, we demonstrate that XLFB1 is present not only in the liver but also in the stomach, intestine, colon, and kidney. In an analysis of the expression of XLFB1 in the developing Xenopus embryo, XLFB1 transcripts appear at the gastrula stage. The XLFB1 protein can be identified in regions of the embryo in which the liver diverticulum, stomach, gut, and pronephros are localized. The early appearance of XLFB1 expression during embryogenesis suggests that the tissue-specific transcription factor XLFB1 is involved in the determination and/or differentiation of specific cell types during organogenesis.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andres A. C., Muellener D. B., Ryffel G. U. Persistence, methylation and expression of vitellogenin gene derivatives after injection into fertilized eggs of Xenopus laevis. Nucleic Acids Res. 1984 Mar 12;12(5):2283–2302. doi: 10.1093/nar/12.5.2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach I., Mattei M. G., Cereghini S., Yaniv M. Two members of an HNF1 homeoprotein family are expressed in human liver. Nucleic Acids Res. 1991 Jul 11;19(13):3553–3559. doi: 10.1093/nar/19.13.3553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumhueter S., Mendel D. B., Conley P. B., Kuo C. J., Turk C., Graves M. K., Edwards C. A., Courtois G., Crabtree G. R. HNF-1 shares three sequence motifs with the POU domain proteins and is identical to LF-B1 and APF. Genes Dev. 1990 Mar;4(3):372–379. doi: 10.1101/gad.4.3.372. [DOI] [PubMed] [Google Scholar]
- Blumenfeld M., Maury M., Chouard T., Yaniv M., Condamine H. Hepatic nuclear factor 1 (HNF1) shows a wider distribution than products of its known target genes in developing mouse. Development. 1991 Oct;113(2):589–599. doi: 10.1242/dev.113.2.589. [DOI] [PubMed] [Google Scholar]
- Cascio S., Zaret K. S. Hepatocyte differentiation initiates during endodermal-mesenchymal interactions prior to liver formation. Development. 1991 Sep;113(1):217–225. doi: 10.1242/dev.113.1.217. [DOI] [PubMed] [Google Scholar]
- Chouard T., Blumenfeld M., Bach I., Vandekerckhove J., Cereghini S., Yaniv M. A distal dimerization domain is essential for DNA-binding by the atypical HNF1 homeodomain. Nucleic Acids Res. 1990 Oct 11;18(19):5853–5863. doi: 10.1093/nar/18.19.5853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke J., Smith J. C. Measurement of developmental time by cells of early embryos. Cell. 1990 Mar 23;60(6):891–894. doi: 10.1016/0092-8674(90)90336-d. [DOI] [PubMed] [Google Scholar]
- Courtois G., Morgan J. G., Campbell L. A., Fourel G., Crabtree G. R. Interaction of a liver-specific nuclear factor with the fibrinogen and alpha 1-antitrypsin promoters. Science. 1987 Oct 30;238(4827):688–692. doi: 10.1126/science.3499668. [DOI] [PubMed] [Google Scholar]
- De Simone V., De Magistris L., Lazzaro D., Gerstner J., Monaci P., Nicosia A., Cortese R. LFB3, a heterodimer-forming homeoprotein of the LFB1 family, is expressed in specialized epithelia. EMBO J. 1991 Jun;10(6):1435–1443. doi: 10.1002/j.1460-2075.1991.tb07664.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finney M. The homeodomain of the transcription factor LF-B1 has a 21 amino acid loop between helix 2 and helix 3. Cell. 1990 Jan 12;60(1):5–6. doi: 10.1016/0092-8674(90)90708-m. [DOI] [PubMed] [Google Scholar]
- Frain M., Swart G., Monaci P., Nicosia A., Stämpfli S., Frank R., Cortese R. The liver-specific transcription factor LF-B1 contains a highly diverged homeobox DNA binding domain. Cell. 1989 Oct 6;59(1):145–157. doi: 10.1016/0092-8674(89)90877-5. [DOI] [PubMed] [Google Scholar]
- Gorski K., Carneiro M., Schibler U. Tissue-specific in vitro transcription from the mouse albumin promoter. Cell. 1986 Dec 5;47(5):767–776. doi: 10.1016/0092-8674(86)90519-2. [DOI] [PubMed] [Google Scholar]
- Hardon E. M., Frain M., Paonessa G., Cortese R. Two distinct factors interact with the promoter regions of several liver-specific genes. EMBO J. 1988 Jun;7(6):1711–1719. doi: 10.1002/j.1460-2075.1988.tb03000.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbomel P., Rollier A., Tronche F., Ott M. O., Yaniv M., Weiss M. C. The rat albumin promoter is composed of six distinct positive elements within 130 nucleotides. Mol Cell Biol. 1989 Nov;9(11):4750–4758. doi: 10.1128/mcb.9.11.4750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopwood N. D., Pluck A., Gurdon J. B. MyoD expression in the forming somites is an early response to mesoderm induction in Xenopus embryos. EMBO J. 1989 Nov;8(11):3409–3417. doi: 10.1002/j.1460-2075.1989.tb08505.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson P. F. Transcriptional activators in hepatocytes. Cell Growth Differ. 1990 Jan;1(1):47–52. [PubMed] [Google Scholar]
- Kaling M., Kugler W., Ross K., Zoidl C., Ryffel G. U. Liver-specific gene expression: A-activator-binding site, a promoter module present in vitellogenin and acute-phase genes. Mol Cell Biol. 1991 Jan;11(1):93–101. doi: 10.1128/mcb.11.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearney J. F., Radbruch A., Liesegang B., Rajewsky K. A new mouse myeloma cell line that has lost immunoglobulin expression but permits the construction of antibody-secreting hybrid cell lines. J Immunol. 1979 Oct;123(4):1548–1550. [PubMed] [Google Scholar]
- Klein-Hitpass L., Schorpp M., Wagner U., Ryffel G. U. An estrogen-responsive element derived from the 5' flanking region of the Xenopus vitellogenin A2 gene functions in transfected human cells. Cell. 1986 Sep 26;46(7):1053–1061. doi: 10.1016/0092-8674(86)90705-1. [DOI] [PubMed] [Google Scholar]
- Krieg P. A. Improved synthesis of full-length RNA probe at reduced incubation temperatures. Nucleic Acids Res. 1990 Nov 11;18(21):6463–6463. doi: 10.1093/nar/18.21.6463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kugler W., Kaling M., Ross K., Wagner U., Ryffel G. U. BAP, a rat liver protein that activates transcription through a promoter element with similarity to the USF/MLTF binding site. Nucleic Acids Res. 1990 Dec 11;18(23):6943–6951. doi: 10.1093/nar/18.23.6943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kugler W., Wagner U., Ryffel G. U. Tissue-specificity of liver gene expression: a common liver-specific promoter element. Nucleic Acids Res. 1988 Apr 25;16(8):3165–3174. doi: 10.1093/nar/16.8.3165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo C. J., Conley P. B., Chen L., Sladek F. M., Darnell J. E., Jr, Crabtree G. R. A transcriptional hierarchy involved in mammalian cell-type specification. Nature. 1992 Jan 30;355(6359):457–461. doi: 10.1038/355457a0. [DOI] [PubMed] [Google Scholar]
- Kuo C. J., Conley P. B., Hsieh C. L., Francke U., Crabtree G. R. Molecular cloning, functional expression, and chromosomal localization of mouse hepatocyte nuclear factor 1. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9838–9842. doi: 10.1073/pnas.87.24.9838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai E., Darnell J. E., Jr Transcriptional control in hepatocytes: a window on development. Trends Biochem Sci. 1991 Nov;16(11):427–430. doi: 10.1016/0968-0004(91)90169-v. [DOI] [PubMed] [Google Scholar]
- Lazzaro D., De Simone V., De Magistris L., Lehtonen E., Cortese R. LFB1 and LFB3 homeoproteins are sequentially expressed during kidney development. Development. 1992 Feb;114(2):469–479. doi: 10.1242/dev.114.2.469. [DOI] [PubMed] [Google Scholar]
- Lichtsteiner S., Schibler U. A glycosylated liver-specific transcription factor stimulates transcription of the albumin gene. Cell. 1989 Jun 30;57(7):1179–1187. doi: 10.1016/0092-8674(89)90055-x. [DOI] [PubMed] [Google Scholar]
- Melton D. A. Pattern formation during animal development. Science. 1991 Apr 12;252(5003):234–241. doi: 10.1126/science.1672778. [DOI] [PubMed] [Google Scholar]
- Mendel D. B., Hansen L. P., Graves M. K., Conley P. B., Crabtree G. R. HNF-1 alpha and HNF-1 beta (vHNF-1) share dimerization and homeo domains, but not activation domains, and form heterodimers in vitro. Genes Dev. 1991 Jun;5(6):1042–1056. doi: 10.1101/gad.5.6.1042. [DOI] [PubMed] [Google Scholar]
- Nicosia A., Monaci P., Tomei L., De Francesco R., Nuzzo M., Stunnenberg H., Cortese R. A myosin-like dimerization helix and an extra-large homeodomain are essential elements of the tripartite DNA binding structure of LFB1. Cell. 1990 Jun 29;61(7):1225–1236. doi: 10.1016/0092-8674(90)90687-a. [DOI] [PubMed] [Google Scholar]
- Nielsen D. A., Shapiro D. J. Preparation of capped RNA transcripts using T7 RNA polymerase. Nucleic Acids Res. 1986 Jul 25;14(14):5936–5936. doi: 10.1093/nar/14.14.5936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastore A., De Francesco R., Barbato G., Castiglione Morelli M. A., Motta A., Cortese R. 1H resonance assignment and secondary structure determination of the dimerization domain of transcription factor LFB1. Biochemistry. 1991 Jan 8;30(1):148–153. doi: 10.1021/bi00215a022. [DOI] [PubMed] [Google Scholar]
- Raney A. K., Easton A. J., Milich D. R., McLachlan A. Promoter-specific transactivation of hepatitis B virus transcription by a glutamine- and proline-rich domain of hepatocyte nuclear factor 1. J Virol. 1991 Nov;65(11):5774–5781. doi: 10.1128/jvi.65.11.5774-5781.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rey-Campos J., Chouard T., Yaniv M., Cereghini S. vHNF1 is a homeoprotein that activates transcription and forms heterodimers with HNF1. EMBO J. 1991 Jun;10(6):1445–1457. doi: 10.1002/j.1460-2075.1991.tb07665.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupp R. A., Weintraub H. Ubiquitous MyoD transcription at the midblastula transition precedes induction-dependent MyoD expression in presumptive mesoderm of X. laevis. Cell. 1991 Jun 14;65(6):927–937. doi: 10.1016/0092-8674(91)90545-a. [DOI] [PubMed] [Google Scholar]
- Ryffel G. U., Kugler W., Wagner U., Kaling M. Liver cell specific gene transcription in vitro: the promoter elements HP1 and TATA box are necessary and sufficient to generate a liver-specific promoter. Nucleic Acids Res. 1989 Feb 11;17(3):939–953. doi: 10.1093/nar/17.3.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schorpp M., Kugler W., Wagner U., Ryffel G. U. Hepatocyte-specific promoter element HP1 of the Xenopus albumin gene interacts with transcriptional factors of mammalian hepatocytes. J Mol Biol. 1988 Jul 20;202(2):307–320. doi: 10.1016/0022-2836(88)90460-3. [DOI] [PubMed] [Google Scholar]
- Slack J. M. Regional biosynthetic markers in the early amphibian embryo. J Embryol Exp Morphol. 1984 Apr;80:289–319. [PubMed] [Google Scholar]
- Taylor M. V., Gurdon J. B., Hopwood N. D., Towers N., Mohun T. J. Xenopus embryos contain a somite-specific, MyoD-like protein that binds to a promoter site required for muscle actin expression. Genes Dev. 1991 Jul;5(7):1149–1160. doi: 10.1101/gad.5.7.1149. [DOI] [PubMed] [Google Scholar]
- Toniatti C., Demartis A., Monaci P., Nicosia A., Ciliberto G. Synergistic trans-activation of the human C-reactive protein promoter by transcription factor HNF-1 binding at two distinct sites. EMBO J. 1990 Dec;9(13):4467–4475. doi: 10.1002/j.1460-2075.1990.tb07897.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsutsumi K., Ito K., Ishikawa K. Developmental appearance of transcription factors that regulate liver-specific expression of the aldolase B gene. Mol Cell Biol. 1989 Nov;9(11):4923–4931. doi: 10.1128/mcb.9.11.4923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahli W., Dawid I. B., Wyler T., Jaggi R. B., Weber R., Ryffel G. U. Vitellogenin in Xenopus laevis is encoded in a small family of genes. Cell. 1979 Mar;16(3):535–549. doi: 10.1016/0092-8674(79)90028-x. [DOI] [PubMed] [Google Scholar]
- Westley B., Wyler T., Ryffel G., Weber R. Xenopus laevis serum albumins are encoded in two closely related genes. Nucleic Acids Res. 1981 Aug 11;9(15):3557–3574. doi: 10.1093/nar/9.15.3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xanthopoulos K. G., Prezioso V. R., Chen W. S., Sladek F. M., Cortese R., Darnell J. E., Jr The different tissue transcription patterns of genes for HNF-1, C/EBP, HNF-3, and HNF-4, protein factors that govern liver-specific transcription. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3807–3811. doi: 10.1073/pnas.88.9.3807. [DOI] [PMC free article] [PubMed] [Google Scholar]