Abstract
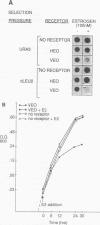
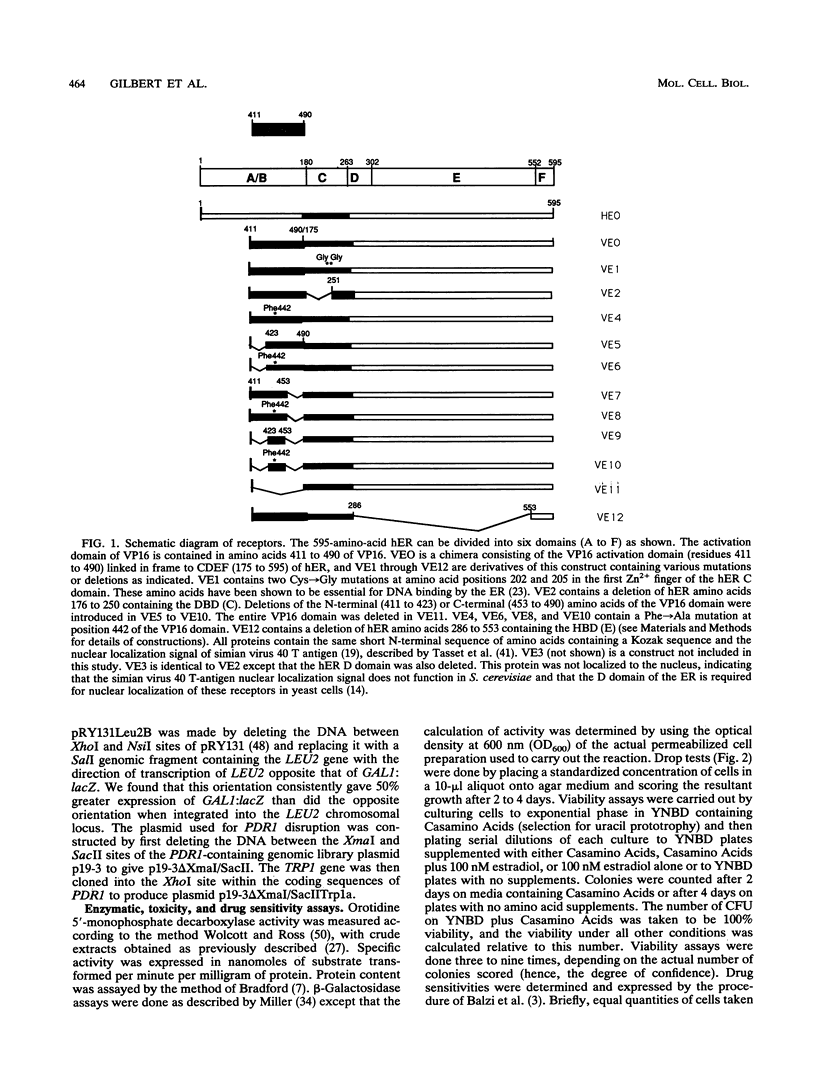
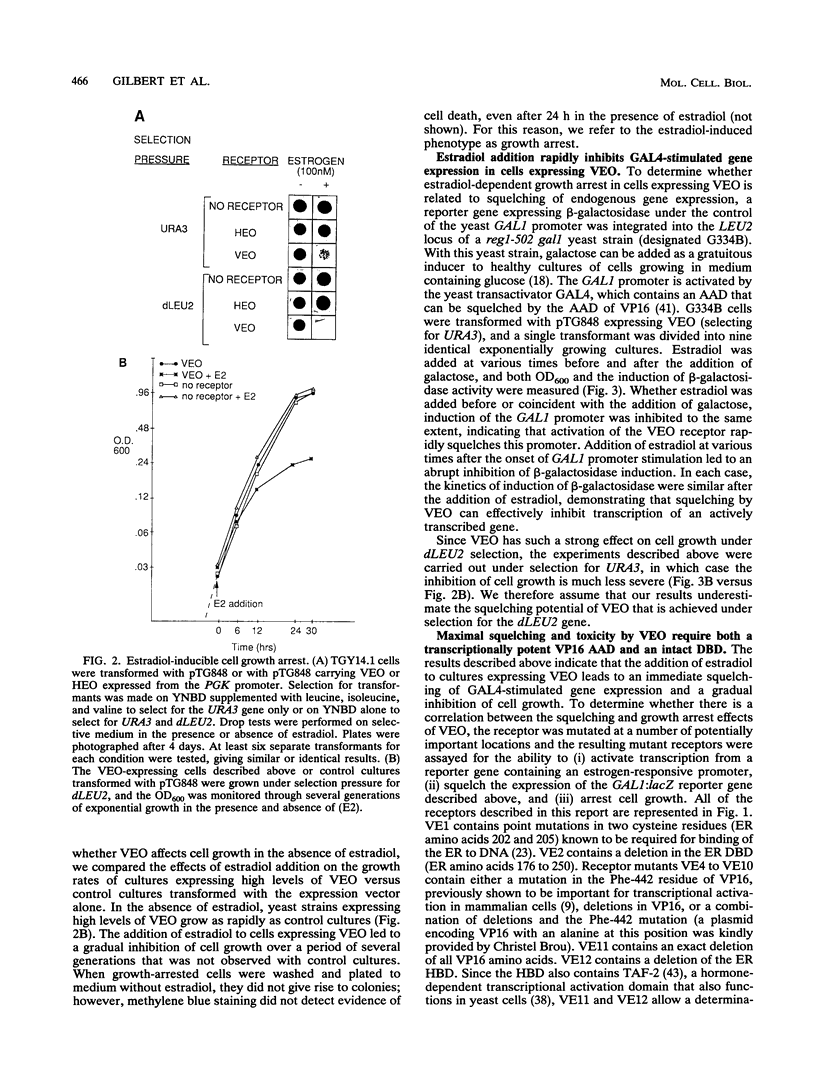
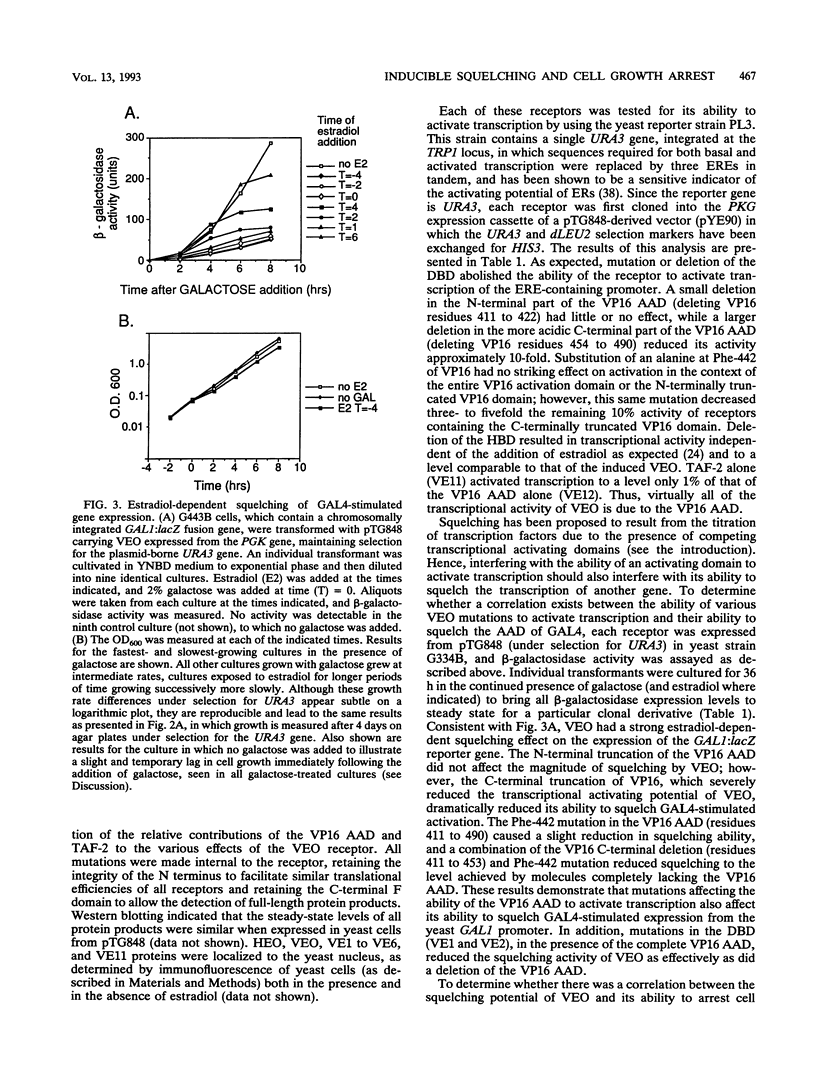
We have constructed and characterized a flexible system for analyzing the phenomenon of squelching and estrogen receptor function in the yeast Saccharomyces cerevisiae. The A/B region of the human estrogen receptor was replaced with the transcriptional activating domain of VP16 and expressed in yeast cells from high-copy-number plasmids. Addition of hormone resulted in an immediate inhibition of expression (squelching) of a chromosomally integrated GAL1:lacZ reporter gene and the eventual arrest of cell growth (toxicity). In order to determine whether a relationship exists between toxicity and squelching, mutations were made in this chimeric receptor (VEO) and their effects on transcriptional activation, squelching, and toxicity were compared. A direct correlation was found between mutations in VEO that reduced VP16 transactivation ability in yeast cells and those that reduced both squelching and toxicity. Surprisingly, mutations in the DNA binding domain (DBD) of VEO dramatically reduced squelching and completely relieved toxicity, suggesting a role for the DBD in squelching and strengthening the correlation between squelching and toxicity. To demonstrate the utility of this system for carrying out genetic selection, a plasmid-based yeast genomic bank was screened for genes that can relieve the toxicity of VEO by means of an elevated copy number, resulting in the repeated cloning of an allele of the PDR1 (pleiotropic drug resistance) gene. We present evidence that mutations in PDR1 can modulate the intracellular availability of estradiol by the same mechanism that leads to multiple drug resistance in yeast cells. Taken together, our results provide evidence that cell growth arrest occurs when squelching exceeds a certain threshold and that strong squelching requires both a DBD and a transcriptional activating domain. Furthermore, we show that growth arrest can provide a useful phenotype for carrying out the genetic analysis of both squelching and estrogen receptor function in yeast cells.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bach M. L., Lacroute F., Botstein D. Evidence for transcriptional regulation of orotidine-5'-phosphate decarboxylase in yeast by hybridization of mRNA to the yeast structural gene cloned in Escherichia coli. Proc Natl Acad Sci U S A. 1979 Jan;76(1):386–390. doi: 10.1073/pnas.76.1.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balzi E., Chen W., Ulaszewski S., Capieaux E., Goffeau A. The multidrug resistance gene PDR1 from Saccharomyces cerevisiae. J Biol Chem. 1987 Dec 15;262(35):16871–16879. [PubMed] [Google Scholar]
- Balzi E., Goffeau A. Multiple or pleiotropic drug resistance in yeast. Biochim Biophys Acta. 1991 Mar 4;1073(2):241–252. doi: 10.1016/0304-4165(91)90128-4. [DOI] [PubMed] [Google Scholar]
- Berger S. L., Cress W. D., Cress A., Triezenberg S. J., Guarente L. Selective inhibition of activated but not basal transcription by the acidic activation domain of VP16: evidence for transcriptional adaptors. Cell. 1990 Jun 29;61(7):1199–1208. doi: 10.1016/0092-8674(90)90684-7. [DOI] [PubMed] [Google Scholar]
- Berger S. L., Piña B., Silverman N., Marcus G. A., Agapite J., Regier J. L., Triezenberg S. J., Guarente L. Genetic isolation of ADA2: a potential transcriptional adaptor required for function of certain acidic activation domains. Cell. 1992 Jul 24;70(2):251–265. doi: 10.1016/0092-8674(92)90100-q. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chevallier M. R., Bloch J. C., Lacroute F. Transcriptional and translational expression of a chimeric bacterial-yeast plasmid in yeasts. Gene. 1980 Oct;11(1-2):11–19. doi: 10.1016/0378-1119(80)90082-7. [DOI] [PubMed] [Google Scholar]
- Cress W. D., Triezenberg S. J. Critical structural elements of the VP16 transcriptional activation domain. Science. 1991 Jan 4;251(4989):87–90. doi: 10.1126/science.1846049. [DOI] [PubMed] [Google Scholar]
- Dynlacht B. D., Hoey T., Tjian R. Isolation of coactivators associated with the TATA-binding protein that mediate transcriptional activation. Cell. 1991 Aug 9;66(3):563–576. doi: 10.1016/0092-8674(81)90019-2. [DOI] [PubMed] [Google Scholar]
- Eilers M., Picard D., Yamamoto K. R., Bishop J. M. Chimaeras of myc oncoprotein and steroid receptors cause hormone-dependent transformation of cells. Nature. 1989 Jul 6;340(6228):66–68. doi: 10.1038/340066a0. [DOI] [PubMed] [Google Scholar]
- Elliston J. F., Tsai S. Y., O'Malley B. W., Tsai M. J. Superactive estrogen receptors. Potent activators of gene expression. J Biol Chem. 1990 Jul 15;265(20):11517–11521. [PubMed] [Google Scholar]
- Gill G., Ptashne M. Negative effect of the transcriptional activator GAL4. Nature. 1988 Aug 25;334(6184):721–724. doi: 10.1038/334721a0. [DOI] [PubMed] [Google Scholar]
- Green S., Issemann I., Sheer E. A versatile in vivo and in vitro eukaryotic expression vector for protein engineering. Nucleic Acids Res. 1988 Jan 11;16(1):369–369. doi: 10.1093/nar/16.1.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hovland P., Flick J., Johnston M., Sclafani R. A. Galactose as a gratuitous inducer of GAL gene expression in yeasts growing on glucose. Gene. 1989 Nov 15;83(1):57–64. doi: 10.1016/0378-1119(89)90403-4. [DOI] [PubMed] [Google Scholar]
- Kalderon D., Roberts B. L., Richardson W. D., Smith A. E. A short amino acid sequence able to specify nuclear location. Cell. 1984 Dec;39(3 Pt 2):499–509. doi: 10.1016/0092-8674(84)90457-4. [DOI] [PubMed] [Google Scholar]
- Kambadur R., Culotta V., Hamer D. Cloned yeast and mammalian transcription factor TFIID gene products support basal but not activated metallothionein gene transcription. Proc Natl Acad Sci U S A. 1990 Dec;87(23):9168–9172. doi: 10.1073/pnas.87.23.9168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelleher R. J., 3rd, Flanagan P. M., Kornberg R. D. A novel mediator between activator proteins and the RNA polymerase II transcription apparatus. Cell. 1990 Jun 29;61(7):1209–1215. doi: 10.1016/0092-8674(90)90685-8. [DOI] [PubMed] [Google Scholar]
- Kilmartin J. V., Adams A. E. Structural rearrangements of tubulin and actin during the cell cycle of the yeast Saccharomyces. J Cell Biol. 1984 Mar;98(3):922–933. doi: 10.1083/jcb.98.3.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar V., Chambon P. The estrogen receptor binds tightly to its responsive element as a ligand-induced homodimer. Cell. 1988 Oct 7;55(1):145–156. doi: 10.1016/0092-8674(88)90017-7. [DOI] [PubMed] [Google Scholar]
- Kumar V., Green S., Stack G., Berry M., Jin J. R., Chambon P. Functional domains of the human estrogen receptor. Cell. 1987 Dec 24;51(6):941–951. doi: 10.1016/0092-8674(87)90581-2. [DOI] [PubMed] [Google Scholar]
- Lewin B. Commitment and activation at pol II promoters: a tail of protein-protein interactions. Cell. 1990 Jun 29;61(7):1161–1164. doi: 10.1016/0092-8674(90)90675-5. [DOI] [PubMed] [Google Scholar]
- Loison G., Vidal A., Findeli A., Roitsch C., Balloul J. M., Lemoine Y. High level of expression of a protective antigen of schistosomes in Saccharomyces cerevisiae. Yeast. 1989 Nov-Dec;5(6):497–507. doi: 10.1002/yea.320050609. [DOI] [PubMed] [Google Scholar]
- Mak P., McDonnell D. P., Weigel N. L., Schrader W. T., O'Malley B. W. Expression of functional chicken oviduct progesterone receptors in yeast (Saccharomyces cerevisiae). J Biol Chem. 1989 Dec 25;264(36):21613–21618. [PubMed] [Google Scholar]
- Martin K. J., Lillie J. W., Green M. R. Evidence for interaction of different eukaryotic transcriptional activators with distinct cellular targets. Nature. 1990 Jul 12;346(6280):147–152. doi: 10.1038/346147a0. [DOI] [PubMed] [Google Scholar]
- Metzger D., White J. H., Chambon P. The human oestrogen receptor functions in yeast. Nature. 1988 Jul 7;334(6177):31–36. doi: 10.1038/334031a0. [DOI] [PubMed] [Google Scholar]
- Meyer M. E., Gronemeyer H., Turcotte B., Bocquel M. T., Tasset D., Chambon P. Steroid hormone receptors compete for factors that mediate their enhancer function. Cell. 1989 May 5;57(3):433–442. doi: 10.1016/0092-8674(89)90918-5. [DOI] [PubMed] [Google Scholar]
- Murray J. A., Scarpa M., Rossi N., Cesareni G. Antagonistic controls regulate copy number of the yeast 2 mu plasmid. EMBO J. 1987 Dec 20;6(13):4205–4212. doi: 10.1002/j.1460-2075.1987.tb02768.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson M. G., Tanese N., Pugh B. F., Tjian R. Functional domains and upstream activation properties of cloned human TATA binding protein. Science. 1990 Jun 29;248(4963):1625–1630. doi: 10.1126/science.2363050. [DOI] [PubMed] [Google Scholar]
- Picard D., Salser S. J., Yamamoto K. R. A movable and regulable inactivation function within the steroid binding domain of the glucocorticoid receptor. Cell. 1988 Sep 23;54(7):1073–1080. doi: 10.1016/0092-8674(88)90122-5. [DOI] [PubMed] [Google Scholar]
- Pierrat B., Heery D. M., Lemoine Y., Losson R. Functional analysis of the human estrogen receptor using a phenotypic transactivation assay in yeast. Gene. 1992 Oct 1;119(2):237–245. doi: 10.1016/0378-1119(92)90277-v. [DOI] [PubMed] [Google Scholar]
- Pugh B. F., Tjian R. Mechanism of transcriptional activation by Sp1: evidence for coactivators. Cell. 1990 Jun 29;61(7):1187–1197. doi: 10.1016/0092-8674(90)90683-6. [DOI] [PubMed] [Google Scholar]
- Sadowski I., Ma J., Triezenberg S., Ptashne M. GAL4-VP16 is an unusually potent transcriptional activator. Nature. 1988 Oct 6;335(6190):563–564. doi: 10.1038/335563a0. [DOI] [PubMed] [Google Scholar]
- Tasset D., Tora L., Fromental C., Scheer E., Chambon P. Distinct classes of transcriptional activating domains function by different mechanisms. Cell. 1990 Sep 21;62(6):1177–1187. doi: 10.1016/0092-8674(90)90394-t. [DOI] [PubMed] [Google Scholar]
- Thukral S. K., Morrison M. L., Young E. T. Alanine scanning site-directed mutagenesis of the zinc fingers of transcription factor ADR1: residues that contact DNA and that transactivate. Proc Natl Acad Sci U S A. 1991 Oct 15;88(20):9188–9192. doi: 10.1073/pnas.88.20.9188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tora L., White J., Brou C., Tasset D., Webster N., Scheer E., Chambon P. The human estrogen receptor has two independent nonacidic transcriptional activation functions. Cell. 1989 Nov 3;59(3):477–487. doi: 10.1016/0092-8674(89)90031-7. [DOI] [PubMed] [Google Scholar]
- Triezenberg S. J., Kingsbury R. C., McKnight S. L. Functional dissection of VP16, the trans-activator of herpes simplex virus immediate early gene expression. Genes Dev. 1988 Jun;2(6):718–729. doi: 10.1101/gad.2.6.718. [DOI] [PubMed] [Google Scholar]
- Vickers P. J., Dickson R. B., Shoemaker R., Cowan K. H. A multidrug-resistant MCF-7 human breast cancer cell line which exhibits cross-resistance to antiestrogens and hormone-independent tumor growth in vivo. Mol Endocrinol. 1988 Oct;2(10):886–892. doi: 10.1210/mend-2-10-886. [DOI] [PubMed] [Google Scholar]
- Wada T., Nogi Y., Handa H., Fukasawa T. Strain-specific lethal effect of the adenovirus E1a protein on Saccharomyces cerevisiae. Biochem Biophys Res Commun. 1990 Jul 31;170(2):470–476. doi: 10.1016/0006-291x(90)92115-g. [DOI] [PubMed] [Google Scholar]
- Webster L. C., Ricciardi R. P. trans-dominant mutants of E1A provide genetic evidence that the zinc finger of the trans-activating domain binds a transcription factor. Mol Cell Biol. 1991 Sep;11(9):4287–4296. doi: 10.1128/mcb.11.9.4287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West R. W., Jr, Yocum R. R., Ptashne M. Saccharomyces cerevisiae GAL1-GAL10 divergent promoter region: location and function of the upstream activating sequence UASG. Mol Cell Biol. 1984 Nov;4(11):2467–2478. doi: 10.1128/mcb.4.11.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White J. H., Brou C., Wu J., Burton N., Egly J. M., Chambon P. Evidence for a factor required for transcriptional stimulation by the chimeric acidic activator GAL-VP16 in HeLa cell extracts. Proc Natl Acad Sci U S A. 1991 Sep 1;88(17):7674–7678. doi: 10.1073/pnas.88.17.7674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright A. P., McEwan I. J., Dahlman-Wright K., Gustafsson J. A. High level expression of the major transactivation domain of the human glucocorticoid receptor in yeast cells inhibits endogenous gene expression and cell growth. Mol Endocrinol. 1991 Oct;5(10):1366–1372. doi: 10.1210/mend-5-10-1366. [DOI] [PubMed] [Google Scholar]