Abstract
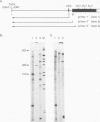
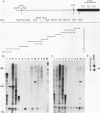
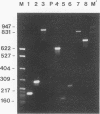
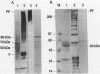
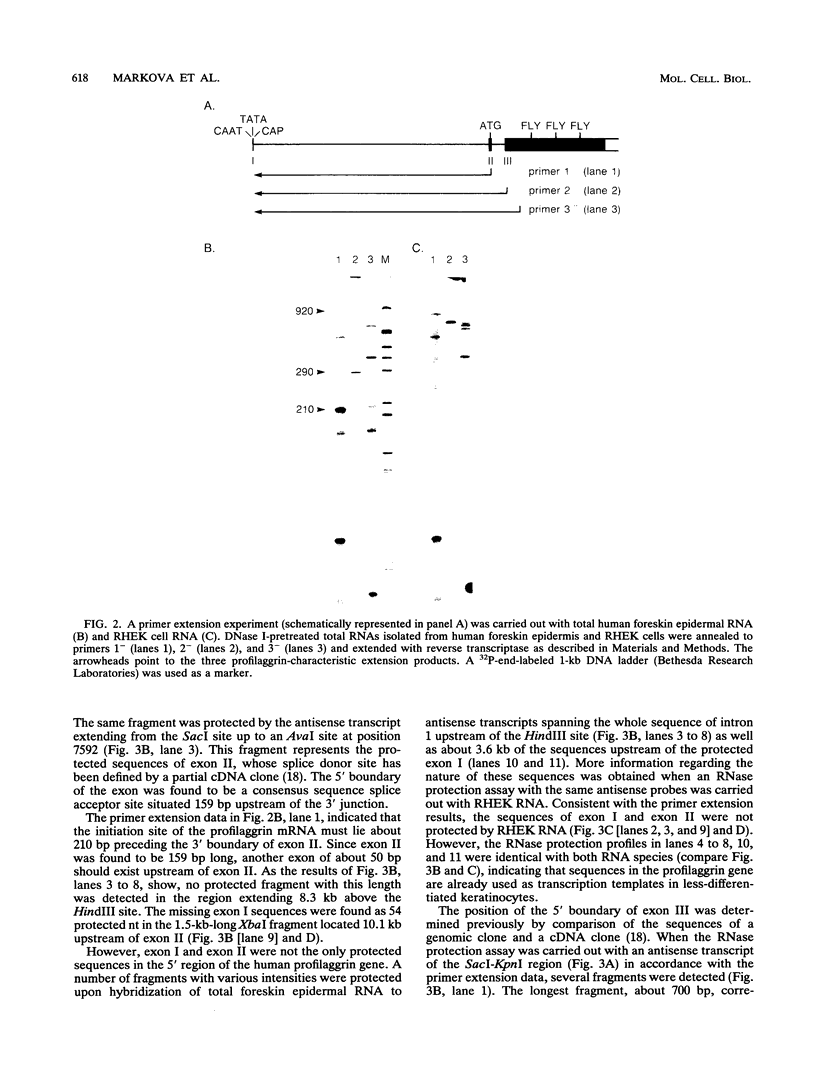
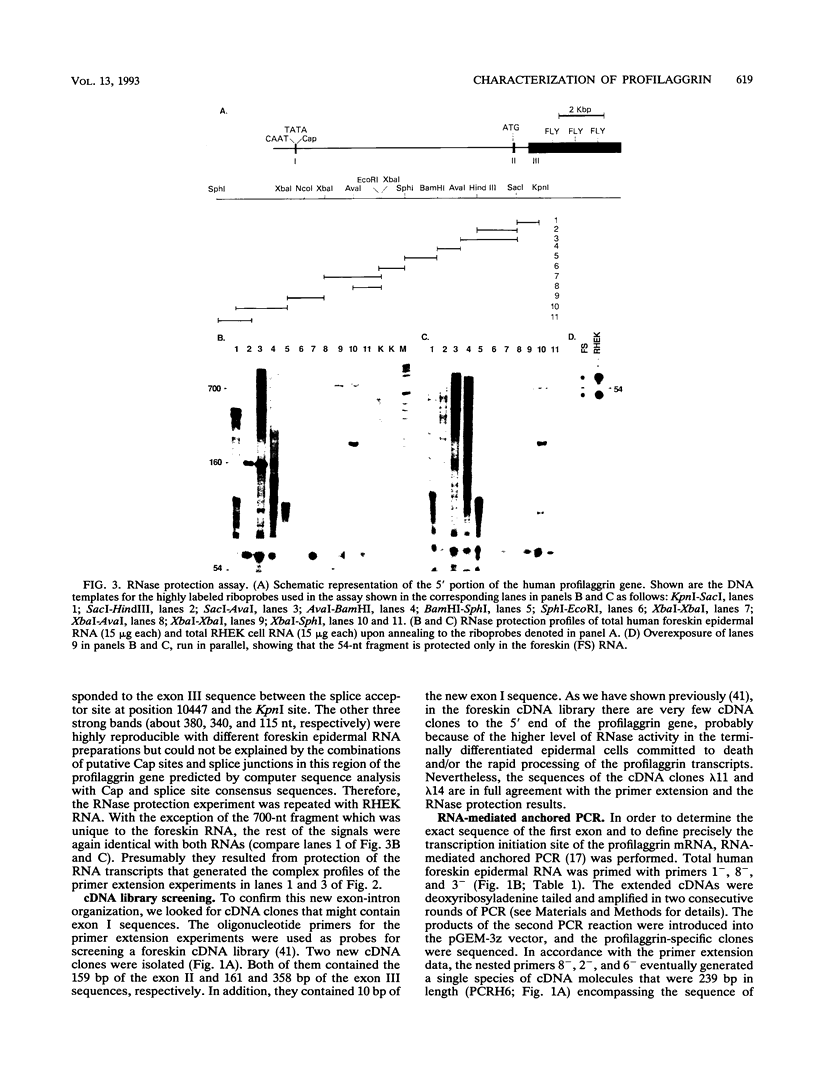
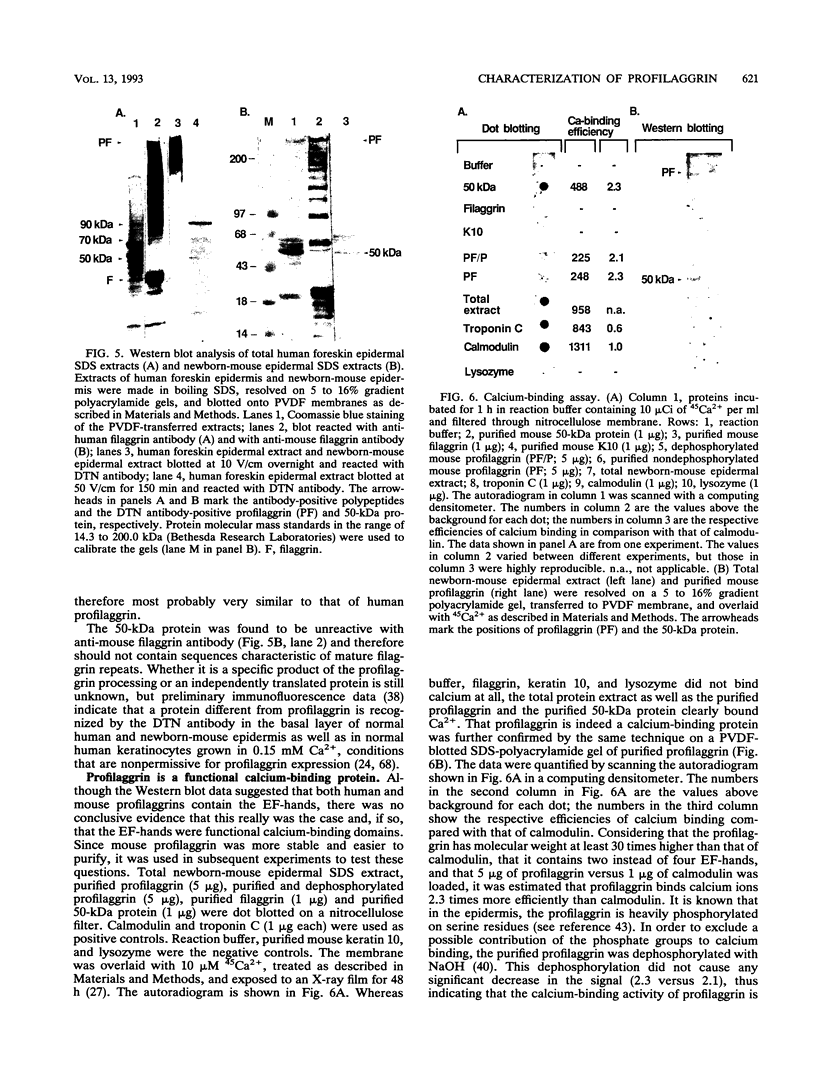
Profilaggrin is a major highly phosphorylated protein component of the keratohyalin granules of mammalian epidermis. It contains 10 to 12 tandemly repeated filaggrin units and is processed into the intermediate filament-associated protein filaggrin by specific dephosphorylation and proteolysis during terminal differentiation of the epidermal cells. Later, filaggrin itself is degraded to free amino acids that participate in maintenance of epidermal flexibility. The present paper describes the structural organization of the 5' region of the human profilaggrin gene as well as the amino terminus of the profilaggrin protein. The primary profilaggrin transcript consists of three exons and two introns. The first exon (exon I) is only 54 bp and is untranslated. The coding sequences are distributed between exon II (159 bp) and exon III, which contains the information for 10 to 12 filaggrin repeats (972 bp each) and the 3' noncoding sequences. A very large intron separates exons I and II. The combination of a very short exon I with an unusually long intron 1 makes the structure of the profilaggrin gene unique among the epidermally expressed genes investigated so far. Comparison of the expression patterns revealed by primer extension and RNase protection analysis of foreskin epidermal and cultured keratinocyte RNAs suggests that alternately spliced messages, which are different from profilaggrin mRNA, are transcribed from the profilaggrin gene system at earlier stages of epidermal differentiation. The amino terminus of profilaggrin exhibits a significant homology to the small calcium-binding S100-like proteins. It contains two alpha-helical regions, termed EF-hands, that bind calcium in vitro. This is the first example of functional calcium-binding domains fused to a structural protein. We suggest that in addition to its role in filament aggregation and the maintenance of epidermal flexibility, profilaggrin may play an important role in the differentiation of the epidermis by autoregulating its own processing in a calcium-dependent manner or by participating in the transduction of calcium signal in epidermal cells.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andresen K., Tom T. D., Strand M. Characterization of cDNA clones encoding a novel calcium-activated neutral proteinase from Schistosoma mansoni. J Biol Chem. 1991 Aug 15;266(23):15085–15090. [PubMed] [Google Scholar]
- Blessing M., Jorcano J. L., Franke W. W. Enhancer elements directing cell-type-specific expression of cytokeratin genes and changes of the epithelial cytoskeleton by transfections of hybrid cytokeratin genes. EMBO J. 1989 Jan;8(1):117–126. doi: 10.1002/j.1460-2075.1989.tb03355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucher P., Trifonov E. N. Compilation and analysis of eukaryotic POL II promoter sequences. Nucleic Acids Res. 1986 Dec 22;14(24):10009–10026. doi: 10.1093/nar/14.24.10009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chodosh L. A., Baldwin A. S., Carthew R. W., Sharp P. A. Human CCAAT-binding proteins have heterologous subunits. Cell. 1988 Apr 8;53(1):11–24. doi: 10.1016/0092-8674(88)90483-7. [DOI] [PubMed] [Google Scholar]
- Dale B. A., Gown A. M., Fleckman P., Kimball J. R., Resing K. A. Characterization of two monoclonal antibodies to human epidermal keratohyalin: reactivity with filaggrin and related proteins. J Invest Dermatol. 1987 Mar;88(3):306–313. doi: 10.1111/1523-1747.ep12466185. [DOI] [PubMed] [Google Scholar]
- Dale B. A., Holbrook K. A., Kimball J. R., Hoff M., Sun T. T. Expression of epidermal keratins and filaggrin during human fetal skin development. J Cell Biol. 1985 Oct;101(4):1257–1269. doi: 10.1083/jcb.101.4.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale B. A., Holbrook K. A., Steinert P. M. Assembly of stratum corneum basic protein and keratin filaments in macrofibrils. Nature. 1978 Dec 14;276(5689):729–731. doi: 10.1038/276729a0. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorin J. R., Emslie E., van Heyningen V. Related calcium-binding proteins map to the same subregion of chromosome 1q and to an extended region of synteny on mouse chromosome 3. Genomics. 1990 Nov;8(3):420–426. doi: 10.1016/0888-7543(90)90027-r. [DOI] [PubMed] [Google Scholar]
- Dorin J. R., Novak M., Hill R. E., Brock D. J., Secher D. S., van Heyningen V. A clue to the basic defect in cystic fibrosis from cloning the CF antigen gene. Nature. 1987 Apr 9;326(6113):614–617. doi: 10.1038/326614a0. [DOI] [PubMed] [Google Scholar]
- Eckert R. L., Green H. Structure and evolution of the human involucrin gene. Cell. 1986 Aug 15;46(4):583–589. doi: 10.1016/0092-8674(86)90884-6. [DOI] [PubMed] [Google Scholar]
- Ferrari S., Calabretta B., deRiel J. K., Battini R., Ghezzo F., Lauret E., Griffin C., Emanuel B. S., Gurrieri F., Baserga R. Structural and functional analysis of a growth-regulated gene, the human calcyclin. J Biol Chem. 1987 Jun 15;262(17):8325–8332. [PubMed] [Google Scholar]
- Gan S. Q., McBride O. W., Idler W. W., Markova N., Steinert P. M. Organization, structure, and polymorphisms of the human profilaggrin gene. Biochemistry. 1990 Oct 9;29(40):9432–9440. doi: 10.1021/bi00492a018. [DOI] [PubMed] [Google Scholar]
- Gerke V., Weber K. The regulatory chain in the p36-kd substrate complex of viral tyrosine-specific protein kinases is related in sequence to the S-100 protein of glial cells. EMBO J. 1985 Nov;4(11):2917–2920. doi: 10.1002/j.1460-2075.1985.tb04023.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton E. H., Payne R. E., Jr, O'Keefe E. J. Trichohyalin: presence in the granular layer and stratum corneum of normal human epidermis. J Invest Dermatol. 1991 May;96(5):666–672. doi: 10.1111/1523-1747.ep12470590. [DOI] [PubMed] [Google Scholar]
- Harding C. R., Scott I. R. Histidine-rich proteins (filaggrins): structural and functional heterogeneity during epidermal differentiation. J Mol Biol. 1983 Nov 5;170(3):651–673. doi: 10.1016/s0022-2836(83)80126-0. [DOI] [PubMed] [Google Scholar]
- Harper J. F., Sussman M. R., Schaller G. E., Putnam-Evans C., Charbonneau H., Harmon A. C. A calcium-dependent protein kinase with a regulatory domain similar to calmodulin. Science. 1991 May 17;252(5008):951–954. doi: 10.1126/science.1852075. [DOI] [PubMed] [Google Scholar]
- Hohl D., Lichti U., Breitkreutz D., Steinert P. M., Roop D. R. Transcription of the human loricrin gene in vitro is induced by calcium and cell density and suppressed by retinoic acid. J Invest Dermatol. 1991 Apr;96(4):414–418. doi: 10.1111/1523-1747.ep12469779. [DOI] [PubMed] [Google Scholar]
- Hohl D., Mehrel T., Lichti U., Turner M. L., Roop D. R., Steinert P. M. Characterization of human loricrin. Structure and function of a new class of epidermal cell envelope proteins. J Biol Chem. 1991 Apr 5;266(10):6626–6636. [PubMed] [Google Scholar]
- Jensen R., Marshak D. R., Anderson C., Lukas T. J., Watterson D. M. Characterization of human brain S100 protein fraction: amino acid sequence of S100 beta. J Neurochem. 1985 Sep;45(3):700–705. doi: 10.1111/j.1471-4159.1985.tb04048.x. [DOI] [PubMed] [Google Scholar]
- Kawasaki H., Kasai H., Okuyama T. Protein analyses and reagents: microscale assay of calcium-binding activity of proteins and peptides using a nitrocellulose membrane. Anal Biochem. 1985 Aug 1;148(2):297–302. doi: 10.1016/0003-2697(85)90232-5. [DOI] [PubMed] [Google Scholar]
- Kligman D., Hilt D. C. The S100 protein family. Trends Biochem Sci. 1988 Nov;13(11):437–443. doi: 10.1016/0968-0004(88)90218-6. [DOI] [PubMed] [Google Scholar]
- Kretsinger R. H. Structure and evolution of calcium-modulated proteins. CRC Crit Rev Biochem. 1980;8(2):119–174. doi: 10.3109/10409238009105467. [DOI] [PubMed] [Google Scholar]
- Krisinger J., Darwish H., Maeda N., DeLuca H. F. Structure and nucleotide sequence of the rat intestinal vitamin D-dependent calcium binding protein gene. Proc Natl Acad Sci U S A. 1988 Dec;85(23):8988–8992. doi: 10.1073/pnas.85.23.8988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lagasse E., Clerc R. G. Cloning and expression of two human genes encoding calcium-binding proteins that are regulated during myeloid differentiation. Mol Cell Biol. 1988 Jun;8(6):2402–2410. doi: 10.1128/mcb.8.6.2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leask A., Byrne C., Fuchs E. Transcription factor AP2 and its role in epidermal-specific gene expression. Proc Natl Acad Sci U S A. 1991 Sep 15;88(18):7948–7952. doi: 10.1073/pnas.88.18.7948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leask A., Rosenberg M., Vassar R., Fuchs E. Regulation of a human epidermal keratin gene: sequences and nuclear factors involved in keratinocyte-specific transcription. Genes Dev. 1990 Nov;4(11):1985–1998. doi: 10.1101/gad.4.11.1985. [DOI] [PubMed] [Google Scholar]
- Lonsdale-Eccles J. D., Haugen J. A., Dale B. A. A phosphorylated keratohyalin-derived precursor of epidermal stratum corneum basic protein. J Biol Chem. 1980 Mar 25;255(6):2235–2238. [PubMed] [Google Scholar]
- Martensen T. M. Chemical properties, isolation, and analysis of O-phosphates in proteins. Methods Enzymol. 1984;107:3–23. doi: 10.1016/0076-6879(84)07003-8. [DOI] [PubMed] [Google Scholar]
- McKinley-Grant L. J., Idler W. W., Bernstein I. A., Parry D. A., Cannizzaro L., Croce C. M., Huebner K., Lessin S. R., Steinert P. M. Characterization of a cDNA clone encoding human filaggrin and localization of the gene to chromosome region 1q21. Proc Natl Acad Sci U S A. 1989 Jul;86(13):4848–4852. doi: 10.1073/pnas.86.13.4848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontremoli S., Melloni E. Extralysosomal protein degradation. Annu Rev Biochem. 1986;55:455–481. doi: 10.1146/annurev.bi.55.070186.002323. [DOI] [PubMed] [Google Scholar]
- Resing K. A., Dale B. A., Walsh K. A. Multiple copies of phosphorylated filaggrin in epidermal profilaggrin demonstrated by analysis of tryptic peptides. Biochemistry. 1985 Jul 16;24(15):4167–4175. doi: 10.1021/bi00336a053. [DOI] [PubMed] [Google Scholar]
- Resing K. A., Walsh K. A., Dale B. A. Identification of two intermediates during processing of profilaggrin to filaggrin in neonatal mouse epidermis. J Cell Biol. 1984 Oct;99(4 Pt 1):1372–1378. doi: 10.1083/jcb.99.4.1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhim J. S., Jay G., Arnstein P., Price F. M., Sanford K. K., Aaronson S. A. Neoplastic transformation of human epidermal keratinocytes by AD12-SV40 and Kirsten sarcoma viruses. Science. 1985 Mar 8;227(4691):1250–1252. doi: 10.1126/science.2579430. [DOI] [PubMed] [Google Scholar]
- Risse G., Jooss K., Neuberg M., Brüller H. J., Müller R. Asymmetrical recognition of the palindromic AP1 binding site (TRE) by Fos protein complexes. EMBO J. 1989 Dec 1;8(12):3825–3832. doi: 10.1002/j.1460-2075.1989.tb08560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roeder R. G. The complexities of eukaryotic transcription initiation: regulation of preinitiation complex assembly. Trends Biochem Sci. 1991 Nov;16(11):402–408. doi: 10.1016/0968-0004(91)90164-q. [DOI] [PubMed] [Google Scholar]
- Rogers G. E., Fietz M. J., Fratini A. Trichohyalin and matrix proteins. Ann N Y Acad Sci. 1991 Dec 26;642:64–81. [PubMed] [Google Scholar]
- Rothnagel J. A., Steinert P. M. The structure of the gene for mouse filaggrin and a comparison of the repeating units. J Biol Chem. 1990 Feb 5;265(4):1862–1865. [PubMed] [Google Scholar]
- Saris C. J., Kristensen T., D'Eustachio P., Hicks L. J., Noonan D. J., Hunter T., Tack B. F. cDNA sequence and tissue distribution of the mRNA for bovine and murine p11, the S100-related light chain of the protein-tyrosine kinase substrate p36 (calpactin I). J Biol Chem. 1987 Aug 5;262(22):10663–10671. [PubMed] [Google Scholar]
- Scott I. R., Harding C. R. Filaggrin breakdown to water binding compounds during development of the rat stratum corneum is controlled by the water activity of the environment. Dev Biol. 1986 May;115(1):84–92. doi: 10.1016/0012-1606(86)90230-7. [DOI] [PubMed] [Google Scholar]
- Scott I. R., Harding C. R. Studies on the synthesis and degradation of a high molecular weight, histidine-rich phosphoprotein from mammalian epidermis. Biochim Biophys Acta. 1981 Jun 29;669(1):65–78. doi: 10.1016/0005-2795(81)90224-5. [DOI] [PubMed] [Google Scholar]
- Steinert P. M., Cantieri J. S., Teller D. C., Lonsdale-Eccles J. D., Dale B. A. Characterization of a class of cationic proteins that specifically interact with intermediate filaments. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4097–4101. doi: 10.1073/pnas.78.7.4097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinert P. M., Roop D. R. Molecular and cellular biology of intermediate filaments. Annu Rev Biochem. 1988;57:593–625. doi: 10.1146/annurev.bi.57.070188.003113. [DOI] [PubMed] [Google Scholar]
- Steinert P., Zackroff R., Aynardi-Whitman M., Goldman R. D. Isolation and characterization of intermediate filaments. Methods Cell Biol. 1982;24:399–419. doi: 10.1016/s0091-679x(08)60667-6. [DOI] [PubMed] [Google Scholar]
- Steven A. C., Bisher M. E., Roop D. R., Steinert P. M. Biosynthetic pathways of filaggrin and loricrin--two major proteins expressed by terminally differentiated epidermal keratinocytes. J Struct Biol. 1990 Jul-Sep;104(1-3):150–162. doi: 10.1016/1047-8477(90)90071-j. [DOI] [PubMed] [Google Scholar]
- Strynadka N. C., James M. N. Crystal structures of the helix-loop-helix calcium-binding proteins. Annu Rev Biochem. 1989;58:951–998. doi: 10.1146/annurev.bi.58.070189.004511. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Eldik L. J., Zendegui J. G., Marshak D. R., Watterson D. M. Calcium-binding proteins and the molecular basis of calcium action. Int Rev Cytol. 1982;77:1–61. doi: 10.1016/s0074-7696(08)62463-8. [DOI] [PubMed] [Google Scholar]
- Williams M. L., Elias P. M. Genetically transmitted, generalized disorders of cornification. The ichthyoses. Dermatol Clin. 1987 Jan;5(1):155–178. [PubMed] [Google Scholar]
- Wood L., Carter D., Mills M., Hatzenbuhler N., Vogeli G. Expression of calcyclin, a calcium-binding protein, in the keratogenous region of growing hair follicles. J Invest Dermatol. 1991 Mar;96(3):383–387. doi: 10.1111/1523-1747.ep12466230. [DOI] [PubMed] [Google Scholar]
- Yoneda K., Hohl D., McBride O. W., Wang M., Cehrs K. U., Idler W. W., Steinert P. M. The human loricrin gene. J Biol Chem. 1992 Sep 5;267(25):18060–18066. [PubMed] [Google Scholar]
- Yuspa S. H., Kilkenny A. E., Steinert P. M., Roop D. R. Expression of murine epidermal differentiation markers is tightly regulated by restricted extracellular calcium concentrations in vitro. J Cell Biol. 1989 Sep;109(3):1207–1217. doi: 10.1083/jcb.109.3.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]